Abstract
Autism spectrum disorder (ASD) is very heterogeneous and multiple subtypes and etiologies likely exist. The maternal immune system has been implicated in the pathogenesis of some forms of ASD. Previous studies have identified the presence of specific maternal IgG autoantibodies with reactivity to fetal brain proteins at 37 and 73KDa in up to 12% of mothers of children with ASD. The current study evaluates the presence of these autoantibodies in an independent cohort of mothers of 181 preschool-aged male children (131 ASD, 50 typically developing [TD] controls). We also investigated whether ASD children born to mothers with these autism-specific maternal IgG autoantibodies exhibit a distinct neural phenotype by evaluating total brain volume using structural magnetic resonance imaging (MRI). Of the 131 ASD children, 10 (7.6%) were born to mothers with the 37/73Kda IgG autoantibodies (ASD-IgG). The mothers of the remaining ASD children and all TD controls were negative for these paired autoantibodies. While both ASD groups exhibited abnormal brain enlargement that is commonly observed in this age range, the ASD-IgG group exhibited a more extreme 12.1% abnormal brain enlargement relative to the TD controls. In contrast, the remaining ASD children exhibited a smaller 4.4% abnormal brain enlargement relative to TD controls. Lobar and tissue type analyses revealed that the frontal lobe is selectively enlarged in the ASD-IgG group and that both gray and white matter are similarly affected. These results suggest that maternal autoantibodies associated with autism spectrum disorder may impact brain development leading to abnormal enlargement.
Keywords: Autism spectrum disorder, MRI, structural neuroimaging, maternal antibody, autoantibody
INTRODUCTION
Autism spectrum disorder (ASD) is a lifelong neurodevelopmental disorder characterized by core deficits in social interaction, communication, and restricted interests and repetitive behaviors (APA, 1994). ASD is a heterogeneous disorder with varied behavioral presentations, as well as multiple co-morbid disorders such as developmental delay, epilepsy, ADHD, and anxiety (Geschwind and Levitt, 2007; Amaral et al., 2008). It is likely that multiple etiologies exist, and immune dysfunction has been implicated in the pathogenesis of a subset of children with ASD.
There is a growing body of evidence suggesting that the maternal immune response may play a role in the etiology of ASD (Zimmerman et al., 2007; Singer et al., 2009). During gestation, the fetal immune system is immature and the fetus relies on maternal IgG autoantibodies to defend against pathogens (Garty et al., 1994). Although this is normally protective, some maternal IgG autoantibodies react with fetal brain tissue and may impact neural development (Braunschweig and Van de Water, 2012). Van de Water and colleagues have identified an association between a specific banding pattern of maternal IgG autoantibodies and the presence of ASD in the offspring (Braunschweig et al., 2008; Braunschweig et al., 2011). Maternal IgG reactivity to 37KDa and 73KDa proteins in fetal brain tissue was observed in 7-12% of mothers of children with ASD, but not in any mothers of typically developing controls or children with other developmental delays.
While these observational studies highlight the potential risk associated with these specific maternal autoantibodies, the pathologic significance of these autoantibodies remains to be determined. Do these autoantibodies impact neurodevelopment in the fetus? In the current study, we investigated whether a specific neural phenotype in ASD males is associated with the presence of the 37/73kDa maternal IgG autoantibodies. Participants were enrolled in the Autism Phenome Project (APP), a large-scale, multidisciplinary study aimed at identifying and characterizing different biological phenotypes of ASD. Serum IgG from the mothers of 131 preschool-aged males with ASD and 50 age-matched typically developing (TD) male controls was evaluated for the presence of 37/73 kDa reactivity with fetal brain tissue. All children underwent magnetic resonance imaging and were evaluated for total cerebral volume as well as lobar (frontal, parietal, temporal, occipital) volume and gray/white matter volumes within each lobe.
METHODS
Participants
Participants were enrolled in the Autism Phenome Project. This study was approved by the University of California (UC) Davis Institutional Review Board, and informed consent was obtained by the parent or guardian of each participant. At study entry, height and weight were measured by trained study personnel. Maternal plasma samples and MRIs were acquired for 181 male participants (131 ASD, 50 TD). Data from a subset of these participants has been reported previously (Nordahl et al., 2011; Nordahl et al., 2012a).
All diagnostic assessments were conducted by trained, licensed clinical psychologists who specialize in autism. Two clinicians agreed on diagnosis for all included participants. Diagnostic instruments included the Autism Diagnostic Observation Schedule – Generic (ADOS-G) (DiLavore et al., 1995; Lord et al., 2000) and the Autism Diagnostic Interview – Revised (ADI-R) (Lord et al., 1994). Diagnostic criteria for ASD were based on DSM IV criteria as further defined by the Collaborative Programs of Excellence in Autism network. An ADOS severity score was calculated ranging from 1-10 (Gotham et al., 2009), which allows for comparison of autism severity across participants tested with different ADOS-G modules. History of regression was determined from parent report, based on the ADI-R and validated by the Early Development Questionnaire (Nordahl et al., 2011).
Overall developmental quotients were determined for all participants using the Mullen Scales of Early Development (MSEL) (Mullen, 1995). A Developmental Quotient (DQ) was calculated as the average of the age equivalent scores on the Visual Reception, Fine Motor, Receptive Language, and Expressive Language scales, divided by chronological age and multiplied by 100.
Typically developing children were screened and excluded for ASD using the Social Communication Questionnaire (scores ≥ 11) (SCQ - Lifetime Edition). Inclusion criteria for typically developing children also included developmental scores within two standard deviations on all scales of the MSEL. All children, both TD controls and children with ASD, were native English speakers, ambulatory, and had no suspected vision or hearing problems or known genetic disorders and/or other neurological conditions. Additional exclusionary criteria included physical contraindications to MRI.
Maternal Plasma Collection
Blood was collected from mothers of APP participants at the time of study enrollment in yellow-top acid-citrate-dextrose tubes (BD Diagnostic, Franklin Lakes, NJ). Plasma was separated from cells, coded, aliquoted to minimize freeze/thaw cycles, and stored at -80°C until use. The samples used in this study had not previously undergone a freeze/thaw cycle.
Protein Preparation
Rhesus macaque brain tissue was prepared as previously described (Braunschweig et al., 2008; Braunschweig et al., 2011). After homogenization, the sample was then centrifuged 10 min at 3,000 × g to remove insoluble material. The supernatant was concentrated 5-fold by ultrafiltration (Mr cutoff 10,000) and diluted in 50 mM Tris-HCl pH 6.8 containing 25% glycerol and 1% lithium dodecyl sulfate. The protein sample concentration was then adjusted to 3.5 mg/ml, as determined by BCA assay (Pierce). The prepared homogenate was stored at -70°C until use.
Western Blotting
Western blot analysis using fetal monkey brain extract was performed as previously described (Cabanlit et al., 2007), with modifications detailed below. Briefly, following SDS-PAGE under reducing conditions, the gels were blotted to nitrocellulose membranes. The membranes were cut into strips and all blots were blocked with Blocker Casein in PBS (Thermo Fisher) for 10 min and probed with maternal plasma diluted 1:400 for 2 hrs at room temperature, incubated with goat anti-human secondary antibody (Invitrogen), and imaged on a FluorChem 8900 imager (Alpha Innotech) following incubation with Super Signal West (Pierce). Scoring for the presence and apparent molecular weight of a positive band was performed by individuals blinded to diagnosis, using internal positive and negative controls on each blot.
Imaging
The majority of MRI scanning was carried out during natural nocturnal sleep (Nordahl et al., 2008) at the UC Davis Imaging Research Center on a 3T Siemens TIM Trio MRI system (Siemens Medical Solutions, Erlangen, Germany) using an 8-channel head coil. The success rate for acquiring images during natural sleep was 85%. For each subject, a T1-weighted three-dimensional (3D) MPRAGE (TR 2170 ms; TE 4.86 ms; matrix 256 × 256, slice thickness 1.0 mm; voxel size 1 mm isotropic, sagittal acquisition) was obtained. A T2-weighted scan was also obtained for clinical evaluation.
For children with ASD, the option of using general anesthesia was an alternative if scanning during sleep was unsuccessful. Ten ASD children were scanned under general anesthesia (2 ASD-IgG, 8 ASD). These scans were carried out at the UC Davis Children’s Hospital under the care of a pediatric anesthesiologist on a GE 3.0T Excite HD MRI system. A T1-weighted 3D-SPGR sequence (TI 1100 ms, TE minimum, matrix 256 × 256, slice thickness 1.0 mm, voxel size 1 mm isotropic, sagittal acquisition) was acquired. Sequences were selected to give comparable studies and image quality. There were no statistically significant differences in DQ (p = .80), autism severity (p = .20), and total cerebral volume (p = .88) between ASD children who were imaged using sedation and those imaged during natural sleep.
All T1-weighted and available T2-weighted scans were reviewed by a pediatric neuroradiologist and screened for significant, unexpected clinical findings. None of the participants were excluded on this basis.
Image distortion correction
Hardware-induced variations have been recognized as an important source of error in volumetric measurements of brain regions obtained from MRI (Fox and Freeborough, 1997). In order to combine MRI data from the two different scanners, a calibration phantom (Magphan Quantitative Imaging Phantom, Phantom Laboratories, Inc.) was utilized to monitor and correct for hardware-induced geometric distortion using procedures described in detail previously (Nordahl et al., 2011; Nordahl et al., 2012a). Volumetric measurements were made on distortion corrected images.
Volumetric Measurements
Total cerebral volume (TCV) was measured using a template-based method described previously (Nordahl et al., 2011; Nordahl et al., 2012a). Cerebral lobes were delineated using a similar automated template-based method. In brief, the template was processed through Freesurfer 5.1 image analysis suite (http://surfer.nmr.mgh.harvard.edu/) and cortical parcellations were carried out based on gyral and sulcal structure (Desikan et al., 2006). Cortical parcellations were exported into Analyze 11.0 software (Robb et al., 1989), and regions of interest (ROIs) for frontal, parietal, temporal, and occipital lobes were defined (http://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation). The ROIs were then manually edited to correct for minor errors, transformed back to native image space using previously acquired deformation parameters, and used to define the four cerebral lobes. Gray and white matter volumes were obtained using FMRIB’s Automated Segmentation Tool (Zhang et al., 2001).
Statistical analyses
A generalized linear model approach was utilized to test for group differences in TCV and to evaluate age effects. A fixed-effect model was fit with TCV as the dependent variable and group (ASD, ASD-IgG, TD) and age (in months) as predictors, allowing the groups to have unequal variances and employing an appropriate adjustment (Satterthwaite) for the degrees of freedom. Tukey-Kramer adjustment for multiple comparisons was used for testing pair-wise group differences.
To examine if the pattern of group differences was generalized to all the cerebral lobes, a repeated measure mixed-effects model (Laird and Ware, 1982) was employed. Lobar volumes were treated as repeated observation on the same child, and the model contained fixed effects for age, group (ASD, ASD-IgG, and TD), lobe (frontal, occipital, parietal, and temporal), and the interaction between group and lobe and a random effect for child which accounted for the correlated nature of the data due to the repeated measurements. P-values for lobar group differences were adjusted for multiple comparisons. A similar repeated measures approach was used to test whether the pattern of enlargement of lobes was different in gray and white matter.
Residual analyses and graphical diagnostics were used to check the validity of all model assumptions. All statistical analyses were implemented in SAS Version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
Participants included 131 male ASD children (mean age 39.8 months, sd 5.9, range 25.7-53.6 months) and 50 male TD controls (mean age 39.1 months, sd 5.5, range 28.0-50.6). The two groups did not differ on age, but as expected, TD controls had significantly higher DQ (TD 107.5, sd 13.9; ASD 57.7, sd 26.3, p < .0001). The ADOS severity score for ASD participants ranged from 4 to 10 (mean 7.9, sd 1.8).
Maternal IgG banding patterns
Of the 131 children in the ASD group, 10 (7.6%) had mothers with 37/73kDa IgG autoantibodies reactive to fetal brain tissue (ASD-IgG). The remaining 121 ASD children had mothers negative for the 37/73KDa IgG autoantibodies (ASD). None of the mothers in the TD group exhibited these autoantibodies. Characteristics for the participants in these three subgroups are presented in Table 1. There were no differences in age, height, autism severity, or DQ between the two ASD groups. Furthermore, the two groups did not differ in the rate of parent reported history of regression. The ASD-IgG group did have a higher rate of megalencephaly (defined as 2 SD above the mean TCV for TD controls) and the difference approached statistical significance (Fisher’s exact test p = .07).
Table 1.
Participant characteristics for maternal IgG subgroups
| ASD-IgG | ASD | TD | |
|---|---|---|---|
| n | 10 | 121 | 50 |
| Age (months) | 39.1 (6.0) | 39.8 (5.9) | 39.1 (5.5) |
| Height (inches) | 37.4 (2.1) | 37.5 (2.7) | 36.5 (2.03) |
| DQ | 57.3 (26.5) | 57.7 (26.4) | 107.5 (13.4) |
| ADOS severity score | 8.0 (1.5) | 7.9 (1.8) | -- |
| History of regression (%) | 50% | 42% | -- |
| Rate of megalencephaly (%) | 30% | 9% | 2% |
| Total cerebral volume (cm3) | 1114.7 (69.4) | 1038.0 (79.8) | 994.7 (74.9) |
Continuous variables are summarized as mean (sd)
Volumetric brain measurements
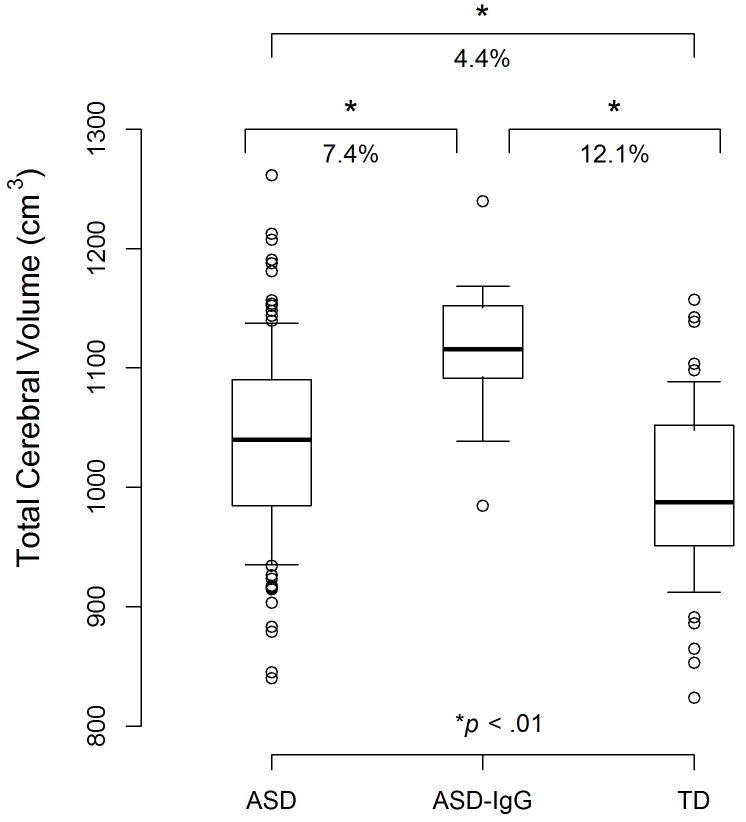
TCV increased significantly with age in all three groups (estimate = 3.9 cm3/month, SE = 1.0, F1,180.2 = 16.8, p < .0001). The overall test for group differences was also significant (F2,29.8 = 15.2, p < .0001). Even after adjusting for multiple comparisons, all three pair-wise group differences remained significant (Figure 1). The ASD group exhibited abnormal brain enlargement relative to TD controls; (estimated difference = 40.7 cm3, SE = 12.0, p = .006). The ASD-IgG group, however, exhibited a substantially greater brain enlargement relative to TD controls (estimated difference = 120.2 cm3, SE = 22.9, p < .0001). The ASD-IgG group also had greater brain enlargement than the remaining ASD children (estimated difference = 79.5 cm3, SE = 21.8, p = .003).
Figure 1.
Total cerebral volume in ASD males born to mothers with 37/73kDa IgG autoantibodies (ASD-IgG), ASD males born to mothers negative for the 37/73KDa IgG antibodies (ASD), and typically developing (TD) male controls. The box plots depict the median, 25th and 75th percentiles. The whiskers denote the 10th and 90th percentiles. Values above the 90th and below the 10th percentile are plotted as points. As a subgroup, the ASD-IgG group exhibits a more extreme brain enlargement relative to TD controls than the ASD group
Table 2 depicts the mean lobar volumes in the three groups and summarizes the results of the mixed-effects model comparing lobe volumes in these groups. The interaction term between group and lobe was significant (F6,180 = 2.85, p = .01), suggesting that the pattern of enlargement was different across lobes. Relative to the other ASD group, only the frontal lobe was selectively enlarged (Table 2) in the ASD-IgG group after correcting for multiple comparisons. Relative to TD controls, the ASD-IgG group exhibited abnormal enlargement in all four cerebral lobes, and the differences remained significant after adjusting for multiple comparisons. In the mixed-effect model fitted to lobar tissue volumes, there was no significant group*lobe*tissue interaction, suggesting that group differences observed in lobar measurements were consistent across gray and white matter.
Table 2.
Summary (mean, standard deviation) of volumes (in cm3) by lobe and estimated group differences (standard errors)
| ASD (n = 121) |
ASD IgG (n = 10) |
TD (n = 50) |
ASD IgG - ASD Difference1 |
ASD IgG - TD Difference1 |
|||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Estimate (SE) | p-value | Estimate (SE) | p-value | ||||
| Frontal | 351.9 (31.3) | 386.3 (26.7) | 341.6 (29.2) | 34.8 (8.3) | < .0001 | 44.8 (8.7) | < .0001 |
| Occipital | 96.4 (11.0) | 106.2 (9.3) | 91.0 (8.5) | 10.2 (5.0) | 0.06 | 15.2 (5.2) | .008 |
| Parietal | 241.3 (20.3) | 252.7 (18.6) | 229.7 (20.0) | 11.8 (5.6) | 0.04 | 23.0 (5.9) | < .001 |
| Temporal | 168.7 (14.0) | 178.9 (13.0) | 160.1 (14.1) | 10.7 (4.4) | 0.03 | 18.9 (4.6) | < .0001 |
Estimated from a mixed-effects regression model fitted to lobar volumes and adjusted for age. Reported p-values are unadjusted for multiple comparisons. Only the p-values in bold are still significant after controlling for multiple comparisons (Holm-Tukey).
DISCUSSION
The results from this study provide further evidence that gestational exposure to maternal antibodies to proteins in the fetal brain may play a role in the pathogenesis of ASD for a subset of children. As in previous studies (Braunschweig et al., 2008; Braunschweig et al., 2012), we found that 7.6% of the mothers of boys with ASD who were enrolled in the Autism Phenome Project exhibit antibodies with reactivity to proteins of 37 and 73KDa molecular weight. These were not observed in any mothers of TD children. Maternal plasma samples were collected at the time of study entry, when children were 2-3 years of age. Although it is possible that antibody titers may have decreased over time, other investigations have shown that maternal antibodies remain present postnatally up to 18 years (Zimmerman et al., 2007).
There was no a priori expectation that children exposed to the antibodies would have a particular neural phenotype. Therefore, it was quite striking that as a group, the brains of these children were 12% larger than their typically developing counterparts, and even 7% larger than the group of other ASD boys whose mothers did not have the 37/73kDa IgG autoantibodies. Indeed, 30% of the ASD-IgG group were classified as megaencephalic compared to 9% of remaining ASD children. Furthermore, the frontal lobe was selectively enlarged in the ASD-IgG children relative to other ASD children, and both gray and white matter were similarly affected. These data suggest that maternal autoantibodies associated with autism spectrum disorder may impact brain development and be an important factor leading to abnormal brain enlargement in ASD. Of course, given the correlational nature of these data, it is possible that another factor associated with the gestational exposure to the antibodies may actually be causal.
Abnormal brain enlargement is a consistent group observation in preschool-aged children with ASD (Courchesne et al., 2001; Hazlett et al., 2005; Schumann et al., 2010), but there is considerable individual variability within ASD. Brain volume in ASD ranges from macrocephaly or megalencephaly to brain volumes within the range of typically developing controls to a small group of individuals with microencephaly. On average, the magnitude of abnormal brain enlargement reported in young children with ASD is about 6% (Amaral et al., 2008). In the current study, the ASD-IgG group exhibited a much larger 12% enlargement relative to TD controls. The remaining ASD children showed a more modest 4.4% enlargement, more consistent with previous literature on brain volume in young children with ASD. The underlying etiology of precocious brain growth is currently unknown, though PTEN mutations have been identified in a small subset of children with macrocephaly (Butler et al., 2005; Zhou and Parada, 2012). Our data suggest that in a subset of approximately 7% of ASD children, the underlying etiology may begin prenatally with exposure to abnormal maternal antibodies.
Additional evidence for the pathogenic nature of these antibodies comes from parallel studies carried out in the nonhuman primate. Groups of pregnant rhesus monkeys have been treated with IgG purified from women who are positive for the 37/73kDa maternal IgG autoantibodies and have given birth to a child with autism or women without these antibodies who have given birth to typically developing children. Interestingly, male monkeys exposed prenatally to the 37/73kDa autoantibodies also exhibit abnormal brain enlargement relative to control monkeys (Nordahl et al., 2012b). These studies provide additional support for the hypothesis that the 37/73kDa autoantibodies, either directly or indirectly, affect brain development leading to abnormal enlargement and the neurobiological alterations leading to autism spectrum disorder.
Obviously, several questions remain: What are the brain antigens recognized by the 37/73 kDa maternal IgG autoantibodies, and what is their role normal neurodevelopment? What induces the production of these antibodies in some women but not in others? What is the mechanism by which these maternal autoantibodies alter brain development? Are there processes that could be implemented to block the deleterious effects of the antibodies? Studies are currently underway to address each of these issues and they will undoubtedly shed more light on the role that maternal autoantibodies may play in ASD and abnormal brain enlargement in ASD.
Additional characterization of ASD-IgG children will also be of interest. Previous reports have indicated higher rates of regression and poorer expressive language scores in children born to mothers with 37/73 kDa IgG autoantibodies, (Braunschweig et al., 2008; Braunschweig et al., 2011). In the current study, however, we did not observe any differences in autism severity, onset status, or general cognitive ability between the ASD-IgG and other ASD children. Additional studies with larger sample sizes will be necessary to elucidate whether there are behavioral characteristics specific to ASD-IgG children. Future studies would also benefit from additional medical characterization of ASD-IgG children, including an investigation of whether there are specific prenatal or perinatal factors associated with gestational exposure to 37/73 kDa IgG autoantibodies. Finally, one question of particular interest is the sibling status of ASD-IgG children, particularly in younger siblings. Presumably, once the maternal autoantibodies are present in the mother, they persist for subsequent pregnancies as well. It is therefore, of great interest to determine whether younger siblings of ASD-IgG children also develop ASD.
In summary, we identified a distinct neural phenotype of extreme brain enlargement, particularly of the frontal lobe of 7% of male children with ASD exposed prenatally to unusual maternal antibodies. As more evidence accumulates implicating these specific autoantibodies in the pathogenesis of ASD, strategies can be developed to blunt their effects and potentially prevent ASD in this subset of children.
This research describes an association between certain maternal IgG autoantibodies and abnormal brain enlargement in a subset of boys with autism.
Acknowledgments
Funding was provided by the NIMH (R01MH089626, U24MH081810, R00MH085099) and the UC Davis M.I.N.D. Institute. The authors would like to acknowledge the Autism Phenome Project staff for helping in the logistics of family visits and data collection. We especially thank all of the families and children who participated in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Braunschweig D, Van de Water J. Maternal autoantibodies in autism. Arch Neurol. 2012;69:693–699. doi: 10.1001/archneurol.2011.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29:226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, Hertz-Picciotto I, Van de Water J. Behavioral Correlates of Maternal Antibody Status Among Children with Autism. J Autism Dev Disord. 2011 doi: 10.1007/s10803-011-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, Hertz-Picciotto I, Van de Water J. Behavioral correlates of maternal antibody status among children with autism. J Autism Dev Disord. 2012;42:1435–1445. doi: 10.1007/s10803-011-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton R, Pilarski R, Eng C. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–321. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- DiLavore PC, Lord C, Rutter M. The pre-linguistic autism diagnostic observation schedule. J Autism Dev Disord. 1995;25:355–379. doi: 10.1007/BF02179373. [DOI] [PubMed] [Google Scholar]
- Fox NC, Freeborough PA. Brain atrophy progression measured from registered serial MRI: validation and application to Alzheimer’s disease. J Magn Reson Imaging. 1997;7:1069–1075. doi: 10.1002/jmri.1880070620. [DOI] [PubMed] [Google Scholar]
- Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol. 1994;1:667–669. doi: 10.1128/cdli.1.6.667-669.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. American Guidance Service, Inc; 1995. [Google Scholar]
- Nordahl CW, Simon TJ, Zierhut C, Solomon M, Rogers SJ, Amaral DG. Brief report: methods for acquiring structural MRI data in very young children with autism without the use of sedation. J Autism Dev Disord. 2008;38:1581–1590. doi: 10.1007/s10803-007-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Scholz R, Yang X, Buonocore MH, Simon T, Rogers S, Amaral DG. Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: a longitudinal study. Arch Gen Psychiatry. 2012a;69:53–61. doi: 10.1001/archgenpsychiatry.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Bauman MD, Braunschweig D, Iosif AM, Ashwood P, Van de Water J, Amaral DG. The presence of specific maternal IgG antibodies is associated with abnormal brain enlargement in ASD and in nonhuman primate model of ASD. International Meeting for Autism Research; Toronto, Canada. 2012b. [Google Scholar]
- Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH, Simon TJ, Rogers S, Ozonoff S, Amaral DG. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc Natl Acad Sci U S A. 2011;108:20195–20200. doi: 10.1073/pnas.1107560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph. 1989;13:433–454. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, Pierce K, Hagler D, Schork N, Lord C, Courchesne E. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. J Neuroimmunol. 2009;211:39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhou J, Parada LF. PTEN signaling in autism spectrum disorders. Curr Opin Neurobiol. 2012;22:873–879. doi: 10.1016/j.conb.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, Pearce DA. Maternal antibrain antibodies in autism. Brain Behav Immun. 2007;21:351–357. doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]