Abstract
Several studies have examined levels of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6. This meta-analysis was conducted to examine the association between obsessive-compulsive disorder (OCD) and plasma serum levels of proinflammatory cytokines. Twelve studies met inclusion criteria. The meta-analysis demonstrated a significant reduction in IL-1β levels in OCD. No significant difference in plasma levels of IL-6 or TNF-α was demonstrated. Stratified subgroup analysis revealed possible moderating effects of age and medication use on IL-6 levels. Studies including children on psychotropic medication had lower plasma IL-6 levels. Stratified subgroup analysis revealed a moderating effect of comorbid depression on TNF-α levels. Elevated TNF-α levels were reported in studies that included individuals with comorbid depression. Future studies examining immune function in OCD should adjust for potential confounding due to medication use and comorbid depression. Further studies assessing cerebrospinal fluid cytokine levels in OCD are also needed.
Keywords: Obsessive-compulsive disorder, OCD, Cytokine, Proinflammatory, Meta-analysis, Interleukin, IL-1β, IL-6, Tumor necrosis factor, TNF-α, Anxiety disorders, Depression, PANDAS, Review
Introduction
Obsessive-compulsive disorder (OCD) is a psychiatric illness with a lifetime prevalence of 1.6% to 2.3% in the general adult population [1–3]. The DSM-IV defines OCD as the presence of either obsessions or compulsions that cause significant distress; consume more than 1 h/d; or interfere with normal functioning at work, home, social activities, or personal relationships [4]. The National Comorbidity Survey Replication reported an average age at onset in OCD patients of 19.5 years, with males experiencing a significantly younger age at onset than females [2]. The prevalence of comorbid lifetime DSM-IV disorders among adult patients with OCD is 90% [2]. The most common comorbidities in OCD patients are anxiety disorders (75.8%), mood disorders (63.3%), and impulse control disorders (55.9%) [2].
Immune dysregulation has been hypothesized to be important in the development and pathophysiology of OCD. Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections (PANDAS) is a controversial potential subgroup of patients with OCD and tic disorders characterized by the presence of neurological abnormalities and prepubertal onset that is temporally related to a group A streptococcal infection [5•, 6, 7]. PANDAS is conceptually similar to Sydenham chorea, a movement disorder characterized by choreatic movement and emotional lability that develops after acute rheumatic fever [5•, 8]. Sydenham chorea is the most common acquired childhood chorea and is often associated with obsessive-compulsive symptoms [5•]. Data supporting PANDAS as a diagnostic entity include case reports describing a potential temporal link between group A streptococcal infection and exacerbation of OCD symptoms, the improvement of OCD symptoms in patients treated with plasmapheresis or intravenous immunoglobulin therapy, identification of antibasal ganglia antibodies in OCD patients, and neuroimaging studies that seem to support PANDAS [6, 9•]. Despite this evidence, significant controversy regarding PANDAS remains [10]. The criteria for diagnosis remain to be validated, and evidence for autoimmunity is weak, as demonstrated by a recent study that failed to demonstrate changes in markers of brain autoimmunity in clinical exacerbations of OCD in children diagnosed with PANDAS [5•, 11]. Another recent study has shown that 85% of OCD exacerbations in patients meeting PANDAS criteria were not related to group A streptococcal infection [11]. Although it is clear that many of the findings supporting PANDAS need to be replicated, the immune markers for and pathogenesis of this clinical entity have been postulated to be important by many respected investigators, and therefore warrant close attention.
A role for immune-mediated pathophysiology has emerged in other psychiatric conditions, including major depression [12•, 13–15] and schizophrenia [16–19], in which many studies have evaluated the link between plasma levels of proinflammatory cytokines and disease. Meta-analysis has shown that proinflammatory cytokines, including tumor necrosis factor (TNF)-α and interleukin (IL)-6, are elevated in depressed patients relative to controls, and that the levels of some inflammatory cytokines, such as IL-1β and possibly IL-6, are reduced in patients successfully treated with antidepressant medication [12•, 20•]. Meta-analysis of inflammatory cytokines in schizophrenia has shown that in vivo circulating IL-6, IL-1RA, and sIL-2R are elevated, supporting a role for inflammation and immune dysregulation in schizophrenia, although there were no significant changes in levels of circulating IL-1β and TNF-α [19]. Levels of plasma cytokines have also been investigated in OCD patients, although no systemic review of this literature exists to date.
Cytokines are small soluble proteins secreted by cells to influence the behavior of other cells, specifically including the regulation of cellular immunity and the inflammatory response [21, 22]. Together, IL-1β, IL-6, and TNF-α are often viewed as proinflammatory or alarm cytokines, commonly being induced together. These cytokines are produced both peripherally and in the central nervous system (CNS), typically by microglia [23]. TNF-α stimulates vagal afferents and is produced by neurons and glial cells in an activity-dependent manner [24, 25]. IL-6 and IL-1 are also produced in the brain, where they mediate neuroinflammation and response to injury [23, 24, 26]. Interestingly, IL-1β, IL-6, and TNF-α also mediate neuroprotection to excitotoxicity [27]. Because of their role in the CNS, these cytokines have been the subject of much investigation in psychiatric disease [24].
IL-6, discovered in the 1980s as a lymphocyte-derived signal for B-cell maturation, functions as both a pro- and anti-inflammatory cytokine and has been implicated in a variety of inflammatory and autoimmune disorders [28–30]. IL-6 is a 184–amino acid cytokine produced by both immune cells, including macrophages, B cells, and T cells, and nonimmune cells, such as endothelial cells and fibroblasts, in response to homeostatic disturbances such as infection and injury [30, 31]. IL-6 is a critical cytokine for the differentiation and growth of T and B cells [32, 33]. As a proinflammatory cytokine, IL-6 is important in neuroinflammation, the brain-specific activation of microglia and astrocytes that accompanies neurodegenerative disorders [29]. Like other proinflammatory cytokines, including TNF-α and IL-1β, IL-6 induces the acute-phase reaction, the body’s systemic anti-inflammatory response to local inflammation designed to limit tissue damage and prevent inflammation at uninvolved, distant organs [34]. The acute-phase reaction consists of fever, the production of acute-phase proteins such as protease inhibitors by the liver, and systemic release of corticosterone [31]. Despite being viewed classically as a proinflammatory cytokine, IL-6 has also been shown to inhibit neutrophil accumulation and functions to block the actions of IL-1β and TNF-α in vitro [29, 35]. In this manner, IL-6 functions to feedback on other proinflammatory cytokines to control the local and systemic inflammatory response [31]. Interestingly, IL-6 protects against excitotoxicity in the brain and is capable of reversing the neurotoxic effects of many drugs [29].
IL-1β is a 153–amino acid cytokine produced by activated blood monocytes and tissue macrophages such as microglia that affects most cell types and promotes inflammation by indirectly promoting lymphocyte function and activating macrophages [36, 37]. IL-1β upregulates endothelial cell expression of the adhesion molecules VCAM-1 and ICAM-1, which bind to leukocyte integrins and promote infiltration of inflammatory cells into tissue [36, 38, 39]. IL-1β induces expression of inflammatory mediators such as COX-2 and iNOS, increases circulating neutrophils through the IL-17–driven T-helper type 17 (Th17) response, induces fever, and is a potent stimulator of IL-6 production by endothelial cells [36, 40]. IL-1β is also important for T-cell–dependent antibody production, possibly through IL-1β induction of IL-6 production, and may contribute to stimulating Th2 cell–mediated immune responses, as demonstrated in a murine model of asthma [41, 42]. These many and diverse proinflammatory functions of IL-1β demonstrate its critical role in the inflammatory response and host defense against pathogens. However, similar to IL-6, IL-1β has an important role in mediating the anti-inflammatory response, most notably through stimulation of the acute-phase reaction [34].
TNF-α is a 157–amino acid cytokine produced by macrophages, natural killer cells, and T cells that was first identified in the late-1960s and early-1970s as a factor that induces apoptosis of tumor cells [21, 43]. First synthesized as a monomeric transmembrane protein, TNF-α is later cleaved by a matrix metalloprotease to its soluble form [44]. Since its early identification and characterization, TNF-α has emerged as a potent proinflammatory cytokine responsible for mediating protective and pathological processes through its role in the activation of vascular endothelium, recruitment of immune cells, and mediation of apoptosis [45]. In response to local infection, macrophages secrete TNF-α, leading to recruitment of phagocytes and lymphocytes to infected tissue, increased expression of cell-adhesion molecules on endothelial cells, and increased vascular permeability to proteins and cells [21, 45, 46]. Though TNF-α is protective in local infection, systemic release of TNF-α by activated macrophages of the liver and spleen in response to sepsis causes shock, disseminated intravascular coagulation, and ultimately multiorgan failure. In response to injury, TNF-α production is increased in the brain, where it may act with nitric oxide to regulate the blood–brain barrier [44]. Besides its role in endothelial activation and phagocytosis, TNF-α mediates activation of the adaptive immune system by stimulating dendritic cell (DC) migration to regional lymph nodes, where DCs mature and present antigen to lymphocytes [21]. Like other proinflammatory cytokines, TNF-α stimulates its own production and that of IL-1β [45].
The goal of this systematic review is to examine cytokine abnormalities in OCD. We specifically conduct a meta-analysis to examine the association between plasma serum proinflammatory cytokine levels and OCD. We specifically focus on the proinflammatory cytokines IL-1β, IL-6, and TNF-α, which have been well studied in OCD. Through stratified secondary analysis, we analyze the potential moderating effects of medication use, comorbid depression, and patient age on cytokine levels in OCD. Additionally, we systematically review studies that have examined proinflammatory cytokine levels in the cerebrospinal fluid (CSF) and lipopolysaccharide (LPS)-induced lymphocyte cytokine production in OCD.
Methods
Search Strategy and Inclusion Criteria
PubMed was searched by two reviewers (SMG, MHB) for relevant citations using the search terms “cytokines” AND “obsessive-compulsive disorder.” The bibliography of review articles and included articles were also searched for additional eligible citations. Authors of some articles were contacted for missing information when necessary. There were no limitations based on the language of publication. Studies were included in this meta-analysis if they measured cytokine levels or cytokine production in OCD patients compared with controls.
Meta-Analysis Procedure
To extract data from included articles, we used Excel spreadsheets. Data extracted included number of OCD subjects and controls, average age of subjects, source of cytokine measure (blood, CSF, or LPS-induced lymphocyte cytokine production), measures of proinflammatory cytokines (mean and SD), and whether subjects on medications or with comorbid depression were included or excluded from the sample. We stratified trials based on the source of cytokine measures—plasma, CSF and LPS-induced cytokine production. Because of the relative paucity of studies examining CSF cytokine levels and LPS-induced lymphocyte cytokine production, we did not conduct meta-analysis in these data and instead only present a systematic review of these studies. By contrast, we were able to conduct a meta-analysis comparing plasma serum levels of proinflammatory cytokines IL-1β, IL-6, and TNF-α in OCD subjects compared with controls.
Our main outcome was the difference in proinflammatory cytokine levels between OCD subjects and controls. We chose standardized mean differences (SMDs) as our summary measures because absolute measures of cytokines are highly variable between laboratories and can suffer from batch effects. SMD allowed us to place results on a common metric. Meta-analytic results were computed using a fixed-effects model in Comprehensive Meta-Analysis version 2 Comprehensive Meta-Analysis (Version 2) (Biostat, Englewood, NJ). We conducted a sensitivity analysis to examine our decision to use a random-effects rather than fixed-effects model for meta-analysis.
Due to the small number of studies included in this meta-analysis, we were unable to test for publication bias. Heterogeneity between trials was assessed using I2 test in CMA as well as the χ2 test for heterogeneity. We considered there to be evidence of possible heterogeneity between trials when the I2 was greater than 30% or the P value for the χ2 test for heterogeneity was less than 0.1. We conducted additional stratified subgroup analyses to examine possible sources of heterogeneity. Stratified subgroup analyses were done to examine the effects of (1) age of subjects (child vs adult), and inclusion or exclusion of subjects with (2) comorbid depression or taking (3) psychoactive medications. We stratified studies as adult or child based on whether the average age of subjects was greater or less than 18 years in studies. It should be noted that no included studies had an average age of subjects between 15 and 33 years of age. We used the test for subgroup differences in CMA to determine whether subgroups reduced overall heterogeneity [47]. We set P<0.05 as our threshold for statistical significance for all primary and stratified subgroup analyses.
Results
Our PubMed search identified 14 articles, 12 of which met the inclusion criteria for our review [24, 48–60]. Two studies were excluded because they did not use controls [56, 60]. The characteristics of included trials are shown in Table 1. Seven studies involving 169 OCD subjects and 215 controls examined plasma levels of proinflammatory cytokines. Six studies examined plasma levels of TNF-α, four studies examined plasma levels of IL-1β, and five studies examined serum levels of IL-6. Additionally, one included study examined CSF levels of proinflammatory cytokines, and four studies examined LPS-induced lymphocyte cytokine production.
Table 1.
Included studies on obsessive-compulsive disorder
| Cytokines examineda | |||||||
|---|---|---|---|---|---|---|---|
| Study | Year | Medication use |
Mood disorder |
Age group | IL-1β | IL-6 | TNF-α |
| Plasma cytokine levels | |||||||
| Brambilla et al. [48] | 1997 | Excluded | Excluded | Adult | ↓ | NA | ↓ |
| Konuk et al. [24] | 2007 | Excluded | Included | Adult | NA | ↑ | ↑ |
| Maes et al. [49] | 1994 | Excluded | Excluded | Adult | ~ | ~ | NA |
| Monteleone et al. [50] | 1998 | Excluded | Excluded | Adult | ~ | ~ | ↓ |
| Leckman et al. [51] | 2005 | Included | Included | Child | NA | ~ | ~ |
| Gabbay et al. [52] | 2009 | Included | Excluded | Child | ~ | ~ | ~ |
| Bos-Veneman et al. [53] | 2010 | Included | Not reported | Child | NA | NA | ~ |
| Cytokine production | |||||||
| Weizman et al. [54] | 1996 | Excluded | Excluded | Adult | ~ | NA | NA |
| Denys et al. [55] | 2004 | Excluded | Excluded | Adult | NA | ↓ | ↓ |
| Fluitman et al. [57] | 2010 | Excluded | Excluded | Adult | NA | ↓ | ~ |
| Fluitman et al. [58] | 2010 | Included | Excluded | Adult | NA | ~ | ~ |
| Cerebrospinal fluid cytokine levels | |||||||
| Carpenter et al. [59] | 2002 | Excluded | Includedb | Adult | NA | ~ | NA |
↑, increased in OCD; ↓, decreased in obsessive-compulsive disorder; ~, unchanged
Included comorbid major depression, tic disorder, dysthymia, panic disorder, social phobia, anorexia nervosa, schizotypal, and schizoid personality disorder, but excluded psychoses, pervasive developmental disorders, substance dependence, and primary psychiatric disorder
IL, interleukin; NA, not assessed; TNF, tumor necrosis factor
Interleukin-1β
Four studies involving 77 OCD subjects and 76 controls measured IL-1β levels in plasma serum. Meta-analysis demonstrated a significant reduction in IL-1β plasma levels in OCD subjects compared with controls (SMD, −0.60 [95% CI, −0.93 to −0.28]; z=−3.6; P<.001). Figure 1 depicts a forest plot of included trials. There was no evidence of heterogeneity between trials (χ2=1.6; df=3; P=0.66; I2=0). Using a random-effects model rather than a fixed-effects model for meta-analysis did not affect the results of the overall meta-analysis. We did not conduct stratified subgroup analysis for this outcome, as there was no evidence of heterogeneity between trials.
Fig 1.
Association between interleukin (IL)-1β plasma levels and obsessive-compulsive disorder (OCD). This forest plot demonstrates a significant reduction in IL-1β levels in OCD subjects compared with controls (standardized mean difference [SMD], −0.60 [95% CI, −0.93 to −0.28]; z=−3.6; P<0.001)
One study involving 11 OCD subjects and 11 controls measured in vitro production of IL-1β by peripheral blood mononuclear cells (PBMCs) isolated from heparinized venous blood [54]. The PBMCs were stimulated with 10 µof LPS and incubated for 24 hours [54]. Cytokine levels were assayed by radioimmunoassay (Advanced Magnetic, Cambridge, MA). IL-1β production by PBMC was not statistically significantly different between OCD patients and control subjects (P=0.09) [54].
No studies have investigated IL-1β levels in the CSF of patients with OCD.
Interleukin-6
Five studies involving 109 OCD subjects and 111 controls measured IL-6 levels in plasma serum. Meta-analysis failed to demonstrate a significant association between IL-6 plasma levels and OCD (SMD, 0.15 [95% CI, −0.12–0.41]; z=1.1; P=0.28). There remained no association when a random-effects model (SMD, 0.14 [95% CI, −0.20–0.49]; z=0.8; P=0.42) was used instead of a fixed-effects model for meta-analysis. There was a large degree of heterogeneity between trials that failed to reach statistical significance (χ2=6.2; df=4; P=0.18; I2=36%). In stratified subgroup analysis, there was a significant moderating effect of subject age and inclusion of subjects with current medication use on the association between IL-6 levels and OCD (χ2 test for subgroup differences=5.2; df=1; P=0.02). Studies involving children and those that included subjects on psychoactive medication (SMD, −0.22 [95% CI, −0.63–0.20]; z=−1.0; P=0.30) demonstrated significantly lower levels of IL-6 (in reference to controls) than did studies involving adult subjects who were not currently taking psychoactive medications (SMD, 0.41 [95% CI, 0.06–0.76]; z=2.3; P=0.02). Figure 2 depicts a forest plot of IL-6 levels in OCD subjects stratified by subject age group and medication use. We were not able to disentangle the effects of medication use and subject age in studies measuring IL-6, as there were no studies in children that did not allow medication use and no studies in adults that included subjects on medications. Inclusion of subjects with comorbid depression did not significantly affect the relationship between serum IL-6 levels and OCD (χ2 test for subgroup differences=0.04; df=1; P=0.85).
Fig 2.
Association between interleukin (IL)-6 plasma levels and obsessive-compulsive disorder (OCD) stratified by age and medication use. Meta-analysis failed to demonstrate a significant association between IL-6 plasma levels and OCD (standardized mean difference [SMD], 0.15 [95% CI, −0.12–0.41]; z=1.1; P=0.28). There was a large degree of heterogeneity between trials that failed to reach statistical significance (χ2=6.2; df=4; P=0.18; I2=36%). In stratified subgroup analysis, there was a significant moderating effect of subject age and inclusion of subjects with current medication use on the association between IL-6 levels and OCD (χ2 test for subgroup differences=5.2; df=1; P=0.02). We were not able to disentangle the possible influence of these two factors, as there were no adult studies that included subjects with current medication use and no children’s studies that excluded subjects with medication use
Three studies measured baseline ex vivo production of IL-6 in whole blood cultures of OCD patients and control subjects [55, 57, 58]. Heparinized venous blood was collected, diluted, stimulated with 2 ng/mL of Escherichia coli 0127:B8 LPS, and incubated for 24 hours [55, 57, 58]. Cytokine levels were assayed by ELISA (CLB, Amsterdam). In a study of 50 OCD patients and 25 healthy controls, investigators found a decrease in IL-6 production (P=0.004) in OCD patients relative to controls that failed to reach statistical significance when adopting the Bonferroni correction (significance level set at P=0.0034 when adopting the Bonferroni correction) [55]. In a study of 10 OCD patients and 10 healthy controls, investigators found no statistically significant difference for IL-6 production between OCD patients and control subjects [58]. In a study of 26 OCD patients and 26 healthy controls, IL-6 production was statistically significantly lower in OCD patients relative to controls (P=0.016) [57]. Overall, these studies suggest LPS-induced lymphocyte production of IL-6 is likely decreased in subjects with OCD.
One study involving 26 OCD patients and 26 healthy controls measured levels of IL-6 in the CSF [59]. CSF was obtained by lumbar puncture, and levels of IL-6 were measured using the Quantikine high sensitivity immunoassay kit (R&D Systems, Minneapolis, MN). CSF levels of IL-6 were not statistically significantly different between OCD patients and control subjects [59].
Tumor Necrosis Factor-α
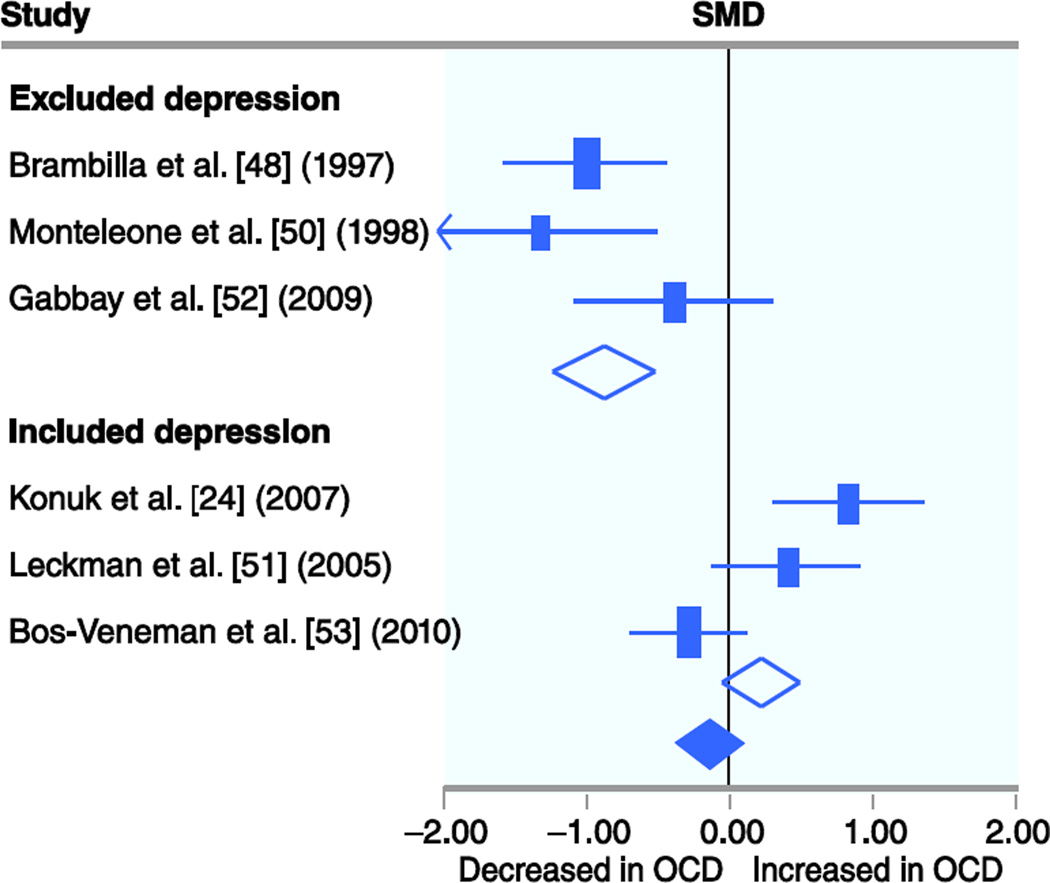
Six studies involving 150 OCD subjects and 196 controls measured TNF-α levels in plasma serum. Meta-analysis failed to demonstrate a significant association between TNF-α plasma levels and OCD (SMD, −0.14 [95% CI, −0.37–0.08]; z=−1.3; P=0.21). There remained no association when a random-effects model (SMD, −0.25 [95% CI, −0.86–0.35]; z=−0.8; P=0.41) was used instead of a fixed-effects model for meta-analysis. There was a large and statistically significant degree of heterogeneity between trials (χ2=35.0; df=5; P<0.001; I2=86%). In stratified subgroup analysis, there was a significant moderating effect of inclusion of subjects with comorbid depression on the association between TNF-α levels and OCD (χ2 test for subgroup differences=20.4; df=1; P<0.001). Studies that included subjects with comorbid depression (SMD, 0.22 [95% CI, −0.05–0.49]; z=−1.6; P=0.11) demonstrated significantly higher levels of TNF-α (in reference to controls) than did studies excluding those subjects (SMD, −0.87 [95% CI, −1.25 to −0.48]; z=−4.4; P<0.001). Figure 3 depicts a forest plot of TNF-α levels in OCD subjects stratified by inclusion of subjects with comorbid mood disorders. Age (χ2 test for subgroup differences=0.49; df=1; P=0.49) and medication use (χ2 test for subgroup differences=0.49; df=1; P=0.49) in studies did not significantly affect the relationship between serum TNF-α levels and OCD. We were not able to disentangle the effects of medication use and subject age in studies measuring TNF-α, as there were no studies in children that did not allow medication use and no studies in adults that included subjects on medications.
Fig 3.
Association between tumor necrosis factor (TNF)-α plasma levels and obsessive-compulsive disorder (OCD) stratified by inclusion of subjects with comorbid depression. Meta-analysis failed to demonstrate a significant association between TNF-α plasma levels and OCD (standardized mean difference [SMD], −0.14 [95% CI, −0.37–0.08]; z=−1.3; P=0.21). There was a large and statistically significant degree of heterogeneity between trials (χ2=35.0; df=5; P<0.001; I2=86%). In stratified subgroup analysis, there was a significant moderating effect of inclusion of subjects with comorbid depression on the association between TNF-α levels and OCD (χ2 test for subgroup differences=20.4; df=1; P<0.001).
Three studies measured ex vivo production of TNF-α in whole blood cultures of OCD patients and controls [55, 57, 58]. Heparinized venous blood was collected, diluted, stimulated with 2 ng/mL of E. coli 0127:B8 LPS, and incubated for 24 hours [55, 57, 58]. Cytokine levels were assayed by ELISA (CLB, Amsterdam). In a study of 50 OCD patients and 25 healthy controls, investigators found that TNF-α production was statistically significantly lower in OCD patients relative to controls (P<0.001) [55]. In a study of 10 OCD patients and 10 healthy controls, no statistically significant difference for TNF-α production between OCD patients and controls was reported [58]. In an additional study of 26 OCD patients and 26 healthy controls, no statistically significant difference for TNF-α production between OCD patients and controls (P=0.13) was found [57]. No studies have investigated TNF-α levels in the CSF of patients with OCD.
Conclusions
Meta-analysis of studies measuring plasma cytokine levels demonstrated a significant reduction in IL-1β levels in OCD subjects compared with controls. In overall meta-analysis, no significant difference in plasma levels of other proinflammatory cytokines, specifically IL-6 and TNF-α, was demonstrated. However, in stratified subgroup analysis, significant moderating effects of age and inclusion of subjects with current medication use and comorbid mood disorders were demonstrated. Stratified subgroup analysis demonstrated that IL-6 levels were significantly increased in medication-free adults with OCD (in reference to controls). In contrast, IL-6 levels were significantly lower in children with OCD who were potentially taking psychoactive medication (in reference to controls). Stratified subgroup analysis also revealed that plasma levels of TNF-α in OCD patients were reported to be significantly higher (relative to controls) in studies that included subjects with comorbid depression.
Our findings highlight that importance of properly accounting for the potential confounding effects of medication use and comorbid mood disorder in patients with OCD. Approximately two thirds of adults with OCD have a lifetime history of a comorbid mood disorder. Selective serotonin reuptake inhibitor pharmacotherapy is currently the first-line pharmacologic treatment for children and adults with OCD. Meta-analysis has previously demonstrated that several proinflammatory cytokines, including TNF-α and IL-6, are elevated in depressed patients. We reported in this meta-analysis that studies that included subjects with comorbid depression reported significantly higher levels of TNF-α in OCD (compared to controls) than studies that excluded subjects with comorbid depression. Meta-analyses have also demonstrated reduction in some inflammatory cytokines, specifically IL-1β and IL-6, with antidepressant medication use in depression [12•, 20•]. We report that studies including subjects on medications reported significantly lower plasma levels of IL-6 in OCD patients than studies that required no medication use in subjects.
Systematic analysis of ex vivo cytokine production by lymphocytes was completed for IL-1β, IL-6, and TNF-α and revealed fairly few studies [54]. Three studies inconsistently reported reduced IL-6 production in OCD subjects relative to controls [55, 57, 58]. Only one study measured IL-1β production and failed to show a statistically significant difference between OCD patients and controls [54, 55, 57, 58]. Only one of three studies found that TNF-α production was significantly lower in OCD patients relative to controls. Only one study has measured CSF levels of proinflammatory cytokines in OCD subjects relative to controls, and it found no difference in IL-6 levels [59].
Although we have found some interesting possible effects of age, mood, and medication use on cytokine levels in OCD patients in the examined studies, our analysis and findings are limited by several factors. First, relatively few studies for each individual cytokine existed for us to analyze, and the sample sizes were small. As such, the quality of our results is limited by the quality of the original studies we analyzed. Second, we were unable to analyze individual patient data from studies. Individual patient data would be much more powerful in assessing moderating effects of age, medication use, and depression. Third, the few number of studies on cytokine production and CSF cytokine levels prevented us from conducting a meta-analysis in this area. In light of the low number of studies, our stratified subgroup analysis must be considered exploratory. Finally, a major limitation of the included studies on cytokines levels, whether in the plasma, CSF, or produced by ex vivo lymphocytes, is their analysis of only one or two samples from each patient. Cytokine secretion is not constant throughout the day. IL-6 is known to have a biphasic secretion pattern in healthy adults, and the secretion of proinflammatory cytokines can be transiently stimulated or suppressed by many phenomena, including stress [61, 62]. In the absence of an integrated measure of cytokine levels throughout the day, the interpretation of these results is difficult.
In summary, this meta-analysis showed that overall plasma levels of IL-1β are reduced in OCD patients relative to controls, while there is no overall difference in TNF-α and IL-6 plasma levels. Additionally, moderating effects of age, mood, and medication appear to have an effect on the plasma levels of TNF-α and IL-6. Finally, the relatively few number of studies of CSF cytokine levels and ex vivo lymphocyte production of cytokines prevented us from performing a meta-analysis in the area. These limited studies suggested that IL-6 production may be decreased ex vivo in OCD, while TNF-α and IL-1β remain unchanged. Future research evaluating immune function in OCD should control for potential confounding of comorbid depression and medication use, involve larger sample sizes, and involve multiple sample collections from subjects to adjust for within-subject variation. Further studies on cytokine response to stress in OCD and measuring CSF cytokine levels in OCD would be of significant value to the field.
Acknowledgments
The authors acknowledge the National Institute of Mental Health support of the Yale Child Study Center Research Training Program (Dr. Bloch), the National Institutes of Health (NIH) 1K23MH091240 (Dr. Bloch), the APIRE/Eli Lilly Psychiatric Research Fellowship (Dr. Bloch), the AACAP/Eli Lilly Junior Investigator Award (Dr. Bloch), the Trichotillomania Learning Center (Dr. Bloch), NARSAD (Dr. Bloch), and UL1 RR024139 from the National Center for Research Resources, a component of the NIH, and NIH roadmap for Medical Research (Dr. Bloch).
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15:53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DuPont RL, Rice DP, Shiraki S, Rowland CR. Economic costs of obsessive-compulsive disorder. Medical interface. 1995;8:102–109. [PubMed] [Google Scholar]
- 4.First MB. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 5. de Oliveira S, Pelajo C. Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infection (PANDAS): a Controversial Diagnosis. Current Infectious Disease Reports. 2010;12:103–109. doi: 10.1007/s11908-010-0082-7. This review highlights important criticism of the PANDAS hypothesis.
- 6.da Rocha FF, Correa H, Teixeira AL. Obsessive-compulsive disorder and immunology: a review. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32:1139–1146. doi: 10.1016/j.pnpbp.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Swedo SE, Grant PJ. Annotation: PANDAS: a model for human autoimmune disease. Journal of Child Psychology and Psychiatry. 2005;46:227–234. doi: 10.1111/j.1469-7610.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 8.Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. The American Journal of Psychiatry. 1998;155:264–271. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- 9. Murphy TK, Kurlan R, Leckman J. The Immunobiology of Tourette's Disorder, Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcus, and Related Disorders: A Way Forward. Journal of Child and Adolescent Psychopharmacology. 2010;20:317–331. doi: 10.1089/cap.2010.0043. This review provides an overview of PANDAS, including diagnostic criteria and evidence supporting the PANDAS hypothesis.
- 10.Araki K, Turner AP, Shaffer VO, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shulman ST. Pediatric autoimmune neuropsychiatric disorders associated with streptococci (PANDAS): update. Current Opinion in Pediatrics. 2009;21 doi: 10.1097/MOP.0b013e32831db2c4. [DOI] [PubMed] [Google Scholar]
- 12. Dowlati Y, Herrmann N, Swardfager W, et al. A Meta-Analysis of Cytokines in Major Depression. Biological Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. This article demonstrates that cytokine levels are altered by mood disorders, which are highly comorbid in OCD.
- 13.Maes M, Bosmans En, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 14.Penninx BWJH, Kritchevsky SB, Yaffe K, et al. Inflammatory markers and depressed mood in older persons: results from the health, aging and body composition study. Biological Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 15.Empana JP, Sykes DH, Luc G, et al. Contributions of Depressive Mood and Circulating Inflammatory Markers to Coronary Heart Disease in Healthy European Men. Circulation. 2005;111:2299–2305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- 16.Kim YK, Suh IB, Kim H, et al. The plasma levels of interleukin-12 in schizophrenia, major depression, and bipolar mania: effects of psychotropic drugs. Molecular Psychiatry. 2002;7:1107. doi: 10.1038/sj.mp.4001084. [DOI] [PubMed] [Google Scholar]
- 17.Jones AL, Mowry BJ, Pender MP, Greer JM. Immune dysregulation and self-reactivity in schizophrenia: do some cases of schizophrenia have an autoimmune basis? Immunol Cell Biol. 2005;83:9–17. doi: 10.1111/j.1440-1711.2005.01305.x. [DOI] [PubMed] [Google Scholar]
- 18.Strous RD, Shoenfeld Y. Schizophrenia, autoimmunity and immune system dysregulation: a comprehensive model updated and revisited. Journal of Autoimmunity. 2006;27:71–80. doi: 10.1016/j.jaut.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Potvin Sp, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory Cytokine Alterations in Schizophrenia: A Systematic Quantitative Review. Biological Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 20. Hannestad J, DellaGioia N, Bloch M. The Effect of Antidepressant Medication Treatment on Serum Levels of Inflammatory Cytokines: A Meta-Analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. This article demonstrates that psychotropic medication alters inflammatory cytokine levels in major depressive disorder.
- 21.Murphy K, Travers P, Walport M. Janeway's Immunobiology. 7ed. New York: Garland Science; 2008. [Google Scholar]
- 22.Steinke JW, Borish L. 3. Cytokines and chemokines. Journal of Allergy and Clinical Immunology. 2006;117:S441–S445. doi: 10.1016/j.jaci.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Brough D, Tyrrell PJ, Allan SM. Regulation of interleukin-1 in acute brain injury. Trends in Pharmacological Sciences. 2011;32:617–622. doi: 10.1016/j.tips.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Konuk N, Tek #x131, et al. Plasma Levels of Tumor Necrosis Factor-Alpha and Interleukin-6 in Obsessive Compulsive Disorder. Mediators of Inflammation. 2007;2007 doi: 10.1155/2007/65704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan W, Zadina JE, Harlan RE, Weber JT, Banks WA, Kastin AJ. Tumor Necrosis Factor-alpha: a neuromodulator in the CNS. Neuroscience & Biobehavioral Reviews. 1997;21:603–613. doi: 10.1016/s0149-7634(96)00047-4. [DOI] [PubMed] [Google Scholar]
- 26.Schöbitz B, de Kloet ER, Sutanto W, Holsboer F. Cellular Localization of Interleukin 6 mRNA and Interleukin 6 Receptor mRNA in Rat Brain. European Journal of Neuroscience. 1993;5:1426–1435. doi: 10.1111/j.1460-9568.1993.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 27.Carlson NG, Wieggel WA, Chen J, Bacchi A, Rogers SW, Gahring LC. Inflammatory Cytokines IL-1α, IL-1β, IL-6, and TNF-α Impart Neuroprotection to an Excitotoxin Through Distinct Pathways. The Journal of Immunology. 1999;163:3963–3968. [PubMed] [Google Scholar]
- 28.Hirano T, Yasukawa K, Harada H, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 29.Spooren A, Kolmus K, Laureys G, et al. Interleukin-6, a mental cytokine. Brain Research Reviews. 2011;67:157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Tadamitsu K. Interleukin-6 and its receptor in autoimmunity. Journal of Autoimmunity. 1992;5(Supplement A):123–132. doi: 10.1016/0896-8411(92)90027-n. [DOI] [PubMed] [Google Scholar]
- 31.Xing Z, Gauldie J, Cox G, et al. IL-6 is an anti-inflammatory cytokine required for controlling local or systemic acute inflammatory responses. Journal of Clinical Investigation. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uyttenhove C, Coulie PG, Van Snick J. T cell growth and differentiation induced by interleukin-HP1/IL-6, the murine hybridoma/plasmacytoma growth factor. The Journal of Experimental Medicine. 1988;167:1417–1427. doi: 10.1084/jem.167.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lotz M, Jirik F, Kabouridis P, et al. B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes. The Journal of Experimental Medicine. 1988;167:1253–1258. doi: 10.1084/jem.167.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng H, Fletcher D, Kozak W, et al. Resistance to fever induction and impaired acute-phase response in interleukin-1β-deficient mice. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 35.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- 36.Dinarello CA. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annual Review of Immunology. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 37.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 38.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 39.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) The Journal of Immunology. 1986;137:245–254. [PubMed] [Google Scholar]
- 40.Sironi M, Breviario F, Proserpio P, et al. IL-1 stimulates IL-6 production in endothelial cells. The Journal of Immunology. 1989;142:549–553. [PubMed] [Google Scholar]
- 41.Wang CC, Fu CL, Yang YH, et al. Adenovirus expressing interleukin-1 receptor antagonist alleviates allergic airway inflammation in a murine model of asthma. Gene Ther. 2006;13:1414–1421. doi: 10.1038/sj.gt.3302798. [DOI] [PubMed] [Google Scholar]
- 42.Nakae S, Asano M, Horai R, Iwakura Y. Interleukin-1β, but not interleukin-1α, is required for T-cell-dependent antibody production. Immunology. 2001;104:402–409. doi: 10.1046/j.1365-2567.2001.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfeffer K. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine & Growth Factor Reviews. 2003;14:185–191. doi: 10.1016/s1359-6101(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 44.McCoy M, Tansey M. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. Journal of Neuroinflammation. 2008;5:1–13. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cairns CB, Panacek EA, Harken AH, Banerjee A. Bench to Bedside Tumor Necrosis Factor-alpha: From Inflammation to Resuscitation. Academic Emergency Medicine. 2000;7:930–941. doi: 10.1111/j.1553-2712.2000.tb02077.x. [DOI] [PubMed] [Google Scholar]
- 46.Graham AL, Allen JE, Read AF. Evolutionary causes and consequences of immunopathology. Annual Review of Ecology, Evolution, and Systematics. 2005;36:373–397. [Google Scholar]
- 47.Deeks J, Higgins J, Altman D. The Cochrane Library. Chinchester, UK: John Wiley & Sons, Ltd; 2004. Cochrane Reviewer's Handbook 4.2.2. [Updated March 2004] [Google Scholar]
- 48.Brambilla F, Perna G, Bellodi L, et al. Plasma Interleukin-1β and Tumor Necrosis Factor Concentrations in Obsessive-Compulsive Disorders. Biological Psychiatry. 1997;42:976–981. doi: 10.1016/s0006-3223(96)00495-7. [DOI] [PubMed] [Google Scholar]
- 49.Maes M, Meltzer HY, Bosmans E. Psychoimmune Investigation in Obsessive-Compulsive Disorder: Assays of Plasma Transferrin, IL-2 and IL-6 Receptor, and IL-1β and IL-6 Concentrations. Neuropsychobiology. 1994;30:57–60. doi: 10.1159/000119136. [DOI] [PubMed] [Google Scholar]
- 50.Monteleone P, Catapano F, Fabrazzo M, Tortorella A, Maj M. Decreased Blood Levels of Tumor Necrosis Factor-Alpha in Patients with Obsessive-Compulsive Disorder. Neuropsychobiology. 1998;37:182–185. doi: 10.1159/000026500. [DOI] [PubMed] [Google Scholar]
- 51.Leckman JF, Katsovich L, Kawikova I, et al. Increased serum levels of interleukin-12 and tumor necrosis factor-alpha in Tourette's syndrome. Biological Psychiatry. 2005;57:667–673. doi: 10.1016/j.biopsych.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Gabbay V, Coffey BJ, Guttman LE, et al. A cytokine study in children and adolescents with Tourette's disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33:967–971. doi: 10.1016/j.pnpbp.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bos-Veneman NGP, Bijzet J, Limburg PC, Minderaa RB, Kallenberg CG, Hoekstra PJ. Cytokines and soluble adhesion molecules in children and adolescents with a tic disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:1390–1395. doi: 10.1016/j.pnpbp.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 54.Weizman R, Laor N, Barber Y, et al. Cytokine production in obsessive-compulsive disorder. Biological Psychiatry. 1996;40:908–912. doi: 10.1016/0006-3223(95)00520-X. [DOI] [PubMed] [Google Scholar]
- 55.Denys D, Fluitman S, Kavelaars A, Heijnen C, Westenberg H. Decreased TNF-α and NK activity in obsessive-compulsive disorder. Psychoneuroendocrinology. 2004;29:945–952. doi: 10.1016/j.psyneuen.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Denys D, Fluitman S, Kavelaars A, Heijnen C, Westenberg HGM. Effects of paroxetine and venlafaxine on immune parameters in patients with obsessive compulsive disorder. Psychoneuroendocrinology. 2006;31:355–360. doi: 10.1016/j.psyneuen.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 57.Fluitman S, Denys D, Vulink N, Schutters S, Heijnen C, Westenberg H. Lipopolysaccharide-induced cytokine production in obsessive-compulsive disorder and generalized social anxiety disorder. Psychiatry Research. 2010;178:313–316. doi: 10.1016/j.psychres.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Fluitman SBAHA, Denys DAJP, Heijnen CJ, Westenberg HGM. Disgust affects TNF-alpha, IL-6 and noradrenalin levels in patients with obsessive-compulsive disorder. Psychoneuroendocrinology. 2010;35:906–911. doi: 10.1016/j.psyneuen.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Carpenter LL, Heninger GR, McDougle CJ, Tyrka AR, Epperson CN, Price LH. Cerebrospinal fluid interleukin-6 in obsessive-compulsive disorder and trichotillomania. Psychiatry Research. 2002;112:257–262. doi: 10.1016/s0165-1781(02)00233-0. [DOI] [PubMed] [Google Scholar]
- 60.Mittleman BB, Castellanos FX, Jacobsen LK, Rapoport JL, Swedo SE, Shearer GM. Cerebrospinal fluid cytokines in pediatric neuropsychiatric disease. The Journal of Immunology. 1997;159:2994–2999. [PubMed] [Google Scholar]
- 61.Elenkov IJ, Chrousos GP. Stress Hormones, Proinflammatory and Antiinflammatory Cytokines, and Autoimmunity. Annals of the New York Academy of Sciences. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 62.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and Its Circadian Secretion in Humans. Neuroimmunomodulation. 2005;12:131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]