SUMMARY
Host antibacterial responses include mechanisms that kill bacteria, but also those that protect or tolerize the host to potentially damaging antibacterial effects. We determined that Chitinase 3-like-1 (Chi3l1), a conserved prototypic chitinase-like protein, is induced by Streptococcus pneumoniae and plays central roles in promoting bacterial clearance and mediating host tolerance. S. pneumoniae-infected Chi3l1 null mice exhibit exaggerated lung injury, inflammation and hemorrhage, more frequent bacterial dissemination, decreased bacterial clearance, and enhanced mortality compared to controls. Chi3l1 augments macrophage bacterial killing by inhibiting caspase-1-dependent macrophage pyroptosis and augments host tolerance by controlling inflammasome activation, ATP accumulation, expression of ATP receptor P2×7R, and production of thymic stromal lymphopoietin and type 1, type 2, and type 17 cytokines. These data demonstrate that Chi3l1 is induced during infection, where it promotes bacterial clearance while simultaneously augmenting host tolerance, and that these roles likely contributed to the retention of Chi3l1 over species and evolutionary time.
INTRODUCTION
The immune system has evolved to allow a host to control and eliminate pathogens. It has long been assumed that the majority of a host’s antipathogen defense strategy involves “resistance” mechanisms that attack the pathogen to block invasion and kill and eliminate the offending organism (Schneider and Ayres, 2008). However, there is mounting evidence that the antipathogen response can also cause critical and even fatal tissue injury. This is particularly problematic in dysregulated and/or exaggerated innate immune responses, which because of their nonspecific nature are well known to induce immunopathology (Gonçalves et al., 2001; Heimesaat et al., 2006; Peiris et al., 2010; Snelgrove et al., 2011). In keeping with this concept, there is an evolving appreciation that the host can also respond to pathogens with responses that limit this host-induced bystander damage (Gatton and Cheng, 2002; Ma et al., 1998; Schneider and Ayres, 2008; Sinton, 1938). The mechanisms that regulate the self-harm that can be caused by a host antipathogen immune response have been referred to as mechanisms of host tolerance (different than immune tolerance), and the sum of a host’s resistance and host tolerance can be thought of as a host’s defensive capacity (Gatton and Cheng, 2002; Ma et al., 1998; Schneider and Ayres, 2008). Although a great deal is known about the molecular mechanisms that are used to kill and clear pathogens, a systematic understanding of how a host regulates the production, repair, and avoidance of the tissue damage that accumulates during an infection is limited (Schneider and Ayres, 2008). In particular, mechanisms that augment bacterial killing and clearance while controlling innate immune response-induced tissue injury have not been appropriately defined.
The glycosyl hydrolase 18 proteins (GH18) are members of an ancient gene family and exist in species as diverse as plants, insects, and man (Aerts et al., 2008; Funkhouser and Aronson, 2007). This gene family contains true chitinases (Cs), which degrade chitin polysaccharides and chitinase-like proteins (CLPs), which bind to but do not degrade chitin. Interestingly, these GH18 moieties have evolved during speciation, with a particularly impressive increase in CLP coinciding with the appearance of mammals (Funkhouser and Aronson, 2007). This retention over species and evolutionary time has led to the belief that these moieties play an essential role(s) in biology. However, the role(s) that have allowed for this conservation and evolution have not been defined, and the biology of CLPs in normal homeostasis and disease pathogenesis is poorly understood.
Chitinase 3-like-1 (Chi3l1, also called breast regression protein 39 [BRP-39] in the mouse and YKL-40 in man) is the prototypic mammalian CLP. It is expressed by a variety of cells, including macrophages, neutrophils, and epithelial cells in the lung and digestive tract (Homer et al., 2006; Lee and Elias, 2010; Mizoguchi, 2006). It is also highly regulated, being stimulated by a number of mediators including IL-13 and IFN-γ and being detected in exaggerated quantities in the circulation and or biologic fluids from patients with a spectrum of diseases including asthma, COPD, rheumatoid arthritis, cancer, diabetes, and atherosclerosis (Chupp et al., 2007; Lee et al., 2011; Matsuura et al., 2011). YKL-40/Chi3l1 is elevated in the serum of patients with community-acquired pneumonia requiring hospitalizations (Nordenbaek et al., 1999), patients with cystic fibrosis and acute lung infections (Aris et al., 2000), and control subjects following endotoxin injection (Johansen et al., 2005). Elevated levels of Chi3l1 are especially prominent in patients with Streptococcus pneumoniae (Sp) infections where they correlate positively with mortality (Kronborg et al., 2002; Østergaard et al., 2002). In combination these studies suggest that elevated levels of Chi3l1 can be an indicator of and a diagnostic and prognostic marker of specific bacterial infections (Coffman, 2008). However, the role(s) of Chi3l1 in the pathogenesis of antipathogen responses have not been defined.
We hypothesized that Chi3l1 has been retained over species and time because it plays a major role in antimicrobial responses. Specifically, we hypothesized that Chi3l1 augments bacterial resistance and contributes to the ability of the host to tolerate antibacterial responses. To test this hypothesis, we evaluated the responses that were induced by Sp, the most common cause of acute bacterial pneumonia in the USA (Lynch and Zhanel, 2009), in wild-type (WT) and Chi3l1−/− mice. These studies demonstrate that Chi3l1 plays a critical role in anti-Sp responses, with Chi3l1 null mice manifesting exaggerated lung injury, inflammation, and hemorrhage, more frequent systemic bacterial dissemination, decreased bacterial clearance, and enhanced mortality compared to WT controls. They also demonstrate that these effects are due to the ability of Chi3l1 to (1) increase bacterial resistance by augmenting macrophage bacterial killing while inhibiting a caspases-1-dependent pyroptosis-like macrophage cell death response and (2) augment host tolerance by controlling Sp-induced inflammasome activation and regulating type 1, type 2, and type 17 immune responses.
RESULTS
S. pneumoniae Stimulates Chi3l1
To determine if Chi3l1 is regulated during bacterial infection, we established a mouse pneumonia model using intratracheal Sp. Uninfected wild-type (WT) C57BL/6 and heterozygous Chi3l1+/− mice had baseline levels of bronchoalveolar lavage (BAL) and serum Chi3l1 in the 50 ng/ml range (Figures 1A and 1B). Infection of WT mice markedly increased the levels of Chi3l1 with levels of BAL and serum Chi3l1 in the 3–4 μg/ml range and 150 ng/ml range, respectively (Figures 1A and 1B). Immunohistochemistry (IHC) demonstrated the enhanced Chi3l1 accumulation in airway epithelial cells and recruited airway inflammatory cells (macrophages and neutrophils) in the lungs of infected mice (Figure 1C). Double labeling IHC demonstrated that this induction was prominent in CD68+ macrophages and CC10+ airway epithelial cells in infected lungs (Figures 1D and 1E). This induction was not specific for alveolar macrophages because Sp also stimulated peritoneal macrophage production of Chi3l1 in a dose- and time-dependent manner (Figures 1F and 1G). Thus, Chi3l1 is prominently induced after Sp infection.
Figure 1. Chi3l1 Is Induced During Streptococcus pneumoniae Infection.
(A–C) WT, Chi3l1+/−, and Chi3l1−/− mice were infected with 105 cfu/mouse of Sp intratracheally or control (−). The levels of Chi3l1 protein were analyzed by ELISA in the bronchoalveolar lavage fluid (BAL) (A) and serum (B). Immunohistochemistry was used to localize Chi3l1 (C, closed arrows depict macrophages and open arrows, airway epithelial cells).
(D and E) Double-label immunohistochemistry with cell-specific antibodies (CD68, macrophages; CC10, airway epithelial cells). These are representative composites of five similar evaluations. Arrows depict double-labeled cells.
(F) Peritoneal macrophages were infected with Sp at 2.5 × 104 cfu/well and incubated for 0, 1, 6, or 12 hr. Chi3l1 was evaluated by ELISA.
(G) Peritoneal macrophages were infected with 0, 104, 2.5 × 104, or 105 cfu/well of bacteria, supernatants were collected 6 hr later, and Chi3l1 was measured by ELISA. The values in (A), (B), (F), and (G) are the mean (±SEM) of evaluations of a minimum of eight mice and are representative of three experiments. Scale bar, 50 μm. n.d., none detected. *p < 0.05, **p < 0.01, ***p < 0.001.
Chi3l1 Is a Critical Regulator of Acute Pneumococcal Pneumonia
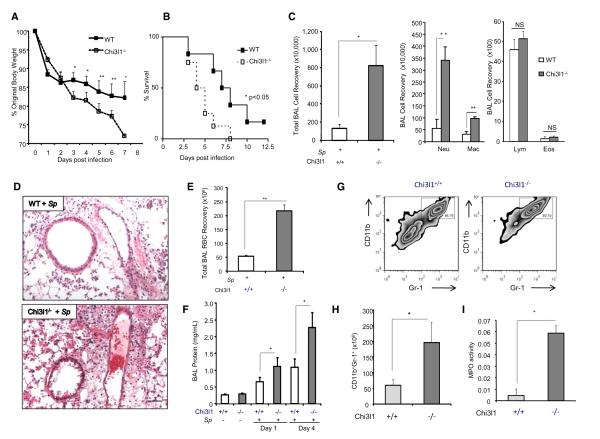
WT and Chi3l1−/− mice were inoculated with 5 × 105 cfu Sp, and animals were monitored over time. Compared to infected WT mice, infected Chi3l1−/− mice manifest enhanced toxicity with significantly greater body weight loss and augmented mortality (Figures 2A and 2B). As regards to the latter, 50% of the null mutant and WT mice expired approximately 4 and 8 days after infection, respectively (Figure 2B). Similarly, 100% of the infected Chi3l1−/− mice died within 8 days of bacterial inoculation compared to 60% peak mortality in infected WT controls (Figure 2B). The enhanced sensitivity of null mutant mice was associated with exaggerated lung injury manifested by augmented neutrophil- and macrophage-rich BAL and tissue inflammation (Figure S1), increased levels of hemorrhage (tissue and BAL red blood cells), increased BAL protein accumulation, and increased levels of CD11b+Gr-1+ neutrophils and myeloperoxidase (Figures 2C–2I). Importantly, the enhanced sensitivity of Chi3l1 null mice could be ameliorated with recombinant (r) Chi3l1. This can be seen in Figures 3A and 3B, which demonstrate that the addition of rChi3l1 to our bacterial inoculum decreased the weight loss and mortality of null mutant but not WT animals. This was not due to a direct antibacterial effect of Chi3l1 because incubation of Sp and rChi3l1 (from 16 ng/ml to 10 μg/ml) in vitro did not alter the titers of Sp bacteria in our infectious inoculum (Figure 3C). These studies demonstrate that Chi3l1 is a critical regulator of the severity of acute pneumococcal pneumonia and its morbid consequences. They also demonstrate that rChi3l1 can rescue the exaggerated sensitivity of Chi3l1−/− mice to Sp infection.
Figure 2. Chi3l1 Plays a Critical Role in the Control of Acute Pneumococcal Infection and Regulates Lung Inflammation and Injury.
(A and B) WT or Chi3l1−/− mice were infected intratracheally with Sp, and body weight (expressed as % original body weight) (A) and mortality (B) were assessed. (C–I) WT or Chi3l1−/− mice were infected intratracheally with Sp. Total BAL cell recovery and differential cell recoveries were assessed (C). Tissue histology (H&E stain) (D), RBC recovery (E), BAL protein (F), and the percentage and numbers of CD11b+Gr-1+ cells (G and H) were assessed via FACS analysis of single lung cell suspensions, as well as the levels of lung lysate myeloperoxidase (MPO) (I). Unless otherwise noted the data were obtained 4 days after infection. The values in (A), (C), (E), (F), (H), and (I) represent the mean (±SEM) of evaluations in a minimum of five animals and are representative of three experiments. NS, not significant. Scale bar, 100 μm. *p < 0.05, **p < 0.01. (See also Figure S1.)
Figure 3. Effects of Recombinant Chi3l1.
(A and B) WT and Chi3l1−/− mice were infected intratracheally with S. pneumoniae (Sp) or with Sp incubated with 10 μg/ml of rChi3l1. Daily weights of infected mice were recorded (A). Survival of WT or Chi3l1−/− mice infected with Sp or with Sp incubated with rChi3l1 was assessed (B). Black boxes, WT mice + Sp; white boxes, WT mice + Sp+rChi3l1; black triangles, Chi3l1−/− mice + Sp; white triangles, Chi3l1−/− mice + Sp+rChi3l1.
(C) Sp was incubated for 16 hr in the presence and absence of 5-fold dilutions of rChi3l1 (from 10 μg/ml to 16 ng/ml), doxycycline (1 μg/ml), or media control, and bacterial growth was assessed.
(D–F) WT and Chi3l1−/− mice were infected intratracheally with 5 × 105 cfu/mouse Sp. Lungs from infected WT and Chi3l1−/− mice were harvested 1 or 4 days postinfection, and tissue bacterial counts were determined (D). BAL from infected WT and Chi3l1−/− mice were prepared 4 days after inoculation, and bacterial counts were determined (E). Spleen lysates from WT and Chi3l1−/− mice were prepared 4 days after bacterial inoculation, and the percentage of mice with positive spleen cultures was assessed (F).
(G) WT and Chi3l1−/− mice were infected with 105 cfu Sp in the presence or absence of 10 μg/ml rChi3l1. One day later, lung lysate recovered bacterial load was determined by plating on blood agar.
(H) Peritoneal macrophages from WT or Chi3l1−/− mice were treated for 60 min with 10:1 and 2.5:1 ratios of fluorescein-labeled Sp to cells. Cells were gated on macrophages according to forward and side scatter properties. Average fluorescein units are shown for WT or Chi3l1−/− cells (n = 5).
(I) Peritoneal macrophages from WT or Chi3l1−/− mice were treated with a 10:1 ratio of unlabeled Sp to cells in media first without antibiotics for 60 min, followed by media with antibiotics for 60 min. The cells were then washed and lysed, and phagocytosed bacteria were quantitated (n = 4).
(J) Peritoneal macrophages from WT or Chi3l1−/− mice were treated with 10:1 ratio of unlabeled Sp to cells in media without antibiotics for 60 min, then incubated with gentamicin to kill extracellular bacteria, and viable bacteria were assessed 6 hr later (n = 4).
(K) Peritoneal macrophages from WT or Chi3l1−/− mice were treated with Sp in the absence or presence of rChi3l1, and at 6 hr, viable intracellular bacteria were assessed. The values in this figure represent the mean (±SEM) of evaluations in a minimum of five animals and are representative of three experiments. NS, not significant. *p < 0.05, **p < 0.01. (See also Figure S2.)
Chi3l1 Regulates Bacterial Clearance and Dissemination
To begin to define the mechanism(s) by which Chi3l1 regulates pneumococcal infection, WT and Chi3l1−/− mice were infected with Sp, and pulmonary bacterial titers and bacterial dissemination were evaluated 1 and 4 days later. At day 1, Chi3l1−/− mice displayed increased pulmonary bacterial loads compared to WT animals (Figure 3D). These differences were even more prominent 4 days postinfection, where the Chi3l1−/− infected mice exhibited approximately 100× greater BAL and lung bacterial burdens compared to infected WT controls (Figures 3D and 3E). In addition, ~20% of the WT infected mice displayed positive Sp spleen cultures. In contrast, >70% of the comparably infected Chi3l1−/− mice had detectable splenic bacteria (Figure 3F). In accord with the findings noted above, Chi3l1−/− mice that received Sp in the presence of rChi3l1 protein exhibited decreased pulmonary bacterial burdens after infection compared to Chi3l1−/− mice that received bacteria alone (Figure 3G). These studies demonstrate that null mutations of Chi3l1 are associated with decreased Sp clearance and enhanced systemic Sp dissemination. They also demonstrate that the defective clearance of bacteria in Chi3l1−/− mice can be rescued with rChi3l1 protein.
Chi3l1 Does Not Alter Bacterial Phagocytosis
Because Chi3l1−/− mice manifest a defect in bacterial clearance, and Chi3l1 did not manifest direct bactericidal activity, studies were undertaken to determine if Chi3l1 plays a role in bacterial phagocytosis. First, we used peritoneal macrophages harvested from WT and Chi3l1−/− mice, incubated for 1 hr with flourescein-labeled Sp in vitro, and analyzed using flow cytometry and Amnis evaluations. Second, macrophages from WT and null mice were incubated in vitro with unlabeled bacteria for 1 hr, washed, and incubated with antibiotic for an additional hour before cells were lysed and bacterial titers assessed by plating on blood agar. In the former experiments using labeled bacteria, similar levels of labeled Sp were seen in macrophages from WT and Chi3l1−/− mice (Figure 3H). Importantly, the Amnis evaluations demonstrated that the Sp was intracellular and not adherent to the surface of the macrophages (Figure S2). Similarly, in the latter experiments studying lysed infected cells, similar numbers of Sp were seen in the cells from the infected and antibiotic-treated macrophages (Figure 3I). Thus, these studies demonstrate that the Chi3l1−/− and WT macrophages exhibited similar abilities to ingest Sp.
Chi3l1 Regulates Bacterial Killing
To assess macrophage bacterial killing, macrophages from WT and null mice were incubated with unlabeled bacteria for 6 hr in vitro, washed, and lysed, and bacterial titers were assessed after plating on blood agar. Chi3l1−/− macrophages contained higher titers of bacteria than cells from WT controls, demonstrating that the null cells have a defect in intracellular bacterial killing (Figure 3J). Importantly, addition of rChi3l1 protein to our bacterial aliquots rescued the bacterial killing response in Chi3l1−/− macrophages (Figure 3K). These studies demonstrate that Chi3l1 plays a critical role in intracellular Sp killing.
Chi3l1 Regulates a Caspase-1-Dependent Macrophage Cell Death Response
We demonstrated previously that Chi3l1 is an important inhibitor of macrophage apoptosis (Lee et al., 2009). Thus, studies were undertaken to determine if the impaired bacterial killing in Chi3l1−/− macrophages was due to an exaggerated Sp-induced cell death response. In these experiments, macrophages from WT and null mice were incubated with varying doses of Sp (moi 0, 1, 5, 20), and apoptosis was assessed using TUNEL staining and Annexin V evaluations by flow cytometry (Figure 4A). At baseline, the levels of TUNEL staining and Annexin V expression were higher in null versus WT cells. However, these differences did not achieve statistical significance (Figure 4A, white open bar). In contrast, after infection with all dosages of bacteria, the levels of TUNEL staining and Annexin V expression were significantly higher in null versus WT cells (Figures 4A and 4B). The levels of LDH release were also higher in null versus WT cells after infection (Figure 4C). In all cases, the addition of rChi3l1 protein to the Sp aliquots rescued these bacterially induced exaggerated cell death responses in Chi3l1−/− cells (Figures 4C, 4D, and S3). These studies demonstrate that Chi3l1 is a critical regulator of macrophage survival.
Figure 4. Chi3l1 Regulates Macrophage Cell Death.
(A) Peritoneal macrophages from WT or Chi3l1−/− mice were incubated with 0, 104, 2.5 × 104, and 105 cfu Sp/well for 12 hr. Cells were fixed and TUNEL stains were performed.
(B) Infected peritoneal macrophages were harvested and stained with Annexin V-FITC, and the percentage of cells that expressed Annexin V was determined.
(C) Macrophages from WT or Chi3l1−/− mice treated with Sp in the absence or presence of rChi3l1 and LDH release were assessed. The values are expressed as the percentage of the LDH released by similar numbers of cells treated with Triton-X.
(D) Macrophages from WT or Chi3l1−/− mice were treated with Sp in the presence or absence of rChi3l1, and the percentage of cells expressing Annexin V by flow cytometry was assessed.
(E) Peritoneal macrophages from WT or Chi3l1−/− mice were treated with Sp in the presence or absence of rChi3l1, and lysate caspase-1 bioactivity was measured using a colorimetric assay.
(F) CD45+ adherent cells from infected WT or Chi3l1−/− lungs were measured for their caspase-1 bioactivity.
(G) Annexin V staining by flow cytometry of macrophages (WT or Chi3l1−/−) infected with Sp in the presence of DMSO (vehicle control) or YVAD-CHO caspase-1 inhibitor.
(H) Annexin V staining by flow cytometry of macrophages from WT, Chi3l1−/−, Chi3l1−/− × Caspase-1−/−, or Caspase-1−/− mice infected with Sp. The data shown in (A)–(H) represent the mean ± SEM of a minimum of five mice in each assessment. NS, not significant. *p < 0.05. (See also Figure S3.)
To further understand the exaggerated cell death response in infected Chi3l1 null macrophages, we assessed the regulation and role(s) of caspase-1 in these responses. These studies demonstrated that the levels of caspase-1 activity were higher in infected peritoneal macrophages from null versus WT mice in vitro (Figure 4E). In accord with this finding, the caspase-1 activity in isolated macrophages from infected lungs of Chi3l1 null mice was greater than WT controls (Figure 4F). These studies also demonstrated that the increased induction of Annexin V+ cell death was abrogated by the specific caspase-1 inhibitor YVAD-CHO compared to its DMSO vehicle control (Figure 4G). Accordingly, the levels of Annexin V+ staining after bacterial infection were diminished in Chi3l1−/− × caspase-1−/− double knockout macrophages compared to comparably treated cells from Chi3l1−/− mice (Figure 4H). When viewed in combination, these studies demonstrate that Chi3l1 is an important inhibitor of a Sp-induced, caspase-1-dependent, macrophage pyroptosis-like cell death response.
Chi3l1 Regulates Inflammasome Activation and the Purinergic Pathway In Vivo
Because the studies noted above demonstrate that caspase-1 is activated in cells from Chi3l1 null mice, studies were undertaken to determine if Chi3l1 regulates the production of caspase-1/inflammasome-dependent cytokines such as IL-1β and IL-18. As can be seen in Figures 5A and 5B, the levels of these cytokines in BAL from infected null mice was significantly greater than in BAL from similarly challenged WT mice. These findings were at least partially specific for these moieties because the levels of other proinflammatory cytokines (TNF-α and IL-6) were not similarly altered (Figures 5C and S4). We also found that Sp-stimulated IL-33 secretion was increased in Chi3l1−/− mice versus wild-type controls. However, this cytokine response was not caspase-1 inflammasome dependent, as demonstrated in studies comparing Chi3l1−/− and Chi3l1/caspase-1 double null animals (Figure S4). In keeping with the concept that inflammasome activity is enhanced in Chi3l1−/− mice, comparisons of lysates, BAL, and mRNA from lungs from infected null and WT mice demonstrated that the null mice manifest enhanced levels of the mature form of IL-1β and enhanced levels of expression of Nlrp3 and the inflammasome adaptor Asc in the lungs (Figures 5D and 5E) and macrophages (Figures 5F and 5G). Because it is known that extracellular ATP, which is often increased in settings characterized by cellular damage, can act as a cofactor in inflammasome activation, the levels of ATP and ATP receptors were also evaluated. These studies demonstrate that bacteria-infected Chi3l1−/− mice have significantly higher levels of measurable BAL ATP (Figure 5H), as well as increased levels of the P2×7r ATP receptor compared to WT controls (Figure 5I). The P2×4r was not similarly regulated (Figure 5J). Importantly, these alterations played a significant role in the exaggerated Sp-induced responses in Chi3l1−/− mice, because BAL inflammation and IL-1β production were decreased in Chi3l1−/− infected mice treated with siRNA against P2×7R (Figures 6A, 6B, and S6). When viewed in combination, these studies demonstrate that the inflammasome, inflammasome activity, and the production of inflammasome-dependent cytokines are increased after Sp infection in the absence of Chi3l1. They also demonstrate that ATP accumulation and P2×7R expression are exaggerated in this setting and play an important role(s) in this response.
Figure 5. Chi3l1 Regulates Inflammasome Activation and the Purinergic Pathway.
(A–C) WT and Chi3l1−/− mice were infected intratracheally with Sp, and BAL fluid IL-1β (A) and IL-18 (B) were assessed 1 or 4 days after inoculation. The levels of BAL TNF and IL-6 were assessed 4 days after inoculation (C). BAL fluids of mock infected animals were obtained at day 4.
(D–G) WT and Chi3l1−/− mice were infected with Sp and 1 or 4 days later the levels of mRNA encoding Nlrp3 (D) and ASC (E) in isolated lung lysates were assessed by real-time RT-PCR. Similarly, isolated macrophages from WT and Chi3l1−/− mice were infected in vitro with Sp and measured for Nlpr3 (F) and ASC (G) expression.
(H) WT and Chi3l1−/− mice were infected with Sp, and the levels of BAL ATP were assessed 4 days later.
(I and J) WT and Chi3l1−/− mice were infected with Sp, and the levels of mRNA encoding the purinergic receptors P2×7R (I) and P2×4R (J) were assessed using real-time RT-PCR. Expression is normalized to a β-actin control and presented as the fold increase compared to mock treated WT mice. Error bars represent mean ± SEM. NS, not significant. *p < 0.05, **p < 0.01. (See also Figure S4.)
Figure 6. Chi3l1 Regulates an Inflammasome that Utilizes Caspase-1 and Nlpr3.
(A and B) BAL cell numbers (A) and IL-1b levels in BAL (B) in Sp-infected WT mice, Chi3l1−/− mice, and mice treated with scrambled siRNA, Nlrp3 siRNA, and P2×7R siRNA.
(C) Peritoneal macrophages from WT or Chi3l1−/− mice were infected with Sp at 10:1 bacteria to cell ratio, and supernatant IL-1β was assessed by ELISA at the noted intervals thereafter.
(D) Macrophages from WT or Chi3l1−/− mice were treated with Sp as indicated for 16 hr, and lysates and supernatant (SN) IL-1β were assessed via western blot analysis. Pro-IL-1β and the mature form of IL-1β are indicated. A control immunoblot using antibodies against β-tubulin is included.
(E and F) Peritoneal macrophages from WT or Chi3l1−/− mice were treated with LPS for 12 hr followed by ATP (5 mM) for 30 min. The levels of supernatant IL-1β were assessed by ELISA (E), and supernatant and lysate IL-1β protein were assessed by immunoblot (F).
(G) Macrophages from WT or Chi3l1−/− mice treated with or without Sp, and 16 hr later caspase-1 was assessed via western blot analysis. Pro-caspase-1 and cleaved caspase-1 (p10) are shown. Control immunoblot using antibodies against β-tubulin is included.
(H) Macrophages from WT mice, Chi3l1−/− mice, caspase-1−/− mice, and Chi3l1−/− × Caspase-1−/− mice were infected with Sp, and supernatant IL-1β was assessed by ELISA 16 hr later.
(I) Macrophages from WT mice, Chi3l1−/− mice, Nlrp3−/− mice, and Chi3l1−/− × Nlrp3−/− mice were infected with Sp, and supernatant IL-1β was assessed by ELISA 16 hr later.
(J) BAL IL-1β levels in infected WT, Chi3l1−/− mice, and Chi3l1−/− × Caspase-1−/− mice. Error bars represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001. (See also Figure S5.)
The Chi3l1-Regulated Inflammasome Utilizes Caspase-1 and Nlrp3
To further understand the interaction of Chi3l1 and the inflammasome, in vitro approaches were employed. These studies demonstrate that macrophages from Chi3l1−/− mice, when infected with Sp, exhibited an exaggerated time- and dose-dependent increase in IL-1β production compared to macrophages from infected WT mice (Figure 6C and data not shown). Increased levels of pro-IL-1β and mature IL-1β in cell lysates and increased secretion of mature IL-1β into cell supernatants were also observed in comparisons of infected WT and Chi3l1−/− macrophage cultures (Figure 6D). These effects were not Sp specific because similar results were seen when other inflammasome activators were employed. Specifically, we noted that Chi3l1−/− macrophages produced more mature IL1-β than cells from WT mice when treated with the prototypic activating combination of LPS plus ATP (Figures 6E and 6F). We also noted that Sp-infected Chi3l1−/− macrophages contained increased levels of activated (cleaved) caspase-1 compared to similarly infected WT cells (Figure 6G). Importantly, macrophage production of mature IL-1β after Sp infection was dependent on Nlrp3 and caspase-1 because the production of this cytokine was significantly diminished in WT macrophages and Chi3l1 null macrophages that lacked Nlrp3 or caspase-1 (Figures 6H and 6I). Moreover, BAL inflammation and IL-1b production were decreased in Chi3l1−/− infected mice treated with siRNA against Nlrp3 (Figures 6A, 6B, and S5) and Chi3l1−/− infected mice that were also deficient in caspase-1 (Figure 6J). When viewed in combination, these studies demonstrate that Chi3l1 is an important inhibitor of the activation of an Nlrp3 inflammasome in vivo and in vitro.
Chi3l1 Regulation of Cytokine Profile In Vivo
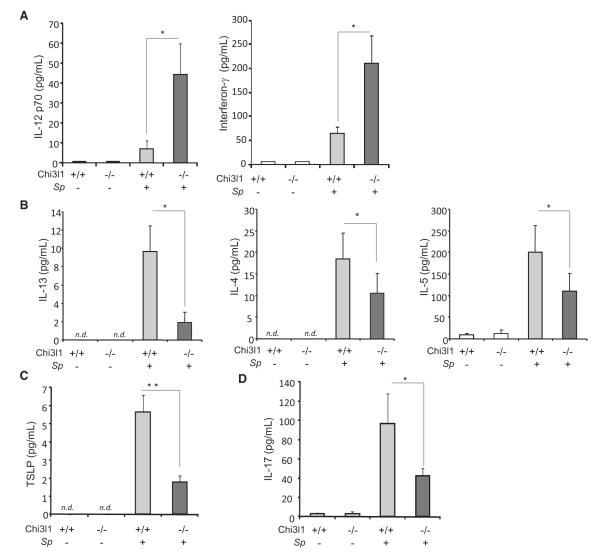
Studies were next undertaken in vivo to determine if the presence or absence of Chi3l1 altered the spectrum and quantities of cytokines that were induced by Sp. As shown in Figure 7, Sp infection caused a significant increase in the prototypic type 1 cytokines IL-12 and IFN-γ and decreases in the classic type 2 cytokines IL-13, IL-4, and IL-5, the T helper 2 (Th2) augmenting cytokine thymic stromal lymphopoietin (TSLP), and the prototypic type 17 cytokine IL-17A. When compared to WT mice, Chi3l1 null mice produced significantly greater levels of IL-12, IFN-γ, and IL-23p19 (Figures 7A and S6). In contrast, these mice manifest impressively decreased levels of IL-13, IL-4, IL-5, TSLP, and IL-17A (Figures 7B–7D). Thus, Chi3l1 plays a major role in the regulation of Sp-induced cytokine responses, where it augments the production of type 2 cytokines, type 17 cytokines, and TSLP while decreasing type 1 cytokine responses.
Figure 7. Chi3l1 Regulation of Cytokine Production In Vivo.
(A–D) WT and Chi3l1−/− mice were infected with or without Sp. BAL was undertaken 4 days later, and Th1 cytokines (IL-12p70 and interferon-γ) (A), Th2 cytokines (IL-13, IL-4, and IL-5) (B), TSLP (C), and IL-17 (D) were measured using ELISA. The values in (A)–(D) represent the mean (±SEM) of evaluations in a minimum of eight animals and are representative of two experiments. NS, not significant. Scale bar, 100 um. *p < 0.05, **p < 0.01. (See also Figure S6.)
DISCUSSION
To further our understanding of the biology of CLPs, we tested the hypothesis that CLPs play an essential role in antibacterial responses. To test this hypothesis, we evaluated the antimicrobial responses in WT and Chi3l1−/− mice infected with Sp. These studies demonstrate that Sp is a powerful stimulator of macrophage and epithelial Chi3l1 production and that null mutations of Chi3l1 result in exaggerated lung hemorrhage, injury and inflammation, systemic bacterial dissemination, and diminished survival after infection. They also demonstrate that these alterations are associated with a defect in pulmonary bacterial clearance and macrophage bacterial killing. Lastly, they highlight mechanisms that underlie these responses by demonstrating that Chi3l1 augments macrophage bacterial killing by inhibiting macrophage pyroptosis and controls inflammation and injury by inhibiting Sp-induced Nlrp3 inflammasome activation, ATP accumulation, and P2×7R expression and regulating type 1, type 2, type 17 cytokine, and TSLP elaboration. These studies demonstrate that Chi3l1 is a critical regulator of antibacterial responses that augments antimicrobial resistance by contributing to bacterial killing and controlling bacterial dissemination. They also demonstrate that Chi3l1 is a critical regulator of host tolerance that inhibits inflammasome and purinergic activation and regulates cytokine balance. When viewed in combination, they suggest that the stimulation of Chi3l1 that is seen during bacterial infections augments bacterial clearance and feeds back to diminish bystander immunopathology. They also raise the possibility that these essential antibacterial roles have contributed to the retention of Chi3l1 over species and evolutionary time.
S. pneumoniae has been described as an “extracellular” pathogen, which is generally considered to be killed by phagocytic ingestion, which can be facilitated by opsonic antibodies (Lu et al., 2008). Our studies provide insights into the complexities that underlie this response. Specifically, they demonstrate that Chi3l1 null mice manifest exaggerated injury and inflammation and enhanced mortality compared with WT controls after Sp infection. They also demonstrate that these responses are associated with increased bacterial titers and that macrophages from Chi3l1−/− mice ingest Sp but fail to kill it in an appropriate manner. This microbicidal failure was associated with augmented macrophage cell death, and this cell death response was associated with caspase-1 activation and mediated by a caspase-1-dependent mechanism. These studies demonstrate that Sp induces macrophage pyroptosis and that Chi3l1 inhibits this cell death response. In accord with the proinflammatory nature of macrophage pyroptosis (Kepp et al., 2010;Khare et al., 2010), our studies demonstrate that Sp-induced pyroptosis is seen in the context of intense inflammation and the exaggerated production of IL-1β and IL-18, which are caspase-1-dependent cytokines (Kepp et al., 2010; Miggin et al., 2007). There is, however, one aspect of our findings and prior reports that deserves additional comment. Specifically, in its present conceptualization, pyroptosis is believed to be antibacterial where it counters bacterial replication and clears infected cells (Lamkanfi and Dixit, 2011). In contrast, our studies demonstrate that in the absence of Chi3l1, pyroptosis decreases Sp killing. These studies demonstrate that pyroptosis must be appropriately regulated to confer its antibacterial effects. In combination, these observations suggest that pyroptosis, like inflammasome activation, can have protective effects or contribute to disease pathogenesis (Bortoluci and Medzhitov, 2010). In accord with this concept, although a variety of pathogens including Listeria monocytogenes, Staphylococcus aureus, and Candida albicans activate caspase-1 via an Nlrp3 inflammasome, direct evidence that Nlrp3-mediated pyroptosis contributes to the control of these pathogens is lacking (Bortoluci and Medzhitov, 2010). In addition, in Plasmodium chabaudi infections, instead of parasite control, inflammation induced by Nlrp3 is implicated in the development of pathology including fever, weight loss, and mortality (Bortoluci and Medzhitov, 2010; Shio et al., 2009). At present it is not clear when pyroptosis and caspase-1 activation are beneficial versus pathologic. It is tempting, however, to speculate that these responses contribute to bacterial clearance when appropriately regulated by molecules such as Chi3l1 and contribute to disease pathogenesis when dysregulated, as seen in the Chi3l1−/− mice in this report.
S. pneumoniae is the leading cause of pneumonia causing hospitalization and an important cause of meningitis and sepsis (Lynch and Zhanel, 2009). In keeping with its importance, the cellular and molecular events that are involved in antipneumococcal responses have been extensively investigated. The studies have highlighted a number of important events, including NF-κB activation and the production of TNF, IL-1β, and IL-6 (Calbo and Garau, 2010; Paterson and Orihuela, 2010). They have also highlighted the importance of a controlled immune response, which clears the pathogen without inducing excess tissue injury (Austrian and Gold, 1964; Calbo and Garau, 2010). This includes studies that demonstrate that mice that lack the p50 subunit of NF-κB (which inhibits the elaboration of proinflammatory cytokines) manifest enhanced inflammation and death despite appropriate bacterial clearance, studies high-lighting the importance of the anti-inflammatory cytokine IL-10 in antipneumococcal responses, and studies that demonstrate that the exaggerated morbidity and mortality that is seen with the simultaneous infection with influenza and Sp are the result of dysregulated inflammation (Calbo and Garau, 2010; Paterson and Mitchell, 2006). The studies of pneumococcal vaccine responses have also highlighted the importance of appropriately balanced type 1, type 2, and type 17 immunity in these protective responses (Ferreira et al., 2008; Littmann et al., 2011; Moffitt et al., 2011; Schmid et al., 2011). Our studies add to these observations in a number of important ways. First, they demonstrate that Sp is a potent inflammasome activator, augments the elaboration of ATP and the expression of the P2×7 ATP receptor, and stimulates a mixed type 1, type 2, and type 17 responses and TSLP elaboration. They also demonstrate that Chi3l1 plays an important role in bacterial clearance and is an important regulator of the appropriateness of anti-Sp responses and that it does this by augmenting bacterial killing, controlling macrophage pyroptosis and inflammasome and purinergic activation, and by regulating the profile of cytokine elaboration.
As noted above, our studies demonstrate that Chi3l1 is an important regulator of the intensity of Sp-induced cytokine responses. The demonstration that type 2 responses are diminished in infected mice that lack Chi3l1 is in accord with studies from our laboratory that demonstrated that Chi3l1 plays a critical role in aeroallergen-induced pulmonary inflammation (Lee et al., 2009). The present studies add to our understanding of the biology of Chi3l1 by demonstrating that Chi3l1 also inhibits type 1, type 17, and IL-1-β and IL-18 responses. Although the mechanisms that underlie these regulatory events have not been fully elucidated, our data provide a number of insights. First, they demonstrate that Chi3l1 regulates the production of inflammasome-dependent moieties (IL-1β and IL-18) and cytokines that have not been associated with inflammasome activation (Th1, Th2, and TSLP cytokines). Although IL-33 has been reported to be produced by inflammasome-dependent and -independent mechanisms (Schmitz et al., 2005; Zhao and Hu, 2010), our studies highlight its caspase-1 inflammation-independent nature in this setting. Importantly, our studies also provide insight into mechanism(s) that may mediate the regulation of IL-17 in this setting. IL-1β and IL-23 are both potent stimulators (Brereton et al., 2009; Sutton et al., 2009) while type 1 immune responses inhibit IL-17 production (Cobb and Smeltz, 2012; Durrant and Metzger, 2010). Interestingly, in this experimental system, the levels of IL-17 were decreased while the IL-1β, type 1 cytokine, and IL-23 responses were increased. This demonstrates that the inhibition of IL-17 in Chi3l1−/− mice is mediated via an IL-1β- and IL-23-independent mechanism. These findings also allow for the interesting speculation that the inhibition is mediated by the accompanying exaggerated type 1 cytokine response. Additional investigation will be required to fully understand each of these important regulatory events.
The immune system provides protection from a wide range of pathogens. One component, the phylogenetically ancient innate immune response, fights infection from the moment of contact and is a fundamental weapon of multicellular organisms (Kimbrell and Beutler, 2001). Innate immune activation, however, can be a double-edged sword that contributes to tissue injury, chronic inflammation, and autoimmunity when unchecked (Liew et al., 2005; Sirisinha, 2011). Studies over recent years have described the numerous negative regulators that control innate immunity, including soluble receptors, transmembrane immunoreceptor tyrosine-based inhibitory motif (ITIM) proteins, and intracellular TLR regulators (Liew et al., 2005; Sirisinha, 2011). Our studies add to this understanding by demonstrating that Chi3l1 is also a powerful inhibitor of innate immunity and that it does this by controlling inflammasome activation, the production of the damage-associated molecular pattern (DAMP) ATP, and the expression of the P2×7 ATP receptor. Recent studies demonstrated that interventions that prevent cell death, decrease the phagocytosis of dying cells, and prevent ATP release or regulate MAP kinase and/or AKT pathway signaling can inhibit inflammasome activation (Ali et al., 2011;Hu et al., 2010). Although the mechanism(s) by which Chi3l1 inhibits inflammasome activation are unknown, it is tempting to speculate that one or more of these mechanisms are involved because studies from our laboratory and others have demonstrated that Chi3l1 inhibits apoptosis and pyroptosis and is known to regulate MAPK and AKT activation (Lee et al., 2009,2011; Lee and Elias, 2010). Our studies have also demonstrated that Chi3l1 augments adaptive Th2 inflammation, TGF-β1 elaboration, and the healing and repair process (Lee et al., 2009). In combination these studies suggest that Chi3l1 is a key regulator of antibacterial responses that augments bacterial killing, regulates the appropriateness and intensity of antibacterial innate immune responses, and facilitates the transition to adaptive immunity while augmenting healing and repair. Additional investigation of the roles of Chi3l1 in bacterial clearance and immunity and the utility of rChi3l1 in these infectious events is warranted.
EXPERIMENTAL PROCEDURES
Animals
Chi3l1−/− (or BRP-39−/−) mice on a C57BL/6 background have been generated and described by our laboratory (Lee et al., 2009). Caspase-1−/− and Nlrp3−/− mice were obtained from Dr. Richard Flavell, Yale University. All animal studies were approved by the Yale IACUC.
Bacteria
Ply+ S. pneumoniae type 3 was used in this study (ATCC #6303). It was stored at −80°C in Todd-Hewitt Broth (THB) containing 10% glycerol. After overnight incubation on 5% sheep blood agar plates (BD Biosciences), freshly grown colonies were suspended in THB and incubated for 2 hr at 37°C to logarithmic growth, pelleted, and resuspended in PBS. Bacterial concentrations were assessed by serial dilutions. Fluorescein-labeled bacteria were prepared as described (Vander Top et al., 2006). Bacteria were incubated at 37°C for 30 min with equal volume of 2.0 μM solution of 5- (and 6-) carboxyfluorescein diacetate succinimidyl ester (CFDA/SE, Molecular Probes).
In Vivo and In Vitro Sp Infection Model
Under anesthesia (ketamine/xylazine), WT or Chi3l1−/− mice were intratracheally inoculated with 105 cfu of Sp or PBS. Infected mice were observed for weights and survival data. Infected mice were sacrificed 1 and 4 days after infection. Blood samples were collected. Single-cell suspensions of spleens were prepared and plated on blood agar plates to determine the presence of bacteria. Elicited peritoneal macrophages from WT and Chi3l1−/− mice were harvested 5 days after intraperitoneal inoculation of 10% thioglycollate broth (Difco Laboratories) and infected with bacteria at various multiplicities of infection (moi) as indicated or treated with LPS (Invivogen) and ATP (Sigma).
BAL and Lung Inflammation
Lung inflammation was assessed by BAL as described previously (Kang et al., 2007). Total RBCs, nucleated cell numbers, and differentials were assessed. Cells were stained with Diff-Quik for cell differentiations. Cell-free BAL fluid was used for cytokine measurements. Lungs were removed, inflated at 25 cm pressure with 0.5% low melting agarose, fixed and embedded in paraffin, sectioned, and stained. H&E stain was performed in the Yale Research Histology. BAL protein concentration was determined by BCA assay (Pierce). Myeloperoxidase activity in lung homogenates was assayed using 3,3′,5,5′-tetramethylbenzidine (Sigma).
Quantitation of Chi3l1 and Cytokines
The concentrations of Chi3l1 in BAL were evaluated by ELISA using anti-Chi3l1 rabbit polyclonal IgG and detecting with biotinylated anti-Chi3l1 antibody (MedImmune) and horseradish peroxidase-labeled streptavidin (Amersham). Concentrations of cytokines were assessed using commercially available ELISA Kits (R&D Systems and eBioscience) according to manufacturer’s instructions.
mRNA Analysis
mRNA concentrations were assessed using real-time RT-PCR as described by our laboratory (Kang et al., 2008; Matsuura et al., 2011). The sequences for the primers used in real-time RT-PCR experiments were obtained from PrimerBank (http://pga.mgh.harvard.edu/primerbank).
Immunoblot Analysis
Precipitated media supernatants or cell extracts were analyzed by standard immunoblot techniques. Primary antibodies used were polyclonal goat antibody to mouse-IL-1β (anti-mouse IL-1β; R&D Systems, Santa Cruz), polyclonal rabbit anti-caspase-1 (Santa Cruz), and polyclonal rabbit antibody to β-tubulin (Cell Signaling). Fifty micrograms of supernatant fluids and/or cell lysates were subjected to immunoblot analysis, fractionated by PAGE, and transferred to membranes.
Immunohistochemistry, Immunofluorescence, and Amnis Imaging
Immunohistochemistry of Chi3l1 was performed as described (Lee et al., 2009;Matsuura et al., 2011) using rabbit anti-Chi3l1 polyclonal antibody (MedImmune). Double-labeled immunofluorescence was performed using antibodies against CD68 (eBioscience) and CC10 (Santa Cruz) to identify macrophages and airway epithelial cells. ToPro-3 was used to stain the nucleus (Invitrogen). Fluorescent images were captured using the LEICA DMIRB microscope. For Amnis experiments, peritoneal macrophages were incubated with labeled bacteria (moi 25:1) and incubated for 2 hr at 37°C in serum-free media, followed by PBS washes prior to staining with anti-mouse F4/80-PE (BD Biosciences), washes, and fixation with 1% formaldehyde. Data was acquired on an Image Stream Multi-Spectral Flow Cytometer. At least 50,000 cells were acquired.
FACS Analysis
Whole-lung cell suspensions were obtained by a previously described method (Lee et al., 2009). Briefly, lung tissue was digested using dispase (Stem Cell Technologies), collagenase (Sigma), and DNaseI (Sigma). Cells were put through varying sizes of strainers (from 100 μm to 40 μm), and were resuspended in PBS/2% FCS. Cells were incubated with purified rat anti-mouse CD16/CD32 monoclonal antibody (1 μg/105 cells; BD Biosciences) to prevent nonspecific binding to Fc receptors. Cells were incubated for 30 min at 4°C with specific antibodies for surface marker staining for Gr-1 and CD11b to identify neutrophils. Surface markers CD45 and F4/80 were used to identify lung macrophages. Markers CD45, SiglecF, and CD11c were used to identify eosinophils. Cell populations were analyzed using FACSCalibur. At least 10,000 cells per sample were analyzed.
Macrophage Apoptosis/Cell Death Evaluation
TUNEL-positive cells on paraffin-embedded tissue were identified using terminal deoxynucleotidyl transferase dUTP nick-end labeling In Situ Cell Death Detection Kit (Roche). Cell death was assessed using Annexin V staining (BD Biosciences) as previously described by our laboratory (Lee et al., 2009). LDH release was assayed using the Cytotoxicity Detection Kit as described by the manufacturer’s protocol (Roche). Ten percent Triton-X-treated cells serve as 100% for LDH release.
Silencing RNA
Nlrp3 and P2×7R siRNAs were synthesized that were deprotected, desalted, and BioRPTM purified (Bioneer). For each of the gene targets, we used three pooled siRNA sequences. See Supplemental Information for siRNA sequences used for Nlrp3 and P2×7r. Nlrp3, P2×7r, and irrelevant (scrambled control) siRNAs were delivered intranasally. We used 60 μg dose of siRNA per mouse given 12 hr prior to Sp infection. Lung tissues were collected 24 hr after infection.
Statistical Analysis
Statistical evaluations were undertaken with GraphPad Prism software. Normally distributed data are expressed as mean ± SEM and were assessed for significance using a Student’s t test or ANOVA as appropriate. Data that were not normally distributed were assessed for significance using the Kruskal-Wallis test, followed by the Mann-Whitney U test to compare individual groups. Statistical significance was defined as a p value less than 0.05.
Supplementary Material
ACKNOWLEDGMENTS
C.S.D.C. is supported by the Parker Francis Fellowship, FAMRI Award, and NHLBI KO8 (HL1K08HL103770). J.A.E. is supported by FAMRI and NIH grants (R01 HL 093017, R01 HL 079328, and U01 HL108638).
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes six figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.chom.2012.05.017.
REFERENCES
- Aerts JM, van Breemen MJ, Bussink AP, Ghauharali K, Sprenger R, Boot RG, Groener JE, Hollak CE, Maas M, Smit S, et al. Biomarkers for lysosomal storage disorders: identification and application as exemplified by chitotriosidase in Gaucher disease. Acta Paediatr. Suppl. 2008;97:7–14. doi: 10.1111/j.1651-2227.2007.00641.x. [DOI] [PubMed] [Google Scholar]
- Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Nizet V, Karin M. Anthrax toxin induces macrophage death by p38 MAPK inhibition but leads to inflammasome activation via ATP leakage. Immunity. 2011;35:34–44. doi: 10.1016/j.immuni.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aris RM, Stephens AR, Ontjes DA, Denene Blackwood A, Lark RK, Hensler MB, Neuringer IP, Lester GE. Adverse alterations in bone metabolism are associated with lung infection in adults with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2000;162:1674–1678. doi: 10.1164/ajrccm.162.5.2002100. [DOI] [PubMed] [Google Scholar]
- Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann. Intern. Med. 1964;60:759–776. doi: 10.7326/0003-4819-60-5-759. [DOI] [PubMed] [Google Scholar]
- Bortoluci KR, Medzhitov R. Control of infection by pyroptosis and autophagy: role of TLR and NLR. Cell Mol Life Sci. 2010 doi: 10.1007/s00018-010-0335-5. in press. Published on-line March 24, 2010. http://dx.doi.org/10.1007/s00018-010-0290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brereton CF, Sutton CE, Lalor SJ, Lavelle EC, Mills KH. Inhibition of ERK MAPK suppresses IL-23- and IL-1-driven IL-17 production and attenuates autoimmune disease. J. Immunol. 2009;183:1715–1723. doi: 10.4049/jimmunol.0803851. [DOI] [PubMed] [Google Scholar]
- Calbo E, Garau J. Of mice and men: innate immunity in pneumococcal pneumonia. Int. J. Antimicrob. Agents. 2010;35:107–113. doi: 10.1016/j.ijantimicag.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N. Engl. J. Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- Cobb D, Smeltz RB. Regulation of proinflammatory Th17 responses during Trypanosoma cruzi infection by IL-12 family cytokines. J. Immunol. 2012;188:3766–3773. doi: 10.4049/jimmunol.1103478. [DOI] [PubMed] [Google Scholar]
- Coffman FD. Chitinase 3-Like-1 (CHI3L1): a putative disease marker at the interface of proteomics and glycomics. Crit. Rev. Clin. Lab. Sci. 2008;45:531–562. doi: 10.1080/10408360802334743. [DOI] [PubMed] [Google Scholar]
- Durrant DM, Metzger DW. IL-12 can alleviate Th17-mediated allergic lung inflammation through induction of pulmonary IL-10 expression. Mucosal Immunol. 2010;3:301–311. doi: 10.1038/mi.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira DM, Darrieux M, Oliveira ML, Leite LC, Miyaji EN. Optimized immune response elicited by a DNA vaccine expressing pneumococcal surface protein a is characterized by a balanced immunoglobulin G1 (IgG1)/IgG2a ratio and proinflammatory cytokine production. Clin. Vaccine Immunol. 2008;15:499–505. doi: 10.1128/CVI.00400-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkhouser JD, Aronson NN., Jr Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol. Biol. 2007;7:96. doi: 10.1186/1471-2148-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatton ML, Cheng Q. Evaluation of the pyrogenic threshold for Plasmodium falciparum malaria in naive individuals. Am. J. Trop. Med. Hyg. 2002;66:467–473. doi: 10.4269/ajtmh.2002.66.467. [DOI] [PubMed] [Google Scholar]
- Gonçalves NS, Ghaem-Maghami M, Monteleone G, Frankel G, Dougan G, Lewis DJ, Simmons CP, MacDonald TT. Critical role for tumor necrosis factor alpha in controlling the number of lumenal pathogenic bacteria and immunopathology in infectious colitis. Infect. Immun. 2001;69:6651–6659. doi: 10.1128/IAI.69.11.6651-6659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- Homer RJ, Zhu Z, Cohn L, Lee CG, White WI, Chen S, Elias JA. Differential expression of chitinases identify subsets of murine airway epithelial cells in allergic inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L502–L511. doi: 10.1152/ajplung.00364.2005. [DOI] [PubMed] [Google Scholar]
- Hu Y, Mao K, Zeng Y, Chen S, Tao Z, Yang C, Sun S, Wu X, Meng G, Sun B. Tripartite-motif protein 30 negatively regulates NLRP3 inflammasome activation by modulating reactive oxygen species production. J. Immunol. 2010;185:7699–7705. doi: 10.4049/jimmunol.1001099. [DOI] [PubMed] [Google Scholar]
- Johansen JS, Krabbe KS, Møller K, Pedersen BK. Circulating YKL-40 levels during human endotoxaemia. Clin. Exp. Immunol. 2005;140:343–348. doi: 10.1111/j.1365-2249.2005.02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MJ, Homer RJ, Gallo A, Lee CG, Crothers KA, Cho SJ, Rochester C, Cain H, Chupp G, Yoon HJ, Elias JA. IL-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J. Immunol. 2007;178:1948–1959. doi: 10.4049/jimmunol.178.3.1948. [DOI] [PubMed] [Google Scholar]
- Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, Elias JA. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J. Clin. Invest. 2008;118:2771–2784. doi: 10.1172/JCI32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepp O, Galluzzi L, Zitvogel L, Kroemer G. Pyroptosis - a cell death modality of its kind? Eur. J. Immunol. 2010;40:627–630. doi: 10.1002/eji.200940160. [DOI] [PubMed] [Google Scholar]
- Khare S, Luc N, Dorfleutner A, Stehlik C. Inflammasomes and their activation. Crit. Rev. Immunol. 2010;30:463–487. doi: 10.1615/critrevimmunol.v30.i5.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrell DA, Beutler B. The evolution and genetics of innate immunity. Nat. Rev. Genet. 2001;2:256–267. doi: 10.1038/35066006. [DOI] [PubMed] [Google Scholar]
- Kronborg G, Ostergaard C, Weis N, Nielsen H, Obel N, Pedersen SS, Price PA, Johansen JS. Serum level of YKL-40 is elevated in patients with Streptococcus pneumoniae bacteremia and is associated with the outcome of the disease. Scand. J. Infect. Dis. 2002;34:323–326. doi: 10.1080/00365540110080233. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Modulation of inflammasome pathways by bacterial and viral pathogens. J. Immunol. 2011;187:597–602. doi: 10.4049/jimmunol.1100229. [DOI] [PubMed] [Google Scholar]
- Lee CG, Elias JA. Role of breast regression protein-39/YKL-40 in asthma and allergic responses. Allergy Asthma Immunol. Res. 2010;2:20–27. doi: 10.4168/aair.2010.2.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J. Exp. Med. 2009;206:1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Littmann M, Hiew J, Mellroth P, Henriques Normark B, et al. Human monocytes promote Th1 and Th17 responses to Streptococcus pneumoniae. Infect. Immun. 2011;79:4210–4217. doi: 10.1128/IAI.05286-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, 3rd, Zhanel GG. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin. Respir. Crit. Care Med. 2009;30:189–209. doi: 10.1055/s-0029-1202938. [DOI] [PubMed] [Google Scholar]
- Ma Y, Seiler KP, Eichwald EJ, Weis JH, Teuscher C, Weis JJ. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect. Immun. 1998;66:161–168. doi: 10.1128/iai.66.1.161-168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura H, Hartl D, Kang MJ, Dela Cruz CS, Koller B, Chupp GL, Homer RJ, Zhou Y, Cho WK, Elias JA, Lee CG. Role of breast regression protein-39 in the pathogenesis of cigarette smoke-induced inflammation and emphysema. Am. J. Respir. Cell Mol. Biol. 2011;44:777–786. doi: 10.1165/rcmb.2010-0081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miggin SM, Pålsson-McDermott E, Dunne A, Jefferies C, Pinteaux E, Banahan K, Murphy C, Moynagh P, Yamamoto M, Akira S, et al. NF-kappaB activation by the Toll-IL-1 receptor domain protein MyD88 adapter-like is regulated by caspase-1. Proc. Natl. Acad. Sci. USA. 2007;104:3372–3377. doi: 10.1073/pnas.0608100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi E. Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology. 2006;130:398–411. doi: 10.1053/j.gastro.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Moffitt KL, Gierahn TM, Lu YJ, Gouveia P, Alderson M, Flechtner JB, Higgins DE, Malley R. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe. 2011;9:158–165. doi: 10.1016/j.chom.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenbaek C, Johansen JS, Junker P, Borregaard N, Sørensen O, Price PA. YKL-40, a matrix protein of specific granules in neutrophils, is elevated in serum of patients with community-acquired pneumonia requiring hospitalization. J. Infect. Dis. 1999;180:1722–1726. doi: 10.1086/315050. [DOI] [PubMed] [Google Scholar]
- Østergaard C, Johansen JS, Benfield T, Price PA, Lundgren JD. YKL-40 is elevated in cerebrospinal fluid from patients with purulent meningitis. Clin. Diagn. Lab. Immunol. 2002;9:598–604. doi: 10.1128/CDLI.9.3.598-604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson GK, Mitchell TJ. Innate immunity and the pneumococcus. Microbiology. 2006;152:285–293. doi: 10.1099/mic.0.28551-0. [DOI] [PubMed] [Google Scholar]
- Paterson GK, Orihuela CJ. Pneumococci: immunology of the innate host response. Respirology. 2010;15:1057–1063. doi: 10.1111/j.1440-1843.2010.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris JS, Hui KP, Yen HL. Host response to influenza virus: protection versus immunopathology. Curr. Opin. Immunol. 2010;22:475–481. doi: 10.1016/j.coi.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid P, Selak S, Keller M, Luhan B, Magyarics Z, Seidel S, Schlick P, Reinisch C, Lingnau K, Nagy E, Grubeck-Loebenstein B. Th17/Th1 biased immunity to the pneumococcal proteins PcsB, StkP and PsaA in adults of different age. Vaccine. 2011;29:3982–3989. doi: 10.1016/j.vaccine.2011.03.081. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shio MT, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, Olivier M. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 2009;5:e1000559. doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinton JA. Immunity or Tolerance in Malarial Infections: (Section of Comparative Medicine) Proc. R. Soc. Med. 1938;31:1298–1302. doi: 10.1177/003591573803101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirisinha S. Insight into the mechanisms regulating immune homeostasis in health and disease. Asian Pac. J. Allergy Immunol. 2011;29:1–14. [PubMed] [Google Scholar]
- Snelgrove RJ, Godlee A, Hussell T. Airway immune homeostasis and implications for influenza-induced inflammation. Trends Immunol. 2011;32:328–334. doi: 10.1016/j.it.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Vander Top EA, Perry GA, Gentry-Nielsen MJ. A novel flow cytometric assay for measurement of in vivo pulmonary neutrophil phagocytosis. BMC Microbiol. 2006;6:61. doi: 10.1186/1471-2180-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Hu Z. The enigmatic processing and secretion of interleukin-33. Cell. Mol. Immunol. 2010;7:260–262. doi: 10.1038/cmi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.