Introduction
Social decisions depend on reliable information about others. Consequently, social primates are motivated to acquire information about the identity, social status, and reproductive quality of others[1]. Neurophysiological[2] and neuroimaging[3, 4] studies implicate the striatum in the motivational control of behavior. Neuroimaging studies specifically implicate ventromedial striatum in signaling motivational aspects of social interaction[5]. Despite this evidence, precisely how striatal neurons encode social information remains unknown. Therefore, we probed the activity of single striatal neurons in monkeys choosing between visual social information at the potential expense of fluid rewards. We show for the first time that a population of neurons located primarily in medial striatum selectively signals social information. Surprisingly, representation of social information was unrelated to simultaneously expressed social preferences. A largely non-overlapping population of neurons that was not restricted to the medial striatum signaled information about fluid rewards. Our findings demonstrate that information about social context and nutritive rewards are maintained largely independently in striatum, even when both influence decisions to execute a single action.
Results and Discussion
Neuroimaging studies implicate the striatum in processing myriad rewarding or motivating stimuli[3, 4] including both simple (e.g., attractive faces[5]) and complex (e.g., cooperation[6]) social rewards. These studies typically report positive monotonic relationships between desirability of an outcome and BOLD fMRI signals [for exceptions see 7, 8]. Furthermore, neuroimaging studies simultaneously utilizing multiple types of rewards [9–11] indicate overlapping areas of striatum signal information about disparate types of rewards, independent of reward modality.
Such findings endorse a ‘common currency’ model wherein activity levels in striatum specify reinforcer strength independent of reinforcer modality. Electrophysiological studies, however, have revealed heterogeneous encoding in striatal neurons that belies any simple monotonic relationship between neuronal activity and reinforcer value[2, 12, 13]. A limitation of these studies is their focus on nutritive and drug-based reinforcers. Thus, whether neurons in primate striatum signal socially motivating information, and if so, how this information is encoded, remains completely unknown.
We addressed this gap by probing the activity of single neurons in rhesus macaque striatum during choices based on social and fluid reinforcers[cf. 14, 15, 16]. Previous work using the same task demonstrated that male monkeys typically value some classes of social stimuli over others, and that individual neurons in lateral intraparietal cortex (LIP), a brain area important in visual attention and saccade planning[17], signal the combined value of social and fluid outcomes associated with orienting to a particular location[14]. One attractive hypothesis is that a “common currency” of value may be computed in striatum and eventually relayed to premotor areas, such as LIP, to bias action selection[18, 19].
In contrast, we show that, when monkeys choose to acquire social information, largely separate, topographically segregated populations of neurons in striatum signal social and fluid information. Moreover, these neurons signal the identity of specific classes of fluid or social outcomes more strongly than they encode the values of these outcomes. These findings constrain striatal-based models of reinforcement, learning, and decision-making, particularly in the social domain.
Two rhesus macaques performed a two-alternative forced choice task while we recorded firing rates of individual neurons in anterior striatum. Monkeys indicated choices by shifting gaze to one of two visual targets. Choice of the left target (image target, contralateral to the recording site) yielded a juice reward followed by a picture of a monkey, whereas choosing the right target (blank target) yielded only juice (Fig. 1A). Images were drawn from pools containing photographs of the faces of familiar dominant or subordinate male monkeys, hindquarters of familiar female monkeys, and a gray square. Image and juice outcomes varied in a nested block design in which 3–5 blocks of varying juice outcomes made up a single image block (Fig. 1A). Changes in outcome contingencies were unsignaled.
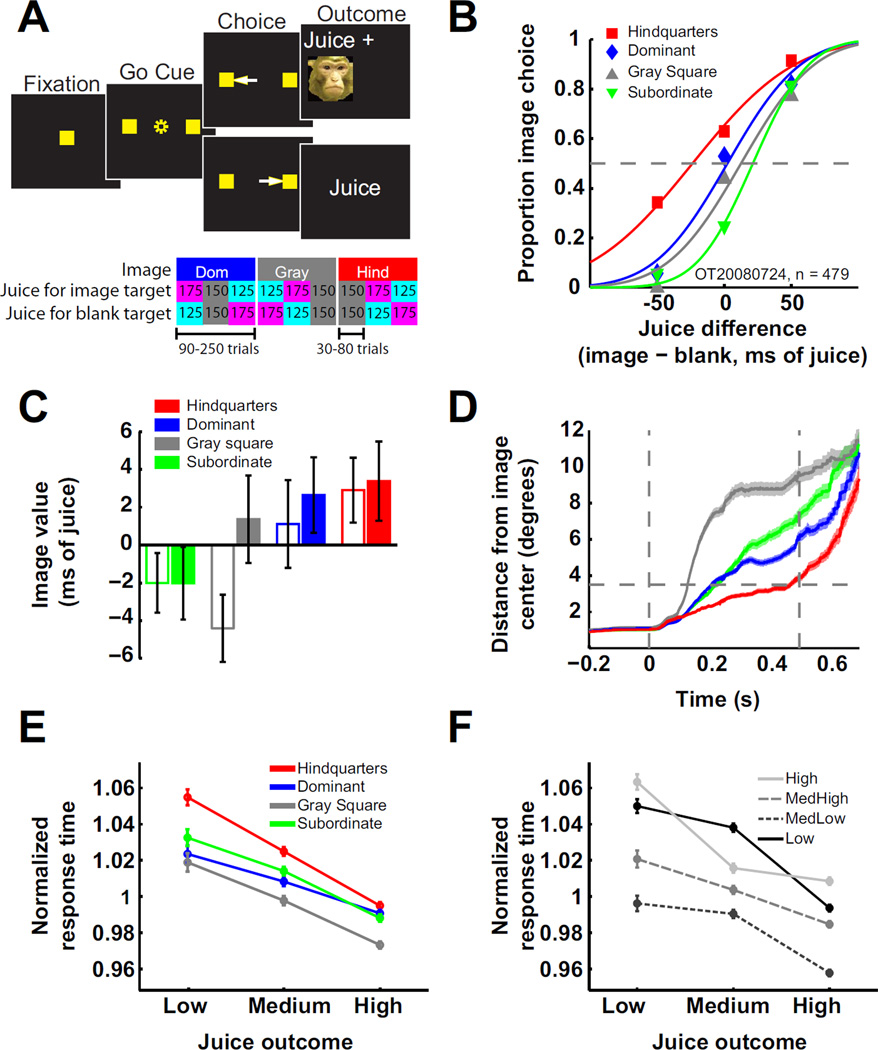
Figure 1. Monkeys value social information.
A) Choice task. Monkeys began the trial by fixating a central target. Next, the central target disappeared and two peripheral targets appeared 15° to the left and right of center. If the monkey shifted gaze to one of these targets, juice was delivered. If he chose the left target (contralateral to the recording site), immediately following juice delivery an image appeared for 500 ms centered on the site of the chosen target. For non-image choices (right), 500 ms of blank screen followed juice delivery. Ten to 20% of trials were single target trials, which were identical except that only one target appeared and only movements to that target were rewarded. The lower panel illustrates outcome contingency changes over three example image blocks. B) Estimating social image value. For all choice trials, the frequency of choosing the image was plotted against the relative juice volume delivered for that choice. These data were fit with cumulative normal functions, and horizontal shifts in those functions were used to estimate image value (Supplemental Experimental Procedures). Example psychometric functions from four image blocks of one behavioral session are shown. C) Monkeys differentially valued image classes (data pooled across all sessions: one way ANOVA, F(3, 355) = 2.67, p < .05; individually: monkey Ot [closed bars] and Os [open bars]: p = .23, p < .03, respectively). D) Viewing duration varied with social image class. For image target choices, the average distance of gaze from the center of the image is plotted as a function of time. Vertical lines depict image onset and offset. The horizontal line is placed at half the height (or width) of the image (3.5°). Shading represents SEM. Due to a data collection error, looking time data is restricted to 36 of 71 behavioral sessions (12 sessions from monkey Os). E,F) Response times were inversely related to juice value and directly related to image valence. Response times separated by juice outcome and image class (E), or juice outcome and image value quartiles (F). The image value quartile boundaries were −8.47, −0.09, and 8.20 ms open juice valve time (Supplementary Experimental Procedures, see also Fig. S1).
We plotted the proportion of image choices against the difference in juice offered for each target and fit these data with cumulative normal functions. We used horizontal shifts of the resulting psychometric curves to estimate the subjective value of each image class during each image block in units of juice reward [Experimental Procedures, Fig. 1B, cf. 14, 15, 16].
Image values varied significantly across the four image categories (Fig. 1C). Although these effects were modest relative to the size of the juice rewards, post-hoc tests collapsing across subjects revealed a significant preference for female sexual signals (t-test against 0, p<.04) and a significant preference for faces of dominant compared to subordinate monkeys (t-test, p<.05). These results indicate information about dominance status and reproductive state influence the choice of social images for inspection. However, it should be noted that block-to-block variation in preference for social image categories was high (Fig. S1).
We further analyzed where monkeys looked after choosing to see the image. Fig. 1D shows the average distance of gaze from the center of the picture during image display. Consistent with previous results[14, 15], monkeys viewed images of female hindquarters for longer durations than male faces or the gray square. We interpret the confluence of image value and looking times as reflective of different informational and hedonic profiles for the image categories; that is, monkeys chose to view images of female hindquarters and faces of males because they predict potential reward and threat, respectively[14–16].
Finally, we analyzed the time it took monkeys to indicate their choice as functions of juice and image category (Fig. 1E). A two-way ANOVA with image category and juice volume as factors revealed significant main effects of both and a significant interaction (p<.005 in all cases). We obtained similar results after splitting reaction times into quartiles based on image value (Fig. 1F; p<<.001 for main effects of juice, image value, and interaction). Overall, monkeys were faster to choose targets with higher juice values but slower to choose targets associated with the most and least preferred images, suggesting differential influences of social and juice information on decisions.
We recorded 151 neurons in anterior striatum of two adult male rhesus macaques (114 from monkey Ot, 37 from monkey Os, Fig. 2A and B). To determine the prevalence and overlap of social and fluid information encoding in striatum, we separately analyzed trials in which monkeys chose the image or blank target using sliding window ANOVAs with normalized firing rate as the dependent variable and image category and juice outcome as independent variables (Supplemental Experimental Procedures). These analyses did not make assumptions about the way information might be encoded but only tested whether neuronal firing rates differed significantly depending on outcomes.
Figure 2. Neurons in primate striatum encode social information.
A) Coronal slice MRI of monkey Os with recording chamber and grid attached. The plane of the slice is at the center of the recording chamber (~22 mm anterior to interaural 0). The red square highlights the area of recording. B) Recording locations overlaid on an outline of the striatum [adapted from 40]. When multiple units were recorded in the same session, locations have been staggered slightly (< 0.5 mm) for visualization. C) Example neurons showing differential firing rates for social image category or juice value when the monkey shifted gaze to the image target. Average normalized firing rates separated by image category (left panels) or juice value (right panels) are plotted for 0.5 s or 1 s epochs aligned to acquisition of fixation target, saccade onset and juice or image onset. Gray shading indicates ti mes of significant firing rate differences by sliding-window ANOVA analysis (Supplemental Experimental Procedures). Insets illustrate recording location of each example neuron. D, E) Heterogeneous population coding of social image class and juice value. For each neuron that significantly differentiated image category or juice level during any epoch, the average firing rate for each image category or juice level during all significant time windows was calculated and ordered from least to greatest. The number of cells with each possible ordering is plotted (see also Fig. S2).
These analyses revealed that, when monkeys chose to view the image, many neurons in our sample systematically conveyed social information, and others conveyed fluid reward information. Fig. 2C plots peristimulus time histograms for 2 neurons as functions of image category (left) and juice volume (right). These 2 neurons signaled social or juice information during anticipation and receipt of social stimuli or juice (significant time periods indicated by shaded bars, Supplemental Experimental Procedures).
To investigate whether neuronal firing rates encoded value monotonically, as predicted by “common currency” hypotheses, we examined average firing rates for each image category across all significant time epochs for all cells showing significant modulation by image class. We ordered these averages from least to greatest and plotted the number of cells exhibiting each possible “coding scheme” (Fig. 2D). We repeated this analysis for juice value (Fig. 2E). For a parallel analysis of block-to-block image values, see Fig. S2A. We also performed Spearman rank correlation analyses of the normalized average firing rate for each neuron as functions of juice volume, image category and image value over four epochs during the trial. The means of the distributions of correlation coefficients for all comparisons were not significantly different from 0 (Fig. S2B–D). These analyses indicate that, for the entire population of neurons, and those subpopulations of neurons significantly modulated by social category, image value, or fluid outcome, there was little evidence of monotonic relationships between any measure of outcome value and firing rates.
Next, we plotted the proportions of neurons in the whole population, and within striatal subregions, that signaled social information, juice information or both during four epochs of the trial (Fig. S3). When monkeys selected the image, firing rates of 36% and 30% of neurons in the population encoded social or juice information at any point in the trial, respectively; only 6% of cells encoded information about both image and juice outcomes. Thus, in the whole population, or within any subregion, only a small proportion of neurons encoded both nutritive and social information. These findings argue against striatal encoding of value in a common currency that integrates information about social and fluid outcomes.
To explore anatomical differences in the encoding of reinforcer information, we created a modulation index (MI) from the results of our sliding window ANOVA that measured the relative influence of social and juice information on neuronal firing rates (Supplemental Experimental Procedures); positive and negative MIs indicate greater influences of social image or fluid on firing rates, respectively. We calculated the MI for each neuron in 100ms windows moving in 5ms steps. Fig. 3A shows the results of this analysis with neurons ordered by medial-to-lateral recording position along the ordinate. When monkeys chose the image target, MIs were negatively correlated with mediolateral position (Fig. 3B). When averaged across epochs and separated by subregion, MIs were significantly more positive in ventral striatum (VS) and caudate than in putamen (Fig. 3C; although these results were consistent across monkeys, the low number of neurons recorded in VS of monkey Os warrants caution in interpreting results from the VS). These regional differences were greatly reduced when monkeys chose the blank target (Fig. 3D–E), reflecting an absence of social information coding in medial striatum when monkeys elected not to acquire it (Fig. 4).
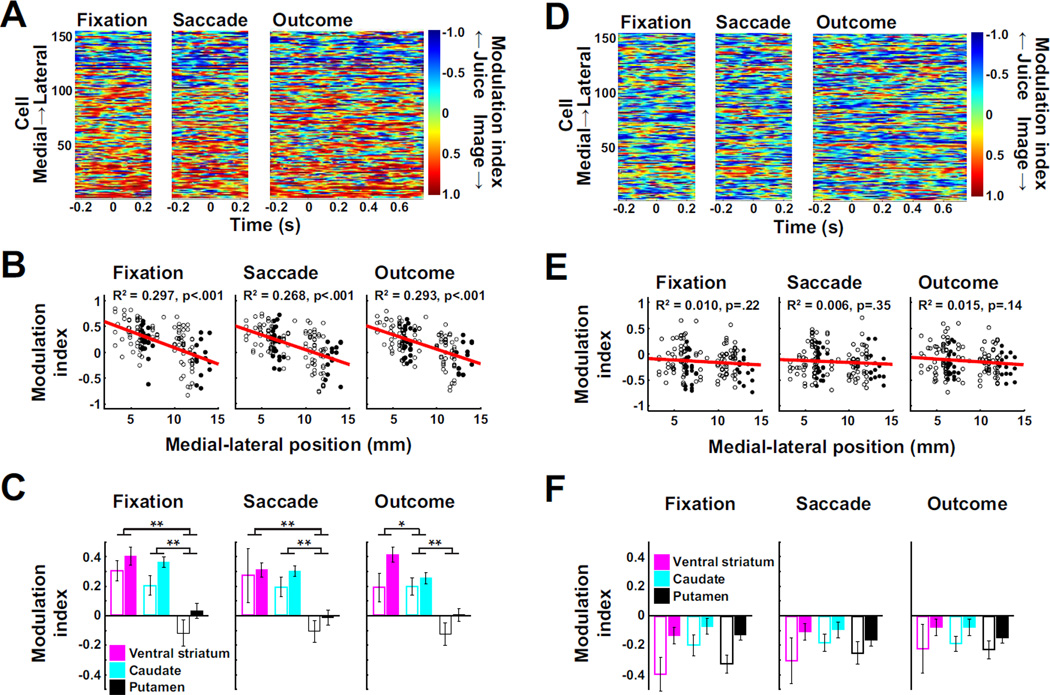
Figure 3. Topography of social and fluid information coding in primate striatum.
A) Pseudocolor plot depicting the modulation index (strength of the influence of social image category relative to that of juice volume on neuronal firing rates), aligned to three trial events when monkeys shifted gaze to the image target. Neurons are ordered from medial to lateral recording position along the y-axis. B) Modulation indices regressed against medial-lateral recording position (linear regression) when monkeys shifted gaze to the image target. Filled circles represent neurons from monkey Os, open circles represent neurons from monkey Ot. All regressions were significant at p < 0.005 when performed for individual monkeys. C) Average modulation indices for each trial event, separated by striatal region (as depicted in Fig. 2B, open bars: monkey Os, closed bars: monkey Ot) when the monkeys shifted gaze to the image target. One way ANOVAs (striatal region × modulation index) collapsing across monkeys: all p values < 10−7. * = p < 0.01, ** = p < 10−4 (post hoc two-tailed t-test). Results were similar when data were separated by monkey. D, E and F) Conventions as in A, B, and C with analyses performed on data from trials in which the monkeys shifted gaze to the blank target (see also Fig. S3).
Figure 4. Neuronal encoding of social information disappears when monkeys do not choose to view the image.
A) Scatterplots and histograms of the variance in firing rate explained by image category for each cell when the monkey shifted gaze to the image target (y-axis) or the blank target (x-axis, Supplemental Experimental Procedures). * indicates the mean of the distribution is significantly above the diagonal (p < 0.001, t-test against 0). B) Scatterplots and histograms of the variance in firing rate for each cell explained by juice outcome when the monkey shifted gaze to the image target or the blank target. None of the means of the distributions were different from zero (p > 0.4).
Discussion
Our findings demonstrate for the first time that neurons in primate striatum respond in anticipation of, and during viewing of, images of conspecifics; further, striatal neurons discriminate between different classes of visual social information. The task required simultaneous evaluation of social and fluid outcomes. While similar proportions of neurons encoded fluid and social information, these two populations were largely distinct. Previous studies of primate and rodent ventral striatum have found similarly non-overlapping populations of neurons encode information about nutritive and drug reinforcers[13, 20, 21], and studies in macaque caudate have found non-overlapping populations of neurons encode distinct aspects of reward[2, 22].
Our findings demonstrate largely independent channels exist in primate striatum for processing social information and fluid rewards, and social information is primarily represented medially. Based on the following observations, the identities of individual neurons within these channels are likely flexible and dependent on task, context, motivation, and outcome: 1) social information coding is diminished when monkeys do not choose to see social images; 2) neurons in rodent ventral striatum respond selectively to cocaine and associated cues upon first exposure[13]; and 3) both the proportion of cells responsive to cocaine and cocaine-seeking behavior increase with prolonged abstinence[23]. Thus, the individual identities and proportions of juice and social category selective cells likely depend on factors including motivation, training, satiety, and the salience and hedonic value of the stimuli. This plasticity notwithstanding, we believe that the medial-to-lateral organization of social and nutritive information coding is a general property of primate striatum for the following reasons: 1) human neuroimaging has found a similar topography [3–5, 7]; 2) the orbitofrontal cortex (OFC) and the anterior cingulate are important for social processing[24, 25] and project primarily to the medial striatum[26]; 3) the caudate is functionally connected to cortical areas that specifically process the social aspects of a competitive bidding game [27]; and 4) the eye- and orofacial motor cortices controlling the movements necessary to acquire social image and fluid rewards project to the medial and lateral striatum, respectively[28].
Numerous human imaging studies have reported striatal BOLD activation scales with the desirability of social, nutritive or monetary outcomes[9–11], and monotonic relationships between the neuronal firing and reward or reward expectation have been observed in several brain structures (e.g., posterior cingulate cortex[29]; LIP[14, 17]; and superior colliculus[30]). Thus, it may seem surprising that firing rates of the striatal neurons we recorded did not vary positively with juice or social image outcome value. However, evidence for such a relationship at the level of individual neurons in striatum is weak. Neurons in ventral striatum that respond in anticipation of cocaine, ethanol, or nutritive rewards[13, 20, 21] do so with excitations, inhibitions, or combinations thereof, and separate populations of caudate neurons respond in anticipation of rewarded or unrewarded eye movements[12]. Taken together, these findings suggest that local spiking activity in striatum does not parallel the BOLD signal. Indeed, the BOLD signal may derive from synaptic potentials reflecting inputs rather than the spiking of local neurons[31].
The context-, action- and/or outcome-specific nature of neuronal information coding observed in t his and other studies[13, 21, 22, 32–34] prompts us to speculate that striatal neurons do not necessarily signal value; rather multiple, unique, small ensembles of striatal neurons each convey idiosyncratic and highly specific information about contexts, cues, outcomes, or combinations thereof. These representations reflect combinatorial processing of incoming information about reward (via dopaminergic neurons[35]); context (via the hippocampus[36]); and social and value information (via cortical inputs[14, 24, 25]) and serve to filter these signals into a form amenable to coherent behavioral output. This speculation finds support from recent work in rats that found neurons in dorsolateral striatum do not encode value, but rather encode information unique to specific movement-outcome contingencies[33]. Similarly, targeted disruption of small ensembles of neurons (2–3%) in ventral striatum that are activated in response to cocaine administration blocks cocaine-induced sensitization in a context-dependent manner[34].
Robust encoding of social information in striatum despite its modest influence on choice behavior suggests reward is not necessarily the best construct for describing the activity of striatal neurons. Indeed, social stimuli are capable of inducing a variety of complex approach or avoidance behaviors, and this complexity is reflected in the activity of individual striatal neurons. Similar results have recently been in OFC[25]. We suggest striatum, along with OFC, are capable of representing social and other behaviorally relevant information in complex and diverse manners when neuronal activity is examined using appropriate tasks. Heterogeneous encoding of information in striatum may reflect the diverse functions this information may serve, including learning[37], action selection[2], attention[38] and motivated behavior[39].
Experimental procedures
All procedures were conducted in compliance with the Public Health Service’s Guide for the Care and Use of Animals and were approved by the Duke University Institutional Animal Care and Use Committee. Individual neurons were recorded from anterior striatum of two adult male rhesus macaques while they performed a pay-per-view decision task [14–16, 25] (Figure 1 and Supplemental Experimental Procedures). Image sets consisted of a uniform gray square, dominant (n = 60) or subordinate faces (n = 60), or female hindquarters (n = 60) presented randomly with replacement. Eye positions were recorded by infrared camera (Eyelink 1000). Neuronal waveforms were discriminated online (Plexon) and firing rates analyzed offline using MATLAB. Firing rates were normalized by dividing raw firing rates by the average firing rate across the whole image block.
Supplementary Material
Highlights.
Single neurons in striatum encode social information.
Typically, striatal neurons signal social or fluid information, but not both.
Topographically distinct information channels process social and fluid rewards.
Acknowledgments
We thank Monica Carlson for primate training and husbandry and Karli Watson and John Pearson for comments on analysis and the manuscript. Funded by MH086712, MH089484 (M.L.P.), F31MH081443 (J.T.K.), and the Autism Speaks Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ghazanfar AA, Santos LR. Primate brains in the wild: the sensory bases for social interactions. Nat Rev Neurosci. 2004;5:603–616. doi: 10.1038/nrn1473. [DOI] [PubMed] [Google Scholar]
- 2.Lau B, Glimcher PW. Action and outcome encoding in the primate caudate nucleus. J Neurosci. 2007;27:14502–14514. doi: 10.1523/JNEUROSCI.3060-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38:339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 4.O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 5.Aharon I, Etcoff N, Ariely D, C habris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 6.King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308:78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- 7.O'Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49:157–166. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Seymour B, Daw N, Dayan P, Singer T, Dolan R. Differential encoding of losses and gains in the human striatum. J Neurosci. 2007;27:4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valentin VV, O'Doherty JP. Overlapping prediction errors in dorsal striatum during instrumental learning with juice and money reward in the human brain. J Neurophysiol. 2009;102:3384–3391. doi: 10.1152/jn.91195.2008. [DOI] [PubMed] [Google Scholar]
- 10.Rademacher L, Krach S, Kohls G, Irmak A, Grunder G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49:3276–3285. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- 11.Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe K, Lauwereyns J, Hikosaka O. Neural correlates of rewarded and unrewarded eye movements in the primate caudate nucleus. J Neurosci. 2003;23:10052–10057. doi: 10.1523/JNEUROSCI.23-31-10052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carelli RM, Wondolowski J. Selective encoding of cocaine versus natural rewards by nucleus accumbens neurons is not related to chronic drug exposure. J Neurosci. 2003;23:11214–11223. doi: 10.1523/JNEUROSCI.23-35-11214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein JT, Deaner RO, Platt ML. Neural correlates of social target value in macaque parietal cortex. Curr Biol. 2008;18:419–424. doi: 10.1016/j.cub.2008.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr Biol. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 16.Watson KK, Ghodasra JH, Platt ML. Serotonin transporter genotype modulates social reward and punishment in rhesus macaques. PLoS One. 2009;4:e4156. doi: 10.1371/journal.pone.0004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- 18.Sugrue LP, Corrado GS, Newsome WT. Choosing the greater of two goods: neural currencies for valuation and decision making. Nat Rev Neurosci. 2005;6:363–375. doi: 10.1038/nrn1666. [DOI] [PubMed] [Google Scholar]
- 19.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson DL, Carelli RM. Distinct subsets of nucleus accumbens neurons encode operant responding for ethanol versus water. European Journal of Neuroscience. 2008;28:1887–1894. doi: 10.1111/j.1460-9568.2008.06464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowman EM, Aigner TG, Richmond BJ. Neural signals in the monkey ventral striatum related to motivation for juice and cocaine rewards. J Neurophysiol. 1996;75:1061–1073. doi: 10.1152/jn.1996.75.3.1061. [DOI] [PubMed] [Google Scholar]
- 22.Lauwereyns J, Takikawa Y, Kawagoe R, Kobayashi S, Koizumi M, Coe B, Sakagami M, Hikosaka O. Feature-based anticipation of cues that predict reward in monkey caudate nucleus. Neuron. 2002;33:463–473. doi: 10.1016/s0896-6273(02)00571-8. [DOI] [PubMed] [Google Scholar]
- 23.Hollander JA, Carelli RM. Abstinence from cocaine self316 administration heightens neural encoding of goal-directed behaviors in the accumbens. Neuropsychopharmacology. 2005;30:1464–1474. doi: 10.1038/sj.npp.1300748. [DOI] [PubMed] [Google Scholar]
- 24.Chang SW, Gariepy JF, Platt ML. Neuronal reference frames for social decisions in primate frontal cortex. Nature neuroscience. 2012;16:243–250. doi: 10.1038/nn.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson KK, Platt ML. Social signals in primate orbitofrontal cortex. Current biology : CB. 2012;22:2268–2273. doi: 10.1016/j.cub.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Bos W, Talwar A, McClure SM. Neural correlates of reinforcement learning and social preferences in competitive bidding. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:2137–2146. doi: 10.1523/JNEUROSCI.3095-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nambu A. Somatotopic organization of the primate Basal Ganglia. Front Neuroanat. 2011;5:26. doi: 10.3389/fnana.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCoy AN, Crowley JC, Haghighian G, Dean HL, Platt ML. Saccade reward signals in posterior cingulate cortex. Neuron. 2003;40:1031–1040. doi: 10.1016/s0896-6273(03)00719-0. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda T, Hikosaka O. Reward-dependent gain and bias of visual responses in primate superior colliculus. Neuron. 2003;39:693–700. doi: 10.1016/s0896-6273(03)00464-1. [DOI] [PubMed] [Google Scholar]
- 31.Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol. 2008;18:631–640. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 32.Lau B, Glimcher PW. Value representations in the primate striatum during matching behavior. Neuron. 2008;58:451–463. doi: 10.1016/j.neuron.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stalnaker TA, Calhoon GG, Ogawa M, Roesch MR, Schoenbaum G. Neural correlates of stimulus-response and response344 outcome associations in dorsolateral versus dorsomedial striatum. Front Integr Neurosci. 2010;4:12. doi: 10.3389/fnint.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nature neuroscience. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultz W. Predictive reward signal of dopamine neurons. Journal of neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behavioural brain research. 2000;110:97–108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- 37.Schultz W, Tremblay L, Hollerman JR. Changes in behavior-related neuronal activity in the striatum during learning. Trends Neurosci. 2003;26:321–328. doi: 10.1016/S0166-2236(03)00122-X. [DOI] [PubMed] [Google Scholar]
- 38.Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature. 2002;418:413–417. doi: 10.1038/nature00892. [DOI] [PubMed] [Google Scholar]
- 39.Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. San Diego: Academic Press; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.