Abstract
Background
Most personalized cancer care strategies involving DNA sequencing are highly reliant on acquiring sufficient fresh or frozen tissue. It has been challenging to comprehensively evaluate the genome of advanced prostate cancer (PCa) because of limited access to metastatic tissue.
Objective
To demonstrate the feasibility of a novel next-generation sequencing (NGS) based platform that can be used with archival formalin-fixed paraffin-embedded (FFPE) biopsy tissue to evaluate the spectrum of DNA alterations seen in advanced PCa.
Design, setting, and participants
FFPE samples (including archival prostatectomies and prostate needle biopsies) were obtained from 45 patients representing the spectrum of disease: localized PCa, metastatic hormone-naive PCa, and metastatic castration-resistant PCa (CRPC). We also assessed paired primaries and metastases to understand disease heterogeneity and disease progression.
Intervention
At least 50 ng of tumor DNA was extracted from FFPE samples and used for hybridization capture and NGS using the Illumina HiSeq 2000 platform.
Outcome measurements and statistical analysis
A total of 3320 exons of 182 cancer-associated genes and 37 introns of 14 commonly rearranged genes were evaluated for genomic alterations.
Results and limitations
We obtained an average sequencing depth of >900X. Overall, 44% of CRPCs harbored genomic alterations involving the androgen receptor gene (AR), including AR copy number gain (24% of CRPCs) or AR point mutation (20% of CRPCs). Other recurrent mutations included transmembrane protease, serine 2 gene (TMPRSS2):v-ets erythroblastosis virus E26 oncogene homolog (avian) gene (ERG) fusion (44%); phosphatase and tensin homolog gene (PTEN) loss (44%); tumor protein p53 gene (TP53) mutation (40%); retinoblastoma gene (RB) loss (28%); v-myc myelocytomatosis viral oncogene homolog (avian) gene (MYC) gain (12%); and phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit α gene (PIK3CA) mutation (4%). There was a high incidence of genomic alterations involving key genes important for DNA repair, including breast cancer 2, early onset gene (BRCA2) loss (12%) and ataxia telangiectasia mutated gene (ATM) mutations (8%); these alterations are potentially targetable with poly(adenosine diphosphate-ribose)polymerase inhibitors. A novel and actionable rearrangement involving the v-raf murine sarcoma viral oncogene homolog B1 gene (BRAF) was also detected.
Conclusions
This first-in-principle study demonstrates the feasibility of performing in-depth DNA analyses using FFPE tissue and brings new insight toward understanding the genomic landscape within advanced PCa.
Keywords: Next-generation sequencing, Castration-resistant prostate cancer, Prostate cancer genome
1. Introduction
Prostate cancer (PCa) is a leading cause of cancer mortality in men in the Western world [1]. Although <5% of patients present with advanced disease, as many as 40% of men eventually develop metastases despite local therapy [2]. Surgical or medical castration is the mainstay of therapy for advanced PCa. However, all patients will eventually experience disease progression, and castration-resistant PCa (CRPC) ensues. There are several approved drugs, as well as promising drugs in clinical development, with significant antitumor effects against metastatic CRPC, including agents targeting the androgen axis [3]. However, despite initial responses, no therapy is curative. We are currently unable to predict which patients may or may not respond to a specific drug.
Somatic genomic alterations contribute to cancer by altering the function of genes or pathways that are important for tumorigenesis, metastasis, and resistance to therapies [4]. Understanding the genomic landscape of PCa and identifying alterations acquired with disease progression can help in the design of new, effective therapies that specifically target altered biologic pathways. The concept of genomic pathway dependency has been validated in other tumor types and has led to the approval of drugs that target specific driving mutations [5].
Recent advances in massively parallel DNA sequencing technologies allow for simultaneous identification of base substitutions, indels, copy number alterations, and structural alterations at much greater sensitivity and cost effectiveness than does screening a large number of genes by traditional Sanger sequencing [6]. Next-generation sequencing (NGS) studies have led to significant advances in our understanding of the cancer genome of several tumor types [5], and current efforts are aimed toward bringing sequencing discoveries into the clinic in the form of biomarkers (diagnostic, prognostic, and predictive) and biomarker-designed clinical trials.
The assessment of the genomic landscape of advanced PCa has been challenging, primarily because of limited access to tissue and technologies that require large amounts of DNA and the fact that most patients with advanced PCa do not undergo biopsies of metastases as part of routine clinical care. The goals of this study are to molecularly characterize metastatic CRPC and to demonstrate the feasibility of performing clinical diagnostics based on deep NGS using a novel platform that requires little DNA and can use tissue that is formalin-fixed and embedded in paraffin.
2. Materials and methods
2.1. Sample collection
Fifty samples were collected under an institutional review board–approved protocol and reviewed by an expert pathologist, who marked 10-μm unstained slides for tumor subtype enrichment. Tissue was collected into extraction tubes and processed using the formalin-fixed paraffin-embedded (FFPE) tissue kit for the Promega Maxwell nucleic acid purification system. Extracted DNA was quantified using a standardized PicoGreen fluorescence assay.
2.2. Library construction and hybrid capture
Molecular barcode indexed ligation-based sequencing libraries were constructed using 200 ng of sheared DNA or total DNA recovered from the sample (if ≥50 ng) when 200 ng was not available. Forty-five cases yielded sufficient DNA for analysis. Libraries were hybrid-captured with custom biotinylated RNA oligo pools (custom SureSelect kit, Agilent) representing 3230 exons in 182 cancer-related genes plus 37 introns from 14 genes often rearranged in cancer [7] (Supplementary Table 1).
2.3. Sequencing and analysis
Paired end sequencing (49 × 49 cycles) was performed using the HiSeq 2000 (Illumina). Sequence data from genomic DNA and complementary DNA were mapped to the reference human genome (hg19) using the Burrows-Wheeler Aligner [8] and were processed using the publicly available SAMtools [9], Picard, and Genome Analysis Toolkit [10]. Genomic base substitutions and indels were detected using custom tools optimized for mutation calling in heterogeneous tumor samples, based on statistical modeling of sequence quality scores and local sequence assembly. Variations were filtered using dbSNP and a custom artifact database and then were annotated for known and likely somatic mutations using the Catalogue of Somatic Mutations in Cancer [10]. Copy number alterations were detected by comparing targeted genomic DNA sequence coverage with a process-matched normal control sample. Genomic rearrangements were detected by clustering chimeric reads mapped to targeted introns.
2.4. Tissue validation studies
The v-ets erythroblastosis virus E26 oncogene homolog (avian) gene (ERG) and the v-raf murine sarcoma viral oncogene homolog B1 gene( BRAF) rearrangement and the phosphatase and tensin homolog gene (PTEN), breast cancer 2, early onset gene (BRCA2), androgen receptor gene (AR) copy number status were assessed by fluorescence in situ hybridization (FISH) on tissue slides from the same tumor nodule used for DNA extraction. Methods for FISH for transmembrane protease, serine 2 gene (TMPRSS2):ERG fusion have been previously described [11]; amplification was defined as more than two copies and deletion as fewer than two copies on average from gene-specific signals per nuclei compared with two reference signals. At least 100 nuclei were evaluated per core/tissue section. Bacterial artificial chromosome probes used are listed in Supplementary Table 2 [12]. Immunohistochemical (IHC) staining of AR was performed on a Bond-Max Autostainer using anti-AR antibody (Biogenex, clone F39.4.1,1:800) according to the manufacturer’s protocol. Speckle-type POZ protein gene (SPOP) and AR mutation status were assessed from residual DNA from same pool used for sequencing by polymerase chain reaction followed by Sanger sequencing.
3. Results
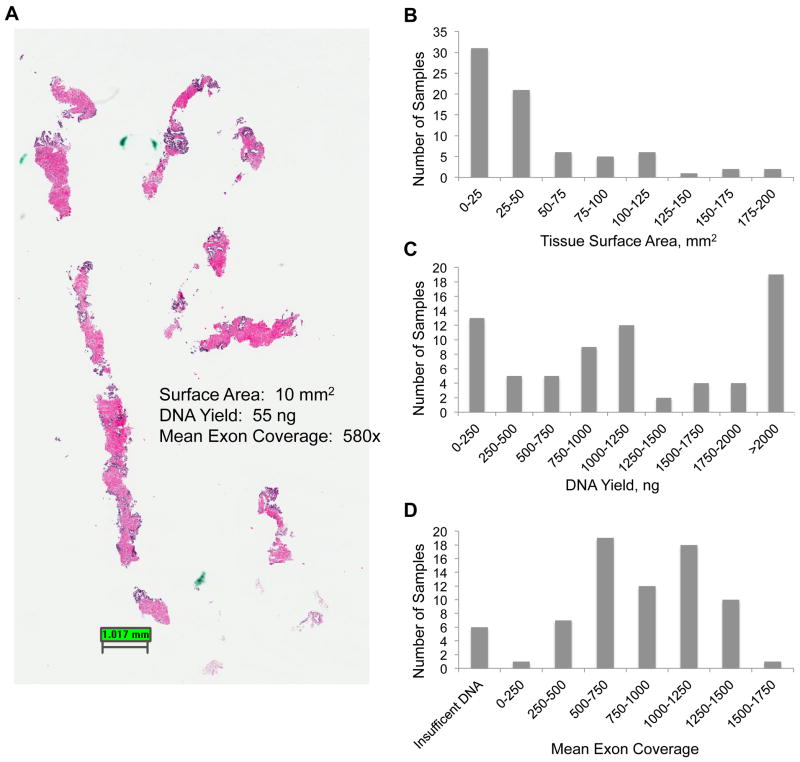
Tumors from 45 patients were evaluated, including 25 metastatic CRPCs (18 with neuroendocrine features), 4 metastatic hormone-naive PCas, and 16 primary localized PCas (including 2 from patients known to later develop CRPC). Matched benign prostate was available in 25 cases (56%). Clinical characteristics are summarized in Supplementary Table 3. Manual dissection was used to enrich 40 μm (4 × 10-μm unstained slides) of FFPE tissue per case, resulting in 90 high-density foci amenable to DNA extraction. These cases included prostatectomy specimens and prostate needle biopsies (Fig. 1a). In four cases of CRPC, enriched adenocarcinoma and neuroendocrine foci from the same tumor were sequenced separately. Prostate biopsies represented a range of tissue sizes, and DNA yield sufficient for library construction (≥50 ng) was obtained from 5 of 8 biopsies (63%) and 79 of 82 prostatectomy foci (96%) (93% success rate) (Fig. 1b and 1c). Paired end sequencing gave an average uniquely mapping sequence coverage of 949X (Fig. 1d).
Fig. 1.
(a) Representative hematoxylin-eosin photomicrograph of needle core biopsy used for sequencing; (b) tissue surface area for all the samples; (c) DNA yield obtained from samples; (d) mean exon coverage obtained from sequencing.
Sequence data were analyzed for base substitutions, small insertions and deletions, copy number changes, and rearrangements (Supplementary Table 4). Recurrent genomic alterations found in CRPC are summarized in Figure 2a and include TMPRSS2:ERG fusion (44%); PTEN loss (44%); tumor protein p53 gene (TP53) mutation (40%); retinoblastoma gene (RB) loss (28%); AR mutation (20%); AR gain (24%); v-myc myelocytomatosis viral oncogene homolog (avian) gene (MYC) gain (12%); BRCA2 loss (12%); catenin (cadherin-associated protein), β 1, 88kDa gene (CTNNB1) mutation (12%); ataxia telangiectasia mutated gene (ATM) mutation (8%); and phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit α gene (PIK3CA) mutation (4%). Metastatic hormone-naive PCa also demonstrated TMPRSS2:ERG gene fusion, BRCA2 deletion, and TP53 mutations, but AR alterations, RB loss, and MYC gain were not seen in any of these cases (Fig. 2c). Genomic alterations were less frequent among the 16 clinically localized prostate tumors (Fig. 2b and 2c) and were higher in intermediate-risk compared with Gleason 3 + 3 tumors. The two primary prostate tumors from patients known to later develop metastatic CRPC harbored a higher number of alterations, including both with TP53 mutations (Fig. 2c). A novel rearrangement was discovered in a clinically localized case with Paneth cell–like differentiation: a gene fusion between BRAF and the erythrocyte membrane protein band 4.1 (elliptocytosis 1, RH-linked) gene (EPB41), which was validated by FISH (Fig. 3a). The structure of the fusion illustrates an intact kinase domain (Fig. 3b), suggesting that it may be a potentially drug-targetable alteration.
Fig. 2.
(a) DNA alterations in castration-resistant prostate cancer (CRPC) (n = 25); (b) DNA alterations in clinically localized prostate cancer (PCa) (n = 16); (c) DNA alterations in metastatic hormone-naive PCa (n = 4). Re-arr = rearrangement; hemiz = hemizygous.
Fig. 3.
(a) Clinically localized prostate cancer Gleason grade 6 (3 + 3) with Paneth cell–like neuroendocrine differentiation (upper inset); BRAF-EPB41 gene fusion was identified in this case by sequencing and was validated by fluorescence in situ hybridization(lower inset showing breakapart of the BRAF gene); (b) diagrammatic representation of novel BRAF rearrangement illustrating preserved kinase domain.
All tumors were independently evaluated by FISH for ERG rearrangement and copy number alterations involving PTEN, AR, and BRCA2, and all positive cases determined by NGS were confirmed (100% positive predictive value)(Supplementary Table 5 and Supplementary Fig. 1). The SPOP gene is commonly mutated in 6–15% of primary PCa [13]. Since SPOP was not included in the genes sequenced by NGS, residual DNA from all NGS cases was amplified by polymerase chain reaction and assessed independently for SPOP status using Sanger sequencing. SPOP mutations were detected in only one case (2%) and occurred in one case without ERG gene rearrangement, consistent with prior observations. The low frequency of SPOP mutation seen in our study may reflect the lower sensitivity of Sanger sequencing compared with NGS assays.
AR mutations included three known activating point mutations involving the ligand-binding domain, as well as a novel variant involving the regulatory domain (Fig. 4). Point mutations occurred in 20% of CRPCs and were mutually exclusive of AR amplification (24%), suggesting a possible alternative mechanism of androgen therapy resistance. Notably, AR protein expression did not always correlate with AR mutation or copy number status, suggesting that IHC may not be a surrogate marker. An AR variant was seen in only one clinically localized case at predicted 100% frequency in both tumor and benign tissues, indicating a likely germline polymorphism in this patient.
Fig. 4.

Somatic mutations involving the androgen receptor gene (AR). Mutations marked in black represent novel alterations, whereas mutations marked in red have been previously described as activating point mutations.
CRPC tumors with neuroendocrine differentiation were evaluated by the study pathologist, who directed enrichment into separate adenocarcinoma and neuroendocrine foci for sequencing in four cases. ERG, PTEN, and TP53 status were concordant between foci for all four cases. High-level, focal AR amplification was observed in one neuroendocrine PCa focus, but this area was negative for AR IHC protein expression. Lack of AR protein expression is expected in neuroendocrine PCa foci and is consistent with prior studies [14]. Together, these data support loss of AR expression as a later event.
Matched primary tumor and metastasis was available for sequencing in a single case. NGS of the primary Gleason 5 + 4 tumor revealed TMPRSS2:ERG gene fusion and TP53 mutation. A soft tissue metastasis removed approximately 3 yr later in the setting of metastatic CRPC revealed adenocarcinoma with neuroendocrine differentiation. The metastatic tumor (both neuroendocrine and adenocarcinoma foci) also harbored TMPRSS2:ERG gene fusion and the TP53 mutation, concordant with the original adenocarcinoma mutation profile (Supplementary Fig. 2). A matched metastatic hormone-naive sample and CRPC metastasis (obtained 3 yr later from another patient) were also evaluated. These tumors demonstrated concordance in ERG gene fusion status (both negative) and BRCA2 deletion status (both positive), but RB loss and TP53 mutation were present in only the CRPC tumor (Supplementary Fig. 3), suggesting that these events occurred later in this case.
4. Discussion
This study provides new insight into the genomic alterations that characterize advanced PCa and complements recent data that have been generated through whole exome sequencing of primary localized PCa and CRPC [13,15]. Some molecular alterations arise early and persist during disease progression, suggesting they may be driving events and potential biomarkers that can be evaluated at the time of cancer diagnosis and used to guide the course of patients’ therapy. This idea is further supported by our results in the series of localized PCa, in which PCa of Gleason grade 6 harbored few genomic alterations, possibly helping to explain the clinical indolence of these tumors.
Other genomic alterations seemed to consistently arise later in PCa, suggesting that they are acquired during progression or in response to therapy. Somatic AR alterations, for example, were seen only in metastatic CRPC (44% in our series, all treated with androgen deprivation therapies), consistent with a recent study published by Grasso et al evaluating whole exomes of 50 CRPC tumors [15]. AR alterations have not been detected in hormone-naive PCa, supporting acquisition of AR alterations as a mechanism of resistance to hormonal therapies. This observation has important clinical implications. We know now that most CRPC tumors remain dependent on AR signaling [17], and this knowledge has led to the clinical development of highly potent drugs that block AR signaling through inhibition of hormone production (eg, abiraterone) or AR receptor binding (eg, MDV3100) [3]. AR point mutations were thought to be uncommon and therefore a minor mechanism of AR dependence; however, through deep sequencing studies, we are observing that these mutations may be more common than previously thought.
It is known that some advanced CRPC tumors evolve to an aggressive neuroendocrine phenotype in late-stage disease [18]. In several of the CRPC cases evaluated in this study, neuroendocrine differentiation was seen mixed with adenocarcinoma (40%), which represents an aggressive subset of tumors. We demonstrated that although AR gene amplification may be retained in neuroendocrine foci (similar to ERG rearrangement), AR protein expression is lost. This finding suggests that these tumors would not be as responsive to AR-targeted drugs. Therefore, understanding the AR status of a patient’s metastatic CRPC tumor may be important in anticipating or interpreting resistance to available hormonal therapies and in guiding subsequent therapy.
Another novel and clinically relevant finding was the discovery of frequent BRCA2 gene deletions and ATM point mutations in CRPC (20%). This finding may have important treatment implications, as tumors with defects in homologous recombination (ie, breast cancer 1, early onset gene [BRCA1]/BRCA2 or ATM mutations) are sensitive to inhibition of poly(adenosine diphosphate-ribose)polymerase (PARP) 1 (involved in base excision DNA repair) [19–21]. The PARP inhibitor olaparib has demonstrated significant antitumor effect in PCa patients with germline BRCA mutations and has also been shown to be effective in breast and ovarian cancer patients with known BRCA alterations [22]. PIK3CA mutations have been previously described in PCa and may identify patients responsive to phosphoinositide 3-kinase/Akt inhibitors [23]. There are several ongoing trials evaluating PARP inhibitors or Akt inhibitors for patients with metastatic CRPC; therefore, incorporating NGS assays into biomarker-designed trials may help stratify patients for treatment and result in improved therapeutic response rates.
Several other novel alterations in CRPC discovered through this NGS study included copy number alterations of cell cycles genes (the cyclin D1 gene [CCND1], cyclin-dependent kinase inhibitor 2A gene [CDKN2A], cyclin-dependent kinase inhibitor 2B gene [p15, inhibits CDK4] [CDKN2B], cyclin-dependent kinase 4 gene [ CDK4], and cyclin-dependent kinase 6 gene [CDK6]) and frequent mutation of the β catenin gene (CTNNB1) in CRPC, as well as less frequent mutations involving the v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog gene (KRAS), fibroblast growth factor receptor 2 gene ( FGFR2), and polycystic kidney and hepatic disease 1 (autosomal recessive) gene (PKHD1). These alterations have been previously described as actionable and warrant clinical investigation. BRAF fusions have been previously identified as uncommon events in PCa but are also potentially targetable [24]. The BRAF fusions, similar to ets variant 1 gene (ETV1) fusions, appear to have variable 5′ partners. In this paper, we show that this focused NGS assay approach is also useful to discover novel 5′ fusion partners for the BRAF kinase domain (ie, EPB41). These findings are hypothesis generating and warrant functional validation, as they may provide further insight into tumor biology and may have clinical implications.
Importantly, this study demonstrates the feasibility of performing robust NGS using FFPE tissue, including needle biopsy material. To date, the requirement of fresh/frozen DNA has been a major barrier in performing such deep-sequencing studies. As little as 55 ng of DNA was needed to achieve deep sequence coverage, which we believe is a major advance in the field of cancer genomics and one step toward bringing such technology realistically into routine clinical practice. Genomic biomarker–based clinical programs centered around precision medicine for cancer patients, such as the Michigan Oncology Sequencing Project [25], are currently being developed and implemented. This focused FFPE DNA-sequencing approach has been validated [26], should be considered to improve cost effectiveness, and can occur in a clinically relevant time frame (average: <21 d) to help guide therapies.
A limitation to this study is the relatively small sample size, which reflects a challenge in studying metastatic tumors in which biopsies are not routinely performed. In addition, because PCa shows wide heterogeneity, larger prospective studies are needed to evaluate the significance of specific mutations for determining prognosis and response to therapies. Although the majority of needle biopsies yielded sufficient DNA for analysis, it was predictably less adequate compared with prostatectomy specimens (63% compared with 96%); future work will need to focus on optimization of these protocols to enhance the clinical utility of using small needle biopsy samples. Additional matched primary-metastatic tumor pairs are needed to help determine the molecular events occurring during progression to advanced disease. Although the gene content in this study was focused on known cancer-associated genes, additional genes will be added as relevant genes are discovered by whole exome/genome approaches, and comprehensive whole exome sequencing is likely to become feasible in the near future as NGS technologies evolve.
5. Conclusions
This first-in-principle study demonstrates the feasibility of performing targeted sequencing using advanced prostate tumor FFPE samples. We show that this targeted content sequencing approach, if designed correctly, can identify mutations and reliably detect gene fusions and copy number alterations all in a single assay. Our results are hypothesis generating, and the spectrum of driver mutations observed highlights the potential value of comprehensive genomic profiling for targeted therapy selection in clinical care. This study marks an exciting step toward designing targeted assays to detect driving mutations and has potential to lead to new biomarkers, drug targets, and general applicability in guiding the development of future therapies.
Take-home message.
We describe and validate an approach for performing next-generation sequencing at >900X coverage that is amenable to formalin-fixed paraffin-embedded biopsy material. This work identifies novel targetable alterations in advanced prostate cancer and mutations associated with disease progression and tumor resistance.
Acknowledgments
Funding/Support and role of the sponsor: None.
We would like to thank Mirjam Blattner, Dimple Chakravarty, and Kai Wang for assisting in validation studies. We thank John Curran and the Foundation Medicine Clinical Lab Staff for their technical support.
Footnotes
Author contributions: Mark A. Rubin had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Beltran, Yelensky, Stephens, Mosquera, Cronin, Rubin.
Acquisition of data: Downing, Park, MacDonald, Jarosz.
Analysis and interpretation of data: Beltran, Yelensky, Frampton, Park, MacDonald, Lipson, Stephens, Mosquera, Cronin, Rubin.
Drafting of the manuscript: Beltran, Yelensky.
Critical revision of the manuscript for important intellectual content: Beltran, Yelensky, Tagawa, Nanus, Stephens, Mosquera, Cronin, Rubin.
Statistical analysis: Yelensky.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Stephens, Cronin, Rubin.
Other (specify): None.
Financial disclosures: Mark A. Rubin certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Roman Yelensky, Garrett M. Frampton, Sean R. Downing, Mirna Jarosz, Doron Lipson, Philip J. Stephens, and Maureen T. Cronin are employed by and have stock options in Foundation Medicine, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.DeVita VT, Lawrence TS, Rosenberg SA. Cancer: principles and practice of oncology: annual advances in oncology. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2010. [Google Scholar]
- 3.Beltran H, Beer TM, Carducci MA, et al. New therapies for castration-resistant prostate cancer: efficacy and safety. Eur Urol. 2011;60:279–90. doi: 10.1016/j.eururo.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 4.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–24. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macconaill LE, Garraway LA. Clinical implications of the cancer genome. J Clin Oncol. 2010;28:5219–28. doi: 10.1200/JCO.2009.27.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stratton MR. Exploring the genomes of cancer cells: progress and promise. Science. 2011;331:1553–8. doi: 10.1126/science.1204040. [DOI] [PubMed] [Google Scholar]
- 7.Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18:382–4. doi: 10.1038/nm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Handsaker B, Wysoker A, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–9. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forbes SA, Bindal N, Bamford S, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39:D945–50. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 12.Berger MF, Lawrence MS, Demichelis F, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–20. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbieri CE, Baca SC, Lawrence MS, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44:685–9. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1:487–95. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–43. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15:4792–8. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tetu B, Ro JY, Ayala AG, Johnson DE, Logothetis CJ, Ordonez NG. Small cell carcinoma of the prostate, I: a clinicopathologic study of 20 cases. Cancer. 1987;59:1803–9. doi: 10.1002/1097-0142(19870515)59:10<1803::aid-cncr2820591019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Lord CJ, Ashworth A. Targeted therapy for cancer using PARP inhibitors. Curr Opin Pharmacol. 2008;8:363–9. doi: 10.1016/j.coph.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Dantzer F, de La Rubia G, Menissier-De Murcia J, Hostomsky Z, de Murcia G, Schreiber V. Base excision repair is impaired in mammalian cells lacking poly(ADP-ribose) polymerase-1. Biochemistry. 2000;39:7559–69. doi: 10.1021/bi0003442. [DOI] [PubMed] [Google Scholar]
- 21.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 22.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 23.Ihle NT, Lemos R, Jr, Wipf P, et al. Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic Ras is a dominant predictor for resistance. Cancer Res. 2009;69:143–50. doi: 10.1158/0008-5472.CAN-07-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palanisamy N, Ateeq B, Kalyana-Sundaram S, et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat Med. 2010;16:793–8. doi: 10.1038/nm.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roychowdhury S, Iyer MK, Robinson DR, et al. Personalized oncology through integrative high-throughput sequencing: a pilot study. Sci Transl Med. 2011;3:111–21. doi: 10.1126/scitranslmed.3003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yelensky R, Wang K, Dogan S, et al. Next-generation sequencing of FFPE solid tumor specimens for clinical use. J Clin Oncol. 2012;30:10524. [Google Scholar]