Abstract
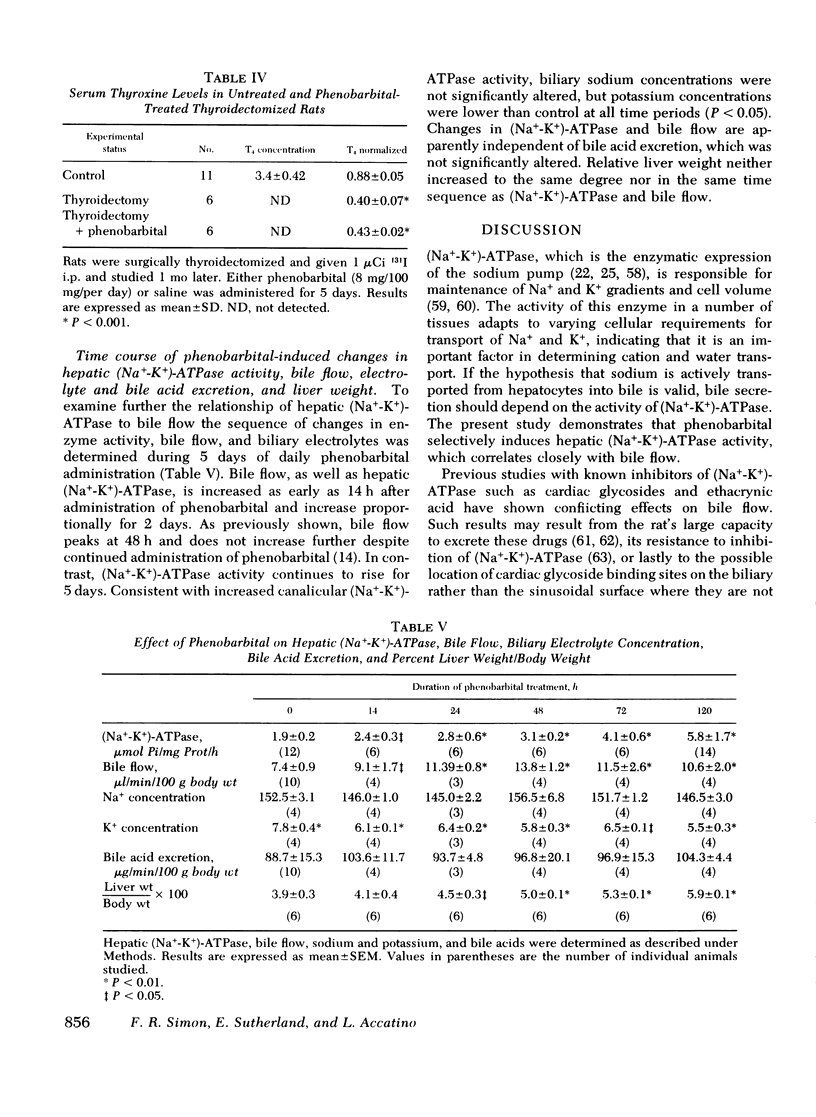
Since phenobarbital administration produces a profound increase in bile flow without changing bile acid secretion, we examined whether this drug increases the activity of hepatic sodium-potassium-activated ATPase [Na+-K+)-ATPase], the postulated regulating enzyme in the secretion of bile salt independent bile flow. After freeze-thawing to increase substrate accessibility, (Na+-K+) ATPase activity was determined by ouabain inhibition of total ATPase activity. Its activity was highest in isolated liver surface membrane fractions enriched in bile canalicult. Phenobarbital administration significatly increased (Na+-K+)-ATPase activity in both liver surface membrane fractions as well as liver homogenates. This enhanced activity is apparently selective for other membrane phosphatases and the enzyme activity in other tissues is either unaltered or decreased. Kinetic analysis of (Ka+-K+)-ATPase indicates that phenobarbital treatment increased maximum velocity and half-maximum activation constant was unchanged, consistent with activation of latent molecules or an increased number of enzyme molecules. The latter process seems more likely because cycloheximide prevented phenobarbital induction and activators were not demonstrated in vitro. Examination of the full time course of phenobarbital induction to determine whether phenobarbital increased synthesis or decreased degradation was consistent with increased synthesis since the apparent degradation rates were similar with or without phenobarbital treatment. The apparent half-life for (Na+-K+)-ATPase was estimated to be approximately 2.5 days, consistent with liver surface membrane protein turnover. The correlation of changes in bile flow with (Na+-K+)-ATPase was examined under several experimental situations. Phenobarbital caused a parallel increase in each during the 1st 2 days of greatment: thereafter other factors become rate limiting for flow, since enzyme activity doesn't reach a new steady state until 4-days. Consistent with increased sodium-potassium exchange, bile sodium was unchanged while potasium concentrations were significantly reduced. Changes in both bile flow and (Na+-K+)-ATPase induced by phenobarbital are independent of thyroid hormone. These studies support the postulate that (Na+-K+)-ATPase is an important factor in regulation of bile flow. In addition, phenobarbital enhancement of both bile flow and (Na+-K+)-ATPase is dependent upon de novo protein synthesis.
Full text
PDF



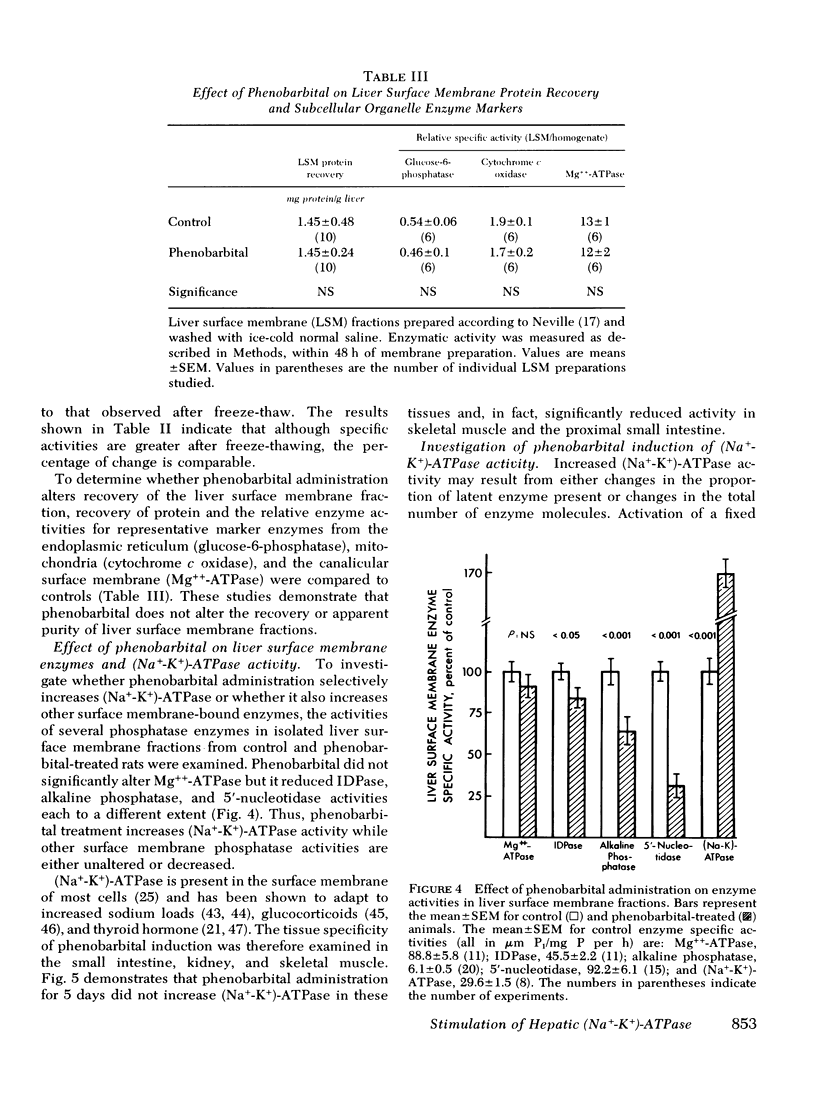







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accatino L., Simon F. R. Identification and characterization of a bile acid receptor in isolated liver surface membranes. J Clin Invest. 1976 Feb;57(2):496–508. doi: 10.1172/JCI108302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. C., Schwartz A. A possible biochemical explanation for the insensitivity of the rat to cardiac glycosides. J Pharmacol Exp Ther. 1969 Jul;168(1):42–46. [PubMed] [Google Scholar]
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Asano Y., Liberman U. A., Edelman I. S. Thyroid thermogenesis. Relationships between Na+-dependent respiration and Na+ + K+-adenosine triphosphatase activity in rat skeletal muscle. J Clin Invest. 1976 Feb;57(2):368–379. doi: 10.1172/JCI108288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkeren J. A., Bonting S. L. Studies on (Na+-K+)-activated ATPase. XX. Properties of (Na+-K+)-activated ATPase in rat liver. Biochim Biophys Acta. 1968 Apr 29;150(3):460–466. doi: 10.1016/0005-2736(68)90145-4. [DOI] [PubMed] [Google Scholar]
- Bakkeren J. A., Bonting S. L. Studies on (Na+-K+)-activated ATPase. XXI. Changes in (Na+-K+)-activated ATPase activity and ouabain-sensitive 86Rb+ uptake rate in regenerating rat liver. Biochim Biophys Acta. 1968 Apr 29;150(3):467–472. doi: 10.1016/0005-2736(68)90146-6. [DOI] [PubMed] [Google Scholar]
- Berlin C. M., Schimke R. T. Influence of turnover rates on the responses of enzymes to cortisone. Mol Pharmacol. 1965 Sep;1(2):149–156. [PubMed] [Google Scholar]
- Bernstein G., Artz S. A., Hasen J., Oppenheimer J. H. Hepatic accumulation of 125I-thyroxine in the rat: augmentation by phenobarbital and chlordane. Endocrinology. 1968 Feb;82(2):406–409. doi: 10.1210/endo-82-2-406. [DOI] [PubMed] [Google Scholar]
- Berthelot P., Erlinger S., Dhumeaux D., Preaux A. M. Mechanism of phenobarbital-induced hypercholeresis in the rat. Am J Physiol. 1970 Sep;219(3):809–813. doi: 10.1152/ajplegacy.1970.219.3.809. [DOI] [PubMed] [Google Scholar]
- Bock K. W., Siekevitz P., Palade G. E. Localization and turnover studies of membrane nicotinamide adenine dinucleotide glycohydrolase in rat liver. J Biol Chem. 1971 Jan 10;246(1):188–195. [PubMed] [Google Scholar]
- Boyer J. L. Canalicular bile formation in the isolated perfused rat liver. Am J Physiol. 1971 Oct;221(4):1156–1163. doi: 10.1152/ajplegacy.1971.221.4.1156. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Klatskin G. Canalicular bile flow and bile secretory pressure. Evidence for a non-bile salt dependent fraction in the isolated perfused rat liver. Gastroenterology. 1970 Dec;59(6):853–859. [PubMed] [Google Scholar]
- Boyer J. L., Reno D. Properties of (Na+ plus K+)-activated ATPase in rat liver plasma membranes enriched with bile canaliculi. Biochim Biophys Acta. 1975 Aug 5;401(1):59–72. doi: 10.1016/0005-2736(75)90341-7. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Bennett V., Cuatrecasas P. Membrane receptors as general markers for plasma membrane isolation procedures. The use of 125-I-labeled wheat germ agglutinin, insulin, and cholera toxin. J Biol Chem. 1975 Jan 25;250(2):488–500. [PubMed] [Google Scholar]
- Charney A. N., Kinsey M. D., Myers L., Gainnella R. A., Gots R. E. Na+-K+-activated adenosine triphosphatase and intestinal electrolyte transport. Effect of adrenal steroids. J Clin Invest. 1975 Sep;56(3):653–660. doi: 10.1172/JCI108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee P. Y., Swick R. W. Effect of dietary protein and tryptophan and the turnover of rat liver ornithine aminotransferase. J Biol Chem. 1976 Feb 25;251(4):1029–1034. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehlinger P. J., Schimke R. T. Effects of phenobarbital, 3-methylcholanthrene, and hematin on the synthesis of protein components of rat liver microsomal membranes. J Biol Chem. 1972 Feb 25;247(4):1257–1264. [PubMed] [Google Scholar]
- Dehlinger P. J., Schimke R. T. Size distribution of membrane proteins of rat liver and their relative rates of degradation. J Biol Chem. 1971 Apr 25;246(8):2574–2583. [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J., BENEDETTI E. L., RUEMKE P. STUDIES ON PLASMA MEMBRANES. I. CHEMICAL COMPOSITION AND ENZYME CONTENT OF PLASMA MEMBRANES ISOLATED FROM RAT LIVER. Biochim Biophys Acta. 1964 Jul 15;90:126–145. doi: 10.1016/0304-4165(64)90125-4. [DOI] [PubMed] [Google Scholar]
- Edelman I. S. Thyroidal regulation of renal energy metabolism and (Na+ + K+)-activated adenosine triphosphatase activity. Med Clin North Am. 1975 May;59(3):605–614. doi: 10.1016/s0025-7125(16)32012-0. [DOI] [PubMed] [Google Scholar]
- Emmelot P., Bos C. J. Studies on plasma membranes. 3. Mg2+-ATPase,(Na+-K+-Mg2+)-ATPase and 5'-nucleotidase activity of plasma membranes isolated from rat liver. Biochim Biophys Acta. 1966 Jul 13;120(3):369–382. doi: 10.1016/0926-6585(66)90304-9. [DOI] [PubMed] [Google Scholar]
- Epstein F. H., Silva P. Role of sodium, potassium-ATPase in renal function. Ann N Y Acad Sci. 1974;242(0):519–526. doi: 10.1111/j.1749-6632.1974.tb19114.x. [DOI] [PubMed] [Google Scholar]
- Erlinger S., Dhumeaux D., Benhamou J. P. Effect on bile formation of inhibitors of sodium transport. Nature. 1969 Sep 20;223(5212):1276–1277. doi: 10.1038/2231276a0. [DOI] [PubMed] [Google Scholar]
- Erlinger S., Dhumeaux D., Berthelot P., Dumont M. Effect of inhibitors of sodium transport on bile formation in the rabbit. Am J Physiol. 1970 Aug;219(2):416–422. doi: 10.1152/ajplegacy.1970.219.2.416. [DOI] [PubMed] [Google Scholar]
- Erlinger S., Dhumeaux D. Mechanisms and control of secretion of bile water and electrolytes. Gastroenterology. 1974 Feb;66(2):281–304. [PubMed] [Google Scholar]
- Evans W. H., Gurd J. W. Biosynthesis of liver membranes. Incorporation of ( 3 H)leucine into proteins and of ( 14 C)glucosamine into proteins and lipids of liver microsomal and plasma-membrane fractions. Biochem J. 1971 Nov;125(2):615–624. doi: 10.1042/bj1250615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDFISCHER S., ESSNER E., NOVIKOFF A. B. THE LOCALIZATION OF PHOSPHATASE ACTIVITIES AT THE LEVEL OF ULTRASTRUCTURE. J Histochem Cytochem. 1964 Feb;12:72–95. doi: 10.1177/12.2.72. [DOI] [PubMed] [Google Scholar]
- Gartner L. M., Arias I. M. Hormonal control of hepatic bilirubin transport and conjugation. Am J Physiol. 1972 May;222(5):1091–1099. doi: 10.1152/ajplegacy.1972.222.5.1091. [DOI] [PubMed] [Google Scholar]
- Glynn I. M. Membrane adenosine triphosphatase and cation transport. Br Med Bull. 1968 May;24(2):165–169. doi: 10.1093/oxfordjournals.bmb.a070620. [DOI] [PubMed] [Google Scholar]
- Graf J., Korn P., Peterlik M. Choleretic effects of ouabain and ethacrynic acid in the isolated perfused rat liver. Naunyn Schmiedebergs Arch Pharmacol. 1972;272(2):230–233. doi: 10.1007/BF00508771. [DOI] [PubMed] [Google Scholar]
- Gumucio J. J., Accatino L., Macho A. M., Contreras A. Effect of phenobarbital on the ethynyl estradiol-induced cholestasis in the rat. Gastroenterology. 1973 Oct;65(4):651–657. [PubMed] [Google Scholar]
- Ismail-Beigi F., Edelman I. S. The mechanism of the calorigenic action of thyroid hormone. Stimulation of Na plus + K plus-activated adenosinetriphosphatase activity. J Gen Physiol. 1971 Jun;57(6):710–722. doi: 10.1085/jgp.57.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Y., Kalant H., Orrego H., Khanna J. M., Videla L., Phillips J. M. Experimental alcohol-induced hepatic necrosis: suppression by propylthiouracil. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1137–1141. doi: 10.1073/pnas.72.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Y., Videla L., Bernstein J. Liver hypermetabolic state after chronic ethanol consumption: hormonal interrelations and pathogenic implications. Fed Proc. 1975 Oct;34(11):2052–2059. [PubMed] [Google Scholar]
- KAMAT V. B., WALLACH D. F. SEPARATION AND PARTIAL PURIFICATION OF PLASMA-MEMBRANE FRAGMENTS FROM EHRLICH ASCITES CARCINOMA MICROSOMES. Science. 1965 Jun 4;148(3675):1343–1345. doi: 10.1126/science.148.3675.1343. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Epstein F. H. Physiologic role of sodium-potassium-activated adenosine triphosphatase in the transport of cations across biologic membranes. N Engl J Med. 1968 Feb 1;278(5):253–261. doi: 10.1056/NEJM196802012780506. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Epstein F. H. The role of sodium-potassium-activated adenosine triphosphatase in the reabsorption of sodium by the kidney. J Clin Invest. 1967 Dec;46(12):1999–2011. doi: 10.1172/JCI105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen C. D. Biliary flow after microsomal enzyme induction. J Pharmacol Exp Ther. 1969 Aug;168(2):218–223. [PubMed] [Google Scholar]
- Kupferberg H. J., Schankl L. S. Biliary secretion of ouabain-3H and its uptake by liver slices in the rat. Am J Physiol. 1968 May;214(5):1048–1053. doi: 10.1152/ajplegacy.1968.214.5.1048. [DOI] [PubMed] [Google Scholar]
- LEAF A. On the mechanism of fluid exchange of tissues in vitro. Biochem J. 1956 Feb;62(2):241–248. doi: 10.1042/bj0620241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laperche Y., Launay A., Oudéa P. Effects of phenobarbital and rose bengal on the ATPases of plasma membranes of rat and rabbit liver. Gut. 1972 Nov;13(11):920–925. doi: 10.1136/gut.13.11.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layden T. J., Boyer J. L. The effect of thyroid hormone on bile salt-independent bile flow and Na+, K+ -ATPase activity in liver plasma membranes enriched in bile canaliculi. J Clin Invest. 1976 Apr;57(4):1009–1018. doi: 10.1172/JCI108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddrey W. C., Boyer J. L. The acute and chronic effects of ethanol administration on bile secretion in the rat. J Lab Clin Med. 1973 Aug;82(2):215–225. [PubMed] [Google Scholar]
- Manitius A., Bensch K., Epstein F. H. (Na+K+)-activated ATPase in kidney cell membranes of normal and methyprednisolone-treated rats. Biochim Biophys Acta. 1968 Jun 11;150(4):563–571. doi: 10.1016/0005-2736(68)90045-x. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B., HEUS M. A microsomal nucleoside diphosphatase. J Biol Chem. 1963 Feb;238:710–716. [PubMed] [Google Scholar]
- Nebert D. W., Gielen J. E. Aryl hydrocarbon hydroxylase induction in mammalian liver cell culture. II. Effects of actinomycin D and cycloheximide on induction processes by phenobarbital or polycyclic hydrocarbons. J Biol Chem. 1971 Sep 10;246(17):5199–5206. [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Obrig T. G., Culp W. J., McKeehan W. L., Hardesty B. The mechanism by which cycloheximide and related glutarimide antibiotics inhibit peptide synthesis on reticulocyte ribosomes. J Biol Chem. 1971 Jan 10;246(1):174–181. [PubMed] [Google Scholar]
- Oppenheimer J. H., Shapiro H. C., Schwartz H. L., Surks M. I. Dissociation between thyroxine metabolism and hormonal action in phenobarbital-treated rats. Endocrinology. 1971 Jan;88(1):115–119. doi: 10.1210/endo-88-1-115. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Roberts R. J., Plaa G. L. Effect of phenobarbital on the excretion of an exogenous bilirubin load. Biochem Pharmacol. 1967 May;16(5):827–835. doi: 10.1016/0006-2952(67)90055-x. [DOI] [PubMed] [Google Scholar]
- Russell J. Q., Klaassen C. D. Biliary excretion of cardiac glycosides. J Pharmacol Exp Ther. 1973 Sep;186(3):455–462. [PubMed] [Google Scholar]
- SCHWARTZ A. A Na K-stimulated adenosine triphosphatase in "microsomal" fractions from rat liver. Biochim Biophys Acta. 1963 Feb 12;67:329–331. doi: 10.1016/0006-3002(63)91834-1. [DOI] [PubMed] [Google Scholar]
- SEGAL H. L., KIM Y. S. GLUCOCORTICOID STIMULATION OF THE BIOSYNTHESIS OF GLUTAMIC-ALANINE TRANSAMINASE. Proc Natl Acad Sci U S A. 1963 Nov;50:912–918. doi: 10.1073/pnas.50.5.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- STRAUS W. Colorimetric determination of cytochrome c oxidase by formation of a quinoedimonium pigment from dimethyl-p-phenylenediamine. Biochim Biophys Acta. 1956 Jan;19(1):58–65. doi: 10.1016/0006-3002(56)90385-7. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Allen J. C., Harigaya S. Possible involvement of cardiac Na+, K+-adenosine triphosphatase in the mechanism of action of cardiac glycosides. J Pharmacol Exp Ther. 1969 Jul;168(1):31–41. [PubMed] [Google Scholar]
- Schwartz A., Lindenmayer G. E., Allen J. C. The sodium-potassium adenosine triphosphatase: pharmacological, physiological and biochemical aspects. Pharmacol Rev. 1975 Mar;27(01):3–134. [PubMed] [Google Scholar]
- Shaw H., Caple I., Heath T. Effect of ethacrynic acid on bile formation in sheep, dogs, rats, guinea pigs and rabbits. J Pharmacol Exp Ther. 1972 Jul;182(1):27–33. [PubMed] [Google Scholar]
- Song C. S., Bodansky O. Subcellular localization and properties of 5'-nucleotidase in the rat liver. J Biol Chem. 1967 Feb 25;242(4):694–699. [PubMed] [Google Scholar]
- Stewart D. J., Semply E. W., Swart G. T., Sen A. K. Induction of the catalytic protein of (Na+ plus K+)-ATPase in the salt gland of the duck. Biochim Biophys Acta. 1976 Jan 8;419(1):150–163. doi: 10.1016/0005-2736(76)90379-5. [DOI] [PubMed] [Google Scholar]
- Szepesi B., Freedland R. A. A possible method for estimating hormone effects on enzyme synthesis. Arch Biochem Biophys. 1969 Aug;133(1):60–69. doi: 10.1016/0003-9861(69)90488-3. [DOI] [PubMed] [Google Scholar]
- TALALAY P. Enzymic analysis of steroid hormones. Methods Biochem Anal. 1960;8:119–143. doi: 10.1002/9780470110249.ch3. [DOI] [PubMed] [Google Scholar]
- Verbin R. S., Goldblatt P. J., Farber E. The biochemical pathology of inhibition of protein synthesis in vivo. The effects of cycloheximide on hepatic parenchymal cell ultrastructure. Lab Invest. 1969 Jun;20(6):529–536. [PubMed] [Google Scholar]
- Wattiaux-De Coninck S., Wattiaux R. Nucleosidediphosphatase activity in plasma membrane of rat liver. Biochim Biophys Acta. 1969 Jun 3;183(1):118–128. doi: 10.1016/0005-2736(69)90135-7. [DOI] [PubMed] [Google Scholar]
- Wheeler H. O., Ross E. D., Bradley S. E. Canalicular bile production in dogs. Am J Physiol. 1968 Apr;214(4):866–874. doi: 10.1152/ajplegacy.1968.214.4.866. [DOI] [PubMed] [Google Scholar]
- Wheeler H. O. Secretion of bile acids by the liver and their role in the formation of hepatic bile. Arch Intern Med. 1972 Oct;130(4):533–541. [PubMed] [Google Scholar]


