Abstract
All eukaryotic cells require efficient trafficking of metabolites between the mitochondria and the rest of the cell. This exchange is carried out by the dominant protein in the outer mitochondrial membrane (OMM), the Voltage Dependent Anion Channel (VDAC), which serves as the primary pathway for the exchange of ions and metabolites between the cytoplasm and the intermembrane space of the mitochondria. Additionally, VDAC provides a scaffold for the binding of modulator proteins to the mitochondria and has been implicated in mitochondria-dependent cell death. We recently determined the structure of the murine VDAC1 (mVDAC1) at 2.3Å resolution crystallized in a native-like bilayer environment. The high-resolution structure provided concise structural details about the voltage-sensing N-terminal domain and catalyzed new hypotheses regarding the gating mechanisms for metabolites and ions that transit the OMM. In this study, the crystal packing of mVDAC1 is analyzed revealing a strong antiparallel dimer that further assemble as hexamers mimicking the native oligomeric packing observed in EM and AFM images of the OMM. Oligomerization has been shown to be important for VDAC regulation and function, and mVDAC1 crystal packing in a lipidic medium reveals insights on how oligomerization is accomplished using protein-protein and protein-lipid interactions. Furthermore, orientation of VDAC in the OMM remains uncertain due to inconsistencies in antibody labeling studies. The physiological implications of a novel antiparallel arrangement are addressed that may clarify these conflicting biochemical data.
Keywords: VDAC, crystal structure, oligomerization, orientation
VDAC serves as an important node in the cellular crosstalk between mitochondria and the rest of the cell, facilitating free exchange of ions and metabolites including ATP across the OMM.1 In addition to its metabolic and energetic functions, VDAC appears to have a more complex role, serving as a scaffold for molecules and proteins that modulate the organelle’s permeability, and thereby its function.2-4 This cell death/survival role has implicated VDAC in the metabolic stresses of cancer and cardiovascular disease specifically – as well as mitochondrial-dependent cell death in general.5-7 Thus, understanding the structure, function and protein interactions of VDAC constitutes a critical objective for basic and medical research.
Recently, three publications reported with increasing detail, the long sought after structure of VDAC. Hiller et al. resolved the solution structure of hVDAC1 by NMR spectroscopy in the presence of the detergent LDAO; Bayrhuber et al. applied a combinatorial approach of NMR spectroscopy and X-ray crystallography to obtain a medium resolution structure (4Å) again of hVDAC1 in the detergent Cymal-5; and our group obtained a high-resolution structure (2.3Å) of mVDAC1 from crystals grown in a more natural lipidic environment (bicelles) by X-ray crystallography.8-10
All three structures reveal an amphipathic β-barrel motif formed by 19 β-strands, representing a new fold of outer membrane β-barrel proteins with an odd number of strands. The high-resolution mVDAC1 crystal structure showed, with the most clarity, the orientation of the N-terminal voltage sensing domain that transverses the entire pore. The α-helix portion of the N-terminal segment is positioned halfway through the pore causing a narrowing of the cavity, where it is ideally situated to regulate metabolite flux. Both N- and C-termini face the same side of the membrane, but inconsistencies in previous, as well as recent, antibody labeling studies preclude the accurate orientation of VDAC within the OMM.11-13
Analysis of the crystal packing of mVDAC1, grown within a native-like lipid medium, revealed a strong antiparallel dimer interface, which further assembles as hexamers matching the native arrangement observed in EM and AFM images of the OMM.14,15 The oligomerization of VDAC has been proposed to have important physiological roles for regulation of VDAC function, binding of proteins to the OMM and mitochondria-dependent apoptosis.16-18 We present crystal packing analysis of mVDAC1 and address a novel antiparallel arrangement that clarifies some conflicting biochemical data about topology and further reveals insights into the packing arrangement of VDAC oligomers.
Packing analysis of mVDAC1 crystals
The oligomerization of VDAC is widely viewed as an essential component for a number of fundamental mitochondrial functions - ranging from scaffolding for the binding of modulator proteins from both sides of the OMM to facilitating the release of cytochrome c during apoptosis.16-18 Biochemical studies have shown VDAC to conform to a number of oligomeric species.18,19 A recent AFM study, on freshly isolated OMM of potato tubers, revealed the most frequently identified VDAC oligomer to be hexamers that may serve as platforms for hexokinase binding to the mitochondria or result in mega-pore formation with pro-apoptotic proteins such as Bax or Bak resulting in the release of cytochrome c in the event of mitochondrial-dependent cell death.15
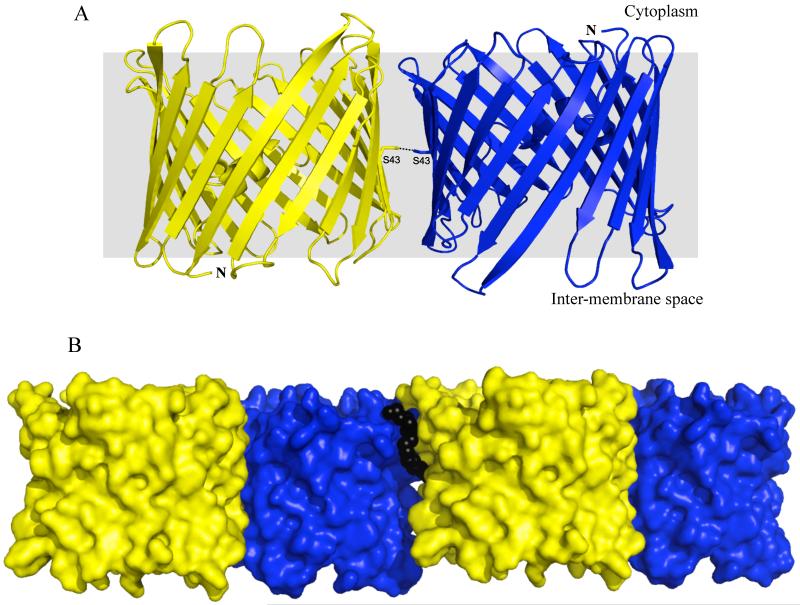
Greater insights into the interaction facilitating oligomerization of VDAC1 can be seen through packing analysis of mVDAC1 (PDB 3EMN) crystals, grown in a native-like bilayer environment using lipidic bicelles. A crystallographic-imposed antiparallel dimer formed by transmembrane β-strands 1, 2, 3, 4, 18 and 19 (Figure 1A) is observed. The dimer interface has a buried surface area of 2,376Å2 composed of 127 inter-monomer contacts contributed by 36 residues per protomer.20 All contacts are formed by van der Waals interactions with the exception of a lone hydrogen bond, situated in the middle of β-strand 2, between Ser-43 of each protomer (Figure 1A). The mVDAC1 antiparallel dimer represents a significantly tighter interface compared to the parallel dimer of hVDAC1 (550Å2),9 and furthermore resembles the values identified from known physiological dimers.21,22
Figure 1.
A. Cartoon representation of the mVDAC1 antiparallel dimer. The protomers are shown in blue and yellow displaying opposite topologies. The N-terminus of each protomer is indicated. The yellow protomer has its N- and C-termini facing the inter-membrane space of the mitochondria while the blue protomer has its N- and C-termini facing the cytoplasm. Ser-43 from each protomer is shown forming a hydrogen bond in the middle of the membrane plane. B. Surface representation of the mVDAC1 dimer-dimer interface. Antiparallel mVDAC1 dimers associate laterally within the membrane plane to generate a hexamer arrangement. The lipid DMPC, shown using black spheres, is sandwiched between the dimers and stabilizes the interface.
This tightly packed antiparallel dimer acts as a building block for additional protein-protein and protein-lipid interactions (Figure 1B), developing a hexamer arrangement that is nearly identical to those observed in EM and AFM studies on native OMM.14,15 Figure 2 shows the mVDAC1 hexamer superimposed on the hexagonal lattice observed in EM images of Neurospora crassa OMM. Each dimer packs laterally within the bicelle membrane plane with a dimer-dimer buried surface area of 1,037Å2, suggesting that it is more dynamic than the individual dimers. This dimer-dimer interface is formed by 51 inter-dimer contacts (7 hydrogen bonds and 44 van der waals interactions), contributed by β-strands 7-10 from dimer-1 and 12-18 from dimer-2. Additionally, the lipid DMPC, sandwiched between the antiparallel dimers, stabilizes the interface by contributing 54 lipid-dimer contacts (31 lipid-dimer1 and 23 lipid-dimer2) formed by van der waals interactions and 3 hydrogen bonds. While lipids have already been shown to regulate VDAC activity, our crystal structure shows that they may also play a critical role in its oligomerization, as seen for other membrane proteins.22-24
Figure 2.
The crystal packing of mVDAC1 hexamer is superimposed on the hexagonal lattice observed in EM images of the OMM (dimensions of the hexagonal lattice are indicated).14 mVDAC1 protomers facing up and down are colored yellow and blue, respectively.
Is the asymmetry physiological?
It is intriguing to speculate as to whether the antiparallel dimer and the resulting hexamer formation seen in the mVDAC1 crystal packing are physiologically relevant. There are a number of comprehensive arguments in support of this scenario: First, mVDAC1 was crystallized in lipidic bicelles that mimic the physiological bilayer environment and possibly represent the native arrangement within the OMM. Second, the antiparallel mVDAC1 dimer interface is considerably tighter than the observed parallel interface of hVDAC1 dimer. Third, the hexamer packing identified in mVDAC1 crystals mimics the native arrangement observed by EM and AFM studies on the OMM.14,15 Thus, an antiparallel arrangement of VDAC1 may be a physiological requirement for a compact dimer suitable for composing a hexameric arrangement seen in native membranes.
Further evidence from previous scientific reports suggests a dual-directionality of VDAC in the outer membrane of the mitochondria. Early studies from Pinto et al.11 labeled intact mitochondria with antibodies generated against the N-terminus of VDAC indicating a cytoplasmic orientation while Stanley et al.12 showed a contradictory result where the N-terminus faces the intermembrane space. It has been widely speculated that the differences between the studies were the result of improper handling and isolation of the mitochondria in the former study. A more recent study further complicates the issue where McDonald et al.13 probed the membrane orientation of yeast VDAC1 with FLAG-epitopes designed using the hVDAC1 and mVDAC1 structures. While epitopes FLAG1 and FLAG5 located on the N-terminal region and C-terminus respectively, suggested an orientation of VDAC with the C-terminus exposed to the cytosol; epitopes FLAG2 and FLAG3 located on loop regions facing opposite sides of the OMM, were more ambiguous (Figure 3). Both VDAC-FLAG2 and VDAC-FLAG3 bound antibody in intact mitochondria, but binding increased significantly upon membrane solubilization, suggesting that both sides of VDAC are exposed to the cytosol and the inter-membrane space. The authors attributed these conflicting results to dynamic behavior of this region formed by β-strands 1-6. It would appear that the topological orientation of VDAC in the OMM is still an ongoing debate.
Figure 3.
Cartoon representation of mVDAC1 showing positions of FLAG-epitope insertion used in the study by McDonald et al.13 The protein is colored from the N-terminus in blue to the C-terminus in red. mVDAC1 residues where FLAG-epitopes were inserted are shown using black spheres and were determined using sequence alignment of mVDAC1 with Saccharomyces cerevisiae VDAC1. FLAG1 and FLAG5 are located on the N-terminal helix and C-terminus respectively. FLAG2 and FLAG3 and located on loop regions facing opposite sides of the OMM. FLAG1 and FLAG5 point to an orientation of VDAC with a cytoplasmic C-terminus; however results from FLAG2 and FLAG3 are ambiguous.
Dual-topology membrane proteins
It is generally believed that integral membrane proteins have only a single orientation within the membrane. Recently, however, dual-topology proteins displaying opposite orientations in the membrane have been reported.25-30 The most well documented of these is the Escherichia coli proton-coupled multidrug transporter EmrE. Supporting evidence for this observation came from structural studies on 2-D and 3-D crystals of EmrE that show an asymmetric dimer with the protomers in an antiparallel topological orientation.25,26 Additionally, von Heijne’s group found using a comparative genomics approach that EmrE has a dual-topology.27,28 Importantly, recent biochemical studies from the Schuldiner group show that EmrE displays unique promiscuous behavior where protomers can adopt different topologies relative to each other29 and to the lipid bilayer (personal communication by Professor Schuldiner), and yet are still capable of transport. The authors show that two EmrE protomers can associate in a parallel Cin-Cin orientation, parallel Cout-Cout orientation or an antiparallel Cin-Cout orientation and remarkably all retain functionality as a homodimer.
In addition to EmrE, von Heijne’s group identified four other dual-topology candidates, SugE (multi-drug resistance protein), CrcB (camphor resistance protein), YdgC (associated with alginate biosynthesis) and YnfA.28 It has been suggested that the flip-flopping of membrane proteins to adopt a mixture of topologies may serve as an effective strategy to satisfy functional requirements.30 Furthermore, it may represent a stage in the topological evolution of membrane proteins resulting from gene duplications and mutations.29 Although the list of identified dual-topology membrane proteins is small, the idea that proteins can adopt multiple topologies in the membrane has been established.
There is increasing evidence to support an antiparallel topology for VDAC in the mitochondria. A dual-topology model will help to explain the inconclusive results of topology studies and will link the hexamers observed in the OMM with those seen in mVDAC1 crystals grown in lipidic bicelles. The hypothesized bi-orientation of VDAC discussed here establishes a model to be tested biochemically.
Acknowledgements
The authors thank: Peipei Ping and Jun Zhang for critical discussion and generous use of resources; The Advance Light Source (P. Zwart and beamline staff at 5.0.2); Advance Photon Source (M. Capel, K. Rajashankar, J. Schuermann and I. Kourinov at NECAT 24-ID-E). Supported by NIH GM07844, HFSP RGY0069 and AHA 0630258N.
Abbreviations
- VDAC
voltage dependent anion channel
- OMM
outer mitochondrial membrane
- hVDAC1
human VDAC1
- mVDAC1
mouse VDAC1
- LDAO
n-dodecyl-N,N-dimethylamine-N-oxide
- EM
electron microscopy
- AFM
atomic force microscopy
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
References
- 1.Rostovtseva T, Colombini M. VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophys J. 1997;72:1954–62. doi: 10.1016/S0006-3495(97)78841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator--thinking outside the box. Biochim Biophys Acta. 2006;1762:181–90. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Tsujimoto Y, Shimizu S. The voltage-dependent anion channel: an essential player in apoptosis. Biochimie. 2002;84:187–93. doi: 10.1016/s0300-9084(02)01370-6. [DOI] [PubMed] [Google Scholar]
- 4.Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora’s box opens. Nat Rev Mol Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- 5.Galluzzi L, Kepp O, Tajeddine N, Kroemer G. Disruption of the hexokinase-VDAC complex for tumor therapy. Oncogene. 2008;27:4633–5. doi: 10.1038/onc.2008.114. [DOI] [PubMed] [Google Scholar]
- 6.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 7.Shoshan-Barmatz V, Keinan N, Zaid H. Uncovering the role of VDAC in the regulation of cell life and death. J Bioenerg Biomembr. 2008;40:183–91. doi: 10.1007/s10863-008-9147-9. [DOI] [PubMed] [Google Scholar]
- 8.Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–10. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, et al. Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci U S A. 2008;105:15370–5. doi: 10.1073/pnas.0808115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, et al. The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci U S A. 2008;105:17742–7. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Pinto V, Prezioso G, Thinnes F, Link TA, Palmieri F. Peptide-specific antibodies and proteases as probes of the transmembrane topology of the bovine heart mitochondrial porin. Biochemistry. 1991;30:10191–200. doi: 10.1021/bi00106a017. [DOI] [PubMed] [Google Scholar]
- 12.Stanley S, Dias JA, D’Arcangelis D, Mannella CA. Peptide-specific antibodies as probes of the topography of the voltage-gated channel in the mitochondrial outer membrane of Neurospora crassa. J Biol Chem. 1995;270:16694–700. doi: 10.1074/jbc.270.28.16694. [DOI] [PubMed] [Google Scholar]
- 13.McDonald BM, Wydro MM, Lightowlers RN, Lakey JH. Probing the orientation of yeast VDAC1 in vivo. FEBS Lett. 2009;583:739–42. doi: 10.1016/j.febslet.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 14.Mannella CA. Structure of the outer mitochondrial membrane: ordered arrays of porelike subunits in outer-membrane fractions from Neurospora crassa mitochondria. J Cell Biol. 1982;94:680–7. doi: 10.1083/jcb.94.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoogenboom BW, Suda K, Engel A, Fotiadis D. The supramolecular assemblies of voltage-dependent anion channels in the native membrane. J Mol Biol. 2007;370:246–55. doi: 10.1016/j.jmb.2007.04.073. [DOI] [PubMed] [Google Scholar]
- 16.Linden M, Gellerfors P, Nelson BD. Pore protein and the hexokinase-binding protein from the outer membrane of rat liver mitochondria are identical. FEBS Lett. 1982;141:189–92. doi: 10.1016/0014-5793(82)80044-6. [DOI] [PubMed] [Google Scholar]
- 17.Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. In self-defence: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem J. 2004;377:347–55. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zalk R, Israelson A, Garty ES, Azoulay-Zohar H, Shoshan-Barmatz V. Oligomeric states of the voltage-dependent anion channel and cytochrome c release from mitochondria. Biochem J. 2005;386:73–83. doi: 10.1042/BJ20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malia TJ, Wagner G. NMR structural investigation of the mitochondrial outer membrane protein VDAC and its interaction with antiapoptotic Bcl-xL. Biochemistry. 2007;46:514–25. doi: 10.1021/bi061577h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372:774–97. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature. 2002;415:287–94. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 22.Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, et al. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science. 1996;272:1136–44. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- 23.Rostovtseva TK, Bezrukov SM. VDAC regulation: role of cytosolic proteins and mitochondrial lipids. J Bioenerg Biomembr. 2008;40:163–70. doi: 10.1007/s10863-008-9145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Essen L, Siegert R, Lehmann WD, Oesterhelt D. Lipid patches in membrane protein oligomers: crystal structure of the bacteriorhodopsin-lipid complex. Proc Natl Acad Sci U S A. 1998;95:11673–8. doi: 10.1073/pnas.95.20.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ubarretxena-Belandia I, Baldwin JM, Schuldiner S, Tate CG. Three-dimensional structure of the bacterial multidrug transporter EmrE shows it is an asymmetric homodimer. Embo J. 2003;22:6175–81. doi: 10.1093/emboj/cdg611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YJ, Pornillos O, Lieu S, Ma C, Chen AP, Chang G. X-ray structure of EmrE supports dual topology model. Proc Natl Acad Sci U S A. 2007;104:18999–9004. doi: 10.1073/pnas.0709387104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daley DO, Rapp M, Granseth E, Melen K, Drew D, von Heijne G. Global topology analysis of the Escherichia coli inner membrane proteome. Science. 2005;308:1321–3. doi: 10.1126/science.1109730. [DOI] [PubMed] [Google Scholar]
- 28.Rapp M, Granseth E, Seppala S, von Heijne G. Identification and evolution of dual-topology membrane proteins. Nat Struct Mol Biol. 2006;13:112–6. doi: 10.1038/nsmb1057. [DOI] [PubMed] [Google Scholar]
- 29.Schuldiner S. EmrE, a model for studying evolution and mechanism of ion-coupled transporters. Biochim Biophys Acta. 2009;1794:748–62. doi: 10.1016/j.bbapap.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Bowie JU. Flip-flopping membrane proteins. Nat Struct Mol Biol. 2006;13:94–6. doi: 10.1038/nsmb0206-94. [DOI] [PubMed] [Google Scholar]