Abstract
Mutations in the leucine-rich repeat kinase 2 gene (LRRK2) are the most frequent genetic cause of Parkinson’s disease (PD) and these mutations play important roles in sporadic PD. The LRRK2 protein contains GTPase and kinase domains and several protein-protein interaction domains. The kinase and GTPase activity of LRRK2 seem to be important in regulating LRRK2-dependent cellular signaling pathways. LRRK2’s GTPase and kinase domains may reciprocally regulate each other to direct LRRK2’s ultimate function. While most LRRK2 investigations are centered on LRRK2’s kinase activity, this review focuses on the function of LRRK2’s GTPase in LRRK2 physiology and pathophysiology.
Keywords: GTPase-activating proteins (GAPs), guanine nucleotide exchange factors (GEFs), GTPase, kinase, LRRK2, Parkinson’s disease
Introduction
PD (Parkinson’s disease) is recognized as the second most common neurodegenerative disorder after Alzheimer’s disease, affecting up to 1% of the population above the age of 60 and 4–5% above the age of 85 [1]. According to the National Institute of Health, 1.5 million people in the U.S.A. are suffering from PD. Patients exhibit a number of characteristic clinical symptoms such as akinesia, resting tremor, muscle rigidity, and postural imbalance [1]. The cardinal symptoms are caused by the progressive degeneration of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNc) [2]. The aetiology of PD is incompletely understood. Although most PD cases appear to be sporadic, identification of mutations in several genes responsible for this chronic, progressive neurodegenerative disease confirms the role of genetics in the disease [3]. To date, seven genes [SNCA (α-synuclein), PARK2 (parkin), PARK7 (DJ-1), PINK1 (phosphatase and tensin homologue deleted on chromosome 10-induced putative kinase 1), VPS35 (vacuolar protein sorting 35), EIF4G1 (eukaryotic initiation factor 4G1) and LRRK2 (leucine-rich repeat kinase 2)] are associated with genetic forms of PD that closely resemble idiopathic PD [3–6]. Mutations in the LRRK2 gene (formerly known as PARK8 or dardarin, OMIM 609007) is the most frequent genetic cause of PD, accounting for 4% of familial PD and 1% of sporadic PD across all populations. Patients with LRRK2 mutations exhibit clinical and neurochemical phenotypes that are indistinguishable from sporadic PD [7, 8].
The LRRK2 protein contains multiple enzymatic and protein-protein interaction domains, including a ROC (Ras of complex proteins) GTPase, a COR (C-terminal of ROC), an LRR (leucine-rich repeat), a protein kinase, a WD40 repeat and an LRRK2-specific repeat domain [9, 10] (Figure 1). LRRK2 has both GTPase and kinase enzyme activity [11]. The kinase domain is most similar to the paralogue LRRK1, but it also has similarity to the receptor-interacting kinases and mitogen-activated protein kinase kinase kinases of the mixed-lineage type [11–13] LRRK2’s interaction domains are thought to serve as protein-binding modules where LRRK2 acts as a signaling scaffold [11]. LRRK2 localizes to a wide range of vesicular and membranous structures in neurons, including mitochondria, the endolysosomal system, the ER (endoplasmic reticulum) and Golgi [14–16]. Many studies suggest that LRRK2 is involved in diverse pathways, including regulation of protein translation, apoptosis, mitochondrial function, vesicle trafficking, neurite outgrowth, autophagy and cytoskeletal dynamics [10, 11]. How LRRK2 mutations cause neurodegeneration in PD is currently unknown, and the underlying mechanisms for the pathogenesis still need to be defined.
Figure 1. Schematic domain structure of the LRRK2 protein.

Residues 1–660 encode LRRK2-specific repeat sequences, residues 984–1278 encode the leucine-rich repeat (LRR) domain, residues1335–1510 encode the Roc GTPase domain, residues 1519–1795 encode the C-terminal of Ras (COR) domain, residues 1879–2138 encode the kinase domain and residues 2138 to 2527 encode the WD40 domain. The positions of mutations clearly segregating with disease are shown in red, whereas the positions of R1441H and N1437H associated with PD are highlighted in blue. The domain boundaries are indicated by the residue numbers in black.
These physiological and pathophysiological cellular processes are probably regulated at multiple levels by LRRK2 through its GTPase and kinase domains as well as the protein- interaction domains. Although most investigations have focused on the kinase function of LRRK2, the present review primarily discusses the GTPase function of LRRK2 and its relationship to LRRK2 kinase activity. We also discuss potential modifiers of the LRRK2 GTPase domain and therapeutic strategies beyond kinase inhibition for LRRK2-associated PD.
Pathogenic mutations in the LRRK2 GTPase domain
A number of point mutations have been found in almost all of domains of LRRK2 in patients with PD. At least seven mutations (I1371V, R1441C/G, Y1699C/G, G2019S, I2020T) segregate with familial PD and are pathogenic [1] (Figure 1). The most common LRRK2 PD-associated mutation, G2019S, is in the kinase domain of LRRK2. Arg1441 in the GTPase domain is the second most common site of pathogenic LRRK2 substitutions after Gly2019 [17]. The R1441C mutation was first reported in two autosomal-dominant PD families and later was also found in sporadic PD [8, 18]. R1441C has been reported in different ethnic races, whereas R1441G is most common in the Basque country [19]. The R1441H mutation appears to be pathogenic and confirms that this amino acid is a mutational hotspot [20]. The I1371V mutation seems to segregate with disease, but little is known about the functional consequences of this mutation [21]. Another mutation in the GTPase domain, N1437H, was recently found in a large Norwegian family with autosomal-dominant PD [22]. The Tyr1699 mutations are within the COR domain, which is important for the GTPase function of LRRK2 [23]. Mutations in the GTPase domain often lack Lewy body pathology and are associated with pure nigral degeneration or pleomorphic neuropathological findings [8, 24].
LRRK2 R1441C knockin and R1441G BAC (bacterial artificial chromosome) transgenic mouse models exhibit mild impairments in nigrostriatal dopaminergic neurotransmission and motor dysfunction, consistent with the pathogenic role of these mutations in PD [25, 26]. Interestingly, in a yeast model of LRRK2 toxicity, the GTPase domain causes more toxicity compared with other domains. The toxicity is closely associated with GTPase activity and defects in endocytic vesicular trafficking and autophagy [27].
LRRK2 GTP binding and GTPase activity
Studies confirm that LRRK2 is a GTP binding protein and that it possesses GTPase activity [28–33], although the intrinsic GTPase activity of LRRK2 is small in comparison with other GTPases. The potential detrimental effects of PD-associated LRRK2 mutations on the kinase domain and GTPase domain are beginning to be clarified. There is universal agreement that the G2019S mutation leads to increased kinase activity and that the toxicity associated with the G2019S mutation is kinase-dependent [34]. On the other hand the R1441C/G and Y1699C mutations cause an increase or no apparent alterations in LRRK2 kinase activity [35]. The effects of LRRK2 R1441C/G mutations on GTP binding either cause an increase or no obvious effect on GTP binding, but consistently lead to decreased GTP hydrolysis [13, 30–32]. Interestingly, the G2019S mutation also exhibits decreased GTPase activity [32, 36]. The Y1699C mutation leads to a comparable decrease in GTPase activity probably, through weakening the dimerization of LRRK2 at the ROC-COR tandem interface [23]. Taken together, these observations suggest that decreased GTPase activity due to mutations in LRRK2 is likely to play a role in LRRK2 toxicity.
Regulation between LRRK2 GTPase and kinase activity
Small GTPases have been well known to control downstream protein kinase activity. The presence of the kinase domain and GTPase domain in the same molecule suggests a functional interaction between both catalytic activities within LRRK2 [37]. GTPases act as molecular switches between an active GTP bound state and an inactive GDP (guanosine diphosphate) bound state (i.e. when the GTP is hydrolyzed to GDP) [38] (Figure 2). In the active state, GTPases activate an effector protein such as a kinase via direct binding. LRRK2 is of special interest as mutations within both the GTPase and kinase domains are associated with PD. Its GTPase activity may regulate its kinase activity, which suggests a novel mechanism of intrinsic control. Indeed, functional mutant forms of LRRK2 K1347A and T1348N in which guanine nucleotide binding is disrupted display very low kinase activity, whereas adding the non-hydrolysable GTP analogue GTPγS (guanosine 5_-[γ -thio]triphosphate) to LRRK2, which mimics the GTP-bound state, increases its kinase activity [13, 27, 28]. How GTP binding to LRRK2 stimulates its kinase activity is not clear. One study suggests that LRRK2 GTP-binding capacity, not GTP binding, stimulates LRRK2 kinase activity [39]. Thus whether direct GTP binding and therefore a simple intramolecular switch stimulates LRRK2 kinase activity or whether the mechanism is indirect needs further investigation.
Figure 2. The GTPase activity cycle.

GTPases cycle between the inactive “Off” GDP-bound state and the active “On” GTP-bound state. The inactive state occurs by stimulation of intrinsic GTPase hydrolysis activity by GTPase activating proteins (GAPs). Activation is facilitated by guanine nucleotide exchange factors (GEFs) to load GTP and dissociate GDP, allowing interaction with downstream effectors and in turn activation of downstream signaling pathways.
Conversely, the LRRK2 kinase domain appears to regulate LRRK2’s intrinsic GTPase activity. Mapping LRRK2’s autophosphorylation sites in vitro reveals a discrete cluster of autophosphorylation sites in its GTPase domain, primarily in the GTP-binding pocket [40–43]. LRRK2 autophosphorylation at these sites serves to propagate LRRK2’s kinase activity by structurally changing the GTPase domain into a configuration that promotes kinase activity [40–43]. Thus the combination of GTP binding and LRRK2 autophosphorylation in the GTPase domain regulates GTPase-dependent activity that controls kinase activity.
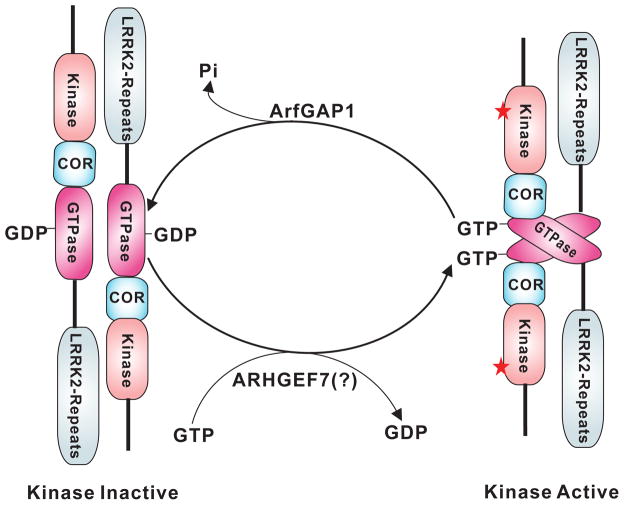
How might this intrinsic regulation mechanism work? Resolving the structure of the GTPase domain of LRRK2 represents an important step toward understanding the underlying molecular mechanism. Crystallization of the ROC GTPase domain of LRRK2 indicates that LRRK2 functions as a dimer, where the ROC GTPase domain cycles between GDP- and GTP-bound states [33, 44] (Figure 3). The COR domain may act as molecular hinge to regulate kinase activity. Pathogenic mutations such as R1441C in the GTPase domain weaken the dimer structure, resulting in decreased GTP hydrolysis that increases kinase activation [33]. Biochemical studies of LRRK2 have shown that LRRK2 kinase activity requires dimeric LRRK2, as kinase activity was not detected in oligomeric or monomeric forms of LRRK2 [45]. Moreover, some PD-associated mutations that increase LRRK2 kinase activity in vitro significantly increase the proportion of LRRK2 dimers [45]. A recent study suggests that the monomeric form LRRK2 is predominant in cells and that dimerization is not required for LRRK2’s enzymatic activity [46]. Thus further work is required to clarify the role of dimerization in LRRK2’s activity and function. How the GTPase and kinase domains communicate is still unclear. It is not known whether these two domains interact physically, although the COR and GTPase domains interact strongly [33]. The elucidation of the crystal structure of the full-length LRRK2 protein will hopefully clarify these mysteries.
Figure 3. Hypothetical model of intrinsic regulation between LRRK2 ROC’s GTPase and kinase activity.
LRRK2 is thought to form homodimers through its ROC GTPase domain. The dimeric ROC GTPase domains cycle between GTP- and GDP- bound states to regulate kinase activation. Upon binding of GTP (Right), the activated ROC domain induces kinase activation and autophosphorylation and phosphorylation of substrates. Through hydrolysis of GTP to GDP, conformational changes in the ROC domain inactivate the kinase (Left). ArfGAP1 is the GTPase activating protein (GAP) for LRRK2. ARHGEF7 may function as the guanine nucleotide exchange factor (GEF) of LRRK2. Both are likely to be involved in regulation of LRRK2’s enzymatic activity.
Potential modifiers of LRRK2’s GTPase domain
Most GTPases are regulated by GAPs (GTPase-activating proteins), which increase the hydrolysis of GTP to GDP, turning the GTPase off, and by GEFs (guanine nucleotide exchange factors), which promote GTP binding, reducing GTP hydrolysis and turning on the activity of the GTPase [38] (Figure 2). LRRK2 requires cofactors for the binding and hydrolysis of nucleotides in the GTPase domain, similar to most small GTPases. Consistent with this notion are the observations that adding either GTPγS or GDP to pure recombinant LRRK2 had no significant effect on kinase activity, but addition of GTPγS to the cell lysate increases LRRK2’s kinase activity and addition of GDP inhibits the activity [13].
In a yeast model of LRRK2 toxicity, a genome-wide screen identified GCS1 as a potential GAP for LRRK2 [27]. Follow-up studies with the human homolog of GCS1, ArfGAP1 (ADP ribosylation factor GTPase activating protein 1), indicates that ArfGAP1 is a GAP for LRRK2. ArfGAP1 enhances both wild type and mutant (G2019S and R1441C) LRRK2 GTP hydrolysis and decreases LRRK2 autophosphorylation and kinase activity, which protects against LRRK2 toxicity in vitro and in vivo [32]. Interestingly, LRRK2 phosphorylates ArfGAP1 and inhibits its GAP activity. ArfGAP1 has intrinsic toxicity that is inhibited by LRRK2 phosphorylation. Thus, ArfGAP1 and LRRK2 act reciprocally to regulate the activity and toxicity of each other [32].
Little is known about the GEFs that regulate LRRK2, other than ARHGEF7 may be a GEF for LRRK2 [47]. Further identification and characterization of LRRK2-specific GEFs will be important, since inhibition of the LRRK2 GEF would be expected to inhibit LRRK2 kinase activity and reduce LRRK2 toxicity.
Therapeutic strategies targeting LRRK2 GTP binding and GTPase activity
The fact that LRRK2 is a kinase and most PD-associated mutations in LRRK2 have pathologic kinase activity indicates that targeting LRRK2 kinase activity is a therapeutic strategy for PD [34]. Indeed, genetic and pharmacological inactivation of LRRK2 kinase activity is neuroprotective [13, 48–50]. However, as discussed above, given the regulation between LRRK2 GTPase and kinase activity, targeting LRRK2 GTPase activity, GTP binding and dimerization offer additional potential therapeutic targets that are beyond kinase inhibition. This is particularly important in light of the recent findings that suggest that long-term inhibition of LRRK2 kinase activity may have untoward side effects, including predisposition for inflammatory bowel disease and kidney atrophy and dysfunction [51–53].
Therapeutic approaches beyond kinase inhibition include developing LRRK2 GTPase inhibitors by targeting LRRK2 GTPase activity directly. A successful example is EHT1864, which is an inhibitor of the Rac1 GTPase. EHT1864 functions by displacing nucleotide binding and preventing association of Rac with the RacGEF [54]. Since a LRRK2 GEF would be expected to decrease LRRK2’s GTPase activity and increase its kinase activity, development of LRRK2 GEF inhibitors may be an impressive means of modulating LRRK2 activity and toxicity. Inhibition of the GEF interaction with LRRK2 or the GEF catalytic activity might be taken into consideration for drug screens. Since LRRK2 and ArfGAP1 reciprocally regulate each other’s activity and toxicity, targeting ArfGAP1 may not be feasible, but, as other LRRK2 GAPs are identified, enhancing their activity could yield new LRRK2 therapies. Another possible target is blocking the GTP-binding pocket of the LRRK2 GTPase domain. Preventing LRRK2 dimerization is another potential route for drug development. Since LRRK2 GEFs and GAPS are likely to regulate other proteins and processes, there may be off-target effects that will need to be considered as agents that target these effectors are developed.
Future perspectives
As the most common genetic link to familial and sporadic PD known to date, LRRK2 has been in the spotlight of the field for the past few years. Genetic and biochemical studies are providing promising and exciting data. However, detailed molecular understanding of LRRK2’s physiological and pathophysiological function is still rudimentary. Identification of GTPase and kinase domain modifiers and physiological and pathophysiological interactors is critical to ultimately decipher LRRK2’s function. Regulation of LRRK2’s GTPase domain by GEFs and GAPs is of particular interest as well as the elucidation of the interplay between LRRK2 GTPase and kinase activity. Modulating the GTPase function of LRRK2 and LRRK2 kinase inhibition are prime targets for small-molecule inhibitors or modulators that hold particular promise for the treatment of PD.
Acknowledgments
This work was supported by grants from the NIH NS38377, the Michael J. Fox Foundation. YX was an American Parkinson’s Disease Association Postdoctoral Fellow. T.M.D. is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases. The authors acknowledge the joint participation by the Adrienne Helis Malvin Medical Research Foundation through its direct engagement in the continuous active conduct of medical research in conjunction with The Johns Hopkins Hospital and the Johns Hopkins University School of Medicine and the Foundation’s Parkinson’s Disease Program No. M-1.
Abbreviations
- PD
Parkinson’s disease
- LRRK2
leucine-rich repeat kinase 2
- ROC
Ras of complex protein
- COR
C terminus of Ras of complex protein
- GEFs
Guanine nucleotide exchange factors
- GAPs
GTPase-activating proteins
- GTP
guanosine triphosphate
- GDP
guanosine diphosphate
References
- 1.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 2.Savitt JM, Dawson VL, Dawson TM. Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest. 2006;116:1744–1754. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin I, Dawson VL, Dawson TM. Recent advances in the genetics of Parkinson’s disease. Annu Rev Genomics Hum Genet. 2011;12:301–325. doi: 10.1146/annurev-genom-082410-101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimprich A, Benet-Pages A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, Rossle SC, Klopp N, Wolf E, Seppi K, Pirker W, Presslauer S, Mollenhauer B, Katzenschlager R, Foki T, Hotzy C, Reinthaler E, Harutyunyan A, Kralovics R, Peters A, Zimprich F, Brucke T, Poewe W, Auff E, Trenkwalder C, Rost B, Ransmayr G, Winkelmann J, Meitinger T, Strom TM. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89:168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chartier-Harlin MC, Dachsel JC, Vilarino-Guell C, Lincoln SJ, Lepretre F, Hulihan MM, Kachergus J, Milnerwood AJ, Tapia L, Song MS, Le Rhun E, Mutez E, Larvor L, Duflot A, Vanbesien-Mailliot C, Kreisler A, Ross OA, Nishioka K, Soto-Ortolaza AI, Cobb SA, Melrose HL, Behrouz B, Keeling BH, Bacon JA, Hentati E, Williams L, Yanagiya A, Sonenberg N, Lockhart PJ, Zubair AC, Uitti RJ, Aasly JO, Krygowska-Wajs A, Opala G, Wszolek ZK, Frigerio R, Maraganore DM, Gosal D, Lynch T, Hutchinson M, Bentivoglio AR, Valente EM, Nichols WC, Pankratz N, Foroud T, Gibson RA, Hentati F, Dickson DW, Destee A, Farrer MJ. Translation initiator EIF4G1 mutations in familial Parkinson disease. Am J Hum Genet. 2011;89:398–406. doi: 10.1016/j.ajhg.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilarino-Guell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, Soto-Ortolaza AI, Cobb SA, Wilhoite GJ, Bacon JA, Behrouz B, Melrose HL, Hentati E, Puschmann A, Evans DM, Conibear E, Wasserman WW, Aasly JO, Burkhard PR, Djaldetti R, Ghika J, Hentati F, Krygowska-Wajs A, Lynch T, Melamed E, Rajput A, Rajput AH, Solida A, Wu RM, Uitti RJ, Wszolek ZK, Vingerhoets F, Farrer MJ. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89:162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Mata IF, Wedemeyer WJ, Farrer MJ, Taylor JP, Gallo KA. LRRK2 in Parkinson’s disease: protein domains and functional insights. Trends Neurosci. 2006;29:286–293. doi: 10.1016/j.tins.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Cookson MR. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat Rev Neurosci. 2010;11:791–797. doi: 10.1038/nrn2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berwick DC, Harvey K. LRRK2 signaling pathways: the key to unlocking neurodegeneration? Trends Cell Biol. 2011;21:257–265. doi: 10.1016/j.tcb.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 12.West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West AB, Moore DJ, Choi C, Andrabi SA, Li X, Dikeman D, Biskup S, Zhang Z, Lim KL, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet. 2007;16:223–232. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- 14.Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 15.Alegre-Abarrategui J, Christian H, Lufino MM, Mutihac R, Venda LL, Ansorge O, Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009;18:4022–4034. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodson MW, Zhang T, Jiang C, Chen S, Guo M. Roles of the Drosophila LRRK2 homolog in Rab7-dependent lysosomal positioning. Hum Mol Genet. 2012;21:1350–1363. doi: 10.1093/hmg/ddr573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biskup S, West AB. Zeroing in on LRRK2-linked pathogenic mechanisms in Parkinson’s disease. Biochim Biophys Acta. 2009;1792:625–633. doi: 10.1016/j.bbadis.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zabetian CP, Samii A, Mosley AD, Roberts JW, Leis BC, Yearout D, Raskind WH, Griffith A. A clinic-based study of the LRRK2 gene in Parkinson disease yields new mutations. Neurology. 2005;65:741–744. doi: 10.1212/01.wnl.0000172630.22804.73. [DOI] [PubMed] [Google Scholar]
- 19.Kumari U, Tan EK. LRRK2 in Parkinson’s disease: genetic and clinical studies from patients. FEBS J. 2009;276:6455–6463. doi: 10.1111/j.1742-4658.2009.07344.x. [DOI] [PubMed] [Google Scholar]
- 20.Ross OA, Spanaki C, Griffith A, Lin CH, Kachergus J, Haugarvoll K, Latsoudis H, Plaitakis A, Ferreira JJ, Sampaio C, Bonifati V, Wu RM, Zabetian CP, Farrer MJ. Haplotype analysis of Lrrk2 R1441H carriers with parkinsonism. Parkinsonism Relat Disord. 2009;15:466–467. doi: 10.1016/j.parkreldis.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seki N, Takahashi Y, Tomiyama H, Rogaeva E, Murayama S, Mizuno Y, Hattori N, Marras C, Lang AE, George-Hyslop PS, Goto J, Tsuji S. Comprehensive mutational analysis of LRRK2 reveals variants supporting association with autosomal dominant Parkinson’s disease. J Hum Genet. 2011;56:671–675. doi: 10.1038/jhg.2011.79. [DOI] [PubMed] [Google Scholar]
- 22.Aasly JO, Vilarino-Guell C, Dachsel JC, Webber PJ, West AB, Haugarvoll K, Johansen KK, Toft M, Nutt JG, Payami H, Kachergus JM, Lincoln SJ, Felic A, Wider C, Soto-Ortolaza AI, Cobb SA, White LR, Ross OA, Farrer MJ. Novel pathogenic LRRK2 p.Asn1437His substitution in familial Parkinson’s disease. Mov Disord. 2010;25:2156–2163. doi: 10.1002/mds.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniels V, Vancraenenbroeck R, Law BM, Greggio E, Lobbestael E, Gao F, De Maeyer M, Cookson MR, Harvey K, Baekelandt V, Taymans JM. Insight into the mode of action of the LRRK2 Y1699C pathogenic mutant. J Neurochem. 2011;116:304–315. doi: 10.1111/j.1471-4159.2010.07105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaig C, Marti MJ, Ezquerra M, Rey MJ, Cardozo A, Tolosa E. G2019S LRRK2 mutation causing Parkinson’s disease without Lewy bodies. J Neurol Neurosurg Psychiatry. 2007;78:626–628. doi: 10.1136/jnnp.2006.107904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Liu W, Oo TF, Wang L, Tang Y, Jackson-Lewis V, Zhou C, Geghman K, Bogdanov M, Przedborski S, Beal MF, Burke RE, Li C. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat Neurosci. 2009;12:826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong Y, Pisani A, Martella G, Karouani M, Yamaguchi H, Pothos EN, Shen J. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci U S A. 2009;106:14622–14627. doi: 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong Y, Coombes CE, Kilaru A, Li X, Gitler AD, Bowers WJ, Dawson VL, Dawson TM, Moore DJ. GTPase activity plays a key role in the pathobiology of LRRK2. PLoS Genet. 2010;6:e1000902. doi: 10.1371/journal.pgen.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito G, Okai T, Fujino G, Takeda K, Ichijo H, Katada T, Iwatsubo T. GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson’s disease. Biochemistry. 2007;46:1380–1388. doi: 10.1021/bi061960m. [DOI] [PubMed] [Google Scholar]
- 29.Guo L, Gandhi PN, Wang W, Petersen RB, Wilson-Delfosse AL, Chen SG. The Parkinson’s disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity. Exp Cell Res. 2007;313:3658–3670. doi: 10.1016/j.yexcr.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis PA, Greggio E, Beilina A, Jain S, Baker A, Cookson MR. The R1441C mutation of LRRK2 disrupts GTP hydrolysis. Biochem Biophys Res Commun. 2007;357:668–671. doi: 10.1016/j.bbrc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Tan YC, Poulose S, Olanow CW, Huang XY, Yue Z. Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson’s disease R1441C/G mutants. J Neurochem. 2007;103:238–247. doi: 10.1111/j.1471-4159.2007.04743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong Y, Yuan C, Chen R, Dawson TM, Dawson VL. ArfGAP1 Is a GTPase Activating Protein for LRRK2: Reciprocal Regulation of ArfGAP1 by LRRK2. J Neurosci. 2012;32:3877–3886. doi: 10.1523/JNEUROSCI.4566-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng J, Lewis PA, Greggio E, Sluch E, Beilina A, Cookson MR. Structure of the ROC domain from the Parkinson’s disease-associated leucine-rich repeat kinase 2 reveals a dimeric GTPase. Proc Natl Acad Sci U S A. 2008;105:1499–1504. doi: 10.1073/pnas.0709098105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee BD, Dawson VL, Dawson TM. Leucine rich repeat kinase 2 (LRRK2) as a potential therapeutic target for Parkinson’s disease. Trends in Pharmacological Sciences. 2012 doi: 10.1016/j.tips.2012.04.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudenko IN, Chia R, Cookson MR. Is Inhibition of Kinase Activity the Only Therapeutic Strategy for LRRK2-associated Parkinson’s Disease? BMC Med. 2012;10:20. doi: 10.1186/1741-7015-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu M, Kang S, Ray S, Jackson J, Zaitsev AD, Gerber SA, Cuny GD, Glicksman MA. Kinetic, mechanistic, and structural modeling studies of truncated wild-type leucine-rich repeat kinase 2 and the G2019S mutant. Biochemistry. 2011;50:9399–9408. doi: 10.1021/bi201173d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss B. ROCO kinase activity is controlled by internal GTPase function. Sci Signal. 2008;1:pe27. doi: 10.1126/scisignal.123pe27. [DOI] [PubMed] [Google Scholar]
- 38.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10:842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taymans JM, Vancraenenbroeck R, Ollikainen P, Beilina A, Lobbestael E, De Maeyer M, Baekelandt V, Cookson MR. LRRK2 kinase activity is dependent on LRRK2 GTP binding capacity but independent of LRRK2 GTP binding. PLoS One. 2011;6:e23207. doi: 10.1371/journal.pone.0023207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gloeckner CJ, Boldt K, von Zweydorf F, Helm S, Wiesent L, Sarioglu H, Ueffing M. Phosphopeptide analysis reveals two discrete clusters of phosphorylation in the N-terminus and the Roc domain of the Parkinson-disease associated protein kinase LRRK2. J Proteome Res. 2010;9:1738–1745. doi: 10.1021/pr9008578. [DOI] [PubMed] [Google Scholar]
- 41.Greggio E, Taymans JM, Zhen EY, Ryder J, Vancraenenbroeck R, Beilina A, Sun P, Deng J, Jaffe H, Baekelandt V, Merchant K, Cookson MR. The Parkinson’s disease kinase LRRK2 autophosphorylates its GTPase domain at multiple sites. Biochem Biophys Res Commun. 2009;389:449–454. doi: 10.1016/j.bbrc.2009.08.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamikawaji S, Ito G, Iwatsubo T. Identification of the autophosphorylation sites of LRRK2. Biochemistry. 2009;48:10963–10975. doi: 10.1021/bi9011379. [DOI] [PubMed] [Google Scholar]
- 43.Webber PJ, Smith AD, Sen S, Renfrow MB, Mobley JA, West AB. Autophosphorylation in the leucine-rich repeat kinase 2 (LRRK2) GTPase domain modifies kinase and GTP-binding activities. J Mol Biol. 2011;412:94–110. doi: 10.1016/j.jmb.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gasper R, Meyer S, Gotthardt K, Sirajuddin M, Wittinghofer A. It takes two to tango: regulation of G proteins by dimerization. Nat Rev Mol Cell Biol. 2009;10:423–429. doi: 10.1038/nrm2689. [DOI] [PubMed] [Google Scholar]
- 45.Sen S, Webber PJ, West AB. Dependence of leucine-rich repeat kinase 2 (LRRK2) kinase activity on dimerization. J Biol Chem. 2009;284:36346–36356. doi: 10.1074/jbc.M109.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito G, Iwatsubo T. Re-examination of the dimerization state of leucine-rich repeat kinase 2: predominance of the monomeric form. Biochem J. 2012;441:987–994. doi: 10.1042/BJ20111215. [DOI] [PubMed] [Google Scholar]
- 47.Haebig K, Gloeckner CJ, Miralles MG, Gillardon F, Schulte C, Riess O, Ueffing M, Biskup S, Bonin M. ARHGEF7 (Beta-PIX) acts as guanine nucleotide exchange factor for leucine-rich repeat kinase 2. PLoS One. 2010;5:e13762. doi: 10.1371/journal.pone.0013762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Lee BD, Shin JH, VanKampen J, Petrucelli L, West AB, Ko HS, Lee YI, Maguire-Zeiss KA, Bowers WJ, Federoff HJ, Dawson VL, Dawson TM. Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson’s disease. Nat Med. 2010;16:998–1000. doi: 10.1038/nm.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Z, Hamamichi S, Lee BD, Yang D, Ray A, Caldwell GA, Caldwell KA, Dawson TM, Smith WW, Dawson VL. Inhibitors of LRRK2 kinase attenuate neurodegeneration and Parkinson-like phenotypes in Caenorhabditis elegans and Drosophila Parkinson’s disease models. Hum Mol Genet. 2011;20:3933–3942. doi: 10.1093/hmg/ddr312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herzig MC, Kolly C, Persohn E, Theil D, Schweizer T, Hafner T, Stemmelen C, Troxler TJ, Schmid P, Danner S, Schnell CR, Mueller M, Kinzel B, Grevot A, Bolognani F, Stirn M, Kuhn RR, Kaupmann K, van der Putten PH, Rovelli G, Shimshek DR. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum Mol Genet. 2011;20:4209–4223. doi: 10.1093/hmg/ddr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z, Lee J, Krummey S, Lu W, Cai H, Lenardo MJ. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat Immunol. 2011;12:1063–1070. doi: 10.1038/ni.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tong Y, Yamaguchi H, Giaime E, Boyle S, Kopan R, Kelleher RJ, 3rd, Shen J. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shutes A, Onesto C, Picard V, Leblond B, Schweighoffer F, Der CJ. Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J Biol Chem. 2007;282:35666–35678. doi: 10.1074/jbc.M703571200. [DOI] [PubMed] [Google Scholar]