Abstract
Echinacea species are used for beneficial effects on immune function, and various prevalent phytochemicals have immunomodulatory effects. Using a commercial E. purpurea (L.) Moench product, we have evaluated the myelopoietic effect on bone marrow of rats treated with various extracts and correlated this with their chemical class composition. Granulocyte/macrophage-colony forming cells (GM-CFCs) from femurs of female Sprague-Dawley rats were assessed at 24 h after 7 daily oral treatments. A 75% ethanolic extract at 50 mg dried weight (derived from 227 mg aerial parts) per kg body weight increased GM-CFCs by 70% but at 100 mg/kg was without effect. Ethanolic extracts from aerial parts of E. angustifolia DC. var. angustifolia and E. purpurea from the US-DA North Central Regional Plant Introduction Station increased GM-CFCs by 3- and 2-fold, respectively, at 200 mg/kg (~ 1400 mg/kg plant material). Extract from another USDA E. angustifolia was inactive. Proton and APT NMR, MS, and TLC indicated alkylamides and caffeic-acid derivatives (CADs) present in ethanolic extracts of both the commercial and USDA-derived material. Cichoric and caftaric acids were prominent in both E. purpurea ethanolic extracts but absent in E. angustifolia. Aqueous extract of the commercial material exhibited polysaccharide and CAD signatures and was without effect on GM-CFCs. A methanol-CHCl3 fraction of commercial source, also inactive, was almost exclusively 1:4 nonanoic:decanoic acids, which were also abundant in commercial ethanolic extract but absent from USDA material. In conclusion, we have demonstrated an ethanol-extractable myelostimulatory activity in Echinacea aerial parts that, when obtained from commercial herbal supplements, may be antagonized by medium-chain fatty acids presumably derived from a non-plant additive.
Keywords: Echinacea angustifolia, Echinacea purpurea, Asteraceae, immune function, herbal supplement, medium-chain triglyceride additive
Introduction
Echinacea species (Asteraceae) are used as immunomodulants in folk medicine, and their prevalent constituent classes – alkylamides, caffeic-acid derivatives (CADs), and polysaccharides – have various effects on the immune system [1,2]. Numerous responses to fractions enriched in specific constituents have been described with mature effector cells. Innate immune-system phagocytes yield anti-inflammatory effects with alkylamides [3–7] and proinflammatory effects with polysac charide fractions [8,9]. Alkylamide inhibition of cyclooxygenase-2 (COX-2) provides another mechanism for suppression of inflammation [10]. Anti-inflammatory activity of Echinacea CADs has been reported [11]. A relatively unexplored mechanism for immune enhancement could entail stimulation of the bone marrow progenitor cells of mature effector cells. Evidence exists that NK prelymphocytes are increased in femurs of mice consuming a commercial Echinacea preparation [12, 13].
Medicinally important species, E. angustifolia, E. purpurea, and E. pallida (Nutt.) Nutt., differ qualitatively and quantitatively in their phytoconstitu ents [1, 14,15]. Differential immune effects for the different species have been correlated with content of their major constituent classes and principal compounds. Bioactivity-directed fractionations coupled with chemical-constituent profiling have led to identification of molecular targets for specific compounds, such as the cannabinoid type-2 receptor [3,16] and peroxisome proliferator-activated receptor-gamma [17,18] as targets of specific alkylamides. Evidence implicating these same receptors in hematopoiesis [19–22] supports the feasibility of Echinacea immunostimulation through modulation of myelopoiesis.
Inconsistent results have been obtained from clinical studies testing Echinacea for immune enhancement; perhaps the most notable one relates to the prevention and/or treatment of the common cold [23]. Relevant procedural differences may include the use of roots versus aerial parts from specific species or mixtures of Echinacea species. A dedicated harvest was processed specifically for one large trial [24], while commercially marketed products were used in others [25]. Some of the latter occasionally contain non-plant additives, such as medium-chain triglycerides (MCT) [26]. Although the composition was analytically verified in most studies, active principles mediating a given outcome are unknown and not necessarily the constituents used for validation or standardization.
This study assessed Echinacea effects on myelomonocytic precursor cells in assays for granulocyte/macrophage-colony forming cells (GM-CFCs). We evaluated activity of ethanolic and aqueous extracts from a commercial preparation of dried aerial parts of E. purpurea on GM-CFCs from bone marrow of treated rats. Active ethanolic extract was compared to those from accessions of E. angustifolia and E. purpurea harvested from the USDA North Central Regional Plant Introduction Station to insure that bioactivity was due to plant-derived constituent(s). We used 1H HMR for source validation since this technique has been used similarly for Echinacea fingerprinting [27,28]. APT NMR and TLC analyses were used to compare principal constituent classes and relate to GM-CFC induction across the various source materials.
Materials and Methods
Plant material
Commercial Echinacea was obtained in capsules (Natural Whole Herb) manufactured by Idea Sphere, Inc. (lots 207079164 and 207438435). Product was labeled as Echinacea purpurea (aerial part) with gelatin, purified water, and medium-chain triglycerides (MCT). Maximum intake is suggested at 3.42 g/d (~ 50 mg/ kg/d for 70 kg body wt). Aerial parts of accessions Echinacea angustifolia DC. var. angustifolia (PI 649026, Minnesota, and PI 649029, North Dakota) and Echinacea purpurea (PI 649040, Alabama), representing source-identified, wild populations, were obtained from the U.S. Department of Agriculture, North Central Regional Plant Introduction Station, Ames, IA. Plants were harvested July 2007, and dried aerial parts were stored dessicated at − 20 °C until use. Voucher specimens are deposited at ISC as J. McCoy s. n., 26 Jun 2007 for E. angustifolia PI 649026 and PI 649029 and J. McCoy s. n., 7 Jul 2007 for E. purpurea PI 649040.
Echinacea extraction
Contents of commercial Echinacea capsules were emptied, and half was macerated with 75% ethanol (0.05 g dry wt/mL; Pharmco-AAPER) at room temperature overnight and then again for 6 h. Extracts were combined, evaporated in a rotary evaporator at 40°C and then lyophilized to give a semisolid residue of 22% yield (8.7 g/39 g of original powder). The second half was steeped in distilled water at room temperature and aqueous phase was strained from solids, washed with 10% (v/v) MeOH (99.9%; Pharmco-AAPER) in CHCl3 (99.8%; Mallinckrodt), dried under vacuum and lyophilized to result in a solid of 30% yield. The MeOH-CHCl3 (1:9, v/v) wash of the aqueous phase was retained, evaporated and lyophilized to yield a pale green liquid (2.6% yield; hereafter referred to as MeOH-CHCl3 wash). Dried aerial parts of E. angustifolia and E. purpurea accessions were pulverized in a blender and extracted with 75% ethanol as described above. Yields of ethanolic extracts from E. angustifolia PI 649026 and PI 649029 and E. purpurea PI 649040 were 12.7, 11.9, and 15.1% by weight, respectively. Ethanol extracts were analyzed for endotoxin using Limulus amebocyte lysate with diazo coupling employing a kit from Associates of Cape Cod, Inc. LPS content was 13 EU/mg, 4 EU/mg, 4 EU/mg, and 9 EU/mg for dried ethanolic extracts from commercial E. purpurea, PI 649040, PI 649026, and PI649029, respectively. Similar values for dried plant material yielded extracts that were inactive in a reporter assay for LPS activation of NF-kB [29]. Specific activity of the kit standard (9 EU/ng) predicts that the highest amount dosed was 0.3µg/kg, which is 105-fold less than the rat oral LD50 for endotoxin.
NMR studies
NMR spectra were acquired using a JEOL Eclipse NMR spectrometer operating at 400 MHz for 1H and 100 MHz for 13C equipped with a 5-mm gradient proton/multifrequency probe with 2H lock and z-gradient. Probe temperature was maintained at 25°C. APT (attached proton test) spectra were acquired using τ = 5 ms and display of quaternary and methylene carbons up and methyl and methine carbons down. Chemical shifts are relative to internal reference tetramethylsilane (TMS, δ = 0.00). Ethanolic extracts were dissolved in 700 µL DMSO (d6) (99.9%; Cambridge Isotope Laboratories). Immediately before analysis, MeOH-CHCl3 wash was dissolved in CDCl3 and aqueous extract in D2O (both solvents 99.8%; Spectrum).
High-performance thin-layer chromatography
HPTLC of ethanolic fractions and MeOH-CHCl3 wash was performed using polar and apolar mobile phases to resolve CADs and alkylamides, respectively [30]. Dried ethanol extract was sonicated with MeOH (0.1 g/mL) for 5 min, then filtered through a 0.2-µm syringe filter. Samples (5 µL) were chromatographed on silica gel 60 F254 plates (10×20 cm, 0.2 mm; E. Merck) with EtOAc-acetone-formic acid-H2O (15:9:1:1) and stained with diphenylborinic acid aminoethylester (Sigma-Aldrich). Caftaric and cichoric acids and echinacoside standards (10 mg/mL; Chroma-Dex) were cochromatographed. Samples and standard β-sitosterol (10 mg/mL; ChromaDex) were chromatographed using toluene-EtOAc-cyclohexane-formic acid (24:6:3:0.9) mobile phase and visualized with p-anisaldehyde-sulfuric acid (Sigma-Aldrich). HPTLC with apolar development was also used for preparative isolation of bands A and B (Fig. 5, Rf = 0.26 and 0.36, resp.) of E. angustifolia PI 649029 for MS analysis.
Fig. 5.
HPTLC of 75 % ethanolic extracts developed with an apolar mobile phase. Extracts of E. angustifolia 649029 and 649026, E. purpurea 649040 (lanes 1,2, and 3, resp.), and 2 lots of commercial E. purpurea were chromatographed (lanes 4, 5). MeOH-CH3Cl wash is in lane 6. Chromatograms were developed with toluene-EtOAc-cyclohexane-formic acid (24:6:3:0.9). β-sitosterol (band E, Rf 0.52) and C9-C10 fatty acids (band H) are indicated by arrows and arrowheads, respectively. p-Anisaldehyde-sulfuric acid was used for visualization.
MS analysis
Mass spectroscopy was conducted using a 3200 Q-trap LC/MS/ MS system (Applied Biosystems). Software used for controlling this equipment, acquiring, and processing data was Analyst version 1.4.1 software (MDS Sciex). Analytes were ionized using electro-spray ionization (ESI) interface operated in the positive mode for alkylamide-enriched samples and negative mode for fatty acids. Analyses were conducted using Q1 scans with direct injection of samples in 0.05% formic acid in MeOH into the MS.
Animal husbandry and treatment
Female Sprague-Dawley (SD) rats (175–240 g) were from the breeding colony maintained at the University of Louisiana at Monroe (ULM). Breeders were from Harlan-Sprague Dawley. Rats had unlimited access to pelleted rodent chow (no. 7001; Harlan/ Teklad) and tap water. Rats were housed under controlled temperature and humidity and a 12-h light/dark cycle. All animal husbandry and handling conditions were maintained in accordance with the 1996 Guide for Use and Care of Animals of the National Research Council. Protocols were preapproved by the ULM Institutional Animal Care and Use Committee.
Rats were weighed and randomly assigned to treatment. Treatments for commercial preparations were vehicle (5% DMSO in corn oil), 75% ethanolic (50 and 100 mg of dry weight/kg/d) and aqueous (69 mg of dry wt/kg/d) extracts, and MeOH-CHCl3 wash (5.9 mg of liquid/kg/d). Dosages of 50, 69, and 5.9 mg/kg of ethanolic, aqueous, and MeOH-CHCl3 fractions, respectively, provided equivalence to 230 mg of encapsulated starting material/kg. Ethanolic extracts of USDA accessions were administered at 50, 100, and 200 mg dry weight/kg/d. All groups of rats (n = 4–6) were treated once daily for 7 days by oral gavage (10 mL/kg). For commercial ethanolic extract, we tested 2 different lots (#207079164 and 207438435). Twenty-four h or 14 d after the last dose, animals were weighed, then euthanized under CO2 anesthesia, and femurs were processed for bone marrow cell isolation.
Bone marrow cell isolation
Femurs were dissected from the carcass, cleaned of tissue and removed of ends. Each marrow cavity was flushed with an 18-g needle with 3 mL of filter (0.2 µm)-sterilized Iscove’s modified Dulbecco medium (IMDM) containing 0.2% bovine serum albumin and 1% antibiotic/antimycotic (Gibco/BRL). Femurs were inverted and flushed again with the same 3 mL medium. Hereafter, sterile tissue culture procedures were used. A single-cell suspension was produced by trituration, filtration through nylon mesh, and centrifugation (250 × g, 10 min). Cells were resuspended in 3 mL medium, counted with a hemocytometer and processed for assay of myeloid lineage colony forming units.
Myeloid lineage colony formation assays (GM-CFCs)
Mononuclear bone marrow cells were isolated by density-gradient centrifugation (400 × g, 30 min) over Histopaque-1077 (1.077 g/mL; Sigma-Aldrich). Cells were collected at the interface, diluted with 10 mL medium, pelleted by centrifugation (400 × g, 10 min), resuspended in 0.25 mL of medium and counted. The GM-CFC assay was performed with a HALO kit (cat # K1-GM2; HemoGenix). In brief, 20000 mononuclear cells in 15 µL IMDM medium were mixed with 60 µL methyl cellulose, 60 µL fetal calf serum, and 15 µL growth factor mix (20 ng/mL GM-CSF, 10 ng/mL IL-3, and 50 ng/mL SCF, all rat recombinant) and plated per well in 96-well plates. After 5 days at 37°C in a humidified 5% CO2 incubator, cellular ATP was measured with luciferase and luciferin substrate and calibrated against a standard curve generated on the same day. Luminescence was read with a Cameleon II plate reader (Hidex Ltd.).
Statistical Analysis
Effects of Echinacea fractions on myelostimulation were determined by one-way ANOVA with Dunnett’s post hoc comparisons of treatment means against vehicle control done with JMP 4.0 (SAS Institute, Inc.). Two-way ANOVA with Tukey-Kramer post hoc tests were performed with SAS, v. 9.1 to determine statistical significance of source and dose on GM-CFCs, bone marrow cellularity, and body weight gain.
Supporting information
Spectra of commercial E. purpurea aqueous extract and effects of 75% ethanolic extract as well as aqueous extract and its MeOH-CHCl3 wash from commercial E. purpurea on femur GM-CFCs are available as Supporting Information.
Results and Discussion
Studies presented here describe a myelostimulatory activity of Echinacea aerial plant parts that is evident when extracted into 75% ethanol. This activity, measured as the number of myeloid progenitor cells (GM-CFCs) of bone marrow from treated rats, was originally observed in the extract of a commercial source of E. purpurea aerial parts formulated with other labeled ingredients. Myelostimulatory activity was also present in ethanolic extracts of accessions of Echinacea aerial parts from the USDA North Central Regional Plant Introduction Station and was present in both E. angustifolia and E. purpurea. Commercial extract was more potent, increasing GM-CFCs at 50 mg/kg/d, but limited, as a higher dose reversed to baseline. USDA E. angustifolia PI 649026 activity threshold was 100 mg/kg/d and yielded a 3-fold increase in GM-CFCs at the highest dose, 200 mg/kg/d, while E. purpurea PI 649040 extract plateaued at a 2-fold increase. Echinacea angustifolia PI 649029 was inactive. These results suggest that the myelostimulatory constituent is not species-specific and varies within species as evidenced by the very different effects of two E. angustifolia accessions. Active constituent is shared between authentic USDA-derived material and the commercial preparation; however, activity of the latter may be limited by a non-plant substance interfering at a higher dose.
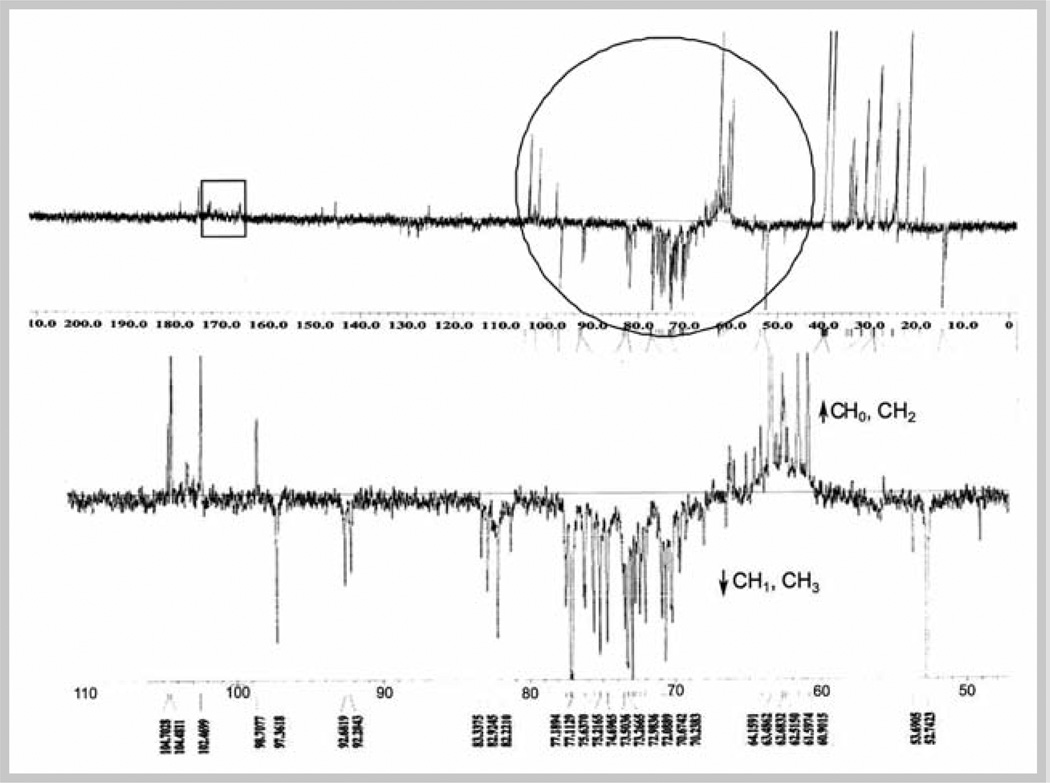
Qualitative assessment of alkylamides in commercial Echinacea ethanolic extract was aided by 13C NMR using APT as shown in Fig. 1. Signals in the 60–80 ppm region indicate the presence of acetylenic quaternary and methine carbons of Echinacea alkylamides. It should be noted that use of τ = 5 ms displays quaternary and methylene carbons up, and methine, including acetylenes, and methyl carbons down. The expected less intense downfield quaternary signals from the amide carbons at 165–176 ppm relative to the acetylenic carbons are due to decreased sensitivity because of their longer relaxation time with respect to the 1 sec delay time used in this experiment. The proton NMR spectrum of ethanolic extract is shown in Fig. 2A. Signals within δ = 3.00–5.00 indicate nitrogenated, acetylenic, oxygenated, and some olefinic protons. Signals within δ = 6.00–7.50 represent some olefinic and the aromatic protons of phenylpropanoids, including CADs.
Fig. 1.
APT spectrum of 75% ethanolic extract of aerial parts of a commercial E. purpurea in DMSO-d6 at 100 MHz. The region circled in the top spectra is expanded below. The region within the square corresponds to amide carbon. DMSO signal is evident at 39.5 ppm.
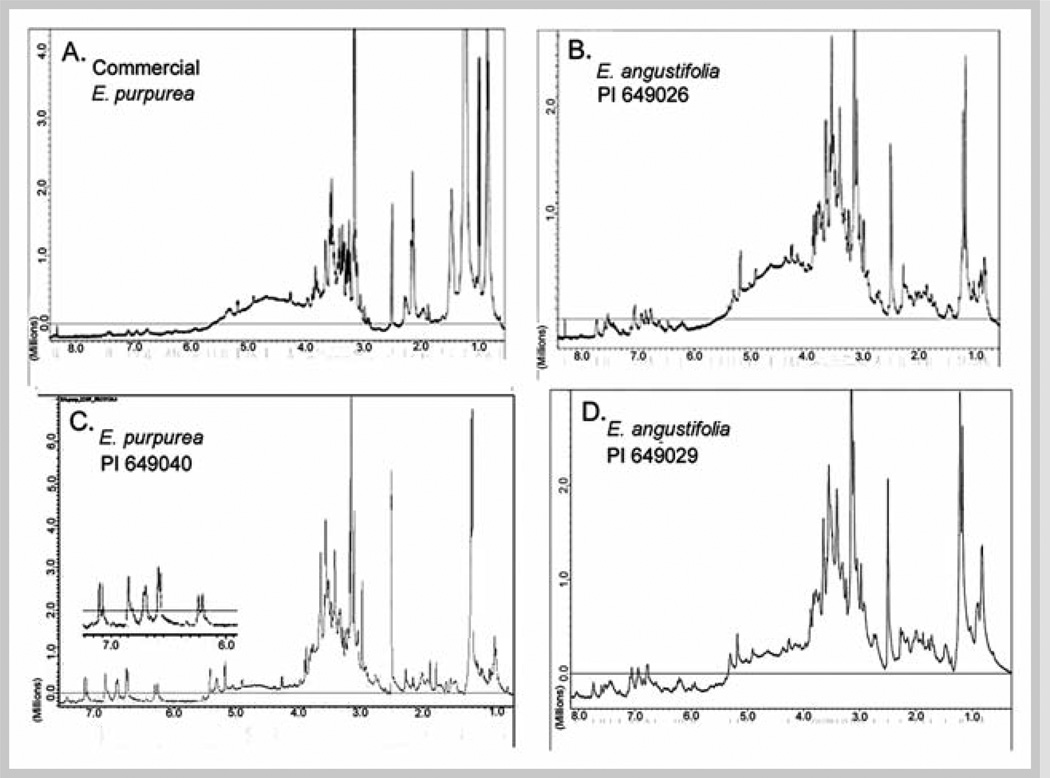
Fig. 2.
1H NMR spectra (400 MHz, in DMSO-d6) of 75% ethanol extracts of aerial parts of a commercial E. purpurea (A), two accessions of E. angustifolia (B and D) and one accession of E. purpurea (C) obtained from the USDA North Central Regional Plant Introduction Station. DMSO signals are evident at 2.50 ppm. Insert in C shows expanded δ = 6.0– 7.30 ppm region to illustrate spin-spin coupling.
The 1H NMR spectrum of the aqueous extract (Fig. 1S; see Supporting Information) exhibits oxygenated polysaccharide protons at δ = 3.00–4.50 and δ = 5.00–5.50, peaks typical of anomeric sugar protons [28]. Oxygenated polysaccharide sugar hydroxy-methylene and methine carbons at 60–75 ppm dominate the APT NMR (Fig. 2S). The MeOH-CHCl3 wash was nearly pure fatty acid as evident from its APT NMR spectrum (Fig. 3S) showing 11 carbons (δ 180.0, qC; 34.2, CH2; 31.7, CH2; 29.5–29.0, 5 CH2s; 24.8, CH2; 22.7, CH2; and 14.1, CH3). 1H NMR was consistent with decanoic acid (δ 0.84, 3H, t, J = 7.3 Hz; 1.25, 12H, m; 1.58, 2H, m; 2.29, 2H, t, J = 7.3 Hz; 8.94, 1H, brs). MS analysis showed a 4:1 mixture of decanoic acid [170.96, (M – H)−, C10H19O2] and nonanoic acid [156.97, (M – H)−, C9H17O2].
NMR profiles of the ethanolic extract of the commercial preparation were compared to those from aerial parts of defined accessions obtained from the USDA to validate the source of the commercial material. 1H NMR spectra for all USDA material contain aromatic signals around 7.00 ppm, characteristic for caffeic acid and other phenylpropanoid derivatives (Fig. 2 B – D). For PI 649040, the spin-spin coupling of the E-oriented olefinic α,β-unsaturated protons (J = 16 Hz) was observed. The δ= 3.00–5.00 region indicates a complex pattern of several nitrogenated, oxygenated, olefinic, and acetylenic protons. APT NMR spectra of the USDA accessions (Fig. 3 A – C) show similar upward-oriented quaternary and downward-oriented methine acetylenic carbons resonating at 60–80 ppm for all three Echinacea accessions. Spectra of commercial ethanolic extract (Figs. 1 and 2A) were qualitatively similar to those from USDA accessions, indicating that alkylamides and CADs were major chemical classes of extracts from both commercial and authentic plant sources. However, the commercial extract exhibited more upfield signals characteristic of alkane protons (δ = 0.50–1.50) and methylene carbons (20–35 ppm).
Fig. 3.
APT spectra of 75% ethanolic extracts (100 Hz in DMSO-d6) of aerial parts of one accession of E. purpurea (A) and two accessions of E. angustifolia (B and C) obtained from the USDA North Central Regional Plant Introduction Station. DMSO signals are evident at 39.5 ppm.
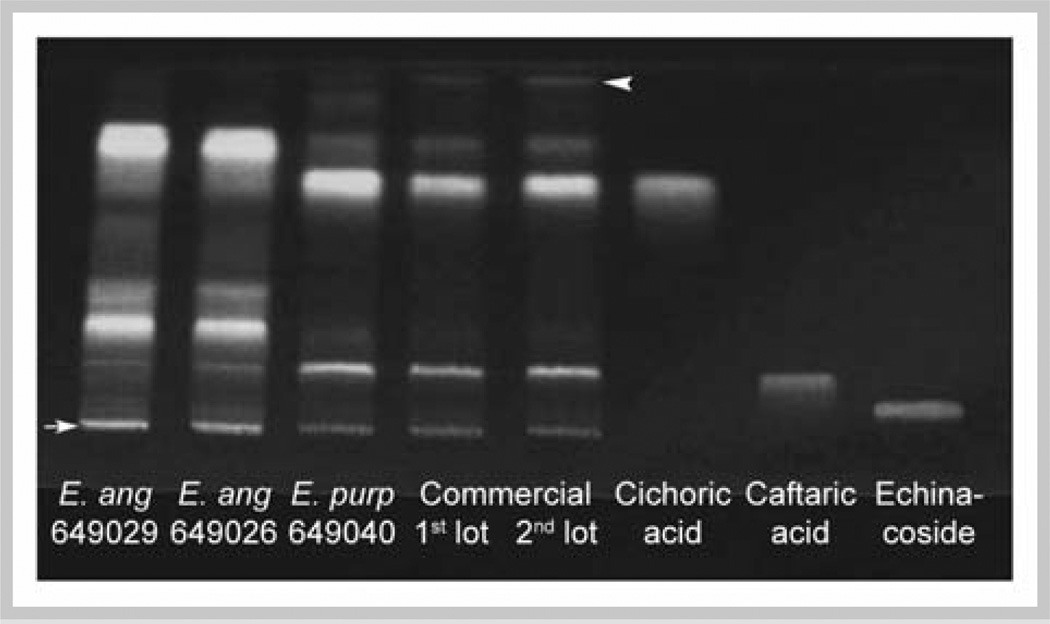
Shown in Figs. 4 and 5 are HPTLC patterns of 75% ethanolic extracts using polar and apolar mobile systems. Phenolic acid standards cichoric acid, caftaric acid, and echinacoside (Fig. 4) were cochromatographed with polar development. Caftaric acid (Rf = 0.17) migrated with a major band of extracts from E. purpurea and a faint band of E. angustifolia. Cichoric acid (Rf = 0.71) was predominant in both lots of commercial E. purpurea and E. purpurea PI 649040. No distinct bands in the ethanolic extracts of aerial parts of any Echinacea sources could be assigned to echinacoside (Rf = 0.08). Unidentified phenolic components with Rf = 0.82 and 0.27 were more abundant in both E. angustifolia sources than in E. purpurea extracts, while another with Rf = 0.38 was unique to E. angustifolia. Greater abundance of cichoric and caftaric acids in E. purpurea and of components with Rf 0.82, 0.38, and 0.27 in E. angustifolia authenticated the labeling of the commercial material as E. purpurea.
Fig. 4.
HPTLC of 75% ethanolic extracts developed with a polar mobile phase. Extracts from USDA E. angustifolia accessions PI 649029 and PI 649026, E. purpurea PI 649040, and 2 lots of commercial E. purpurea were chromatographed. Standards are cichoric acid, caftaric acid, and echinaco-side. Chromatograms were developed with EtOAc-acetone-formic acid-water (15:9:1:1). Origin and mobile phase front are indicated by an arrow and arrowhead, respectively. Diphenylborinic acid aminoethylester spray reagent and UV light, λ366 nm, were used for visualization.
TLC profiles of ethanolic extracts with apolar solvents exhibited band E (Fig. 5, Rf = 0.52) common to all sources that comigrated with β-sitosterol. Bands D, F, and G with Rf = 0.48, 0.65, and 0.71, respectively, were evident in extracts from the USDA accessions (lanes1–3). Band C (Rf = 0.41) was a minor constituent of commercial preparations. Band A (Rf = 0.23) was unique to E. angustifolia, being most evident in PI 649029. Band B (Rf = 0.33) was observed in E. angustifolia and commercial E. purpurea. Isolation of bands A and B by preparative HPTLC and determination of parent-ion mass by MS gave [M + H] + values of 230.2 and 248.2, respectively. Mass of band A constituent and species-specificity suggest that it is undeca-2Z,4E–diene-8,10-diynoic acid isobutyl amide, which has been previously noted in E. angustifolia, but not E. purpurea, flowers [31]. Mass of 248.2 and Rf relative to β-sitosterol are consistent with identification of band B as 2E,4E,8Z,10E/Z-dodecatetraenoic acid isobutyl amides [32,33]. Band H (Rf = 0.74) was present only in commercial extracts and comigrated with the major band of the MeOH-CHCl3 wash, which had been shown by NMR and MS to be nonanoic and decanoic acids. Detection of these medium-chain fatty acids uniquely in the commercial extract was consistent with their NMR fingerprints suggesting saturated alkane constituents derived from non-plant material (Figs. 1 and 2A). We suspect that medium-chain triglyceride additive to the commercial product, which is marketed as a food additive with saturated C9 and C10 esters [26], hydrolyzed during extraction and resulting fatty acids partitioned in the 75% ethanol phase.
Our initial objective was to determine whether Echinacea pretreatment would counter toxicity of MNX, a nitro-reduced product of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), whose myelosuppression we had previously determined required 14 d for expression [34]. Hence, our early studies assessed the effects of various Echinacea fractions 14 d after the last of 7 daily doses. It was noted in these studies that pretreatment of ethanolic extract of commercial Echinacea (50 mg dried extract/kg/d), independent of administration of myelotoxicant, increased GM-CFCs by 50% after the 14 d lag (Fig. 4S). We also tested for myelostimulatory activity of aqueous extract of the commercial material and MeOH-CHCl3 wash of the aqueous extract using the 14-d lag protocol. Both of these fractions, at doses derived from the amount of starting material that yielded 50 mg/kg dose of active ethanolic extract, were without effect (Fig. 4S).
Myelostimulation by ethanolic extract of commercial Echinacea evaluated 24 h after 7 daily doses (50 mg/kg/d) was increased to 70% over vehicle (Fig. 6A). However, a higher dose (100 mg/kg) of this same extract yielded GM-CFCs similar to vehicle control. For all USDA accessions at 50 mg/kg/d, GM-CFCs were not increased to statistical significance. However, higher doses of PI 649026 and PI 649040 caused a significant increase, with E. angustifolia PI 649026 exhibiting the greatest activity at 3 times GM-CFCs of vehicle at 200 mg/kg/d. Echinacea purpurea PI 649040 also was active and plateaued at twice that of the vehicle at 200 mg/kg (Fig. 6A).
Fig. 6.
Effects of 75% ethanolic extract of commercial E. purpurea and cultivars of E. angustifolia 649029 and 649026 and E. purpurea 649040 on femur GM-CFCs (A) and total bone marrow cells (B) of rats treated with 50, 100, and 200 mg/kg/d. Rats received 7 daily doses of extract, and bone marrow cells were processed 24 h after the last dose. Means ± SEM for n = 4 rats are shown. Means that differed from vehicle control (0 mg/kg) by a statistically significant amount are indicated by * p < 0.05 and ** p < 0.01.
The ethanolic (50 mg/kg) and aqueous extracts, and MeOH-CHCl3 wash of commercial E. purpurea were without any effect on total bone marrow cellularity. However, a higher dose (100 mg/kg/d) of commercial ethanolic extract significantly decreased the bone marrow cell number (Fig. 6B). In contrast, ethanolic extracts of USDA accessions did not affect bone marrow cellularity at any dose, including a high dose (200 mg/kg/d) (Fig. 6B). None of the extracts at any dose affected body weight gain over the 7-day treatment (mean ± SEM = 6.4 ± 1.4 g, n = 54). One rat of four treated with 100 mg/kg/d of ethanolic extract of commercial Echinacea died on trial, while no lethality occurred with any dose of extracts from the USDA accessions. These observations support the notion that toxicity of the commercial extract at a higher dose may have limited its myelostimulatory activity.
Results from this study are collectively summarized in Table 1. In summary, our analytical work identified alkylamides and CADs in Echinacea myelostimulatory ethanolic extracts, but presence of the same in an inactive E. angustifolia extract precluded assignment of activity to these chemical classes in general. No identified entity correlated with myelostimulatory activity across the various preparations. Hence, our study has identified a biological activity that is consistent with Echinacea immunostimulation, but more detailed chemical analysis, fractionation, and optimization of efficacy are required to identify responsible constituent(s).
Table 1.
Analytical chemistry results and relative myeloproliferative activity of fractions from various Echinacea sources.
| Source | Fraction | 1H NMR | APT | Chemistry HPTLC Polar |
Apolar | Bioactivitya |
|---|---|---|---|---|---|---|
| Commercial E. purpurea |
75% Ethanolic | CADs, Alkanes | Alkylamides, Alkanes | Cichoric, Caftaric acids |
β-Sitosterol, Decanoic, Nonanoic acids, C12:4N iBub |
1 |
|
E. purpurea PI 649040 |
75% Ethanolic | CADs | Alkylamides | Cichoric, Caftaric acids |
β-Sitosterol | 2 |
|
E. angustifolia PI 649026 |
75% Ethanolic | CADs | Alkylamides | Rf 0.82, 0.38 and 0.27c |
β-Sitosterol, C12:4N iBu C11:2:2N iBud |
3 |
|
E. angustifolia PI 649029 |
75% Ethanolic | CADs | Alkylamides | Rf 0.82, 0.38 and 0.27 |
β-Sitosterol, C12:4N iBu C11:2:2N iBu |
0 |
| Commercial E. purpurea |
MeOH-CHCl3 | Decanoic acid | C11, Fatty acid | nd | β-Sitosterol, Decanoic, Nonanoic acids (4:1)e |
0 |
| Commercial E. purpurea |
Aqueous | PS, CADs | PS | nd | nd | 0 |
Myeloproliferative activity ranked from lowest (1) to highest (3) or as absent (0)
C12:4N iBu = 2E,4E,8Z,10E/Z-dodecatetraenoic acid isobutyl amides
Mobility of bands of unknown identity
C11:2:2N iBu = undeca-2Z,4E–diene-8,10-diynoic acid isobutyl amide
Identities and ratio confirmed by MS
Supplementary Material
Acknowledgements
This research was supported by the U.S. Department of Defense through Congressionally Directed Medical Research Program grant W81XWH0-5-1-0537 (SAM) and by award number P50AT004155 from the National Center for Complementary & Alternative Medicine (MPW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Defense, National Center for Complementary & Alternative Medicine, or the National Institutes of Health. Mention of commercial brand names does not constitute an endorsement of any product by the U.S. Department of Agriculture or cooperating agencies.
Footnotes
Supporting information available online at http://www.thieme-connect.de/ejournals/toc/plantamedica
Conflict of Interest
There are no conflicts of interest to disclose.
References
- 1.Wills RBH, Bone K, Morgan M. Herbal products:active constituents, modes of action and quality control. Nutrition Res Rev. 2000;13:47–77. doi: 10.1079/095442200108729007. [DOI] [PubMed] [Google Scholar]
- 2.Barnes J, Anderson LA, Gibbons S, Phillipson JD. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench):a review of their chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2005;57:929–954. doi: 10.1211/0022357056127. [DOI] [PubMed] [Google Scholar]
- 3.Gertsch J, Schoop R, Kuenzle U, Suter A. Echinacea alkylamides modulate TNF-alpha gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Lett. 2004;577:563–569. doi: 10.1016/j.febslet.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Fu T, Tao T, Yang J, Chang Y, Wang M, Kim L, Qu L, Cassady J, Scalzo R, Wang X. Macrophage activating effects of new alkamides from the roots of Echinacea species. J Nat Prod. 2005;68:773–776. doi: 10.1021/np040245f. [DOI] [PubMed] [Google Scholar]
- 5.Matthias A, Banbury L, Stevenson LM, Bone KM, Leach DN, Lehmann RP. Alkylamides from Echinacea modulate induced immune responses in macrophages. Immunol Invest. 2007;36:117–130. doi: 10.1080/08820130600745786. [DOI] [PubMed] [Google Scholar]
- 6.Birt DF, Widrlechner MP, Lalone CA, Wu L, Bae J, Solco AK, Kraus GA, Murphy PA, Wurtele ES, Leng Q, Hebert SC, Maury WJ, Price JP. Echinacea in infection. Am J Clin Nutr. 2008;87:488S–492S. doi: 10.1093/ajcn/87.2.488S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woelkart K, Bauer R. The role of alkamides as an active principle of Echinacea. Planta Med. 2007;73:615–623. doi: 10.1055/s-2007-981531. [DOI] [PubMed] [Google Scholar]
- 8.Luettig B, Steinmuller C, Gifford GE, Wagner H, Lohmann-Matthes ML. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. J Natl Cancer Inst. 1989;81:669–675. doi: 10.1093/jnci/81.9.669. [DOI] [PubMed] [Google Scholar]
- 9.Brovelli EA, Rua D, Roh-Schmidt H, Chandra A, Lamont E, Noratto GD. Human gene expression as a tool to determine horticultural maturity in a bioactive plant (Echinacea purpurea L. Moench) J Agric Food Chem. 2005;53:8156–8161. doi: 10.1021/jf0505372. [DOI] [PubMed] [Google Scholar]
- 10.Hinz B, Woelkart K, Bauer R. Alkamides from Echinacea inhibit cycloox-ygenase-2 activity in human neuroglioma cells. Biochem Biophys Res Commun. 2007;360:441–446. doi: 10.1016/j.bbrc.2007.06.073. [DOI] [PubMed] [Google Scholar]
- 11.Zhai Z, Solco A, Wu L, Wurtele ES, Kohut ML, Murphy PA, Cunnick JE. Echinacea increases arginase activity and has anti-inflammatory properties in RAW 264.7 macrophage cells, indicative of alternative macrophage activation. J Ethnopharmacol. 2009;122:76–85. doi: 10.1016/j.jep.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delorme D, Miller SC. Dietary consumption of Echinacea by mice afflicted with autoimmune (type I) diabetes:effect of consuming the herb on hemopoietic and immune cell dynamics. Autoimmunity. 2005;38:453–461. doi: 10.1080/08916930500221761. [DOI] [PubMed] [Google Scholar]
- 13.Miller SC, Sun LZ-Y. Effect of dietary administration of Echinacea on immune and hemopoietic cell lineages in murine spleen and bone marrow. FASEB J. 1998;12:A874. [Google Scholar]
- 14.Binns SE, Livesey JF, Arnason JT, Baum BR. Phytochemical variation in Echinacea from roots and flowerheads of wild and cultivated populations. J Agric Food Chem. 2002;50:3673–3687. doi: 10.1021/jf011439t. [DOI] [PubMed] [Google Scholar]
- 15.Wu L, Dixon PM, Nikolau BJ, Kraus GA, Widrlechner MP, Wurtele ES. Metabolic profiling of Echinacea genotypes and a test of alternative taxonomic treatments. Planta Med. 2009;75:178–183. doi: 10.1055/s-0028-1112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raduner S, Majewska A, Chen JZ, Xie XQ, Hamon J, Faller B, Altmann KH, Gertsch J. Alkylamides from Echinacea are a new class of cannabinomimetics Cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects. J Biol Chem. 2006;281:14192–14206. doi: 10.1074/jbc.M601074200. [DOI] [PubMed] [Google Scholar]
- 17.Christensen KB, Petersen RK, Petersen S, Kristiansen K, Christensen LP. Activation of PPARgamma by metabolites from the flowers of purple coneflower (Echinacea purpurea) J Nat Prod. 2009;72:933–937. doi: 10.1021/np900003a. [DOI] [PubMed] [Google Scholar]
- 18.Spelman K, Iiams-Hauser K, Cech NB, Taylor EW, Smirnoff N, Wenner CA. Role for PPARgamma in IL-2 inhibition in T cells by Echinacea-derived undeca-2E-ene-8,10-diynoic acid isobutylamide. Int Immunopharmacol. 2009;9:1260–1264. doi: 10.1016/j.intimp.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Jiang S, Fu Y, Williams J, Wood J, Pandarinathan L, Avraham S, Makriyannis A, Avraham HK. Expression and function of cannabinoid receptors CB1 and CB2 and their cognate cannabinoid ligands in murine embryonic stem cells. PLoS ONE. 2007;2:e641. doi: 10.1371/journal.pone.0000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valk P, Verbakel S, Vankan Y, Hol S, Mancham S, Ploemacher R, Mayen A, Lowenberg B, Delwel R. Anandamide, a natural ligand for the peripheral cannabinoid receptor is a novel synergistic growth factor for hematopoietic cells. Blood. 1997;90:1448–1457. [PubMed] [Google Scholar]
- 21.Patinkin D, Milman G, Breuer A, Fride E, Mechoulam R. Endocannabinoids as positive or negative factors in hematopoietic cell migration and differentiation. Eur J Pharmacol. 2008;595:1–6. doi: 10.1016/j.ejphar.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Prost S, Le Dantec M, Auge S, Le Grand R, Derdouch S, Auregan G, Deglon N, Relouzat F, Aubertin AM, Maillere B, Dusanter-Fourt I, Kirszenbaum M. Human and simian immunodeficiency viruses deregulate early hematopoiesis through a Nef/PPARgamma/STAT5 signaling pathway in macaques. J Clin Invest. 2008;118:1765–1775. doi: 10.1172/JCI33037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woelkart K, Linde K, Bauer R. Echinacea for preventing and treating the common cold. Planta Med. 2008;74:633–637. doi: 10.1055/s-2007-993766. [DOI] [PubMed] [Google Scholar]
- 24.Turner RB, Bauer R, Woelkart K, Hulsey TC, Gangemi JD. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N Engl J Med. 2005;353:341–348. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- 25.Barrett B, Brown R, Rakel D, Mundt M, Bone K, Barlow S, Ewers T. Echinacea for treating the common cold. Ann Intern Med. 2010;153:769–777. doi: 10.7326/0003-4819-153-12-201012210-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heydinger JA. Physical properties of medium-chain triglycerides and application in food. In: Widlak N, editor. Physical properties of fats, oils, and emulsifiers. Champaign, Il: AOCS Press; 1999. pp. 220–225. [Google Scholar]
- 27.Frédérich M, Jansen C, de Tullio P, Tits M, Demoulin V, Angenot L. Metabolomic analysis of Echinacea spp. by 1H nuclear magnetic resonance spectrometry and multivariate data analysis technique. Phytochem Anal. 2010;21:61–65. doi: 10.1002/pca.1156. [DOI] [PubMed] [Google Scholar]
- 28.Politi M, Zloh M, Pintado M, Castro P, Heinrich M, Prieto J. Direct metabolic fingerprinting of commercial herbal tinctures by nuclear magnetic resonance spectroscopy and mass spectrometry. Phytochem Anal. 2009;20:328–334. doi: 10.1002/pca.1131. [DOI] [PubMed] [Google Scholar]
- 29.Tamta H, Pugh ND, Balachandran P, Moraes R, Sumiyanto J, Pasco DS. Variability in in vitro macrophage activation by commercially diverse bulk Echinacea plant material is predominantly due to bacterial lipoproteins and lipopolysaccharides. J Agric Food Chem. 2008;56:10552–10556. doi: 10.1021/jf8023722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reich E, Schibli A, DeBatt A. Validation of high-performance thin-layer chromatographic methods for the identification of botanicals in a cGMP environment. J AOAC Intl. 2008;91:119S–150S. [PMC free article] [PubMed] [Google Scholar]
- 31.Kraus GA, Bae J, Wu L, Wurtele E. Synthesis and natural distribution of anti-inflammatory alkamides from Echinacea . Molecules. 2006;11:758–767. doi: 10.3390/11100758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CAMAG. Application notes F-24A and F-24B–HPTLC. [Accessed January 7, 2011];Identification of Echinacea-phenolics and alkylamides. Available at www.camag.com/downloads/protected/herbals/F-24a_echinacea_phenolics.pdfandF24B_echinacea_alkylamides.pdf.
- 33.Matovic N, Matthias A, Gertsch J, Raduner S, Bone KM, Lehmann RP, Devoss JJ. Stereoselective synthesis, natural occurrence and CB(2) receptor binding affinities of alkylamides from herbal medicines such as Echinacea sp. Org Biomol Chem. 2007;5:169–174. doi: 10.1039/b615487e. [DOI] [PubMed] [Google Scholar]
- 34.Kale VM, Aycock MM, Wilbanks MS, Perkins E, Inouye LS, Meyer SA. Hematotoxicity of munitions compound hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and environmental degradation product MNX. The Toxicologist. 2007;96:35. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.