Abstract
MicroRNAs (miRs) are non-coding RNAs that inhibit expression of their targets in a sequence-specific manner and play crucial roles during oncogenesis. Here we show that miR-7 inhibits p21-activated kinase 1 (Pak1) expression, a widely upregulated signaling kinase in multiple human cancers including breast and gliomas, by targeting the 3′-UTR of Pak1 mRNA. We noticed an inverse correlation between the levels of endogenous miR-7 and Pak1 expression in human cancer cells. We discovered that endogenous miR-7 expression is positively regulated by a homeodomain transcription factor HoxD10, loss of which leads to an increased invasiveness. The HoxD10 directly interacts with the miR-7 chromatin. Accordingly, the levels of Pak1 protein are progressively upregulated while that of miR-7 and its upstream activator HoxD10 are progressively downregulated in a cellular model of breast cancer progression from low to highly invasive phenotypes. Furthermore, HoxD10 expression in highly invasive breast cancer cells resulted in an increased expression of miR-7 but a reduced Pak1 3′UTR-luciferase activity as well as reduced Pak1 protein. Finally, we show that miR-7 introduction inhibits the motility, invasiveness, anchorage-independent growth and tumorigenic potential of highly invasive breast cancer cells. Collectively, these findings establish for the first time that Pak1 is a target of miR-7 and that HoxD10 play a regulatory role in modifying the expression of miR-7, and consequently, functions of miR7 - Pak1 pathway in human cancer cells.
Introduction
MicroRNAs (miRs) are endogenous, non-coding RNAs that regulate gene expression in a sequence-specific manner in plants and animals. MiRs are derived from long transcripts which undergo trimming by Dicer and Drosha complexes into mature miRs before feeding in to the RNA interference pathway (1). In addition to cleaving mRNA molecules, most of the animal miRs pair with their target sequence in the 3′UTR regions of mRNA with imperfect complimentarity and leads to interference with mRNA translation (2). In the recent years, it is becoming apparent that many miRs play crucial roles during development and oncogenesis (1-6). MiRs genes are present in the cancer associated genomic regions or in fragile sites (3). In general, miRs are believed to be part of network wherein a modest change in the level of one will set-up a chain reaction and feed-back pathways involving multiple miRs and affecting multiples targets of the same of different pathways (7). Many miRs are implicated as tumor suppressors or as oncogenes and found to be down- or up-regulated in human tumors, including breast cancer (2-6). In fact, there is considerable excitement about the prospect of using miRs for anti-cancer therapy (8).
The progression of number of human cancers, including breast cancer to more invasive phenotypes and invasiveness of experimental model systems have been linked by the upregulation and activation of p21-activated kinase 1 (Pak1) (9, 10). The Pak family of serine/threonine kinases plays a pivotal role in physiological processes including motility, survival, invasion, and mitosis (10). Paks are widely expressed in a variety of tissues and are often up-regulated or hyper-activated in a variety of human cancers such as breast (9, 10) and gliomas (11). Due to the signal-dependent or constitutive hyperactivation of Pak1, there is an increased emphasis in designing specific inhibitors to interfere with its activation process (12-14). Given the diversity and overlapping nature of Pak regulators and effectors, it is likely that some of these inhibitors will have to overcome the expected problem of specificity and redundancy before clinical development.
In-spite of an established role of Pak1 in normal physiologic or cancerous states, it remains unclear whether Pak1 is targeted by miRs in cancer cells. By computational microRNA prediction tool mirBASE (15), we have identified hsa-mir-7-1 (termed miR-7) as a potential miRNA for targeting Pak1 mRNA. MiR-7 is an intronic miRNA that resides in the first intron of heterogenous ribonuclear protein K gene on chromosome 9 and is conserved across all species. In Drosophila photoreceptor cells, miR-7 controls EGFR signaling and promotes photoreceptor differentiation (16). More recently, miR-7 has been shown to inhibit EGFR expression in glioblostoma (17). We have now discovered an inverse correlation between the levels of endogenous miR-7 and Pak1 expression and that miR-7 downregulates Pak1 expression by targeting the 3′-UTR of Pak1 mRNA and also inhibit the invasiveness of cancer cells. Here we have also defined the cis acting elements in the miR-7 promoter region involving positive regulation by a homeodomain transcription factor HoxD10, a gene product responsible for the invasiveness of cancer cells (6, 18).
Materials and Methods
Cell lines, culture conditions and Transfections
Human cell lines were cultured as described in supplementary methods. Transfections for miRNA mimics and plasmids constructs were done with Oligofectamine or Fugene as described in the Supplementary methods.
Plasmid constructs, Luciferase assay and Western blotting
Pak1 3′- UTR and microRNA-7 promoter region were cloned into pGL3 control vector and pGL3 basic promote less vector respectively as described in supplementary methods. Luciferase assays and western blotting were done as described in the Supplementary methods.
Quantitative real-time PCR analysis of microRNAs
RNA was isolated using mirVana miRNA isolation kit. For quantitative analysis of miRNAs, two-step TaqMan real-time PCR analysis was performed using primers and probes obtained from Applied Biosystems.
Migration, Soft-agar and Confocal Studies
Migration, Invasion, Soft agar assays and Confocal studies were done as described in the Supplementary methods
Chromatin immunoprecipitation (ChIP)
Chromatin immunoprecipitation (ChIP) was performed as described in the Supplementary methods.
Results and Discussion
miR-7 targets 3′-UTR of Pak1 and inhibits its expression
In order to search for microRNAs that might regulate the Pak1 expression, we screened the 3′ -UTR region of human Pak1 mRNA against the public database for possible complementation of a minimum of 8-bp to the seed region of miRNAs (15). This exercise resulted in identification of miR-7 and miR-465 as candidate micro-RNAs for Pak1. MiR-7 is human miRNA (Fig. 1A) while miR-465 is of murine origin and both were predicted to target Pak1 in multiple species cells (Supplementary Fig. 1A). To directly evaluate the effects of miR-7 and -465 on Pak1 expressions, a number of human cancer cell lines were transfected with miR-7 or miR-465 or negative control (designated miR-con) miRNA mimics and expression of Pak1 levels were determined by the Western blotting. MiR-7 and miR-465 inhibited the levels of Pak1 protein and not Pak2, actin or vinculin (Fig. 1B and Supplementary Fig. 1B). To show the direct targeting of the 3′-UTR region by the miRs, we next cloned the 3′-UTR region of Pak1, complementary to miR-7 or miR-465, into the pGL3-luciferase reporter (Pak1 3′UTR-luc). We found that transfection of miR-7 or miR-465 along with respective Pak1 3′-UTR-luc into various human cancer cell lines resulted in a significant inhibition of luc-activity from the reporter, while there was no such inhibitory effect of the control miR-con (Fig. 1C and Supplementary Fig. 1C). These findings suggested that miR-7 or miR-465 directly targets 3′UTR of Pak1 and selectively inhibits the Pak1 expression. To assess the relationship between the endogenous levels of Pak1 and its miRs, we next determined the levels of miR-7 and miR-465 by quantitative polymerase chain-reaction (q-PCR) as well as Pak1 protein in a variety of exponentially growing cancer cell lines. In general, cells with increased levels of miR-7 contain reduced levels of Pak1 protein as compared to cells with low levels of miR-7 and high levels of Pak1 (Fig. 1D). Inspite of repeated attempts, we were unable to detect miR-465 in human cells (data not shown), presumably due to its restricted expression in murine systems. Therefore, we have studied the regulation of Pak1 expression and functions by miR-7 in the subsequent studies.
Fig. 1. miRNA-7 inhibits Pak1 expression.

A, Sites of miRNA-7 seed matches in the Pak1 3′UTR. B, Western blot of Pak1, Pak2 and actin in HeLa, ZR-75 and MDA-231 cells after 48 hr of transfection with miR-7 or miR-con. C, normalized Pak1 3′UTR-luc reporter activity in HeLa, ZR-75 and HEK-293 cells after 48 hr of transfection of miR-7 or miR-con. Mean of three experiments; bars, SD. D, Western blot for Pak1 expression and relative expression of miR-7 by q- PCR in the indicated cell lines. Mean of three experiments; bars, SD.
MiR-7 is directly regulated by the transcription factor HoxD10
To gain insights into the mechanisms by which miR-7 might be regulated in physiologic setting, we analyzed the upstream 2 kb-region of the putative miR-7 promoter for the presence of binding motifs for various transcription factors using the PROMO (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3 and http://www.fruitfly.org/seq_tools/promoter.html) search programs. This analysis suggested that miR-7 resides within the first intron of heterogenous ribonuclear protein K gene on chromosome 9. We found the presence of two perfect consensus motifs for HoxD10 within the -958 to -968 and -1019 to -1028 from the transcriptional initiation site of miR-7 (Fig. 2A). Since HoxD10 has been intimately linked with the invasion and metastatic potential of human breast cancer cells (6, 18) and because HoxD10 expression is lost as a function of invasion (18) and reintroduction of HoxD10 restores non-tumorigenic phenotypes in invasive breast cancer cells (18), we decided to focus on HoxD10 as a potential upstream modular of miR-7 expression.
Fig. 2. HoxD10 regulation of the miR-7 promoter.

A, Schematic representation of miR-7 putative promoter with the binding sites for HoxD10 and Sp1. B, Normalized pGL-miR-7-luc activity in the HeLa cells. Mean of three experiments; bars, SD. C, Normalized pGL-miR-7-luc activity in the indicated cells transfected with HoxD10 or pCMV control vector. Mean of three experiments; bars, SD. D, Recruitment of Myc-HoxD10 onto the miR-7 gene chromatin (240 bp, -1139 to − 899) by ChIP in the HEK-293 and MCF10-AT cells.
To study the impact of HoxD10 on the expression of miR-7, we next cloned the putative miR-7 promoter into a TATA-less basic pGL3-luc reporter (pGLmiR-7) and established the functionality of pGLmiR-7 in the HeLa cells (Fig. 2B). We next demonstrated that transient expression of HoxD10 efficiently but not control vector stimulates the transcription of miR-7 from the pGLmiR-7 reporter in multiple human cancer cell lines (Fig. 2C). To demonstrate the potential recruitment of HoxD10 to the miR-7 gene, we next performed chromatin immunoprecipitation analysis (ChIP) of the miR-7 chromatin. Due to the lack of a good ChIP-grade HoxD10 antibody, we used a myc-tagged HoxD10 expression vector in these studies. We found that Myc-HoxD10 is indeed, recruited to miR-7 promoter region encompassing from -1139 to −899 (240 bp) which included both HoxD10 binding sites. PFK promoter was used as a negative control, which lacks HoxD10 binding sites (Fig. 2D). Together, these observations suggested that HoxD10 directly interacts with the putative miR-7 promoter and that miR-7 expression is positively regulated by HoxD10 transcription factor.
HoxD10 regulation of Pak1 expression via miR-7
Since miR-7 targets Pak1 (this study) and because HoxD10 positively regulates the expression of miR-7 (this study) and the fact that the loss of HoxD10 (6, 18) is a major contributor of cancer cell invasiveness which is also driven by Pak1 (10, 19), the above findings suggested an interesting possibility of a dynamic relationship between the Pak1, miR-7 and HoxD10, and potential role of HoxD10 in the cellular action of the miR7-Pak1 axis in cancer cells. To examine these possibilities in the context of breast cancer progression to more aggressive phenotypes, we next analyzed the levels of Pak1, miR-7, and HoxD10 in exponentially growing cell lines derived from the MCF-10A model system (20). This model consists of four isogenic, human breast cancer cell lines with distinct invasive and metastatic potential. For example, MCF-10A cells are non-malignant human breast cancer cells; MCF10AT cells are weakly tumorigenic; MCF-10CA cells are undifferentiated metastatic carcinomas; and MCF10DCIS cells are highly proliferative, aggressive and invasive in nature (20, 19). Interestingly, we found that the levels of Pak1 protein progressively upregulated while that of miR-7 as well as HoxD10 are progressively downregulated from low invasive MCF10A to highly invasive MCF-10DCIS cells (Fig. 3A). To validate these results, we next showed that transient over expression of HoxD10 leads to upregulation of miR-7 (Fig. 3B) and downregulation of the Pak1 3′UTR-luc activity in the highly invasive MCF-10DCIS cells as well as in the HEK-293 cells (Fig. 3C). The noticed regulation of miR7 and Pak1-UTR activity by HoxD10 was also accompanied by a corresponding down regulation of Pak1 protein in HEK-293 and MCF10DCIS cells (Fig. 3D). In brief, these results suggested a regulatory role of HoxD10 in modifying the expression of miR-7, and consequently, functions of miR-7-Pak1 pathway in human cancer cells.
Fig. 3. Dynamic correlation of Pak1, miR-7 and HoxD10 in breast cancer progression model.

A, Western blot analysis of Pak1 and HoxD10, and q-PCR analysis of the endogenous miR-7 and HoxD10 is MCF10A model system. B, Effect of HoxD10 expression on the levels of miR-7 in MCF-10DICS and HEK-293 cells. Mean of three experiments; bars, SD. C, Normalized Pak1 3′UTR-luc activity in MCF10DCIS and HEK-293 cells transfected with HoxD10 or control vector. Mean of three experiments; bars, SD. D, Western blot analysis of Pak1 and vinculin in MCF10-DCIS and HEK-293 cells transfected with HoxD10 or control vector.
MiR-7 suppresses motility, invasiveness and anchorage-independence of breast cancer cells
To further delineate the potential effects of miR-7 downregulation of Pak1 on the biology of breast cancer cells, we next examined the effects of miR-7 on the biology of MDA-MB231 cells, an established model system for highly invasive and tumorigenic breast cancer cells. We found that miR-7-mediated downregulation of Pak1 in MDA-MB231 cells were accompanied by a profound inhibition of the cell motility, the cell invasiveness, the ability of cells to grow in an anchorage-independent manner and inhibited tumorigenic potential in nude mice (Fig. 4A-C). These findings raise the possibility that miR-7 could be potentially developed as an agent to downregulate the expression, and consequently, tumor-promoting functions of Pak1. This is important as Pak1 or its effectors have been shown to be upregulated in a number of human cancers, including breast and gliomas (10-12). Accordingly, a number of academic groups and pharmaceutics have initiated research programs to target Pak1 kinase activity for cancer therapy (13, 14).
Fig. 4. Biologic effects of miR-7 in breast cancer cells.
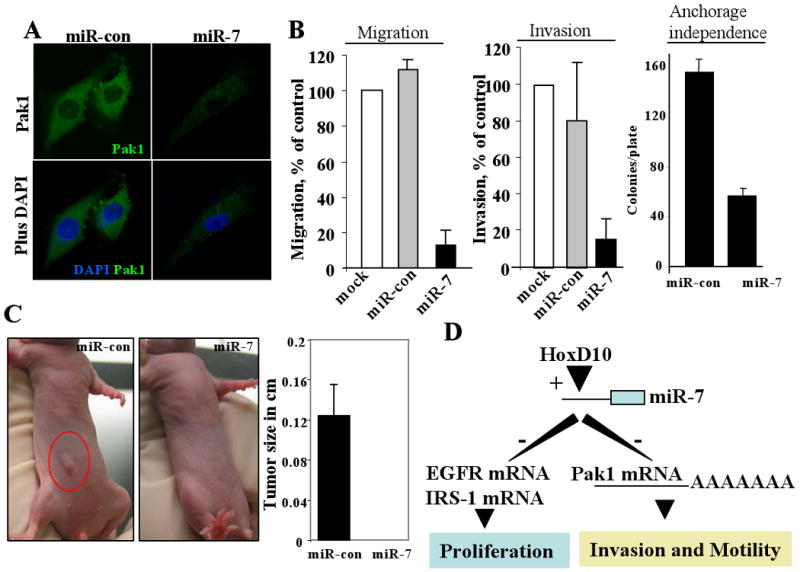
A, Confocal analysis of Pak1 expression in miR-7 transfected MDA-231 cells. B, Effect of miR-7 or miR-con on the motility, invasiveness, and anchorage-independence of the MDA-231 cells. C, Effect of miR-7 on the tumorigenic potential of MDAMB-231 cells in nude mice. D, An integrated working model of the findings presented here.
While this study was near completion, we noticed an interesting report describing the ability of miR-7 to also inhibit the EGFR and IRS1 by directly targeting the 3′-UTR regions of its mRNAs (17). This is understandable but exciting, given the fact that miRs are expected to target multiple mRNAs with desired complementary base-pair matching in their 3′-UTR, and thus, reaffirming the emerging regulatory role of miR-7 in cancer cells. Furthermore, microRNAs are expected to work as part of network and could affect the components of the same pathway at multiple levels (7). Since Pak1 signaling is also affected EGFR (10) and Akt (a target of IRS1), we next examined the levels of EGFR and IRS-1 proteins in the cell lysates used above in this study. As expected, transfection of miR-7 downregulates the levels of EGFR and IRS1 proteins in the Hela, ZR-75 and MDA-231 cells (Supplementary Fig. 2). We further observed that the levels of EGFR and IRS1 correlate well with the levels of Pak1 but inversely related with the endogenous miR-7 status in human cell lines with the exception of IRS1 in the MCF-10A model system (Supplementary Fig. 3A, B). Interestingly, we found that HoxD10 overexpression in the HEK-293 or MCF-10DCIS cells also leads to a distinct downregulation of EGFR and IRS-1 (Supplementary Fig. 4), presumably due to increased expression of miR-7 (this study, Fig. 3B).
In summary, the findings presented here in conjunction of recent reports on miR-7 and HoxD10 suggest a model wherein HoxD10, a known regulator of cancer invasiveness (6, 18), is likely to control the levels of Pak1 protein (and of EGFR and IRS1 and perhaps, other targets yet to be described) and thus, impact the cancerous phenotypes, via miR-7 pathway. Since reintroduction of HoxD10 into MDA-MB231 cells have been previously shown to compromise invasiveness (18), these results now suggest a potential role of the noted reduction in the Pak1 level due to increased expression of miR-7. In addition, our present findings also connect the presently described HoxD10/miR-7/Pak1 pathway with a recently described role of invasion promoting role of Twist regulation of miR-10B which in-turn, has been shown to inhibit the translation of HoxD10 (6). In the light of observations presented here, it is possible that inhibition of HoxD10 will lead to repression of miR-7 level and upregulation of Pak1 expression and its functions (Fig. 4D). In order to have a comprehensive understanding of the emerging significance of microRNA-network and engagement of multiple targets in shaping the behavior of the cancer cells, it will be important to keep investigating the above hypotheses and testing the lead microRNA in more complex model systems in due course.
Supplementary Material
References
- 1.Tomari Y, Zamore PD. Perspective: machines for RNAi. Genes Dev. 2005;19:517–529. doi: 10.1101/gad.1284105. [DOI] [PubMed] [Google Scholar]
- 2.Lai EC. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 3.Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 6.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 7.Makeyev EV, Maniatis T. Multilevel regulation of gene expression by microRNAs. Science. 2008;319:1789–1790. doi: 10.1126/science.1152326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czech MP. MicroRNAs as therapeutic targets. N Engl J Med. 2006;354:1194–1195. doi: 10.1056/NEJMcibr060065. [DOI] [PubMed] [Google Scholar]
- 9.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 11.Holm C, Rayala S, Jirstrom K, Stal O, Kumar R, Landberg G. Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J Natl Cancer Inst. 2006;98:671–680. doi: 10.1093/jnci/djj185. [DOI] [PubMed] [Google Scholar]
- 12.Aoki H, Yokoyama T, Fujiwara K, et al. Phosphorylated Pak1 level in the cytoplasm correlates with shorter survival time in patients with glioblastoma. Clin Cancer Res. 2007;13:6603–6609. doi: 10.1158/1078-0432.CCR-07-0145. [DOI] [PubMed] [Google Scholar]
- 13.Deacon SW, Beeser A, Fukui JA, et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porchia LM, Guerra M, Wang YC, et al. 2-amino-N-{4-[5-(2-phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-phe nyl} acetamide (OSU-03012), a celecoxib derivative, directly targets p21-activated kinase. Mol Pharmacol. 2007;72:1124–1131. doi: 10.1124/mol.107.037556. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Carthew RW. A microRNA mediates EGF receptor signaling and promotes photoreceptor differentiation in the Drosophila eye. Cell. 2005;123:1267–1277. doi: 10.1016/j.cell.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 17.Kefas B, Godlewski J, Comeau L, et al. microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res. 2008;68:3566–3572. doi: 10.1158/0008-5472.CAN-07-6639. [DOI] [PubMed] [Google Scholar]
- 18.Carrio M, Arderiu G, Myers C, Boudreau NJ. Homeobox D10 induces phenotypic reversion of breast tumor cells in a three-dimensional culture model. Cancer Res. 2005;65:7177–7185. doi: 10.1158/0008-5472.CAN-04-1717. [DOI] [PubMed] [Google Scholar]
- 19.Vadlamudi RK, Bagheri-Yarmand R, et al. Dynein light chain 1, a p21-activated kinase 1-interacting substrate, promotes cancerous phenotypes. Cancer Cell. 2004;5:575–585. doi: 10.1016/j.ccr.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Heppner GH, Miller FR, Shekhar PM. Nontransgenic models of breast cancer. Breast Cancer Res. 2000;2:331–334. doi: 10.1186/bcr77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


