Abstract
Fragile X Syndrome (FXS) is a heritable form of mental retardation caused by a non-coding trinucleotide expansion of the FMR1 gene leading to loss of expression of this RNA binding protein. Mutations in this gene are strongly linked to enhanced Group I metabotropic glutamate receptor (mGluR) signaling. A recent report found that mGluR5-dependent endogenous cannabinoid signaling is enhanced in hippocampal slices from fmr1 knockout mice, suggesting a link between FXS and cannabinoid signaling. Alterations in cannabinoid signaling have an impact on learning and memory and may therefore be linked to some aspects of the FXS phenotype.
We have used autaptic hippocampal neurons cultured from fmr1 knockout mice to further explore the interaction between endocannabinoid signaling and FMRP. These neurons express several robust forms of retrograde endocannabinoid signaling including depolarization induced suppression of excitation (DSE) and a metabotropic form (MSE) that results from Group I mGluR activation.
We now report that young fmr1 neurons exhibit considerably enhanced DSE, likely via increased production of 2-AG, rather than enhanced mGluR-MSE. We find that depolarizations as brief as 50ms, which do not ordinarily produce DSE, routinely inhibited glutamate release. Furthermore, as neuronal cultures mature, CB1-receptor signaling strongly desensitizes. Our results suggest that loss of FMRP broadly affects the endocannabinoid signaling system, possibly through local 2-AG over production. Furthermore, the net effect of the loss of FMRP may actually be diminished cannabinoid signaling due to receptor desensitization as an adaptation to 2-AG overproduction.
Keywords: Fmr1, Fragile X, cannabinoid, mGluR, CB1
INTRODUCTION
Fragile X Syndrome (FXS) is the most common heritable form of mental retardation, arising from an expanded and hypermethylated non-coding trinucleotide repeat of the FMR gene (Verkerk et al., 1991). This leads to a deficiency of fragile X mental retardation protein (FMRP), an RNA binding protein. In addition to significant mental retardation, FXS may be associated with neurological abnormalities including seizures (Kerbeshian et al., 1984). Mutations in this gene are linked to enhancement of metabotropic glutamate receptor or (mGluR) Group I signaling (Huber et al., 2002) a change that may be due to increased mGluR protein synthesis (Bear et al., 2004; Dolen et al., 2007; Pfeiffer et al., 2006). The evidence for an mGluR Group I link to FXS gave rise to an “mGluR theory” of FXS (Bear et al., 2004).
The cannabinoid signaling system includes cannabinoid CB1 receptors that are the target of exogenous phytocannabinoids such as Δ9-THC (Gaoni et al., 1964; Matsuda et al., 1990), the chief psychoactive ingredient in marijuana and hashish. This signaling system also includes endogenous cannabinoids, including 2-arachidonoyl glycerol (Stella et al., 1997), a lipid messenger, and the enzymes to produce and break down this messenger (Bisogno et al., 2003; Dinh et al., 2002). Functionally, this CB1-based signaling system has been shown to serve as a retrograde signaling mechanism in many neurons throughout the CNS (Kano et al., 2009; Wilson et al., 2001). 2-AG is produced in response to activation of diacylglycerol lipases in dendrites, either via depolarization (increasing intracellular calcium) or activation of metabotropic receptors (Bisogno et al., 2003). When targeting excitatory neurons, the former form of plasticity is known as depolarization induced suppression of excitation (DSE) whereas the latter is metabotropic suppression of excitation (MSE). The consequence of DSE or MSE in glutamatergic neurons is an inhibition of glutamate release. Analogous processes targeting inhibitory neurons are referred to as DSI/MSI. DSE/DSI typically lasts for tens of seconds and occurs via inhibition of presynaptic calcium channels. MSE/I is driven by post-synaptic Gq-coupled receptor activation of phospholipase C beta (PLCβ). The best-studied Gq-coupled G protein coupled receptors (GPCRs) that drive MSE/I are Group I mGluR’s (which include mGluR5) and muscarinic receptors. The association between FXS and group I mGluR’s as well as the association between group I mGluR’s and endocannabinoids, suggests that forms of endocannabinoid-mediated synaptic plasticity involving group I mGluR’s may be altered in models of FXS. Indeed, using hippocampal slices from fmr1−/− mice, Zhang & Alger showed that mGluR MSI and iLTD (a form of mGluR5/endocannabinoid-mediated long term depression) but not DSI were enhanced in fmr1−/− mice. In contrast, Jung et al. (Jung et al., 2012) report an uncoupling of mGluRs from DGLs in the striatum and prefrontal cortex of fmr1−/− mice.
To further explore the mechanisms of altered endocannabinoid signaling in fmr1−/− mice early in development we made use of autaptic hippocampal neurons from these mice. These neurons express all the machinery necessary for robust DSE and MSE, and have been extensively characterized (Straiker et al., 2009a; Straiker et al., 2005; Straiker et al., 2007). We have found that cultured fmr1−/− hippocampal neurons do not have altered mGluR5-based MSE. Instead they initially exhibit enhanced DSE, which declines with time as the cultures age. Likely this is due to excessive 2-AG production with progressive CB1 receptor desensitization and eventual inhibition of CB1-mediated synaptic plasticity.
Materials & Methods
Animals and Cell culture
All animal care and experimental procedures used in this study were approved by the Institutional Animal Care and Use Committee of Indiana University and conform to the Guidelines of the National Institutes of Health on the Care and Use of Animals. Mouse (FVB.129P2-Pde6b+ Tyrc-ch Fmr1tm1Cgr/J, Jackson Laboratory, Bar Harbor, ME, or WT (CD1)) hippocampal neurons isolated from the CA1–CA3 region were cultured on microislands as previously described (Bekkers et al., 1991; Furshpan et al., 1976). Neurons were obtained from animals (at postnatal day 0–2, killed via rapid decapitation without anesthesia) and plated onto a feeder layer of hippocampal astrocytes that had been laid down previously (Levison et al., 1991). Cultures were grown in high-glucose (20 mM) minimum essential media containing 10% horse serum, without mitotic inhibitors and used for recordings after 8 days in culture and for no more than 3 h after removal from culture medium (Straiker et al., 2005). All electrophysiological experiments were performed exclusively on excitatory neurons. All tests were made on neurons from at least two different preparations.
Electrophysiology
When a single neuron is grown on a small island of permissive substrate, it forms synapses – or ‘autapses’ – onto itself. All experiments were performed on isolated autaptic neurons. Whole-cell, voltage-clamp recordings from autaptic neurons were carried out at room temperature using an Axopatch 200B amplifier (Axon Instruments, Burlingame, CA, USA). The extracellular solution contained (mM) NaCl 119, KCl 5, CaCl2 2, MgCl2 1, glucose 30 and HEPES 20. Continuous flow of solution through the bath chamber (2 mL·min-1) ensured rapid drug application and clearance. Drugs were typically prepared as a stock then diluted into extracellular solution at their final concentration and used on the same day. Recording pipettes of 1.8–3 MΩ were filled with solution containing (mM): potassium gluconate 121.5, KCl 17.5, NaCl 9, MgCl2 1, HEPES 10, EGTA 0.2, MgATP 2 and LiGTP 0.5. Access resistance was monitored and only cells with a stable access resistance were included for data analysis.
EPSC sizes were lower for fmr1−/− neurons in both young and older cultures, with the difference increasing with age (EPSC peak amplitude for young wild type neurons: 2.9 ± 0.04 nA, n=21; for young fmr1−/− neurons: 2.1 ± 0.05 nA, n=9; for older wild type neurons: 3.8 ± 0.5 nA, n=14; for older fmr1−/− neurons: 2.6 ± 0.3 nA, n=9). However the difference was not statistically significant (p=0.11 for older wild type vs. older fmr1−/− neurons.
To test for an effect of the lithium salt used in the internal solution we obtained response curves for DSE and mGluR agonist DHPG using a NaGTP salt and found that the baseline responses (EC50 and maximal) were unaltered (DHPG EC50 LiGTP: 1.6µM (1.4–1.7); NaGTP 2.6µM (1.7–3.9); DSE EC50 LiGTP: 0.76 sec (0.65–0.91); NaGTP: 0.74 sec (0.65–0.84)). We also examined whether passive membrane properties in the form of leak conductance differed between wild type and mutant neurons. We found that leak conductance was unaltered (wild type: 9.0 ± 1.8 nS; fmr1−/−: 10.2 ± 2.8 nS).
Data analysis
Data are reported as mean ± SEM (except EC50 and t1/2 data, which are reported as mean (95% CI)). Non-linear regression was used to fit the concentration response curves. Treatment effects on evoked EPSCs were evaluated using one-way ANOVA with Bonferroni multiple comparison tests where indicated. Statistical significance is indicated as follows: *p < 0.05. All graphs and statistical analyses were generated using GraphPad Prism 4.0 software (Hearne Scientific Software, Chicago, IL, USA).
RESULTS
Depolarization of autaptic hippocampal neurons can result in a form of retrograde inhibition termed depolarization induced suppression of excitation (DSE (Straiker et al., 2005)). This can be quantified by a series of successively longer depolarizations (50ms, 100ms, 300ms, 500ms, 1sec, 3 sec, 10 sec) resulting in progressively greater inhibition of neurotransmission (Straiker et al., 2012). This produces a “depolarization-response curve” that permits the characterization of some pharmacological properties of cannabinoid signaling including the calculation of an effective-depolarization 50 (ED50), corresponding in this case to the duration of depolarization that results in 50% of the maximal response. We found that fmr1−/− neurons between 8 and 10 days in culture were much more sensitive to brief depolarizations than wild-type neurons. As a consequence the ED50 was shifted from 760ms (Fig. 1, 95% CI: 645–906) to 234ms (95% CI: 201–274). The maximal effect was unaltered (Fig 1., Relative EPSC charge after 10 sec depolarization (1.0 = no inhibition) in WT: 0.38 ± 0.06, n=8; and fmr1−/−: 0.36 ± 0.04, n=7). In principle, enhanced endocannabinoid signaling may reflect increased 2-AG production, decreased 2-AG degradation, or increased sensitivity of pre-synaptic CB1 signaling. To test whether the sensitivity of presynaptic CB1 signaling was increased we applied increasing concentrations of 2-AG to WT and fmr1−/− neurons. We found that the concentration-response curves for 2-AG were similar (WT EC50 (95% CI): 261nM (240–283); fmr1−/−: 185nM (106–323), overlapping 95% CI). This indicates that the enhancement of cannabinoid signaling is likely not due to increased CB1 receptor sensitivity. In excitatory autaptic cultures 2-AG is primarily degraded by monoacylglycerol lipase (MGL)(Straiker et al., 2009a). Decreased MGL activity will prolong the time course of DSE (Straiker et al., 2009a), thus we tested whether the DSE recovery time courses were altered in young WT vs. fmr1−/− cultures. However, the half-lives (t½) of DSE in cultures from the two genotypes were identical (t1/2 for young WT neurons: 42.2 sec (95% CI: 37–49); for fmr1−/− neurons: 41.5 sec (95% CI: 36–48)). Thus, the most likely explanation for the increased DSE in fmr1 −/− cultures is an increase in 2-AG production.
Figure 1.
DSE is enhanced in fmr1−/− autaptic hippocampal neurons with no change in CB1 receptor sensitivity. A) Depolarization-response curves for WT and fmr1−/− autaptic neurons. B) Sample time courses for DSE in response to 500ms depolarization in WT and fmr1−/− neurons. Inset shows sample EPSCs from same neurons (WT, top; fmr1−/− bottom) at time points indicated. C) Concentration-response curves for 2-AG in WT and fmr1−/− neurons.
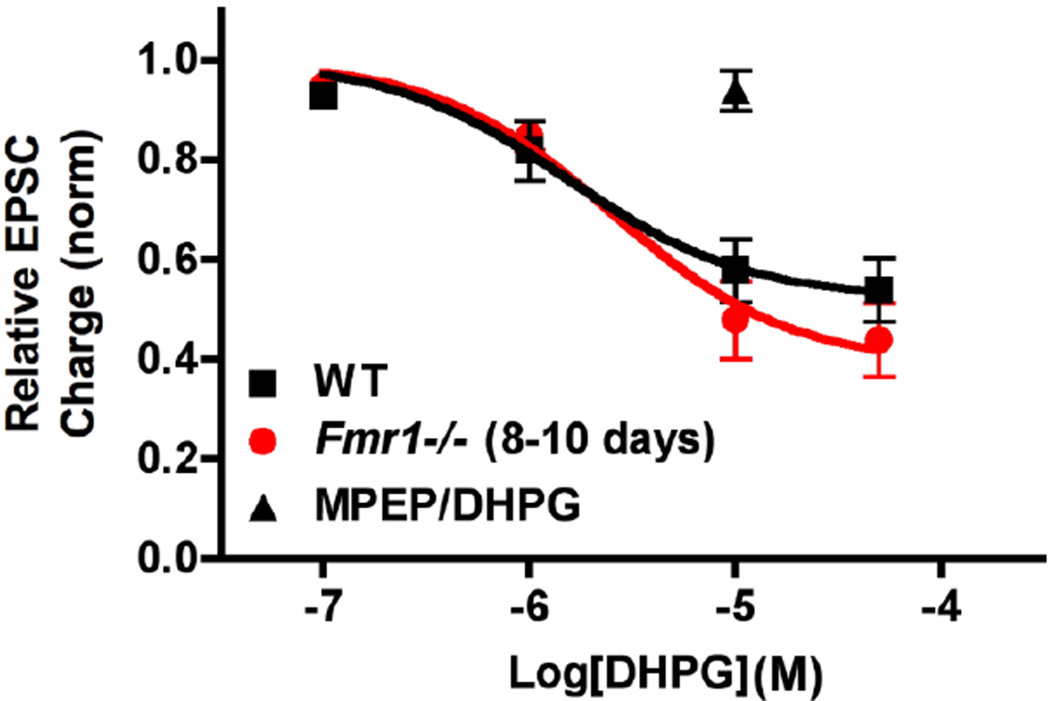
As DHPG-induced MSI has been reported to be enhanced in hippocampal slices from Fmr1−/− mice (2–4 months of age), we also tested autaptic Group I mGluR MSE (Straiker et al., 2007). In contrast to Zhang & Alger who found that the Group I mGluR agonist DHPG more potently elicited MSI, the effect of a low concentration of DHPG (1µM) was unaltered in the autaptic neurons. Surprisingly, the EC50 for DHPG-induced MSE exhibited a small, but statistically significant rightward shift in fmr1−/− cultures (EC50 in WT: 1.56µM (95% CI: 1.40–1.74); in Fmr1−/−: 2.52µM (95% CI: 2.06–3.07)). This suggests that increased Group I mGluR signaling is unlikely to explain the increased sensitivity of DSE. Autaptic MSE in cultures from fmr1−/− continued to be mediated by mGluR5 as it was eliminated by the mGluR5 antagonist, 2-methyl-6-(phenylethynyl)-pyridine (MPEP; 5µM, Figure 2).
Figure 2.
Group I mGluR responses are non-significantly increased in fmr1−/− vs. WT autaptic neurons. Concentration response curves for inhibition in response to varying concentrations of Group I mGluR agonist DHPG in WT (black squares) and fmr1−/− (red circle) neuronal cultures. Black triangle shows DHPG (10µM) response in presence of the mGluR5 blocker, MPEP (5µm in fmr1−/− cultures. fmr1−/− points are non-significantly different vs. WT at each concentration tested, 2-way ANOVA with Bonferroni posthoc test.
In perhaps our most notable finding, we observed that DSE responses diminished as the neuronal cultures from fmr1−/− mice aged. Maximal responses to depolarization after 14–17 days in culture were substantially diminished in Fmr1−/− cultures relative to young (8–10 days in culture) fmr1−/− or WT counterparts (Fig. 3A; Relative EPSC charge after 10 sec depolarization: 0.48 ± 0.09, n=5). The ED50 was shifted to the right (ED50 older culture: 1.32 sec (95% CI: 0.82–2.11 sec); younger culture: 0.23 sec (95% CI: 0.20–0.27)), as was the EC50 for 2-AG (Fig 3B; EC50 older culture: 1.72µM (95% CI: 1.51–1.96µM); younger culture: 185nM (95% CI: 106–322nM). EPSC amplitudes of WT and fmr1−/− neurons did not change with age in culture (see Methods). However, inhibition of glutamate release by other presynaptic Gi/o G protein-coupled receptors remained intact, with GABAB and adenosine A1 responses unchanged with age in culture (Fig 3C, D). This indicates that CB1 responses desensitize, presumably as a consequence of increased 2-AG production.
Figure 3.
CB1 receptors desensitize with age in fmr1−/− neurons. A) Depolarization-response curve in young (8–10 days in culture (DIC)) vs. old (14–17 DIC) fmr1−/− neuronal cultures. B) Concentration response curve for 2-AG in older fmr1−/− cultures. WT and fmr1−/− young cultures shown from Fig 1 for reference. C) EPSC inhibition in young vs. old cultures for the indicated concentrations Gi/o-coupled GPCR agonists: CB1 (2-AG), GABAB (baclofen), and Adenosine A1 (CPA). p<0.05 1 way ANOVA with Bonferroni posthoc test for 2-AG in young vs. older cultures. D) Sample time course from a neuron after 14 days in cultures showing little EPSC inhibition in response to increasing concentrations of 2-AG (1nM-5µM, steps indicate increasing concentrations) followed by robust inhibition from baclofen (25µM) and cyclopentyladenosine (CPA, 1µM).
DISCUSSION
Our chief findings are that CB1-mediated DSE, a form of depolarization-dependent retrograde inhibition, is profoundly enhanced in young fmr1−/− cultures. This is most likely due to enhanced 2-AG production, with the result that CB1 receptors ultimately desensitize as the cultures age. This finding is surprising in several respects. First, the net effect of an absence of FMR protein in this model system is in fact a diminishment of CB1-dependent cannabinoid signaling. Second, we see a clear enhancement of DSE despite the absence of an effect on DSI in brain slices (Zhang et al., 2010). Third, our results run counter to the mGluR model insofar as we see no statistically significant alteration in maximal responses for autaptic mGluR Group I dependent MSE.
The reported enhancement of Group I mGluR signaling in FXS models, and the enhancement of endocannabinoid signaling (mGluR MSI) in slices from fmr1−/− mice (Zhang. et al., 2010) led us to the expectation that autaptic mGluR-dependent MSE would also be enhanced. In particular, Zhang & Alger (2010) studied potential alterations of three forms of cannabinoid signaling in wild type vs. fmr1−/− acute hippocampal slices: DSI, mGluR-initiated eCB short term-depression of evoked IPSCs (MSI) and eCB-dependent LTD of IPSCs (eCBiLTD). DSI was unaltered but MSI was increased and the fmr1−/− neurons were more susceptible to eCB-iLTD. Because levels of mGluR1, mGluR5 and CB1 protein were unaltered, the authors concluded that the changes in MSI and iLTD occurred as a result of enhanced coupling of mGluR’s to eCB mobilization. We also observed an enhancement of 2-AG mobilization, but in contrast to Zhang & Alger it was DSE that was markedly sensitized, while mGluR-dependent signaling was at most modestly altered. Furthermore, with increasing time in culture, the 2-AG responses desensitized, decreasing the influence of endocannabinoids on synaptic signaling.
Given the difference in our findings, enhanced metabotropic 2-AG mobilization in hippocampal slices vs. enhanced depolarization-dependent 2-AG mobilization in cultured hippocampal neurons, it is worth reviewing qualitative differences between these forms of 2-AG mobilization. Under normal circumstances, DSE and MSE each mobilize 2-AG via activation of diacylglycerol lipases (DGLs), but they differ in the way that they bring about DGL activation (Bisogno et al., 2003; Hashimotodani et al., 2007). MSI/MSE occurs via Gq-coupled Group I mGluR and PLCβ activation (Hashimotodani et al., 2005; Maejima et al., 2001). PLCβ in turn forms the 2-AG precursor DAG and IP3, which may contribute to release of calcium from internal stores. In contrast, DSE occurs via depolarization-induced postsynaptic calcium increases that activate DGL, without obligatory PLCβ activation (Brenowitz et al., 2003; Gao et al., 2010; Hashimotodani et al., 2008), though autaptic DSE includes calcium release from intracellular stores (Straiker et al., 2005). Our confirmation that GABAB and adenosine A1 signaling remain intact in the older fmr1−/− neurons shows that Gi/o-mediated inhibition of neurotransmission is not fundamentally altered by the absence of the FMR protein. Instead, in terms of presynaptic modulation, the consequences may be specific to the CB1-based cannabinoid signaling system.
Our finding of potentiated signaling may be seen as complementary to those of Zhang & Alger, who studied cannabinoid modulation of GABA-based inhibitory signaling as opposed to glutamate-based excitatory signaling. Recent work by Jung et al. (Jung et al., 2012) offers a completely different profile in fmr1−/− mice, albeit at excitatory synapses in prefrontal cortex and striatum. Instead of potentiation of 2-AG mobilization they found that mGlu5-dependent 2-AG mobilization was compromised in the fmr1−/− mice. Their findings were supported by evidence that loss of FMRP leads to mistargeting of DGLα mRNA, increasing the distance between mGluR5/PLCβ, and DGLα, which reduces mGluR5—DGLα functional coupling and markedly diminishes endocannabinoid-dependent LTD. However, in our studies with cultured hippocampal neurons, the mGluR-coupling remains intact at excitatory synapses. It is possible that other factors may account for the observed differences. For instance, the fmr1−/− strain used in this work (FVB) differed from that used by Zhang et al. (C57), while Jung et al. used a mix of C57 and FVB-based strains. It should also be noted that our own work made use of two different strains of mice (FVB and CD1), however the core observation of desensitization of CB1 signaling occurred within strain; nor did we observe desensitization in C57 cultures of increasing age (data not shown) indicating that in two wild type strains CB1 desensitization does not occur on its own. It is also possible that DSE is intrinsically more prone to desensitization than DSI as it requires higher concentrations of 2-AG to produce an equivalent suppression of synaptic currents in some preparations (Ohno-Shosaku et al., 2002), though not in autaptic neurons (Straiker et al., 2009b). We have found that the rate of recovery of fast autaptic DSI is due to the cooperative breakdown of 2-AG by monoacylglyceride lipase (MGL) and cyclooxygenase 2 (COX2) in contrast to the slower DSE that relies on MGL. As such, the cellular machinery supporting DSE may be less well equipped to deal with overproduction of 2-AG before CB1 desensitization occurs. It is additionally possible that the age at which autaptic neurons are harvested (early postnatal) contributes to the observed profile and to the differences between our results and other studies. Zhang et al. and Jung et al. both used adult animals (2–4, and 1–3 months, respectively). Because FXS is a developmental disorder, there are advantages to understanding the alterations in endocannabinoid signaling during early stages of postnatal development as was done here. Lastly, it is possible that differences between autaptic DSE and DSE/DSI described thus far in brain slices may go some way to explaining the differences seen. In particular, autaptic DSE appears to rely more heavily on calcium from internal calcium stores rather than on post-synaptic influx through calcium channels. This stands in contrast to characterizations of DSE/DSI in hippocampal slices (Brenowitz et al., 2003; Kreitzer et al., 2001). As a consequence, autaptic DSE may share some qualities in common with mGluR-dependent MSE. The differences between our results and those of Jung et al. and Zhang et al., whether due to the brain region, developmental stage, the preparation, the strain, temperature or some other cause, nonetheless underscores the diversity of consequences of fmr1 deletion. Perhaps this diversity should be expected given that FMR protein serves as a regulator of protein expression, and is consequently well-placed to alter the function of numerous proteins (Bear et al., 2008), which will have diverse cellular effects, even on a signaling pathway utilizing a single neuromodulator.
The modulation of cannabinoid signaling is of particular interest for FXS research, partly because of the ubiquity of cannabinoid CB1 receptors (Herkenham et al., 1990), but also because of their extensive role in neuromodulation and their implication in important physiological processes including learning and memory and seizures (Kano et al., 2009; Lutz, 2004). As such, alterations of the cannabinoid signaling system may participate in the expression of FXS and modulating endocannabinoid signaling may serve as a therapeutic avenue to ameliorate some behavioral symptoms, as recently reported (Jung et al., 2012). In addition, a desensitization-induced decline in cannabinoid inhibition of excitatory synapses may favor an increased propensity to uncontrolled neuronal firing and an increased risk for seizures.
In summary, we have found that initially cannabinoid inhibition of excitatory neurotransmission is enhanced in autaptic hippocampal neurons cultured from fmr1 knockout mice. However, counter to expectations, this enhancement is independent of mGluR5 signaling, instead occurring when endocannabinoids are produced by depolarization (DSE). The long term consequence of this enhanced CB1 signaling is desensitization of CB1 receptors and a decline in (endo)cannabinoid signaling. By identifying a distinct alteration of cannabinoid signaling by fmr1 deletion, our results underscore the breadth of consequences of fmr1 deletion and also offer a novel mechanism for FMRP regulation of neurotransmission.
Highlights.
In a mouse model of Fragile X syndrome (FXS), endocannabinoid signaling is enhanced.
However, this ultimately leads to CB1 cannabinoid receptor desensitization.
The net impact of fmr1 deletion is diminished cannabinoid signaling.
This is the first evidence that fmr1 deletion affects endocannabinoid-mediated DSE.
Because mGluR signaling is unaltered in this model, this runs counter to the ‘mGluR’ model of FXS.
Acknowledgements
This work was supported by grants DA021696 (KM), DA011322 (KM), EY021831(AS)
Abbreviations
- DSE
depolarization-induced suppression of excitation
- FXS
Fragile X Syndrome
- mGluR
metabotropic glutamate receptor
- FMRP
fragile X mental retardation protein
- MSE
metabotropic suppression of excitation
- 2-AG
2-arachidonoyl glycerol
- EPSC
excitatory postsynaptic current
- DHPG
dihydroxyphenyl glycine
- MPEP
2-methyl-6-(phenylethynyl)-pyrydine
- CPA
cyclopentyladenosine
- LTD
long-term depression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
LITERATURE CITED
- Bear MF, Dolen G, Osterweil E, Nagarajan N. Fragile X: translation in action. Neuropsychopharmacology. 2008;33(1):84–87. doi: 10.1038/sj.npp.1301610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27(7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bekkers JM, Stevens CF. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci U S A. 1991;88(17):7834–7838. doi: 10.1073/pnas.88.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163(3):463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J Neurosci. 2003;23(15):6373–6384. doi: 10.1523/JNEUROSCI.23-15-06373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99(16):10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56(6):955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furshpan EJ, MacLeish PR, O'Lague PH, Potter DD. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci U S A. 1976;73(11):4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, et al. Loss of Retrograde Endocannabinoid Signaling and Reduced Adult Neurogenesis in Diacylglycerol Lipase Knock-out Mice. J Neurosci. 2010;30(6):2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Ca(2+)-assisted receptor-driven endocannabinoid release: mechanisms that associate presynaptic and postsynaptic activities. Curr Opin Neurobiol. 2007;17(3):6. doi: 10.1016/j.conb.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Maejima T, Fukami K, Kano M. Pharmacological evidence for the involvement of diacylglycerol lipase in depolarization-induced endocanabinoid release. Neuropharmacology. 2008;54(1):58–67. doi: 10.1016/j.neuropharm.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, et al. Phospholipase Cbeta Serves as a Coincidence Detector through Its Ca(2+) Dependency for Triggering Retrograde Endocannabinoid Signal. Neuron. 2005;45(2):257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99(11):7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nature communications. 2012;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89(1):309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kerbeshian J, Burd L, Martsolf J. A family with fragile-X syndrome. The Journal of nervous and mental disease. 1984;172(9):549–551. doi: 10.1097/00005053-198409000-00007. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29(3):717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Levison SW, McCarthy KD. Characterization and partial purification of AIM: a plasma protein that induces rat cerebral type 2 astroglia from bipotential glial progenitors. J Neurochem. 1991;57(3):782–794. doi: 10.1111/j.1471-4159.1991.tb08220.x. [DOI] [PubMed] [Google Scholar]
- Lutz B. On-demand activation of the endocannabinoid system in the control of neuronal excitability and epileptiform seizures. Biochem Pharmacol. 2004;68(9):1691–1698. doi: 10.1016/j.bcp.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31(3):463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22(10):3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. Current advances in local protein synthesis and synaptic plasticity. J Neurosci. 2006;26(27):7147–7150. doi: 10.1523/JNEUROSCI.1797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388(6644):773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Straiker A, Hu SS, Long JZ, Arnold A, Wager-Miller J, Cravatt BF, et al. Monoacylglycerol lipase limits the duration of endocannabinoid-mediated depolarization-induced suppression of excitation in autaptic hippocampal neurons. Mol Pharmacol. 2009a;76(6):1220–1227. doi: 10.1124/mol.109.059030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Cannabinoid signaling in inhibitory autaptic hippocampal neurons. Neuroscience. 2009b;163(1):11. doi: 10.1016/j.neuroscience.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol. 2005;569(Pt 2):501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Mackie K. Metabotropic suppression of excitation in murine autaptic hippocampal neurons. J Physiol. 2007;578(Pt 3):773–785. doi: 10.1113/jphysiol.2006.117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A, Wager-Miller J, Hutchens J, Mackie K. Differential signalling in human cannabinoid CB1 receptors and their splice variants in autaptic hippocampal neurones. Br J Pharmacol. 2012;165(8):2660–2671. doi: 10.1111/j.1476-5381.2011.01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410(6828):588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Zhang L, Alger BE. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J Neurosci. 2010;30(16):5724–5729. doi: 10.1523/JNEUROSCI.0795-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]