Summary
The tumor suppressor p53 is frequently mutated in human cancer. Common mutant p53 (mutp53) isoforms can actively promote cancer through gain-of-function (GOF) mechanisms. We report that mutp53 prolongs TNF-α-induced NF-κB activation in cultured cells and intestinal organoid cultures. Remarkably, when exposed to dextran sulfate sodium (DSS), mice harboring a germline p53 mutation develop severe chronic inflammation and persistent tissue damage, and are highly prone to inflammation-associated colon cancer. This mutp53 GOF is manifested by rapid onset of flat dysplastic lesions that progress to invasive carcinoma with mutp53 accumulation and augmented NF-κB activation, faithfully recapitulating features frequently observed in human colitis-associated colorectal cancer (CAC). These findings might explain the early appearance of p53 mutations in human CAC.
Introduction
The connection between inflammation and cancer has drawn intensive research (Ben-Neriah and Karin, 2011; Demaria et al., 2010; Hanahan and Weinberg, 2011; Schetter et al., 2010), and has highlighted the context-dependent modulation of inflammation-associated cancer by the transcription factor NF-κB (Ben-Neriah and Karin, 2011; He and Karin, 2011). One well documented link between chronic inflammation and human cancer involves colorectal cancer (CRC) in patients suffering from inflammatory bowel disease (IBD) (Asquith and Powrie, 2010; Ullman and Itzkowitz, 2011). Continuous tissue destruction and renewal, together with persistent oxidative damage inflicted by the inflamed microenvironment, can trigger mutagenic processes that serve as cancer initiating events. Further tumor progression is augmented by the continuous presence of inflammatory cytokines, which may promote excessive cell proliferation and survival through activation of NF-κB and additional signaling pathways.
The p53 tumor suppressor provides powerful intrinsic defense against cancer (Levine and Oren, 2009; Vousden and Prives, 2009). Mutations in the TP53 gene are the most frequent genetic alteration in human cancer. The main selective advantage of such mutations is through abrogation of wild type (WT) p53-mediated tumor suppression. Yet, at least some frequently observed p53 mutations also contribute actively to cancer development through gain-of-function (GOF) activities (Brosh and Rotter, 2009; Oren and Rotter, 2010; Rivlin et al., 2011). This may involve enhancement of invasive properties, attenuation of apoptosis and increased genomic instability. Of note, mutant p53 (mutp53) has been reported to augment NF-κB activation in cultured cells presumably through direct protein-protein interaction (Schneider et al., 2010; Scian et al., 2005; Weisz et al., 2007). Since NF-κB is a master regulator of inflammation and a modulator of inflammation-associated cancer, it is plausible that mutp53 GOF may also impact the latter processes.
In the present study we investigated the conjecture that mutp53 may promote chronic inflammation and inflammation-associated cancer.
Results
Mutp53 prolongs NF-κB activation by TNF-α
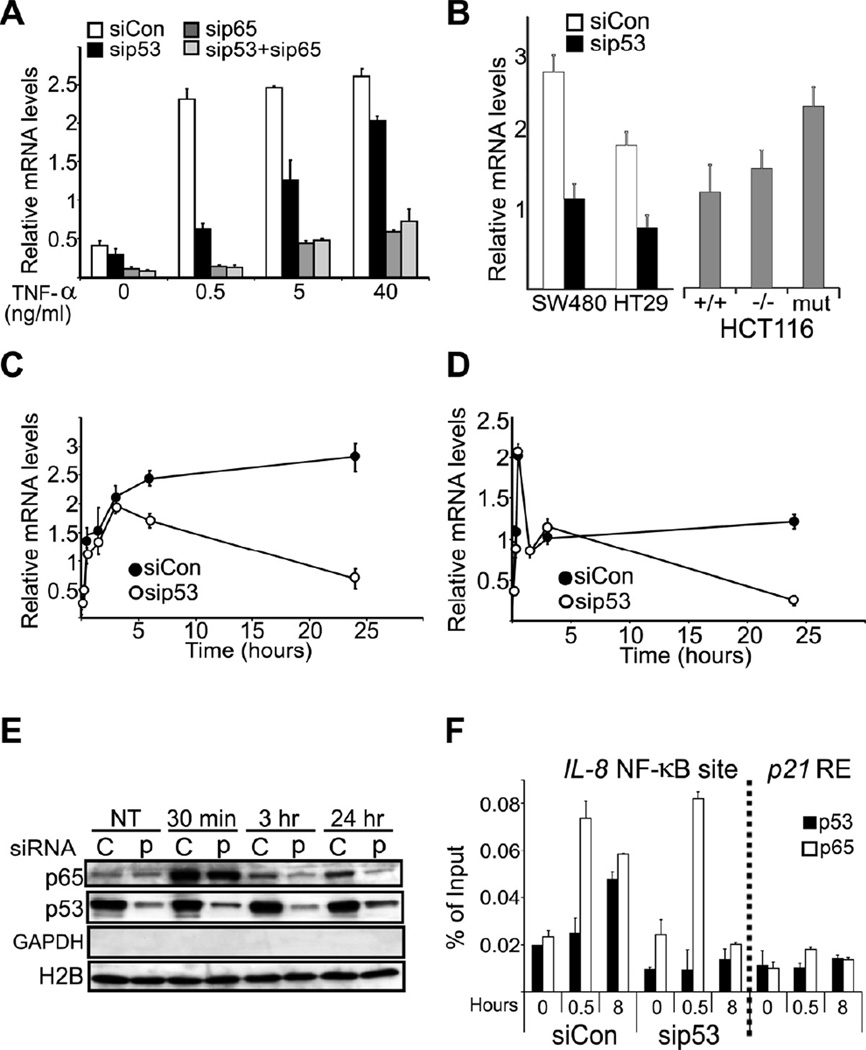
We explored mutp53 GOF activity employing the human pancreatic cancer-derived PANC-1 cell line, which harbors the p53R273H mutant. NF-κB activation was triggered by TNF-α; however, in addition to the usual high concentrations for short durations, we also included longer exposures to lower concentrations to mimic a chronic inflammatory state. Remarkably, siRNA-mediated transient depletion of mutp53 (Figure S1A) strongly attenuated the induction of IL-8, a prototypic NF-κB target gene, by low (0.5 ng/ml) TNF-α concentrations (Figure 1A, compare sip53 to siCon). This was reproduced with different p53 siRNA oligos and shRNA-mediated stable mutp53 knockdown (Figure S1B), making this being an off-target effect unlikely. Knockdown of the p65 NF-κB subunit confirmed that induction was NF-κB-dependent (Figure 1A, sip65). Additional NF-κB targets were similarly affected by mutp53 depletion (Figure S1C). Interestingly, mutp53 dependence was most pronounced at low TNF-a concentrations (Figure 1A). Mutp53 depletion had no further effect in the absence of p65 (sip53+sip65), supporting an epistatic interaction wherein mutp53 boosts IL-8 expression via p65/NF-κB. Similar effects of mutp53 depletion were observed in CRC-derived SW480 and HT29 cell lines (Figure 1B). Conversely, CRC-derived HCT116 cells expressing only mutp53 (mut, Figure 1B) (Sur et al., 2009) displayed elevated IL-8 induction relative to their WT p53 (+/+) or p53 knockout (−/−) counterparts.
Figure 1. Mutp53 prolongs TNFα–induced NF-κB activation.
A. PANC-1 cells were transfected with siRNA oligonucleotides specific for p53 (sip53) or lacZ as control (siCon). 48 hr later TNF-α was added at the indicated final concentration for an additional 24 hr. RNA was extracted and subjected to qRT-PCR analysis with primers specific for IL-8 mRNA. Values were normalized for GAPDH mRNA in the same sample.
B. SW480, HT29, HCT116 (+/+), and HCT116 derivatives without p53 (−/−) or with a knock-in 53 R248W (mut) were transfected as in (A) and exposed to 0.5 ng/ml TNF-α for 24 hr. IL-8 mRNA was quantified as in (A).
C. PANC-1 cells were transfected as in (A) and exposed to 0.5 ng/ml TNF-α for the indicated periods. RNA was extracted and subjected to qRT-PCR analysis with primers specific for IL-8 mRNA. Values were normalized as in (A).
D. The same RNA samples as in (B) were subjected to qRT-PCR analysis with primers derived from the first intron of the IL-8 gene. Values were normalized as in (A).
E. PANC-1 cells were transfected with siRNA oligonucleotides specific for p53 (p) or scrambled oligonucleotides as control (C), treated with 0.5 ng/ml TNF-α for the indicated time periods and harvested. Chromatin-bound proteins (see Experimental Procedures) were subjected to Western blot analysis with the indicated antibodies. GAPDH and H2B served to assess the absence of cytoplasmic contamination and the presence of chromatin, respectively.
F. PANC1 cells were treated with TNF-α (0.5 ng/ml) for 0, 0.5 or 8 hr and subjected to chromatin immunoprecipitation (ChIP) with p65 and p53 antibodies, followed by qPCR analysis with primers flanking the NF-κB site of the IL-8 promoter or the upstream p53-responsive element (RE) of the p21 gene (negative control). Values are presented as percentage of input.
In the entire figure, error bars represent +/− standard deviations (SD). See also Figure S1.
Kinetic analysis of IL-8 mRNA induction by low (0.5 ng/ml) TNF-α revealed that mutp53 was dispensable for the rapid early rise in IL-8 mRNA (Figure 1C). However, whereas in control PANC-1 cells (siCon) high IL-8 mRNA persisted for many hours, mutp53 depletion severely shortened the response duration. Hence, mutp53 prolongs the NF-κB response to limiting amounts of inflammatory cytokine, converting it from transient into chronic. Consistent with earlier observations (Schneider et al., 2010; Weisz et al., 2007), experiments employing a luciferase reporter under an NF-κB-regulated promoter (Figure S1D) confirmed a transcriptional mechanism. This was validated by qRT-PCR analysis of IL-8 pre-mRNA using intron-derived primers (Kuroda et al., 2005; Phelps et al., 2006; Shema et al., 2008) (Figure 1D). Remarkably, this revealed a biphasic transcriptional response, where the first rapid phase was indifferent to mutp53 whereas the second, later phase was highly dependent on mutp53. Thus, IL-8 transcription reverted to basal levels by 24 hours in mutp53-depleted cells, but continued unabated in mutp53-expressing cells. Notably, augmented association of p65 with chromatin, peaking at 30 minutes, remained detectable at 24 hours in mutp53 expressing (C lanes; Figure 1E) but not in mutp53-depleted cells (p lanes), probably underpinning the extended transcriptional response. Although mutp53 binds many NF-κB sites (Dell’Orso et al., 2011), global association of mutp53 with chromatin was unaltered (Figure 1E). However, chromatin immunoprecipitation (ChIP) analysis revealed recruitment of both p65 and mutp53 to the NF-κB site of the IL-8 promoter (Figure 1F). Remarkably, mutp53 depletion resulted in complete loss of p65 occupancy by 8 hours. Prolonged p65 retention coincided with enhanced recruitment of mutp53; this suggests a crucial role for mutp53 in maintaining NF-κB chromatin association, consistent with data obtained using DNA oligonucleotides (Schneider et al., 2010). To further characterize the impact of mutp53, we performed expression microarray analysis on control and mutp53-depleted PANC-1 cells, with or without TNF-α (GEO accession number - GSE43738). Indeed, induction of numerous genes by TNF-α was significantly attenuated by mutp53 knockdown (Figure S1E). To determine whether our findings apply also to non-transformed epithelial cells, 3D intestinal organoid cultures (Sato et al., 2009) (Figure 2A, left) were prepared from p53515A “knock-in” mice carrying a germline mutp53 allele encoding p53R172H, the mouse equivalent of the human hotspot mutant p53R175H (Lang et al., 2004), as well as from WT (+/+) and p53 knockout (−/−) mice. These organoids, comprised purely of epithelial cells, were then challenged with 0.5 ng/ml TNF-α for 24 hours. Remarkably, the presence of mutp53 greatly augmented the expression of KC (a mouse functional homolog of IL-8) and TNF-α mRNA (Figure 2A, right), confirming the trend seen in human cancer cells. A similar pattern was observed when comparing mouse embryonic fibroblasts (MEFs) of different genotypes: WT (+/+), p53 knockout (−/−), and homozygous (m/m) or heterozygous (+/m) for the p53515A allele (Figure S2A). Following exposure to 1 or 20 ng/ml TNF-α, induction of KC mRNA was strongly enhanced and prolonged in +/m and m/m MEFs. Transient p65 knockdown confirmed that this was largely NF-κB-dependent (Figure S2B). Hence, a single mutp53 allele suffices for robust prolongation of the NF-κB response. This did not occur in p53−/− MEFs, clearly demonstrating a mutp53 GOF effect. Additional NF-κB-regulated cytokine genes displayed a similar trend (Figure S2C). Likewise, primary mouse monocytes displayed a robust mutp53-mediated increase in the amount of KC protein secreted upon TNF-α stimulation (Figure S2D).
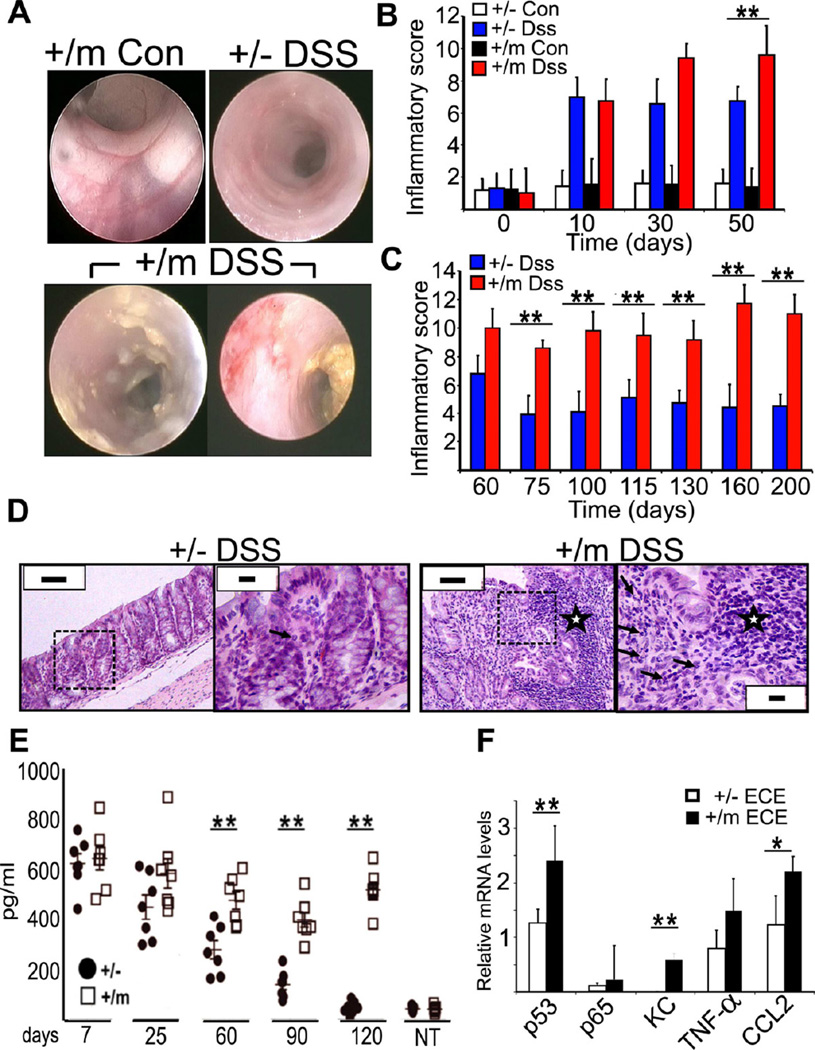
Figure 2. Mice expressing mutp53 are excessively susceptible to DSS.
A. Organoids comprised of intestinal epithelial cells of p53 +/+, −/− and m/m mice were cultured as in Experimental Procedures- left: representative photomicrograph of both low magnification (bar = 500 µm) and higher magnification (bar = 200 µm), then treated with TNF-α (0.5ng/ml) for 24 hr. p53, TNF-α and KC mRNA levels were quantified as in Fig. 1A. Bars represent ± SD.
B. p53+/− and +/m mice were treated with 2% DSS at the indicated time windows or left untreated (Con). Body weight was monitored throughout the indicated period. Values represent average relative weight normalized to the weight at the start of the treatment; bars indicate standard errors. **=p-value< 0.01.
C. p53+/− and +/m mice were treated as in (B). Mice were sacrificed at day 50 and colon lengths were measured. Distribution of individual measurements along with mean and standard deviation are shown. **=p-value< 0.01.
D. Colons of p53+/− and +/m mice, either untreated (Con) or treated with DSS, were collected at day 60 and subjected to histopathological analysis (bars = 100 µm).
See also figure S2.
Thus, in both epithelial and non-epithelial cells, mutp53 enforces an extended NF-κB response to an otherwise self-limiting pro-inflammatory trigger.
Mice expressing mutp53 are excessively susceptible to chronic inflammation and tissue damage
The ability of mutp53 to establish a “chronic” state of NF-κB activation in cultured cells raised the question whether mutp53 might also promote chronic inflammation and inflammation-associated carcinogenesis in vivo. To address these possibilities, p53515A mice were utilized. Of relevance, p53R175H mutations have been detected in neoplastic and preneoplastic lesions of colitis patients (Leedham et al., 2009; Noffsinger et al., 2001). Repeated exposure of mice to the inflammation-inducing agent Dextran Sodium Sulfate (DSS) elicits a condition resembling human IBD, including late onset colorectal tumors (Clapper et al., 2007). We employed DSS either in a chronic setting, involving three intermittent cycles of DSS over a total period of 43 days, or an acute setting involving a single 7-day treatment (Figure S2E; all subsequent numbering relates to day 0). Homozygous mutp53 mice are highly prone to early-onset spontaneous cancer (Lang et al., 2004; Lozano, 2010; Olive et al., 2004). Given the relatively long duration of the standard DSS carcinogenesis protocol and the ability of a single mutp53 allele to induce a prolonged NF-κB response in primary mouse cells (Figure S2A and S2D), we therefore opted to use heterozygous mutp53 mice, whose spontaneous tumors emerge relatively late (Lang et al., 2004; Olive et al., 2004). Specifically, we compared mice carrying a single mutp53 allele (+/m) or a single p53 knockout allele (+/−).
Exposure to DSS inflicts damage to the distal colon, with consequent weight loss. The two genotypes were similarly affected by the first DSS cycle, exhibiting comparable weight loss (Figure 2B). However, upon repeated DSS treatments, a marked difference became apparent: whereas +/− mice recovered fairly well, +/m mice progressively lost weight, suggesting sustained tissue damage. Indeed, determination of colon length at day 50 revealed more severe shortening of the colon in +/m as compared to +/− mice (Figure 2C). Histological examination confirmed chronic tissue damage in +/m colons, including loss of glands, glandular architectural distortion and extensive edema and inflammatory infiltrates (Figure 2D), whereas +/− colons appeared to be undergoing effective tissue repair. This was confirmed by quantitative analysis of crypt loss (Malaterre et al., 2007) (Figure S2F). Notably, mutp53 did not affect significantly intestinal barrier function following either acute or chronic DSS treatment (Figure S2G). Thus, mutp53 hampers tissue recovery and renders mice more susceptible to the deleterious effects of DSS.
The ability of mutp53 to prolong the expression of pro-inflammatory genes, together with the exacerbated response of +/m mice to DSS, suggested that these mice were experiencing accentuated chronic inflammation. This was directly confirmed by endoscopy (Figure 3A). Unlike +/− colons, +/m colons reveal ample evidence of active inflammation, including diffuse mucosal erythema and edema with disappearance of mucosal vessels and shallow ulcers. Systematic endoscopic followup, applying a semiquantitative inflammatory score (see Experimental Procedures), revealed that whereas inflammation was similarly induced in both genotypes at early times (Figure 3B), inflammation was attenuated in +/− colons, with most areas regaining near-normal morphology, but persisted in +/m colons (Figure 3C). Haematoxylin-eosin staining confirmed massive presence of both active and chronic constituents of inflammation in +/m colons (Figure 3D), including lymphoplasmacytic aggregates and neutrophils infiltrating the cryptic epithelium and forming crypt abscesses (grade 5). This was much milder in +/− colons (grade 2). Severity of colitis was graded as described in (Geboes et al., 2000), evaluating structural change, chronic inflammation, lamina propria neutrophils, neutrophils in epithelium, crypt destruction and erosions or ulcers. Quantification of secreted pro-inflammatory cytokines in the colon (Figure 3E and S3A,B) revealed that both genotypes mounted an abundant cytokine response at very early times, which subsided gradually in +/− but remained chronically elevated in +/m mice. To assess the involvement of the colonic epithelial cells in this response, epithelial cells-enriched (ECE) fractions prepared from colons of both genotypes were subjected to RNA analysis. Relative to +/− ECE, +/m ECE expressed significantly higher levels of several pro-inflammatory cytokine mRNAs (Figure 3F), implying augmented NF-κB activation. Unexpectedly, p65 mRNA levels were also higher in the +/m ECE, although p65 protein levels were not significantly elevated (data not shown).
Figure 3. Mice expressing mutp53 are prone to chronic inflammation.
A. Representative colonoscopy images of p53+/− and +/m mice subjected to chronic DSS treatment (DSS) or left untreated (Con) at day 50.
B,C. p53+/− and +/m mice, treated as in (A), were subjected to colonoscopy at the indicated time points and scored blindly. An average inflammatory score was calculated (n = 5 mice/group). *=p-value< 0.05; **=p-value< 0.01
D. Histopathological analysis of mouse colon sections at day 107. Low (bars = 200 µm) and high (bars = 50 µm) magnifications are shown for each specimen; dashed rectangle indicates the enlarged area. Asterisks indicate lymphocytic aggregations; arrows depict neutrophils. Note the existence of an area with microerosion in the +/m DSS case.
E. Colons were obtained from DSS-treated mice at the indicated time points, and levels of secreted TNF-α were assessed as described in Experimental Procedures. Distributions of individual mice together with mean plus standard deviation for each group are shown. NT = non-treated. **=p-value< 0.01.
F. Epithelial cell enriched (ECE) fractions were obtained from DSS-treated mice at day 60. RNA was extracted and subjected to qRT-PCR with primers specific to the indicated genes. Values were normalized to GAPDH mRNA in the same sample. **=p-value< 0.01. *=p-value< 0.05. In the entire figure, error bars represent +/− SD.
See also figure S3.
Thus, mice harboring a germline mutp53 allele display a markedly enhanced propensity for chronic inflammation and failure to resolve inflammation-associated tissue damage. The mice employed here carry mutp53 in all their cells. This might augment NF-κB activation not only in the epithelium but also in other tissues, including the myeloid compartment as suggested by Figure S2D. To evaluate the relative contribution of different tissue compartments to mutp53-driven chronic inflammation, we generated bone marrow chimeras. Briefly, irradiated +/− and +/m mice were reconstituted with bone marrow from non-irradiated animals of either genotype and subjected to chronic DSS treatment. Regardless of donor and recipient genotype, mixed chimeras underwent substantial weight loss, albeit less pronounced than when both donor and recipient were +/m (Figure S3C). However, good recovery was achieved when both donor and recipient were +/−. Concurrently, both types of mixed chimera displayed elevated chronic inflammation (Figure S3D,E). Hence, augmented chronic inflammation is driven by mutp53 GOF in multiple body compartments, probably entailing a mutually reinforcing cytokine-mediated crosstalk between the colonic epithelium and myeloid as well as other stromal cells.
Mutp53 exerts an unequivocal GOF effect on the proinflammatory transcriptional response in cultured mouse cells (Figure S2A–D). Yet, one could still argue that the in vivo effects are not due to true GOF but rather to a dominant negative mechanism, wherein the p53R172H protein inactivates the remaining WT p53 protein in the +/m mice and contributes to chronic inflammation merely by rendering the cells practically devoid of WT p53 activity. We therefore compared the DSS response of homozygous p53−/− and p53m/m mice. Remarkably, weight loss in −/− mice was very similar to +/− and WT mice (Figure 4A), arguing that loss of WT p53 function does not explain the exaggerated DSS hypersensitivity. In stark contrast, +/m mice exhibited enhanced progressive weight loss. Although not statistically significant, this appeared even slightly more severe in m/m mice. The differences between −/− and m/m mice were confirmed by monitoring inflammatory score (Figure 4B). Thus, also in vivo, mutp53 exerts proinflammatory GOF. Indeed, DSS-treated m/m mice suffered increased mortality (Figure 4C), presumably owing to superimposition of persistent tissue damage on the inherent morbidity of mice lacking WT p53.
Figure 4. Gain-of-function effect of mutp53.
A. Mice of the indicated genotypes were treated and analyzed as in Fig. 2B. Only DSS-treated mice are shown. Error bars represent +/− standard error (SE).
B. p53−/− and m/m mice were treated with DSS as in Fig. 2B. and monitored periodically by colonoscopy. Inflammatory scores were determined as in Fig. 3B. **=p-value< 0.01. Error bars represent +/− SD.
C. Kaplan-Mayer plot showing survival of DSS treated p53−/− and m/m mice.**=p-value< 0.01 by Mantel-Cox test.
Mutp53 mice are highly prone to DSS-induced colon carcinoma
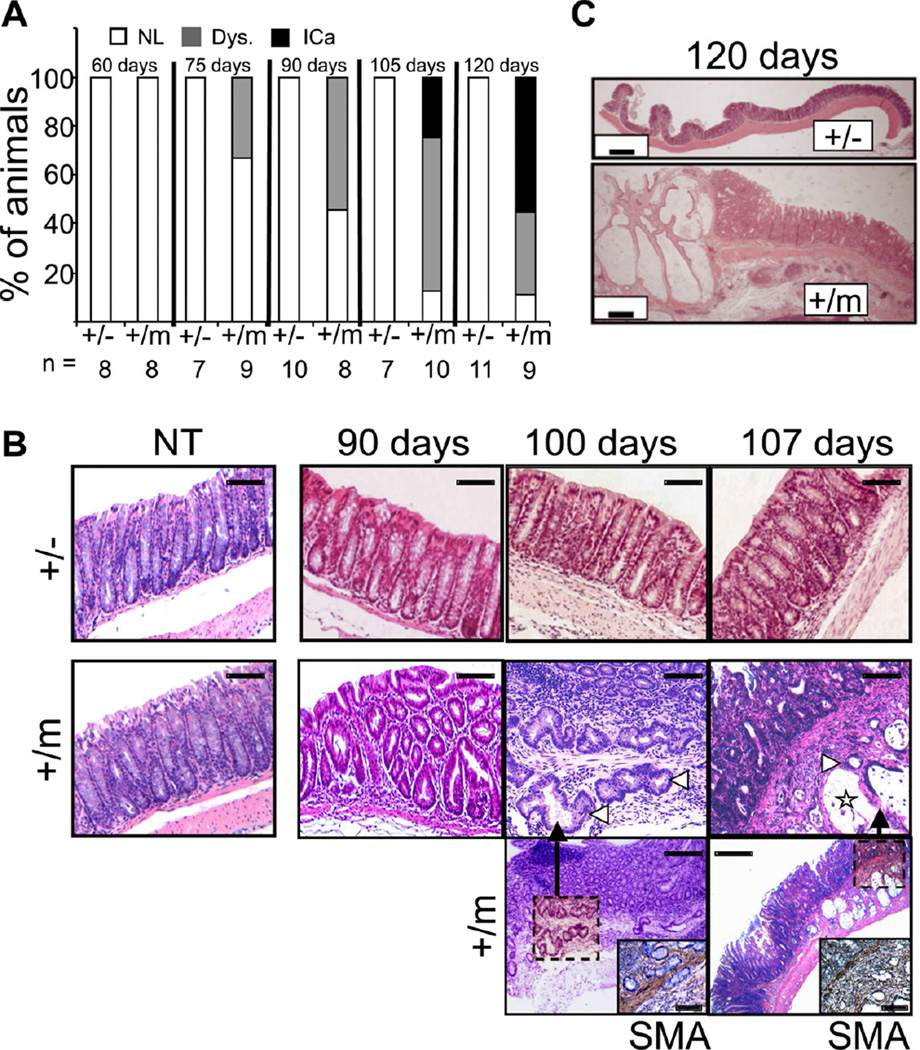
The propensity of mutp53 mice to perpetuate unresolved chronic colonic inflammation raised the question whether they are more prone to inflammation-associated CRC. For up to 60 days (18 days after the last DSS cycle), no neoplastic lesions were observed by histopathological examination in the colons of either +/− or +/m mice (Figure 5A). However, by day 75 low grade dysplastic lesions were discernible in several +/m mice, increasing in number and grade by day 90. By day 100, invasive carcinoma was already apparent in some cases (Figure 5B). Angular irregularly shaped glands of well-differentiated tubuloglandular colorectal adenocarcinoma were observed invading through the muscularis mucosae into the submucosa. By day 120, multifocal adenocarcinoma was seen only in +/m colons, with abundant extracellular mucin production, reminiscent of human mucinous colorectal adenocarcinoma. The cancerous glands penetrated the submucosa, accompanied with desmoplastic reaction of the surrounding stroma. The overlying epithelium was dysplastic, encompassing areas with low and high grade flat dysplasia (Figure 5C). Invasion through the muscularis mucosae was confirmed by smooth muscle actin (SMA) immunostaining (Figure 5B). Flat dysplastic lesions are hard to detect by standard colonoscopy, particularly at early stages; however, at later time points suspicious dysplasia associated lesions or masses (DALM) were sometimes observed (Figure S4A), confirmed by gross assessment of the colon (Figure S4B) and subsequent histopathological examination, which also revealed the presence of invasive carcinoma with extracellular mucin production (Figure S4C,D). Throughout that entire period, no neoplastic lesions were detected in any +/− mouse. As expected, chronic inflammation was suppressed by treatment of +/m mice with the anti-inflammatory drug sulindac, initiated 7 days after the last DSS cycle (Figure S4E,F). Importantly, sulindac inhibited DSS-induced carcinogenesis (Figure S4G,H), confirming that this mutp53 GOF effect relies on its ability to elicit chronic inflammation. Thus, mutp53 not only makes the mice more susceptible to chronic inflammation, but also greatly accelerates inflammation-associated colon cancer.
Figure 5. Mutp53 mice are prone to DSS-induced colon carcinoma.
A. p53+/− and +/m mice were treated with DSS as in Fig. 2B. Mice were sacrificed at the indicated time points and colon sections were subjected to histopathological analysis. Each bar depicts the percentage of mice within a given group scoring positive for the indicated lesions. Numbers of animals/group are noted at the bottom (n=). NL= no lesion; Dys. = dysplasia (low or high grade); ICa = invasive carcinoma.
B. Representative histopathological images of colon sections obtained from non-treated mice (NT) of the indicated genotypes or mice subjected to chronic DSS treatment and sacrificed at the indicated time points. Note the presence of low grade flat dysplasia in p53+/m cases at 90 days and the development of well-differentiated carcinoma and mucinous adenocarcinoma at days 100 and 107, respectively. Cancerous glands (white arrowheads) invade the muscularis mucosae, identified by smooth muscle actin (SMA) staining (insets). Star denotes pool of extracellular mucin. Bar sizes: upper panel = 50µm, middle panel = 200 µm, lower panel = 600 µm.
C. Low magnification images of colon sections from DSS-treated mice of the indicated genotypes, obtained at day 120. Note the progressive multifocal invasive carcinoma with prominent mucin lakes in the p53+/m mouse. Bars = 500 µm.
See also Figure S4.
Mutp53 accumulates in the inflamed colon and in cancerous glands concomitantly with NF-κB activation and sustained DNA damage
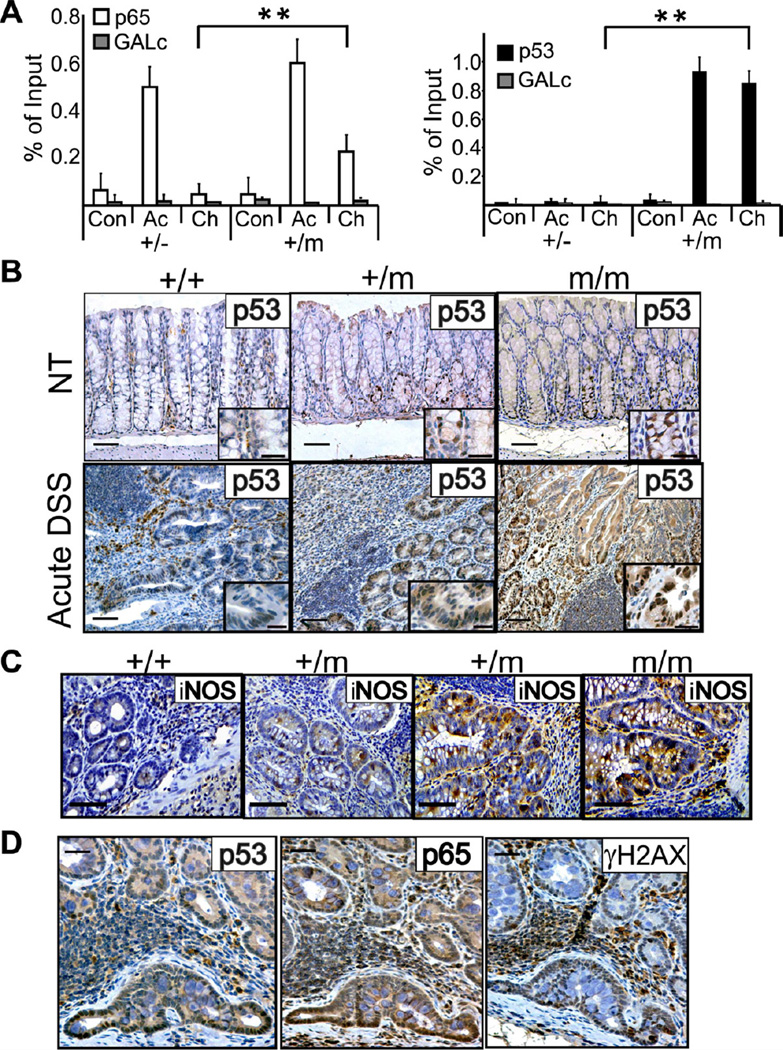
To interrogate molecular events underpinning the GOF effect of mutp53, chromatin from mouse colons was subjected to ChIP analysis. In colons of untreated mice, very little p65 or p53 associated with the NF-κB site of the MIP2 gene, regardless of p53 status (Figure 6A). After a 7-day acute DSS treatment, p65 occupancy increased considerably in both +/− and +/m colons. Importantly, by day 60 of chronic DSS treatment, p65 remained markedly associated with the NF-κB site only in +/m but not +/− colons, as seen also with several additional genes (Figure S5A). Remarkably, mutp53 was recruited to the same genomic region upon DSS treatment, persisting through day 60. This suggests that in the colon, like in cultured cells (Figure 1E,F), mutp53 retains p65 on the chromatin for extended periods.
Figure 6. Analysis of p53, p65, iNOS and γH2AX in DSS-exposed mice.
A. Colons of p53+/− and +/m mice were harvested either without treatment (Con), immediately after acute DSS treatment (Ac), or at day 60 of the chronic DSS protocol (Ch), and subjected to ChIP analysis with antibodies against mouse p65 (left) or mouse p53 (right). Extracted DNA was subjected to qPCR with primers flanking the NF-κB site of the MIP2 gene promoter. Primers corresponding to a region located far from any coding gene (GALc) served as negative control. ChIP values are presented as percentage of input.
B. Colon sections were prepared at day 7 from mice of the indicated genotypes subjected to acute DSS treatment, as well as from non-treated mice (NT), and stained for p53 (bars = 100 µm). Insets display a higher magnification (bars in upper insets are 50 µm and bars in lower insets are 25 µm).
C. Colon sections prepared as in (B) were stained with antibodies against iNOS; sections from two +/m mice are included to show the mouse-to-mouse variability in this genotype. Bars are 50 µm.
D. Staining for p53, p65 and γH2AX in invading gland within the colon of a p53+/m mouse, collected at day 105 of the chronic DSS protocol. Bars are 50 µm.
In the entire figure, error bars represent +/− SD. See also figure S5.
In colonic epithelium of untreated WT p53 mice, p53 staining was practically undetectable by immunohistochemistry (Figure 6B), while some p53-positive nuclei were present in +/m and m/m mice, particularly within the lower part of the crypt. Acute 7 day DSS treatment of WT mice triggered modest nuclear p53 accumulation in some cells (Figure 6B). Notably, this was greatly augmented in +/m mice, and even more in m/m ones. This p53 accumulation was likely driven by reactive oxygen and nitrogen species (ROS and RNS) in the inflamed microenvironment (reviewed by Schetter et al., 2010; Ullman and Itzkowitz, 2011). Indeed, expression of inducible nitric oxide synthase (iNOS) was most pronounced in m/m mice and least in +/+ ones, with +/m mice exhibiting variable intermediate iNOS levels (Figure 6C). A similar pattern, consistent with the differential regulation of iNOS expression by WT p53 versus mutp53 (Forrester et al., 1996), persisted also after cessation of DSS treatment in the chronic protocol (Figure S5B). As expected, induction of p53 in WT colons was accompanied by upregulation of p21, a prototypical WT p53 transcriptional target (Figure S5C,D +/+). In contrast, p21 was hardly detected in p53 m/m colons, confirming that the mutant p53 was transcriptionally inactive.
Intense mutp53 staining persisted throughout the tumor progression sequence. It was particularly prominent in invasive fronts of cancerous glands (Figure 6D), suggesting that in addition to cell-autonomous factors, local microenvironmental cues may also drive persistent mutp53 stabilization. Furthermore, strongest mutp53 staining coincided with intense nuclear p65 staining (Figure 6D). This implies that, like in cultured cells, high mutp53 levels may fuel an augmented and prolonged NF-κB response in the colonic epithelium. Interestingly, many of those cells were also positive for phosphorylated histone H2AX (γH2AX; Figure 6D), indicative of persistent DNA damage. This could simply reflect the DNA damage constantly inflicted on the cells within the chronic inflammatory microenvironment. Alternatively, and not mutually exclusive, defective DNA repair in the mutp53 cells might lead to gradual accumulation of unrepaired double strand breaks and other types of damage. Either way, such exacerbated damage may increase genomic instability, driving the neoplastic lesions towards higher malignancy.
Mutp53 accumulation correlates with NF-kB activation in human colitis-associated cancer and non-neoplastic glands
Human colitis-associated cancer (CAC) involves frequent TP53 mutations, along with abundant mutp53 protein accumulation in the cancerous glands (reviewed in Asquith and Powrie, 2010; Ullman and Itzkowitz, 2011). To determine whether, like in p53515A mice, this is associated with constitutive NF-κB activation, 18 formalin fixed paraffin embedded (FFPE) CAC specimens from two different sources were subjected to TP53 tagged-amplicon Illumina HiSeq 2000 sequencing (Forshew et al., 2012) along with IHC analysis. TP53 mutations were detected in 16 tumors (Figure S6A). Based on their TP53 sequence, these tumors were divided into three groups: no p53 mutations (WT p53; 2/18), missense p53 mutations (8/18), and other p53 mutations (nonsense, frameshift and splicing; 8/18). As expected, intense diffuse nuclear p53 staining was more prominent in the missense mutation cases (Figure 7A,B, Figure S6A,B). Importantly, consistent with our mouse data, abundant p53 staining correlated strongly with augmented nuclear p65 staining (Pearson correlation coefficient = 0.85), as observed also in head-and-neck cancer and sporadic CRC (Weisz et al, 2007; Schwitalla et al, 2013).
Figure 7. Mutp53-p65 correlation in human CAC and IBD.
A. Concurrent strong immunostaining of p53 and nuclear p65 in consecutive serial sections of a representative human CAC case carrying a p53R273H mutation (case 36102, Figure S6A).
B. Staining abundance and intensity (%LI) were calculated for all 18 specimens as described in Figure S6A. The scatter plot depicts the p65 and p53 %LI values for each individual tumor. Pearson correlation coefficient for all groups together = 0.85.
C. Immunostaining for p53, p65 and p21 in the epithelium, within an area of severe acute inflammation, in a human case of severe active colitis. Upper panel displays a low magnification field; two glands (a and b) are indicated by dashed rectangles. Higher magnifications of gland a (middle panel) and gland b (lower panel) are shown below. Arrows indicate cells with intense nuclear staining. Scale bars = 50µm.
See also Figure S6.
Intense p53 staining in tumors is generally considered indicative of missense mutations; yet, CAC cases with positive p53 IHC but no detectable mutation have been reported (Yoshida et al., 2003). Interestingly, four of our cases carried either the R213X nonsense mutation or the chr17:7578370:C>T splice mutation, both observed repeatedly in human tumors (http://p53.iarc.fr), raising the intriguing possibility that such mutations confer an unexpected GOF. Indeed, in all those four cases, p53 staining was considerably greater than in the remainder “other” mutants (38.1 ± 13.9 average p53 % LI vs. 17.8 ± 9.7); this warrants further investigation.
In human CAC, mutp53 accumulation often precedes the appearance of detectable neoplastic lesions (Hussain et al., 2000). To assess whether such early mutp53 accumulation might already drive constitutive NF-κB activation, IHC analysis was performed on specimens from IBD patients experiencing chronic inflammation with no recognizable neoplastic lesions. As exemplified in Figure 7C, the majority of glands displayed relatively weak p53 staining, with occasional areas of stronger positivity (e.g gland a). Typically, p21 also stained more strongly in the same areas, arguing that the p53-positive cells were expressing WT p53, presumably activated by local stress signals. However, a few glands (exemplified by gland b) exhibited a strikingly different pattern, with strong diffuse nuclear p53 but practically no p21 staining; this was retained also in more lumen-proximal sections of the gland (Figure S6C), suggesting the presence of mutp53 throughout gland b. Remarkably, the same gland displayed also robust NF-κB activation. Dysplasia and cancer can arise from patches of chronically inflamed crypts containing p53 mutations (Leedham et al., 2009); gland b might represent such mutp53 crypt, potentially serving as a precursor to subsequent neoplasia. Furthermore, as in the mouse, presence of mutp53 within a chronically inflamed microenvironment appears to kindle persistent NF-κB activation.
In sum, both histologically and molecularly, the p53515A DSS mouse model of IBD-associated cancer closely reproduces features frequently observed in its human counterpart.
AOM bypasses the dependence on mutp53 GOF for accelerated tumorigenesis
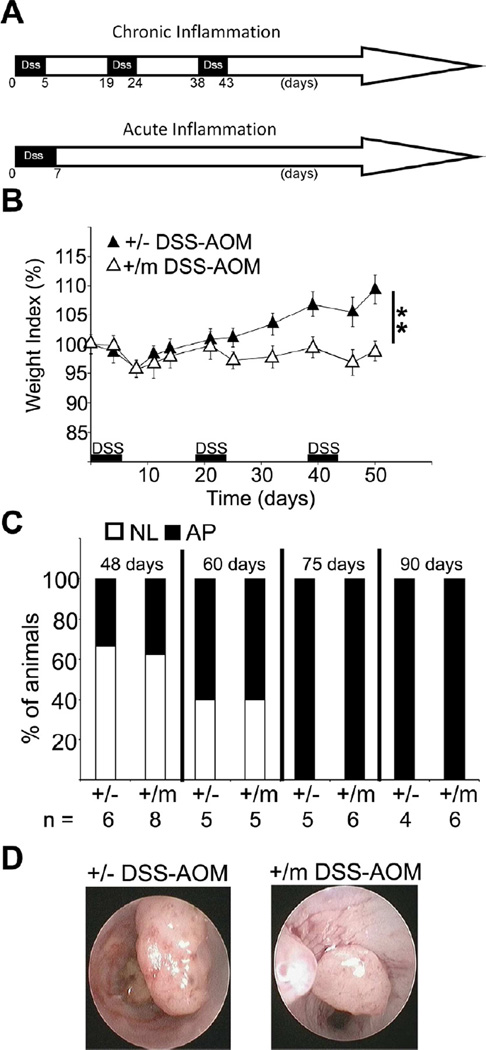
In parallel with DSS only, additional +/− and +/m mice were exposed to a standard protocol (Tanaka et al., 2003) combining DSS with the chemical carcinogen azoxymethane (AOM; Figure 8A). As with DSS alone, +/m mice displayed more severe weight loss (Figure 8B). Surprisingly, tumorigenesis was not noticeably accelerated in these mice. Instead, the entire tumor progression pattern was altered, both genotypes rapidly developing massive adenomatous polyps (Figure 8C,D) at similar numbers and size (Figure S7A). Remarkably, regardless of genotype, nuclear and cytoplasmic β-catenin accumulation predominated in these tumors (Figure S7B,C), consistent with the frequent induction of Ctnnb mutations by AOM (Greten et al, 2004) and indicative of robust Wnt pathway activation. In contrast, in DSS-only tumors of +/m mice β-catenin was retained at the plasma membrane, suggesting absence of Wnt pathway activation. Notably, while Wnt pathway activation is almost unanimous in human sporadic CRC, mostly involving early Adenomatous Polyposis Coli (APC) mutations, such mutations are typically late and markedly less frequent in human CAC (Ullman and Itzkowitz, 2011). Interestingly, in mice treated with AOM only, where tumor development approximates human sporadic CRC, the mere loss of p53 triggers an inflammatory transcription program and promotes invasiveness (Schwitalla et al, 2013). Thus, inclusion of a chemical carcinogen switches the response towards a different course with pathological and molecular features commonly observed in sporadic human CRC. In this setting, early p53 mutations do not appear to exert much impact.
Figure 8. Analysis of mice subjected to DSS+AOM.
A. Scheme of treatment schedule with DSS+AOM. p53+/− and +/m mice were subjected to treatment with DSS+AOM.
B. Weight index was monitored and calculated as in Fig. 2B. **=p-value< 0.01. Error bars represent +/− SE.
C. p53+/− and +/m mice treated with DSS+AOM were periodically monitored by colonoscopy. Each bar depicts the percentage of mice within a given group bearing adenomatous polyps (AP) or no lesion (NL); number of animals/group indicated at the bottom.
D. Representative colonoscopy images of polyps in p53+/− and +/m mice at day 105 of exposure to the DSS+AOM protocol.
See also Figure S7.
Discussion
The course of human IBD-associated neoplasia differs substantially from that of spontaneous CRC. While the latter is characterized by adenomatous polyps, a few percent of which may progress to invasive carcinoma, IBD-associated CRC often involves flat dysplastic lesions that progress from low to high grade and eventually may give rise to invasive carcinoma, although tumor development may skip one of these steps (Fearon, 2011; Terzic et al., 2010; Ullman and Itzkowitz, 2011). Likewise, the underlying genetic events also differ. In sporadic CRC, APC mutational inactivation is very often the initiating event. In contrast, loss of APC function typically occurs late in IBD-associated CRC, in only a minority of tumors. Conversely, even though p53 mutations are highly abundant in both types of CRC, they occur very early in IBD-associated CRC but are considered late events in sporadic CRC (Brentnall et al., 1994; Yin et al., 1993); reviewed in (Fearon, 2011; Kinzler and Vogelstein, 1996; Terzic et al., 2010; Ullman and Itzkowitz, 2011). In fact, p53 mutations are enriched in the inflamed colonic tissue well before neoplastic lesions become detectable (Hussain et al., 2000). Moreover, analysis of individual crypts suggests that p53 mutations have a founder effect in driving colitis-associated carcinogenesis (Leedham et al., 2009). Thus, p53 mutations probably serve an initiating role in IBD-associated cancer, whereas in sporadic CRC they are more important for transition from localized late adenoma to invasive carcinoma. Yet, in both scenarios one might argue that p53 mutations serve solely to abrogate the tumor suppressor effects of WT p53, without the need to implicate mutp53 as having GOF.
We now show that mutp53 exerts a distinct GOF activity through augmenting and prolonging the response of epithelial cells to low amounts of inflammatory cytokine, enforcing a chronic state of NF-κB activation. Furthermore, mutp53 instates a chronic inflammatory condition in animals exposed to tissue damage. In the mouse colon, this spawns invasive carcinoma within less than 4 months. These effects, attainable by even a single mutp53 allele, are not reproduced by loss of WT p53.
The greatly accelerated tumorigenesis likely results from a combination of enforced prolonged inflammation associated with persistent tissue damage, increased genome instability, and augmented capacity of mutp53-containing cells to evade apoptosis in the face of the harsh inflammatory microenvironment. These effects may benefit from mutp53’s ability to perpetuate NF-κB activation through its prolonged stabilization on κB sites associated with pro-inflammatory cytokine genes, probably involving physical interactions between mutp53 and NF-κB (Schneider et al., 2010). Notably, the course of disease in mutp53-expressing mice bears striking resemblance to that observed in many cases of human IBD-associated cancer, progressing from low to high-grade flat dysplasia and finally to invasive carcinoma, without involvement of adenomatous polyps. Our findings support the conjecture that the early presence of p53 mutations in the inflamed colon of IBD patients (Hussain et al., 2000) is not merely a reflection of the mutagenic microenvironment, but may actually be a driver of the subsequent emergence of neoplasia by invigorating inflammation in the immediate microenvironment of cells harboring mutp53.
Loss of WT p53 can by itself increase DSS-induced tumorigenesis, yielding a mixture of polypoid and flat dysplastic lesions, some of which progress to invasive carcinoma (Chang et al., 2007; Fujii et al., 2004). Hence, WT p53 is a relevant tumor suppressor in this setting. Indeed, numerous studies have revealed an intricate crosstalk between WT p53 and NF-κB, wherein WT p53 often inhibits NF-κB activation but can also sometimes cooperate with NF-κB to orchestrate a more vigorous stress response (Ak and Levine, 2010; Ryan et al., 2000; Tergaonkar and Perkins, 2007) (Meylan et al., 2009; Schwitalla et al, 2013). However, in DSS-treated p53−/− mice, cancer develops with long latency (126–160 days) and partial penetrance, even when using high DSS (4%, compared to 2% in our study). By contrast, we show that a single mutp53 allele suffices to elicit a much faster and more aggressive disease course, demonstrating a true GOF effect. Such GOF requires the buildup of high mutp53 concentrations, typically entailing protein stabilization (Brosh and Rotter, 2009; Oren and Rotter, 2010; Rivlin et al., 2011; Suh et al., 2011). Notably, while mutp53 accumulation is usually seen in tumors but not non-transformed mutp53 mouse tissues (Suh et al., 2011; Terzian et al., 2008), we find that under inflammatory conditions this occurs already well before the onset of any observable neoplastic events. This may be due to elevated iNOS expression and higher ROS and RNS (Schetter et al., 2010; Ullman and Itzkowitz, 2011), eliciting genotoxic stress with subsequent p53 stabilization (Suh et al., 2011). A similar mechanism may account for accumulation of mutp53 in seemingly non-neoplastic crypts of human IBD patients, where strong p53 accumulation in the entire crypt suggests that epithelial cells with mutp53 may have gradually taken over the crypt. Repetitive exposure of such crypt to oxidative stress and inflammatory cytokines, driving constitutive NF-kB activation, probably increases its likelihood to progress towards neoplasia.
Mutp53 GOF effects are particularly prominent in the presence of oncogenic Ras mutations, as demonstrated both in vitro and in vivo (Acin et al., 2011; Buganim et al., 2010; Doyle et al., 2010). Indeed, PANC-1, SW480 and HCT116 cells harbor mutant K-Ras. However, similar effects are observable in HT29 cells, which do not carry Ras mutations. Hence, although Ras mutations may cooperate with mutp53, they are not obligatory for its GOF in augmenting NF-κB activation, as reflected also by the fact that NF-κB activation correlates with mutp53 accumulation in CAC tumors regardless of K-Ras mutations.
The mice employed in this study harbor mutp53 not just in their colonic epithelium but rather in all cell types, including adjacent fibroblasts and mobilized inflammatory cells. Experiments employing intestinal organoids, comprising only epithelial cells, suggest that mutp53-driven augmentation of NF-κB activity may suffice to drive a chronic inflammatory process. Indeed, constitutive activation of NF-κB in the mouse intestinal epithelium is sufficient to establish an inflammatory microenvironment and promote tumorigenesis (Vlantis et al., 2011). However, non-epithelial cells are also prone to mutp53-mediated augmented NF-κB activation. Hence, the accelerated tumorigenesis seen in the mutp53 mice may involve a vicious cycle wherein both epithelial and non-epithelial components engage in excessive production of cytokines and an exaggerated response to those cytokines, a conjecture supported by the bone marrow chimera experiments. Stromal p53 mutations have been described in several types of human cancer, including CRC (Patocs et al., 2007; Wernert et al., 2001), although this has subsequently been questioned (Campbell et al., 2009). It is plausible that a chronically inflamed microenvironment, characterized by continuous exposure to mutagenic compounds such as ROS and RNS, could promote stromal p53 mutations, which might potentially contribute to human inflammation-associated cancer. In sum, our findings suggest that by augmenting NF-κB activation, the emergence of p53 mutations in a chronically inflamed microenvironment may both exacerbate the inflammatory response and protect the mutant cells from the deleterious effects of this microenvironment. These GOF effects of mutp53 may account for the apparent early role of p53 mutations in inflammation-associated carcinogenesis.
Experimental procedures
Cell culture, transfection and infection
PANC-1 (generous gift of Yoel Kloog, Tel-Aviv University), SW480, HT29, HCT116 cells and their derivatives somatically knocked out for p53 and knocked in for the R248W p53 mutant (Sur et al., 2009) (generous gift of Bert Vogelstein), mouse intestinal organoids and isolated primary mouse monocytes were maintained at 37°C in a standard 5% CO2 humidified incubator, while mouse embryonic fibroblasts (MEFs) obtained from C57BL/6 mice were maintained at 37°C in a 5% CO2 and 3% O2 humidified incubator (Thermo Scientific). Dulbecco’s modified Eagle’s medium (DMEM) was used for growing PANC1, SW480 and MEFs, McCoy’s medium for the HT29 and HCT116 cells, and DMEM/F12 medium for intestinal organoids. DMEM and McCoy’s were supplemented with 10% heat-inactivated fetal bovine serum (FBS) (HI-FBS, Sigma), 2 mM L-glutamine and antibiotics. Intestinal organoids were cultured in Matrigel with medium added with Glutamax (x100, Gibco), B27 (1:50, Invitrogen), mouse bFGF (10 ng/ml, Peprotech), human EGF (20 ng/ml, Peprotech), mouse noggin (100 ng/ml, Peprotech), human r-spondin (500 ng/ml, Peprotech) and antibiotics.
For siRNA transfections, Dharmafect 1 reagent (Dharmacon) was used according to the manufacturer’s protocol. SiRNAs for p53 and p65, as well as control scrambled siRNA, were purchased from Dharmacon as SMARTpools. To exclude off-target effects, ON-TARGET plus p53 siRNA oligos (Dharmacon) were also used (sip53 II, Fig. S1B).
For stable clones, ecotropic Phoenix packaging cells were transfected with 10 µg DNA of the appropriate retroviral construct by a standard calcium phosphate procedure. Culture supernatants were collected 36–48 h after transfection and filtered. PANC-1 cells were infected with the filtered viral supernatants in the presence of 4µg/ml Polybrene (Sigma) for 12 h, after which the medium was changed. Fresh viral suspensions were added after a 24 h interval for an additional 12 hrs. Infected cells were selected for 5 days in 2.5µg/ml puromycin.
Chromatin immunoprecipitation (ChIP)
Cells were cross-linked with 1% formaldehyde at room temperature for 10 min, washed twice with 10 ml ice-cold phosphate-buffered saline, and then scraped into 0.5 ml of lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.1, 1 mM PMSF, 1 µg/ml leupeptin, 1 µg/ml Aprotinin) and left on ice for 10 min. Samples were then sonicated seven times at 4°C; each sonication was for 20 s, followed by a 40 s pause. Supernatants were recovered by centrifugation at 12,000 rpm in an Eppendorf microfuge for 10 min at 4°C before being diluted 4–8 fold in dilution buffer (1% Triton X-100, 2 mM EDTA, 150 mM NaCl, 20 mM Tris-HCl, pH 8.1). Samples were then pre-cleared for 2 hr at 4°C with 2 µg sheared salmon sperm DNA and 20 µl protein A Sepharose (50% slurry). At this stage, 50µl of the material was kept as input. Immunoprecipitations were performed overnight with specific antibodies (1–2µg), with addition of NP-40 to a final concentration of 0.5%. Immune complexes were captured by incubation with 20 µl protein A Sepharose (50% slurry) and 2 µg salmon sperm DNA for 1 hr at 4°C. Immunoprecipitates were washed sequentially for 5 min each at 4°C in TSE 1 (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 150 mM NaCl), TSE 2 (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 500 mM NaCl), and Buffer 3 (0.25 M LiCl, 1% NP-40, 1% deoxycholate, 1 mM EDTA, 10 mM Tris-HCl, pH 8.1). Beads were washed twice with TE buffer (10mM Tris-HCl, 1mM EDTA) and eluted with 500 µl elution buffer (1% SDS, 0.1 M NaHCO3). To reverse the crosslinks, samples were incubated at 65°C overnight, after which DNA was eluted and subjected to qPCR. When mouse colons were subjected to ChIP, epithelium was scraped as described in (Guma et al., 2011) and subsequently underwent the same procedure as described above. The primers used for qPCR are listed in the supplementary section of the Experimental Procedures.
RNA and real-time quantitative PCR (qPCR)
RNA was isolated with a NucleoSpin RNA II kit (Macherey-Nagel). 1.5 µg aliquots were reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Promega) and random hexamer primers (Amersham). Real-time qPCR was performed using SYBR Green Master Mix (Applied Biosystems) in a StepOnePlus instrument (Applied Biosystems). The primers used for qPCR are listed in the supplementary section of the Experimental Procedures.
Mice
All mouse strains were maintained on a C57BL/6 background. Cohorts were generated by mating p53 +/m p53 or p53 +/− mice. p53515A mice (Lang et al., 2004) harbor a G-to-A substitution at nucleotide 515, resulting in an Arg to His substitution at amino acid position 172 of mouse p53. Genotyping was performed by polymerase chain reaction (PCR) analysis as described (Lang, 2004). Acute colitis was induced by providing 2% dextran sodium sulfate (DSS; Millipore, USA) for seven days in the drinking water of 8 week old male mice weighing 21–25g, whereas the chronic protocol involved three 5 day cycles of 2% DSS separated by 14 day intervals. Mice were monitored 3 times a week for overall weight, stool consistency and gross bleeding. Colonoscopy procedures were conducted using a 1.9mm endoscope (Karl Storz GmbH & Co, Tuttlingen, Germany). Mice were anesthetized with 10% xylasine and 10% ketamine in PBS before colonoscopy. Inflammatory score assessment was as described in (Becker et al., 2006).
Mouse organs were fixed in 4% formalin/PBS for 24 hr, embedded in paraffin, sectioned at 4 µm, dewaxed and stained with haematoxylin and eosin (H&E). Procedures involving animals were approved by the Animal Ethics Committee of the Weizmann Institute (IACUC #05331109-2) and conformed to the guidelines of the Israel Council for Experiments in Animals. Pathological examination was conducted blindly and independently by two molecular pathologists advised by a mouse pathologist.
Human tissues
Primary tumor and colon biopsies were obtained from the “Cooperative Human Tissue Network” (OHSR approval number OHSR3637) and the Mount Sinai School of Medicine , New-York, NY. The project was approved by the institutional review boards of the National Institutes of Health and the Mount Sinai School of Medicine. Serially sectioned slides from formalin fixed, paraffin embedded biopsies of 18 human CAC cases were obtained from the National Cancer Institute, Bethesda, and the Mount Sinai School of Medicine, New York. DNA extracted from tumor cell-enriched areas of each specimen was subjected to TP53 tagged-amplicon Illumina HiSeq 2000 sequencing (Forshew et al., 2012); K-Ras mutations were similarly interrogated. In parallel, additional slides from each specimen were subjected to immunohistochemical analysis (IHC).
Microarray Hybridization and Analysis
For expression microarray analysis, RNA was extracted as described under Experimental Procedures. RNA (10 µg) was labeled and hybridized to Affymetrix GeneChip Human Exon 1.0 ST arrays. For analysis, the Affymetrix Expression Console (parameters: annotation confidence, full; summarization method, iter-PLIER include DABG; background, PM-GCBG; normalization method, none) was used, followed by normalization of all arrays together using a Lowess multiarray algorithm. Intensity-dependent estimation of noise was used for statistical analysis of differential expression. Geo accession number - GSE43738.
Supplementary Material
Significance.
The links between chronic inflammation and cancer are the subject of extensive research. Identification of the underlying molecular mechanisms may be of high relevance for cancer prevention and treatment. Here we demonstrate that a cancer-associated mutant isoform of the p53 tumor suppressor promotes chronic inflammation and inflammation-driven cancer. Specifically, we report that mutant p53 acquires a significant pro-inflammatory activity mediated by NF-κB, which may promote both tumor initiation and tumor progression. Furthermore, we describe a mouse model that faithfully mimics features frequently seen in human colitis-associated colorectal cancer. As p53 mutations occur very early in the course of inflammation-associated human colorectal cancer, targeting those mutations in premalignant lesions may be clinically beneficial.
Highlights.
Mutant p53 promotes chronic NF-κB activation.
Mutant p53 promotes persistent tissue damage and extended inflammation.
Mutant p53 mice are highly prone to inflammation-associated colorectal cancer.
DSS-treated mutant p53 mice faithfully recapitulate human colitis-associated cancer.
Acknowledgements
We thank Ori Brenner and Gilgi Friedlander for help with histopathological analysis and expression microarray analysis, respectively. We also thank Ronnie Apte, Elena Voronov, Derek Mann, Neil Perkins and Yinon Ben-Neriah for helpful advice and suggestions. This work was supported in part by EC FP7 funding (INFLACARE, agreement 223151, and INSPiRE, agreement 284460), grant R37 CA40099 from the National Cancer Institute, a Center of Excellence grant (#1779/11) from the Israel Science Foundation (ISF), the Dr. Miriam and Sheldon Adelson Medical Research Foundation and a Center of Excellence grant from the Flight Attendant Medical Research Institute (FAMRI). MO is incumbent of the Andre Lwoff chair in Molecular Biology. The EC is not liable for any use that may be made of the information contained herein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acin S, Li Z, Mejia O, Roop DR, El-Naggar AK, Caulin C. Gain-of-function mutant p53 but not p53 deletion promotes head and neck cancer progression in response to oncogenic K-ras. J Pathol. 2011;225:479–489. doi: 10.1002/path.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ak P, Levine AJ. p53 and NF-kappaB: different strategies for responding to stress lead to a functional antagonism. FASEB J. 2010;24:3643–3652. doi: 10.1096/fj.10-160549. [DOI] [PubMed] [Google Scholar]
- Asquith M, Powrie F. An innately dangerous balancing act: intestinal homeostasis, inflammation, and colitis-associated cancer. J Exp Med. 2010;207:1573–1577. doi: 10.1084/jem.20101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Neurath MF. High resolution colonoscopy in live mice. Nat Protoc. 2006;1:2900–2904. doi: 10.1038/nprot.2006.446. [DOI] [PubMed] [Google Scholar]
- Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- Brentnall TA, Crispin DA, Rabinovitch PS, Haggitt RC, Rubin CE, Stevens AC, Burmer GC. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology. 1994;107:369–378. doi: 10.1016/0016-5085(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9:701–713. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- Buganim Y, Solomon H, Rais Y, Kistner D, Nachmany I, Brait M, Madar S, Goldstein I, Kalo E, Adam N, et al. p53 Regulates the Ras circuit to inhibit the expression of a cancer-related gene signature by various molecular pathways. Cancer Res. 2010;70:2274–2284. doi: 10.1158/0008-5472.CAN-09-2661. [DOI] [PubMed] [Google Scholar]
- Campbell I, Polyak K, Haviv I. Clonal mutations in the cancer-associated fibroblasts: the case against genetic coevolution. Cancer Res. 2009;69:6765–6768. doi: 10.1158/0008-5472.CAN-08-4253. discussion 6769. [DOI] [PubMed] [Google Scholar]
- Chang WC, Coudry RA, Clapper ML, Zhang X, Williams KL, Spittle CS, Li T, Cooper HS. Loss of p53 enhances the induction of colitis-associated neoplasia by dextran sulfate sodium. Carcinogenesis. 2007;28:2375–2381. doi: 10.1093/carcin/bgm134. [DOI] [PubMed] [Google Scholar]
- Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin. 2007;28:1450–1459. doi: 10.1111/j.1745-7254.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- Dell’Orso S, Fontemaggi G, Stambolsky P, Goeman F, Voellenkle C, Levrero M, Strano S, Rotter V, Oren M, Blandino G. ChIP-on-chip analysis of in vivo mutant p53 binding to selected gene promoters. OMICS. 2011;15:305–312. doi: 10.1089/omi.2010.0084. [DOI] [PubMed] [Google Scholar]
- Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, Trinchieri G, Dubinett SM, Mao JT, Szabo E, et al. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33:335–351. doi: 10.1097/CJI.0b013e3181d32e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle B, Morton JP, Delaney DW, Ridgway RA, Wilkins JA, Sansom OJ. p53 mutation and loss have different effects on tumourigenesis in a novel mouse model of pleomorphic rhabdomyosarcoma. J Pathol. 2010;222:129–137. doi: 10.1002/path.2748. [DOI] [PubMed] [Google Scholar]
- Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC, Felley-Bosco E, Wang XW, Geller DA, Tzeng E, et al. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc Natl Acad Sci U S A. 1996;93:2442–2447. doi: 10.1073/pnas.93.6.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forshew T, Murtaza M, Parkinson C, Gale D, Tsui DW, Kaper F, Dawson SJ, Piskorz AM, Jimenez-Linan M, Bentley D, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003726. 136ra168. [DOI] [PubMed] [Google Scholar]
- Fujii S, Fujimori T, Kawamata H, Takeda J, Kitajima K, Omotehara F, Kaihara T, Kusaka T, Ichikawa K, Ohkura Y, et al. Development of colonic neoplasia in p53 deficient mice with experimental colitis induced by dextran sulphate sodium. Gut. 2004;53:710–716. doi: 10.1136/gut.2003.028779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Lofberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404–409. doi: 10.1136/gut.47.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr., Kastrinakis NG, Levy B, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- Guma M, Stepniak D, Shaked H, Spehlmann ME, Shenouda S, Cheroutre H, Vicente-Suarez I, Eckmann L, Kagnoff MF, Karin M. Constitutive intestinal NF-kappaB does not trigger destructive inflammation unless accompanied by MAPK activation. J Exp Med. 2011;208:1889–1900. doi: 10.1084/jem.20110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- He G, Karin M. NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennett WP, Shields PG, Ham AJ, Swenberg JA, Marrogi AJ, Harris CC. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333–3337. [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Oikawa K, Yoshida K, Takeuchi A, Takeuchi M, Usui M, Umezawa A, Mukai K. Effects of 3-methylcholanthrene on the transcriptional activity and mRNA accumulation of the oncogene hWAPL. Cancer Lett. 2005;221:21–28. doi: 10.1016/j.canlet.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Leedham SJ, Graham TA, Oukrif D, McDonald SA, Rodriguez-Justo M, Harrison RF, Shepherd NA, Novelli MR, Jankowski JA, Wright NA. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology. 2009;136:542–550. doi: 10.1053/j.gastro.2008.10.086. e546. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano G. Mouse models of p53 functions. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a001115. a001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaterre J, Carpinelli M, Ernst M, Alexander W, Cooke M, Sutton S, Dworkin S, Heath JK, Frampton J, McArthur G, et al. c-Myb is required for progenitor cell homeostasis in colonic crypts. Proc Natl Acad Sci U S A. 2007;104:3829–3834. doi: 10.1073/pnas.0610055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, Jacks T. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature. 2009;462:104–107. doi: 10.1038/nature08462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noffsinger AE, Belli JM, Miller MA, Fenoglio-Preiser CM. A unique basal pattern of p53 expression in ulcerative colitis is associated with mutation in the p53 gene. Histopathology. 2001;39:482–492. doi: 10.1046/j.1365-2559.2001.01274.x. [DOI] [PubMed] [Google Scholar]
- Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, Jacks T. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–860. doi: 10.1016/j.cell.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a001107. a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patocs A, Zhang L, Xu Y, Weber F, Caldes T, Mutter GL, Platzer P, Eng C. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med. 2007;357:2543–2551. doi: 10.1056/NEJMoa071825. [DOI] [PubMed] [Google Scholar]
- Phelps ED, Updike DL, Bullen EC, Grammas P, Howard EW. Transcriptional and posttranscriptional regulation of angiopoietin-2 expression mediated by IGF and PDGF in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C352–C361. doi: 10.1152/ajpcell.00050.2005. [DOI] [PubMed] [Google Scholar]
- Rivlin N, Brosh R, Oren M, Rotter V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer. 2011;2:466–474. doi: 10.1177/1947601911408889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KM, Ernst MK, Rice NR, Vousden KH. Role of NF-kappaB in p53-mediated programmed cell death. Nature. 2000;404:892–897. doi: 10.1038/35009130. [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitalla S, Ziegler PK, Horst D, Becker V, Kerle I, Begus-Nahrmann Y, Lechel A, Rudolph KL, Langer R, Slotta-Huspenina J, et al. Loss of p53 in Enterocytes Generates an Inflammatory Microenvironment Enabling Invasion and Lymph Node Metastasis of Carcinogen-Induced Colorectal Tumors. Cancer Cell. 2013;23:93–106. doi: 10.1016/j.ccr.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Schneider G, Henrich A, Greiner G, Wolf V, Lovas A, Wieczorek M, Wagner T, Reichardt S, von Werder A, Schmid RM, et al. Cross talk between stimulated NF-kappaB and the tumor suppressor p53. Oncogene. 2010;29:2795–2806. doi: 10.1038/onc.2010.46. [DOI] [PubMed] [Google Scholar]
- Schramek D, Kotsinas A, Meixner A, Wada T, Elling U, Pospisilik JA, Neely GG, Zwick RH, Sigl V, Forni G, et al. The stress kinase MKK7 couples oncogenic stress to p53 stability and tumor suppression. Nat Genet. 2011;43:212–219. doi: 10.1038/ng.767. [DOI] [PubMed] [Google Scholar]
- Scian MJ, Stagliano KE, Anderson MA, Hassan S, Bowman M, Miles MF, Deb SP, Deb S. Tumor-derived p53 mutants induce NF-kappaB2 gene expression. Mol Cell Biol. 2005;25:10097–10110. doi: 10.1128/MCB.25.22.10097-10110.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema E, Tirosh I, Aylon Y, Huang J, Ye C, Moskovits N, Raver-Shapira N, Minsky N, Pirngruber J, Tarcic G, et al. The histone H2B-specific ubiquitin ligase RNF20/hBRE1 acts as a putative tumor suppressor through selective regulation of gene expression. Genes Dev. 2008;22:2664–2676. doi: 10.1101/gad.1703008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YA, Post SM, Elizondo-Fraire AC, Maccio DR, Jackson JG, El-Naggar AK, Van Pelt CS, Terzian T, Lozano G. Multiple stress signals activate mutant p53 in vivo. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-11-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur S, Pagliarini R, Bunz F, Rago C, Diaz LA, Jr., Kinzler KW, Vogelstein B, Papadopoulos N. A panel of isogenic human cancer cells suggests a therapeutic approach for cancers with inactivated p53. Proc Natl Acad Sci U S A. 2009;106:3964–3969. doi: 10.1073/pnas.0813333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tergaonkar V, Perkins ND. p53 and NF-kappaB crosstalk: IKKalpha tips the balance. Mol Cell. 2007;26:158–159. doi: 10.1016/j.molcel.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Terzian T, Suh YA, Iwakuma T, Post SM, Neumann M, Lang GA, Van Pelt CS, Lozano G. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev. 2008;22:1337–1344. doi: 10.1101/gad.1662908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. e2105. [DOI] [PubMed] [Google Scholar]
- Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–1816. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- Vlantis K, Wullaert A, Sasaki Y, Schmidt-Supprian M, Rajewsky K, Roskams T, Pasparakis M. Constitutive IKK2 activation in intestinal epithelial cells induces intestinal tumors in mice. J Clin Invest. 2011;121:2781–2793. doi: 10.1172/JCI45349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Weisz L, Damalas A, Liontos M, Karakaidos P, Fontemaggi G, Maor-Aloni R, Kalis M, Levrero M, Strano S, Gorgoulis VG, et al. Mutant p53 enhances nuclear factor kappaB activation by tumor necrosis factor alpha in cancer cells. Cancer Res. 2007;67:2396–2401. doi: 10.1158/0008-5472.CAN-06-2425. [DOI] [PubMed] [Google Scholar]
- Wernert N, Locherbach C, Wellmann A, Behrens P, Hugel A. Presence of genetic alterations in microdissected stroma of human colon and breast cancers. Anticancer Res. 2001;21:2259–2264. [PubMed] [Google Scholar]
- Yin J, Harpaz N, Tong Y, Huang Y, Laurin J, Greenwald BD, Hontanosas M, Newkirk C, Meltzer SJ. p53 point mutations in dysplastic and cancerous ulcerative colitis lesions. Gastroenterology. 1993;104:1633–1639. doi: 10.1016/0016-5085(93)90639-t. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Mikami T, Mitomi H, Okayasu I. Diverse p53 alterations in ulcerative colitis-associated low-grade dysplasia: full-length gene sequencing in microdissected single crypts. J Pathol. 2003;199:166–175. doi: 10.1002/path.1264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.