Abstract
Functionally related genes are coregulated by specific RNA–protein interactions that direct transcript-selective translational control. In myeloid cells, interferon (IFN)-γ induces formation of the heterotetrameric, IFN-γ-activated inhibitor of translation (GAIT) complex comprising glutamyl-prolyl tRNA synthetase (EPRS), NS1-associated protein 1 (NSAP1), ribosomal protein L13a and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). This complex binds defined 3′ untranslated region elements within a family of inflammatory mRNAs and suppresses their translation. IFN-γ-dependent phosphorylation, and consequent release of EPRS and L13a from the tRNA multisynthetase complex and 60S ribosomal subunit, respectively, regulates GAIT complex assembly. EPRS recognizes and binds target mRNAs, NSAP1 negatively regulates RNA binding, and L13a inhibits translation initiation by binding eukaryotic initiation factor 4G. Repression of a post-transcriptional regulon by the GAIT system might contribute to the resolution of chronic inflammation.
Transcript-selective translational control of gene expression
Gene expression is regulated at multiple synthetic and degradative steps, including transcription, splicing, mRNA transport, mRNA stability, translation, protein stability and post-translational modification. In many cases, translational control has evolved as an ‘off-switch’ mechanism to modulate protein expression because it exhibits key regulatory advantages. Foremost is the rapid responsiveness of the translational ‘braking’ mechanism due to its location as the final step in the process of genetic information transfer. Also, because translational control is non-destructive, that is, mRNA levels are unaltered, it can act as a ‘rheostat’, rapidly dialing up or down the level of a specific protein in a condition-dependent way. Finally, the decoupling of translational ‘off-switches’ from (mostly) transcriptional ‘on-switches’ permits negative regulation even in the continued presence of initiating factors. In the interferon (IFN)-γ-activated inhibitor of translation (GAIT) system, a single inflammatory stimulus (IFN-γ) activates both transcriptional ‘on-switches’ and translational ‘off-switches’ in a temporally staged manner.
Translational control can be subdivided into two major classes: global and transcript-selective. Global mechanisms regulate the expression of the majority of transcripts, generally by modifying an essential translation factor [1]. By contrast, transcript-selective mechanisms permit the translational regulation of a group of functionally-related mRNAs and are mediated proteins to cognate cis-elements in the 5′ or 3′UTRs (untranslated regions) of target transcripts [2,3]. In eukaryotic cells, initiation is often the regulated step, and protein–mRNA interactions generally repress, rather than activate, translation [4,5]. Early studies focused on the 5′UTR as the site of translational control: for example, ferritin mRNA translation is inhibited by binding of iron regulatory protein to the 5′UTR iron-responsive element. More recently, investigators have recognized the special importance of the 3′UTR in regulating translation by trans-acting proteins and by microRNAs [2,3,6]. The mean length of human 3′UTRs is nearly four times greater than that of human 5′UTRs [7]. Moreover, mean 3′UTR length increases with evolutionary ‘complexity’, but mean 5′UTR lengths remain essentially constant [2]. These observations point towards the enormous potential of 3′UTR-based translational control, particularly in vertebrates. Here, we describe recent studies that have revealed a 3′UTR-mediated, cell-type-specific mechanism to suppress the translation of a family of inflammation-associated transcripts in response to IFN-γ.
Translational control by the GAIT pathway
Monocytes and macrophages provide an early line of defense against infection or injury. They are activated by cytokines to produce and secrete proteins and small molecules that can kill invading microorganisms. Overexpression of these toxic agents can be detrimental to host cells and tissues, contributing to chronic inflammatory disease. Therefore, host responses must be strictly controlled to limit the expression of injurious molecules. The ‘resolution of inflammation’ is an active program of regulated gene expression by which pro-inflammatory processes are restricted after neutralization or elimination of the initial insult [8]. Thus monocytes or macrophages, and their agonists, for example IFN-γ, can exhibit either pro- or anti-inflammatory responses depending on the context [9,10]. Early studies from our laboratory showed marked transcriptional induction of ceruloplasmin (Cp), an acute phase inflammatory protein, in human monocytes upon IFN-γ treatment [11]. However, Cp synthesis, as measured by pulse-labeling, was terminated almost completely after ~16 h, despite the presence of abundant Cp mRNA, a result indicative of translational inhibition. Total protein synthesis was unaltered, indicating transcript-selective inhibition. The inhibition was cell-type specific and observed only in myeloid cells (and related cell lines) [12]. The shift of Cp mRNA from the polyribosomal to non-polyribosomal pool indicated that inhibition occurred at the level of translation initiation.
Analysis of the Cp 3′UTR GAIT element
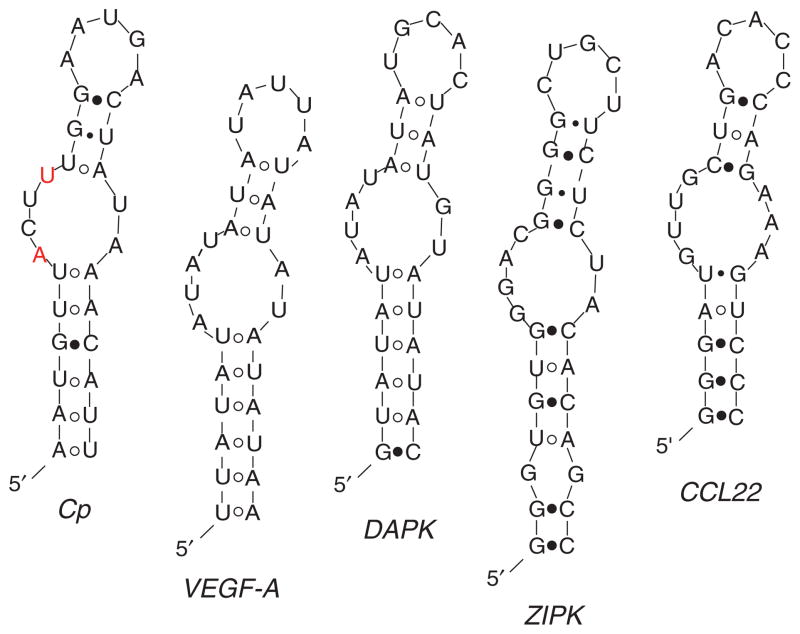
An RNA gel shift assay demonstrated that the Cp 3′UTR binds an IFN-γ-inducible protein/complex, termed the GAIT complex [13]. The functionality of the Cp 3′UTR was verified by in vitro translation; ligation of the 3′UTR downstream of a luciferase reporter conferred the translational silencing response to the heterologous transcript in rabbit reticulocyte lysates supplemented with lysate from IFN-γ-treated U937 cells. From these analyses, a minimal, 29-nt Cp 3′UTR GAIT RNA element was identified. A secondary structure consisting of a stem-loop with an asymmetric internal bulge was predicted by Mfold, an RNA-folding algorithm (http://mfold.bioinfo.rpi.edu/) [14], and was confirmed by mutation analysis (Figure 1). Most loop residues could be mutated without loss of binding or silencing activity; however, two residues in the bulge loop, A84 and U87, are essential, and mutation of either completely inactivated the element. Similar functional GAIT elements containing a stem-loop with an internal bulge were identified in the 3′UTR of multiple transcripts (Figure 1; see below).
Figure 1.
Secondary structures of GAIT elements. Secondary structures of functional 3′UTR GAIT elements as predicted by Mfold: ceruloplasmin (Cp, nt 78–106), vascular endothelial growth factor-A (VEGF-A, nt 358–386), death-associated protein kinase (DAPK, nt 1141–1169), zipper-interacting protein kinase (ZIPK, nt 174–206) and chemokine C-C motif ligand 22 (CCL22, nt 433–462) mRNAs. Nucleotides essential for Cp-GAIT activity are shown in red.
Elucidation of the heterotetrameric GAIT complex
RNA affinity purification and the yeast three-hybrid approach [15] identified the four constituent proteins of the GAIT complex that binds the Cp GAIT RNA element but not an inactive mutant element. This complex is comprised of glutamyl-prolyl tRNA synthetase (EPRS), NS1-associated protein 1 (NSAP1, also known as SYNCRIP or heterogenous nuclear ribonucleoprotein [hnRNP] Q1), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and ribosomal protein L13a [16,17]. The GAIT complex assembles in two distinct steps. In the first step, which occurs ~2 h after IFN-γ treatment, EPRS is released from its residence in the tRNA multisynthetase complex (MSC) and joins NSAP1 to form an inactive pre-GAIT complex that neither binds target mRNAs nor inhibits translation (Figure 2). About 12–14 h later, ribosomal protein L13a is released from the 60S ribosomal subunit and joins GAPDH and the pre-GAIT complex to form the functional GAIT complex. Elucidation of the mechanisms by which protein constituents are inducibly released from ‘stable’ macromolecular complexes remains a particularly exciting challenge [18] (Box 1).
Figure 2.
GAIT-mediated translational control. IFN-γ treatment elicits an intricate translational regulatory pathway in which the GAIT complex modulates the expression of its target mRNAs. (i) IFN-γ induces phosphorylation and release of EPRS (green) from the tRNA multisynthetase complex (yellow). Phosphorylated EPRS (P-EPRS) interacts with NSAP1 (light red) and forms an inactive, pre-GAIT complex. (ii) Later, L13a (dark blue) is phosphorylated and released from the 60S ribosomal subunit. Phosphorylated L13a (P-L13a), with GAPDH (brown), joins the pre-GAIT complex to form the functional GAIT complex. (iii) The complex binds the GAIT element in the 3′UTR of target transcripts circularized by simultaneous interactions of poly(A)-binding protein (PABP, gray) with eIF4G (orange) and the poly(A) tail. (iv) P-L13a interacts with eIF4G in the translation initiation complex to block recruitment of the eIF3 (light blue)-containing 43S ribosomal complex subunit and repress translation. Cap-binding protein, eIF4E, is shown (dark orange).
Box 1. The depot hypothesis of large macromolecular complexes.
Remarkably, two of the four proteins in the GAIT complex are inducibly released from parent macromolecular complexes previously thought to be stable, machine-like assemblies: EPRS from the MSC and L13a from the 60S ribosome subunit [16,17]. This discovery helped to formulate the generalized ‘depot hypothesis’, which holds that large macromolecular complexes can act as ‘depots’ or ‘reservoirs’ for regulatory proteins inducibly released by cellular signals to perform non-canonical functions distinct from those of the parent complexes [18]. The depot hypothesis expands the accepted concept that many (if not most) proteins are multifunctional, exhibiting secondary ‘moonlighting’ functions distinct from its ‘principal’ (or at least best characterized) function [62]. Moreover, certain proteins found in large macromolecular complexes, for example S19 from the ribosome and COP9 signalosome subunit 5 (CSN5, also called Jun activation domain-binding protein or JAB1), can exhibit distinct functions in isolation from the parent complex [63–65]. The novel concept in the depot hypothesis is that daughter proteins are released from the parent macromolecular complex and not derived from an existing pool or from de novo synthesis. Recent studies support the generality of the hypothesis as two other MSC components might undergo stimulus-dependent release: aminoacyl-tRNA synthetase-interacting, multifunctional protein 2 (AIMP2) and lysyl-tRNA synthetase (KRS) [66,67]. Although the molecular mechanisms underlying the release of EPRS and L13a from the MSC and 60S ribosome, respectively, remain unknown, recent insights from structural biology have provided clues. The X-ray crystallographic structure of archaeal 50S ribosomal subunit indicates that L13, the archaeal homolog of human L13a, is localized exclusively on the rRNA surface of the 60S subunit, with minimal penetration into the RNA and little contact with nearby proteins [68]. Similarly, cryo-electron microscopy has revealed EPRS at the MSC exterior, consistent with inducible release [69]. A phosphorylation-dependent alteration in L13a and EPRS conformation might be responsible for a protein–RNA or protein–protein ‘uninteraction’, which frees the protein from the parent macromolecular complexes for auxiliary functions.
Regulation of assembly and function of the GAIT nanomachine
IFN-γ-dependent assembly of the heterotetrameric GAIT complex is tightly regulated. Phosphorylation of both EPRS and L13a are essential events in the formation of a functional GAIT complex. Moreover, the complex is subject to both negative and positive regulation.
Phosphorylation-dependent release of EPRS from the tRNA MSC
EPRS has a crucial role in the GAIT pathway because it recognizes and directly binds GAIT element-bearing target mRNAs [17]. EPRS is a unique, bifunctional tRNA synthetase that consists of two catalytic cores, which catalyze the ligation of Glu and Pro to cognate tRNAs [19,20]. The catalytic domains are joined by a noncatalytic linker containing three tandem WHEP-TRS (or WHEP) domains (named after three tRNA synthetases that contain them, i.e. Trp(W)RS, His(H)RS and EPRS). The function of WHEP domains is unknown, but they bind RNA and protein with low affinity and specificity [19,20]. The presence of EPRS WHEP domains in nearly all metazoan species examined to date suggests an important function that has been retained for half a billion years or more [21,22]. The absence of WHEP domains in the separate ERS and PRS enzymes in bacteria and yeast indicates that they are not essential for synthetase activity but instead might be involved in a noncanonical function of the enzyme.
EPRS phosphorylation at one or more Ser residues is the earliest IFN-γ-inducible event in activating the GAIT translational silencing pathway. Phosphorylation induces EPRS release from the MSC and facilitates binding to cytoplasmic NSAP1; Ser kinase inhibitors prevent EPRS release and its interaction with NSAP1 [17]. Earlier studies showed that tRNA synthetase phosphorylation has little or no effect on synthetase activity [23], supporting a potential role for post-translational modifications in regulating synthetase noncanonical functions.
EPRS–target mRNA binding is subject to negative and positive regulation
EPRS binds the GAIT RNA element but, intriguingly, is unable to do so until ~14 h after its release from the MSC, and a domain analysis of EPRS binding activities provided an important clue as to why. Deletion analysis showed that the upstream pair of WHEP repeats (R1 and R2) in the EPRS linker directed phosphorylation-independent, high-affinity binding to Cp GAIT element RNA [24]. A similar analysis revealed that NSAP1 also bound EPRS in the linker domain; however, binding was phosphorylation-dependent and required the downstream pair of WHEP repeats (R2 and R3). Thus NSAP1 and GAIT element RNA share overlapping binding sites on EPRS, suggesting that NSAP1 might inhibit EPRS–RNA binding. Surface plasmon resonance (SPR) experiments with recombinant, purified GAIT constituent proteins revealed that high-affinity binding of phosphorylated EPRS to GAIT element RNA was completely blocked by pre-incubation with NSAP1. NSAP1 did not bind non-phosphorylated EPRS or inhibit its binding to RNA. Thus, EPRS phosphorylation is not required for binding to the GAIT RNA but is essential for NSAP1-mediated negative regulation.
The presence of NSAP1 inhibitory activity raised a question, namely, how is inhibition overcome 16 h after IFN-γ treatment? The restoration of RNA binding by the remaining components of the GAIT complex was investigated. Addition of either GAPDH or phosphorylated L13a (P-L13a) was ineffective; however, simultaneous addition of both proteins restored EPRS binding to GAIT element RNA even in the presence of NSAP1 [24]. Comparable reconstitution of translational inhibition activity in vitro established that the functional GAIT complex requires only these four proteins [24].
The mechanism underlying regulation of the GAIT complex was investigated by measuring Förster resonance energy transfer (FRET) between NSAP1 and phosphorylated EPRS linker, both fluorescently-labeled at their N-termini. Addition of phospho-L13a and GAPDH induced a conformational shift that increased the distance between NSAP1 and the N-terminus of EPRS linker by ~9 Å [24], a shift that might be sufficient to move NSAP1 away from the upstream WHEP domains and permit RNA binding. We expect that elucidation of the specific EPRS phosphorylation sites and detailed structural analysis of GAIT constituents and their interaction sites will provide additional insight into the conformational switches that regulate GAIT complex activity.
Phosphorylation of ribosomal protein L13a and release from the 60S ribosomal subunit
Phosphorylation of L13a ~12–16 h post-IFN-γ treatment is obligatory for L13a release from the large, 60S ribosomal subunit and is a crucial rate-determining event in GAIT pathway activation [16,25]. Remarkably, nearly the entire cellular complement of L13a exits the ribosome without detectable inhibition of global protein synthesis [26]. Mass spectrometry and mutational studies revealed that IFN-γ-inducible L13a phosphorylation occurs only on Ser77 [25] in an RXXS/T consensus kinase recognition motif [27–29]. In vitro and in vivo screening studies identified zipper-interacting protein kinase (ZIPK also known as death-associated protein kinase-3 or DAPK3), a member of the DAPK family, as the proximal kinase for L13a phosphorylation [25]. Another member of the same family, DAPK1 (referred to as DAPK) was identified as an upstream activator of ZIPK, indicating a mini DAPK–ZIPK signaling cascade, consistent with reports that DAPK can activate ZIPK [30].
Mechanism of L13a-mediated translational inhibition
Binding of proteins (or microRNAs) to 3′UTRs influences translation initiation at the distant 5′UTR. The ‘closed-loop’ model of mRNA translation provides a crucial clue. According to this model, an interaction between the 3′UTR and 5′UTR improves translation efficiency by ensuring that ribosomes released at the 3′ end after the completion of protein synthesis are close to the 5′ end to enable initiation of a new round of synthesis. In this model, the transcripts are ‘circularized’ by interaction of eukaryotic initiation factor 4G (eIF4G) at the 5′ terminus with poly(A)-binding protein (PABP), which binds the poly(A) tail at the 3′ terminus (Figure 2) [31]. Initial studies in the GAIT system sought to understand whether or not the GAIT complex interrupted transcript closure, thereby inhibiting protein synthesis. Despite multiple approaches, the GAIT complex was not found to disrupt any interactions at the termini [32]. Subsequent studies of the alternative hypothesis, that the GAIT complex does not impede circularization but rather depends on it, revealed that PABP depletion prevented translational silencing of a reporter transcript containing the GAIT element. Therefore, transcript circularization not only promotes efficient translation but also can be essential for transcript-selective translational control. The requirement for a poly(A) tail has been shown for translational inhibition by at least one microRNA, but the generality of this requirement for transcript closure has not been established [33,34].
Multiple mechanisms of transcript-selective translation inhibition have been shown, including inhibition of eIF4F (cap-binding complex) activity, recruitment of the 43S pre-initiation complex, scanning of the 43S complex to the initiation codon, and 60S ribosomal subunit joining [2,3,6]. L13a was considered a strong candidate for the translational repressor because its phosphorylation and release from the ribosome temporally coincided with translational silencing of target mRNAs. Indeed, P-L13a targets eIF4G, the component of the 5′ cap-binding, translation-initiation complex that makes initial contact with the ribosome via interaction with eIF3 of the 43S translation preinitiation complex (which contains the 40S small ribosomal subunit) (Figure 2). Its large size, central positioning and manifold interactions render eIF4G a conspicuous target for translational control. Studies with the eIF4G-specific protease HRV-2A (human rhinovirus-2A) complemented by deletion mapping revealed that P-L13a binds eIF4G at its eIF3-binding site [35]. This interaction blocks recruitment of the eIF3-containing 43S ribosomal complex to GAIT element-bearing mRNAs, thereby blocking translation initiation. Inactivation or degradation of eIF4G are well-established RNA-independent mechanisms to achieve global translation inhibition, for example during cellular stress [35]. However, the RNA-dependent eIF4G–P-L13a interaction provides a unique mechanism for transcript-selective translation control.
The GAIT system defines a post-transcriptional regulon of inflammatory genes
Studies from several laboratories have revealed the existence of ‘post-transcriptional regulons’, that is, functionally-related mRNAs containing structural elements in their UTRs that are specifically recognized and regulated by the same RNA-binding protein or complex [36–38]. The high cellular abundance of each of the GAIT proteins (and the robust release of L13a and EPRS from their parent complexes) suggests that sufficient GAIT complex is available for silencing multiple transcripts. Indeed, recent evidence suggests that the GAIT pathway regulates multiple pro-inflammatory mRNAs and thus forms a post-transcriptional regulon that has evolved to limit or resolve inflammation [25,39,40].
Pattern analysis identifies a GAIT element in the 3′UTR of VEGF-A and other mRNAs
PatSearch (http://www.ba.itb.cnr.it/BIG/PatSearch/), a folding energy-independent, pattern analysis algorithm, was used to search a 3′UTR database containing more than 200 000 sequences for a consensus GAIT element pattern, based on sequence and secondary structure of the human Cp GAIT element [41]. The algorithm found 55 human mRNAs with putative 3′UTR GAIT elements; only two mRNAs were predicted to contain 5′UTR GAIT elements, indicating marked positional specificity [39]. A Gene Ontology (GO)-based literature search [42] indicated that many putative GAIT-element-bearing transcripts were associated with the inflammatory response or induced by inflammatory agonists [39].
Vascular endothelial growth factor A (VEGF-A), a hallmark inflammatory protein that induces angiogenesis, was among the candidate mRNAs. Mfold, which calculates the optimal thermodynamic folding structure of RNA [43], revealed a bipartite stem-loop structure in the VEGF-A 3′UTR (nt 358–386) similar to the Cp GAIT element [13]. A series of experiments confirmed the authenticity of the VEGF-A GAIT element. The element conferred translational silencing to a heterologous reporter RNA in an in vitro translation system [39]. IFN-γ shifted VEGF-A mRNA from ribosome-rich, polysomal fractions to a ribosome-poor, ribonucleoprotein fraction. An RNA electrophoretic mobility shift assay (EMSA) ‘supershift’ experiment demonstrated an interaction with the four GAIT proteins. Moreover, suppression was not observed in L13a-deficient cells. In monocytic cells, IFN-γ induced VEGF-A protein expression after 8 h, followed by a return to the basal level after 24 h despite abundant VEGF-A mRNA, indicating delayed translational repression of endogenous VEGF-A. This translational inhibition decreased angiogenic activity of monocytic cells, as shown by reduced formation of capillary-like tubes by endothelial cells cultured in Matrigel™ [39].
Analysis of the kinase cascade (DAPK–ZIPK) responsible for L13a phosphorylation showed that expression and activity of both DAPK and ZIPK plummeted ~16 h after IFN-γ treatment, despite robust mRNA expression, suggesting the possibility of translational control [25]. The PatSearch algorithm revealed a candidate GAIT element in the human DAPK mRNA 3′UTR but not in ZIPK. Secondary structure analysis of the ZIPK 3′UTR by Mfold revealed a split stem-loop structure with some resemblance to the Cp and VEGF-A GAIT element structures (Figure 1). The crucial ‘A’ and ‘U’ nucleotides were present in the bulge of the DAPK GAIT element, as in Cp and VEGF-A, but were missing from the ZIPK GAIT element. Functionality of both the DAPK and ZIPK elements was shown by translational silencing of a heterologous reporter in vitro, by RNA EMSA and by supershift analysis [25]. Transfection of antisense morpholino oligomers complementary to the GAIT elements of both kinases prevented their translational silencing, presumably by blocking GAIT complex binding. The morpholinos maintained long-term expression of the kinases as well as L13a in its active, phosphorylated state. The possibility of restricting macrophage inflammatory activity using morpholinos targeted against DAPK and ZIPK presents an exciting application with potential therapeutic benefit.
Translational silencing of DAPK and ZIPK can prevent the accumulation of these potentially injurious, pro-apoptotic kinases [30,44]. More importantly, DAPK, ZIPK and L13a form a negative-feedback module in which L13a phosphorylation activates the GAIT complex, represses kinase translation and returns L13a to the non-phosphorylated form, thereby restoring the cell to the basal state, and permitting re-stimulation by IFN-γ [25]. This network module could exemplify a principle of parsimonious pathway evolution in which the principal function of an existing system, in this case translational silencing of a regulon of inflammatory transcripts, is co-opted to downregulate the initial stimulus. Likewise, negative-feedback regulation of translation has been observed for the tyrosine kinase c-Src; binding of its phosphorylated substrate, hnRNP K, to the cSrc mRNA 3′UTR inhibits expression during erythroid maturation [45,46]. Thus, inhibition of kinase mRNA translation by binding of a phosphorylated substrate to the 3′UTR might represent a new ‘network motif’ in gene expression [47].
Multiple chemokine ligand and receptor mRNAs are GAIT pathway targets
Genome-wide microarray analysis of polysome-bound mRNAs isolated from IFN-γ-activated monocytic cells identified a family of mRNAs encoding multiple chemokine ligands and receptors as candidate GAIT pathway targets [40]. Structural prediction supported this finding. The presence of functional GAIT elements in transcripts encoding chemokine ligand and receptors CCL22, CCR3, CCR4 and CCR6, and also in APOL2 (apolipoprotein L 2), was validated by their ability to repress the translation of a heterologous reporter RNA in L13a-dependent fashion [40]. Importantly, overexpression of chemokines and their receptors is a major contributor to the pathogenesis of multiple inflammatory conditions, for example atherosclerosis and neuroinflammation [48,49]. Despite the expanding cohort of GAIT-targeted transcripts, consensus on the secondary structure of the element has not yet been achieved, except that all form stems split by their asymmetric bulge loops. Likewise, information is not yet available on the relative binding affinity of the GAIT elements in these transcripts for the GAIT complex, and it remains unknown whether a silencing hierarchy exists whereby certain transcripts are more effectively silenced than others.
Physiological functions of the GAIT system
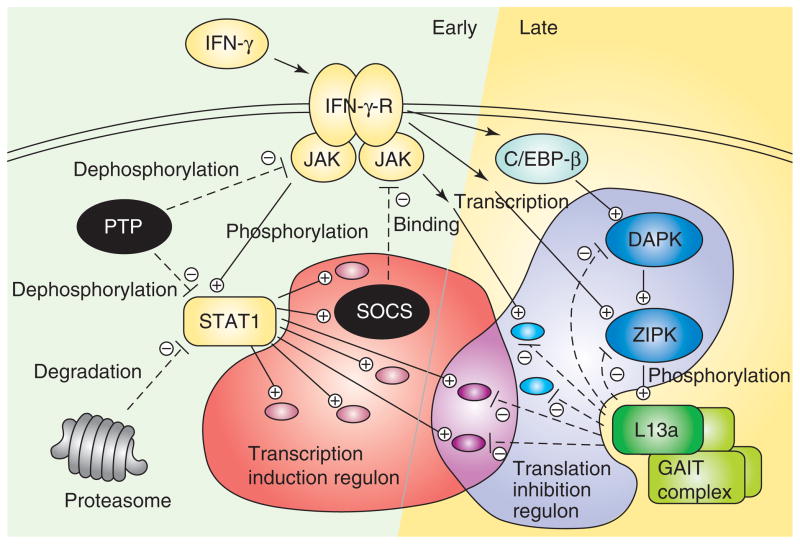
Several unique features of the GAIT-mediated anti-inflammatory system distinguish it from established signaling pathways. Perhaps most obvious is the 12–16 h lag between stimulation by IFN-γ and translational silencing of targets, attributable to the delayed activation of the DAPK–ZIPK cascade that directs L13a phosphorylation. We can envision several advantages of this delayed translational silencing mechanism. A major function of the pathway could be to prevent excessive accumulation of inflammatory proteins that can potentially cause injury to host tissue. Thus the GAIT pathway joins the list of several inhibitory mechanisms that have evolved to restrict IFN-γ-inducible inflammatory gene expression. Previously recognized negative regulatory mechanisms act at the level of transcription inhibition; these mechanisms include phosphatase-mediated inactivation of crucial receptor-activated kinases, for example JAKs (Janus kinases) and MAPKs (mitogen-activated protein kinases) [50], proteasomal degradation of the STAT1 (signal transducer and activator of transcription 1) transcription factor [51], and inhibition of JAKs by interaction with SOCS (suppressors of cytokine signaling) [52]. The GAIT-mediated, post-transcriptional mechanism provides the unique advantage of suppressing mRNAs that avoid early transcriptional blocks, for example transcripts that are highly stable or induced with delayed kinetics (Figure 3). Moreover, the GAIT pathway can silence mRNAs, which are induced by multiple IFN-γ-mediated transcriptional pathways, for example JAK–STAT or C/EBP-β (CCAAT-enhancer-binding protein-β). Early transcriptional induction by IFN-γ, followed by delayed translational suppression, is consistent with its paradoxical pro- and anti-inflammatory effects [9,10].
Figure 3.
Early and late negative regulatory pathways of IFN-γ-driven gene expression programs. STAT1 and the GAIT complex regulate early transcription-induction (light red) and late translation-inhibition (light blue) regulons, respectively, with an intersection in both regulons (light violet). Dark red ellipses within the transcription-induction regulon denote early-IFN-γ-induced transcripts repressed by negative regulators of the JAK–STAT pathway (black); violet ellipses represent stable, IFN-γ-induced transcripts that evade transcriptional inhibition but are repressed by L13a (dark green) in the GAIT complex. Light blue ellipses denote transcripts induced by JAK–STAT-independent pathways, for example transcriptional induction by C/EBP-β. Abbreviations: IFN-γ-R, IFN-γ-receptor; PTP, protein tyrosine phosphatase.
Little is known about physiological or pathological conditions that can influence GAIT pathway activity. Recent studies showed that hypoxic conditions increased the expression of the crucial angiogenic factor [53] and GAIT pathway target VEGF-A [39,54] through transcriptional upregulation, mRNA stabilization and internal ribosome entry site-mediated translation initiation [55]. Therefore, we addressed the consequences of concurrent exposure of monocytic cells to opposing regulatory signals from hypoxia and IFN-γ, stresses that are likely to exist in the cores of avascular, growing tumors or in the thickened intima of atherosclerotic lesions of blood vessels. Interestingly, hypoxia overcame GAIT-mediated VEGF-A mRNA translational silencing without disturbing formation of the active GAIT complex or silencing translation of another GAIT target, namely Cp mRNA [54]. Target-specific override of the GAIT pathway was directed by adjacent elements in the VEGF-A 3′UTR – the GAIT element and a binding site for hnRNP L functioning as a condition-dependent, binary conformational RNA switch. During normoxia, IFN-γ causes proteasomal degradation of hnRNP L and activation of the GAIT complex that stabilizes the conformer that represses VEGF-A translation. By preventing degradation and allowing binding of hnRNP L, hypoxia switches the UTR to the conformer that maintains high-level translation. The switching mechanism allows high-level VEGF-A expression to relieve tissue hypoxia while simultaneously permitting GAIT-mediated repression of harmful inflammatory proteins [54].
IFN-γ-mediated suppression of inflammatory gene expression implicates the DAPK–ZIPK–L13a axis in negative regulation of the inflammatory response of myeloid cells. The GAIT system can potentially fine-tune inflammatory gene expression in the presence of persistent inflammatory stimuli and also contribute to inflammation-resolution after the stimulus is eliminated. These findings provide an unexpected expansion of the function of the DAPK–ZIPK cascade, previously shown to have important roles in apoptosis and vesicle autophagy [30,44,56]. Because inflammation is an important contributor to tumorigenesis [57], the anti-inflammatory function of DAPK might contribute to its established tumor-suppressor activity [56,58]. Genetic defects in components of the GAIT pathway, or defects caused by environmental stress, might contribute to progression of chronic inflammatory disorders. Prolonged inflammatory gene expression contributes to malignant tumor progression [53,57]. Failure of tumor-associated macrophages to suppress VEGF-A expression could be particularly important because these cells regulate the angiogenic switch that drives the transition from small, dormant, avascular tumors to large, rapidly growing, vascularized tumors [59]. Thus, GAIT-mediated translational control of inflammatory transcripts might have a crucial role in protecting cells from inflammation and injury associated with tumorigenesis. In addition, recent studies suggest that genetic variation in two GAIT-pathway-related genes, GAPDH and DAPK, is associated with Alzheimer’s disease [60,61]. Elucidation of the GAIT pathway could present alternative targets for novel anti-inflammatory therapeutic strategies that, owing to their target and cell specificity, might exhibit minimal adverse side effects.
Concluding remarks
In the decade following its discovery, notable progress has been made in understanding certain aspects of the GAIT system, for example its components and their interactions, the upstream activation pathways and several downstream RNA targets. However, there remain far more questions than answers. Among the immediate and future challenges are: (i) the determination of the specific domains involved in protein–protein and protein–RNA interactions and their consequent conformational changes; (ii) the determination of the structures of the pathway intermediates as well as the holo-GAIT complex; (iii) the elucidation of the signaling pathways responsible for EPRS phosphorylation and for the 16-h delay before L13a phosphorylation; (iv) the function of the pre-GAIT complex (if any); (v) the potential role of phosphatases in pathway inactivation; (vi) the function of GAPDH; and (vii) the role of nearby RNA elements, including microRNA-binding sites. Much more experimentation is needed to understand the physiological and pathophysiological importance of the GAIT pathway. Analysis of GAIT pathway evolution could provide insights into its function, as will a comprehensive analysis of the post-transcriptional regulon. Most importantly, we look forward to results from animal models that express genetically modified GAIT proteins and from association studies of variants in GAIT-related genes with human disease. We hope that answers to these and other fundamental questions will elucidate the mechanisms and functions of the GAIT system and, furthermore, that the insight gained from their elucidation will be applicable to depot systems yet to be discovered and to other complex transcript-selective translational control systems.
Acknowledgments
Our work is supported by awards from the National Institutes of Health (to P.L.F.) and by Postdoctoral Fellowships from the American Heart Association, Ohio Valley Affiliate (to A.A. and J.J.).
Glossary
- Cp
ceruloplasmin. A copper protein produced by hepatocytes and cells of the myeloid lineage, including monocytes and macrophages
- DAPK
death-associated protein kinase. A calmodulin-regulated, serine/threonine kinase implicated in apoptosis and tumor suppression
- eIF4G
eukaryotic initiation factor 4G. A component of the cap-binding translation initiation complex that makes initial contact with the ribosome by interaction with eIF3 in the 40S-containing, 43S pre-initiation complex
- EPRS
glutamyl-prolyl tRNA synthetase. A bifunctional tRNA synthetase that catalyzes the ligation of Glu and Pro to their cognate tRNAs. EPRS and 7 other synthetases reside in the cytoplasm within the tRNA multisynthetase complex
- FRET
Förster resonance energy transfer. FRET provides a molecular ruler, accurately determining the distance between two fluorescently-labeled macromolecules
- GAIT complex
interferon-γ activated inhibitor of translation complex. A heterotetrameric complex comprising EPRS, NSAP1, L13a and GAPDH that binds a structural 3′UTR GAIT element of target mRNAs and silences their translation
- GAIT element
a defined structural RNA element present in the 3′UTR of several inflammatory mRNAs; the GAIT complex binds this element and suppresses translation
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase. A housekeeping enzyme that converts glyceraldehyde 3-phosphate to D-glycerate 1,3 bisphosphate during glycolysis
- IFN-γ
interferon-γ. A type II pro- and anti-inflammatory cytokine known for anti-viral, immunoregulatory, and anti-tumor properties
- L13a
ribosomal protein L13a. A 23 kDa, highly basic protein constituent of the 60S ribosomal subunit
- MSC
tRNA multisynthetase complex. A cytosolic, 1.5 mDa complex containing nine tRNA synthetases (counting EPRS as two) and three non-synthetase proteins
- NSAP1
NS1-associated protein (also referred to as SYNCRIP or hnRNP Q1). A splicing factor with several cytoplasmic activities including translational control
- PABP
poly(A)-binding protein. A protein that interacts with both the 3′ poly(A) tail of mRNA and eIF4G at the 5′ terminus to maintain the closed-loop structure of translating mRNA
- RRL
rabbit reticulocyte lysate. An RNA- and cell-free system containing all components required for efficient translation of exogenous mRNA
- SPR
surface plasmon resonance. A sensitive, laser-based method that enables real-time quantitative analysis of protein–protein or protein–RNA binding kinetics
- UTR
untranslated region. Upstream or downstream regions of an mRNA flanking the coding sequence which are not translated but offer post-transcriptional regulation of gene expression
- VEGF
vascular endothelial growth factor. A critical factor involved in blood vessel growth and tumorigenesis
- WHEP domain, a conserved helix-turn-helix domain of ~50 amino acids named after three vertebrate synthetases containing the domain
tryptophanyl (W), histidyl (H), and glutamyl-prolyl (EP) tRNA synthetases
- ZIPK
zipper-interacting protein kinase, also known as DAPK3. A member of the death-associated protein kinase family involved in apoptosis and smooth muscle contractility
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dever TE. Gene-specific regulation by general translation factors. Cell. 2002;108:545–556. doi: 10.1016/s0092-8674(02)00642-6. [DOI] [PubMed] [Google Scholar]
- 2.Mazumder B, et al. Translational control by the 3′-UTR: the ends specify the means. Trends Biochem Sci. 2003;28:91–98. doi: 10.1016/S0968-0004(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 3.Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nat Rev Mol Cell Biol. 2004;5:827–835. doi: 10.1038/nrm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Standart N, Jackson RJ. Regulation of translation by specific protein/mRNA interactions. Biochimie. 1994;76:867–879. doi: 10.1016/0300-9084(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 5.Sachs AB, Varani G. Eukaryotic translation initiation: there are (at least) two sides to every story. Nat Struct Biol. 2000;7:356–361. doi: 10.1038/75120. [DOI] [PubMed] [Google Scholar]
- 6.Sonenberg N, Hinnebusch AG. New modes of translational control in development, behavior, and disease. Mol Cell. 2007;28:721–729. doi: 10.1016/j.molcel.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Pesole G, et al. UTRdb and UTRsite: specialized databases of sequences and functional elements of 5′ and 3′ untranslated regions of eukaryotic mRNAs. Update 2002. Nucleic Acids Res. 2002;30:335–340. doi: 10.1093/nar/30.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 9.Mühl H, Pfeilschifter J. Anti-inflammatory properties of pro-inflammatory interferon-γ. Int Immunopharmacol. 2003;3:1247–1255. doi: 10.1016/S1567-5769(03)00131-0. [DOI] [PubMed] [Google Scholar]
- 10.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci. 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 11.Mazumder B, et al. Induction of ceruloplasmin synthesis by IFN-γ in human monocytic cells. J Immunol. 1997;159:1938–1944. [PubMed] [Google Scholar]
- 12.Mazumder B, Fox PL. Delayed translational silencing of ceruloplasmin transcript in gamma interferon-activated U937 monocytic cells: role of the 3′ untranslated region. Mol Cell Biol. 1999;19:6898–6905. doi: 10.1128/mcb.19.10.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampath P, et al. Transcript-selective translational silencing by gamma interferon is directed by a novel structural element in the ceruloplasmin mRNA 3′ untranslated region. Mol Cell Biol. 2003;23:1509–1519. doi: 10.1128/MCB.23.5.1509-1519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SenGupta DJ, et al. A three-hybrid system to detect RNA-protein interactions in vivo. Proc Natl Acad Sci U S A. 1996;93:8496–8501. doi: 10.1073/pnas.93.16.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazumder B, et al. Regulated release of L13a from the 60S ribosomal subunit as a mechanism of transcript-specific translational control. Cell. 2003;115:187–198. doi: 10.1016/s0092-8674(03)00773-6. [DOI] [PubMed] [Google Scholar]
- 17.Sampath P, et al. Noncanonical function of glutamyl-prolyl-tRNA synthetase: gene-specific silencing of translation. Cell. 2004;119:195–208. doi: 10.1016/j.cell.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 18.Ray PS, et al. Macromolecular complexes as depots for releasable regulatory proteins. Trends Biochem Sci. 2007;32:158–164. doi: 10.1016/j.tibs.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Cahuzac B, et al. A recurrent RNA-binding domain is appended to eukaryotic aminoacyl-tRNA synthetases. EMBO J. 2000;19:445–452. doi: 10.1093/emboj/19.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong EJ, et al. Structural analysis of multifunctional peptide motifs in human bifunctional tRNA synthetase: identification of RNA-binding residues and functional implications for tandem repeats. Biochemistry. 2000;39:15775–15782. doi: 10.1021/bi001393h. [DOI] [PubMed] [Google Scholar]
- 21.Berthonneau E, Mirande M. A gene fusion event in the evolution of aminoacyl-tRNA synthetases. FEBS Lett. 2000;470:300–304. doi: 10.1016/s0014-5793(00)01343-0. [DOI] [PubMed] [Google Scholar]
- 22.Shiba K. Intron positions delineate the evolutionary path of a pervasively appended peptide in five human aminoacyl-tRNA synthetases. J Mol Evol. 2002;55:727–733. doi: 10.1007/s00239-002-2368-3. [DOI] [PubMed] [Google Scholar]
- 23.Clemens MJ. Does protein phosphorylation play a role in translational control by eukaryotic aminoacyl-tRNA synthetases? Trends Biochem Sci. 1990;15:172–175. doi: 10.1016/0968-0004(90)90153-3. [DOI] [PubMed] [Google Scholar]
- 24.Jia J, et al. WHEP domains direct noncanonical function of glutamyl-prolyl tRNA synthetase in translational control of gene expression. Mol Cell. 2008;29:679–690. doi: 10.1016/j.molcel.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukhopadhyay R, et al. DAPK–ZIPK–L13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Mol Cell. 2008;32:371–382. doi: 10.1016/j.molcel.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaudhuri S, et al. Human ribosomal protein L13a is dispensable for canonical ribosome function but indispensable for efficient rRNA methylation. RNA. 2007;13:2224–2237. doi: 10.1261/rna.694007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Songyang Z, et al. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol Cell Biol. 1996;16:6486–6493. doi: 10.1128/mcb.16.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ubersax JA, Ferrell JE. Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 29.Deiss LP, et al. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the γ interferon-induced cell death. Genes Dev. 1995;9:15–30. doi: 10.1101/gad.9.1.15. [DOI] [PubMed] [Google Scholar]
- 30.Shani G, et al. Death-associated protein kinase phosphorylates ZIP kinase, forming a unique kinase hierarchy to activate its cell death functions. Mol Cell Biol. 2004;24:8611–8626. doi: 10.1128/MCB.24.19.8611-8626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells SE, et al. Circularization of mRNA by eukaryotic translation initiation factors. Mol Cell. 1998;2:135–140. doi: 10.1016/s1097-2765(00)80122-7. [DOI] [PubMed] [Google Scholar]
- 32.Mazumder B, et al. Translational silencing of ceruloplasmin requires the essential elements of mRNA circularization: poly(A) tail, poly(A)-binding protein, and eukaryotic translation initiation factor 4G. Mol Cell Biol. 2001;21:6440–6449. doi: 10.1128/MCB.21.19.6440-6449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humphreys DT, et al. MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc Natl Acad Sci U S A. 2005;102:16961–16966. doi: 10.1073/pnas.0506482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pillai RS, et al. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Kapasi P, et al. L13a blocks 48S assembly: role of a general initiation factor in mRNA-specific translational control. Mol Cell. 2007;25:113–126. doi: 10.1016/j.molcel.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown V, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 37.López de Silanes I, et al. Identification and functional outcome of mRNAs associated with RNA-binding protein TIA-1. Mol Cell Biol. 2005;25:9520–9531. doi: 10.1128/MCB.25.21.9520-9531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keene JD. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 2007;8:533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- 39.Ray PS, Fox PL. A post-transcriptional pathway represses monocyte VEGF-A expression and angiogenic activity. EMBO J. 2007;26:3360–3372. doi: 10.1038/sj.emboj.7601774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vyas K, et al. Genome-wide polysome profiling reveals an inflammation-responsive post-transcriptional operon in IFN-γ-activated monocytes. Mol Cell Biol. 2009;29:458–470. doi: 10.1128/MCB.00824-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grillo G, et al. PatSearch: a program for the detection of patterns and structural motifs in nucleotide sequences. Nucleic Acids Res. 2003;31:3608–3612. doi: 10.1093/nar/gkg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doms A, Schroeder M. GoPubMed: exploring PubMed with the Gene Ontology. Nucleic Acids Res. 2005;33:W783–W786. doi: 10.1093/nar/gki470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaeger JA, et al. Improved predictions of secondary structures for RNA. Proc Natl Acad Sci U S A. 1989;86:7706–7710. doi: 10.1073/pnas.86.20.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bialik S, Kimchi A. The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem. 2006;75:189–210. doi: 10.1146/annurev.biochem.75.103004.142615. [DOI] [PubMed] [Google Scholar]
- 45.Ostareck-Lederer A, et al. c-Src-mediated phosphorylation of hnRNP K drives translational activation of specifically silenced mRNAs. Mol Cell Biol. 2002;22:4535–4543. doi: 10.1128/MCB.22.13.4535-4543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naarmann IS, et al. mRNA silencing in human erythroid cell maturation: heterogeneous nuclear ribonucleoprotein K controls the expression of its regulator c-Src. J Biol Chem. 2008;283:18461–18472. doi: 10.1074/jbc.M710328200. [DOI] [PubMed] [Google Scholar]
- 47.Milo R, et al. Network motifs: simple building blocks of complex networks. Science. 2002;298:824–827. doi: 10.1126/science.298.5594.824. [DOI] [PubMed] [Google Scholar]
- 48.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 49.Ubogu EE, et al. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, et al. MAPK phosphatases – regulating the immune response. Nat Rev Immunol. 2007;7:202–212. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- 51.Kim TK, Maniatis T. Regulation of interferon-γ-activated STAT1 by the ubiquitin-proteasome pathway. Science. 1996;273:1717–1719. doi: 10.1126/science.273.5282.1717. [DOI] [PubMed] [Google Scholar]
- 52.Starr R, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 53.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 54.Ray PS, et al. A stress-responsive RNA switch regulates VEGFA expression. Nature. 2009;457:915–919. doi: 10.1038/nature07598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bastide A, et al. An upstream open reading frame within an IRES controls expression of a specific VEGF-A isoform. Nucleic Acids Res. 2008;36:2434–2445. doi: 10.1093/nar/gkn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inbal B, et al. DAP kinase links the control of apoptosis to metastasis. Nature. 1997;390:180–184. doi: 10.1038/36599. [DOI] [PubMed] [Google Scholar]
- 57.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim DH, et al. Promoter methylation of DAP-kinase: association with advanced stage in non-small cell lung cancer. Oncogene. 2001;20:1765–1770. doi: 10.1038/sj.onc.1204302. [DOI] [PubMed] [Google Scholar]
- 59.Lin EY, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, et al. Association of late-onset Alzheimer’s disease with genetic variation in multiple members of the GAPD gene family. Proc Natl Acad Sci U S A. 2004;101:15688–15693. doi: 10.1073/pnas.0403535101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, et al. DAPK1 variants are associated with Alzheimer’s disease and allele-specific expression. Hum Mol Genet. 2006;15:2560–2568. doi: 10.1093/hmg/ddl178. [DOI] [PubMed] [Google Scholar]
- 62.Jeffery CJ. Moonlighting proteins: old proteins learning new tricks. Trends Genet. 2003;19:415–417. doi: 10.1016/S0168-9525(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 63.Warner JR, McIntosh KB, et al. How common are extraribosomal functions of ribosomal proteins? Mol Cell. 2009;34:3–11. doi: 10.1016/j.molcel.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wool IG. Extraribosomal functions of ribosomal proteins. Trends Biochem Sci. 1996;21:164–165. [PubMed] [Google Scholar]
- 65.Wei N, et al. The COP9 signalosome: more than a protease. Trends Biochem Sci. 2008;33:592–600. doi: 10.1016/j.tibs.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Han JM, et al. AIMP2/p38, the scaffold for the multi-tRNA synthetase complex, responds to genotoxic stresses via p53. Proc Natl Acad Sci U S A. 2008;105:11206–11211. doi: 10.1073/pnas.0800297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park SG, et al. Human lysyl-tRNA synthetase is secreted to trigger proinflammatory response. Proc Natl Acad Sci U S A. 2005;102:6356–6361. doi: 10.1073/pnas.0500226102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ban N, et al. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science. 2000;289:905–920. doi: 10.1126/science.289.5481.905. [DOI] [PubMed] [Google Scholar]
- 69.Wolfe CL, et al. A three-dimensional working model of the multienzyme complex of aminoacyl-tRNA synthetases based on electron microscopic placements of tRNA and proteins. J Biol Chem. 2005;280:38870–38878. doi: 10.1074/jbc.M502759200. [DOI] [PubMed] [Google Scholar]