Abstract
The HIV-1 protein Vpr enhances macrophage infection, triggers G2 cell cycle arrest, and targets cells for NK-cell killing. Vpr acts through the CRL4DCAF1 ubiquitin ligase complex to cause G2 arrest and trigger expression of NK ligands. Corresponding ubiquitination targets have not been identified. UNG2 and SMUG1 are the only known substrates for Vpr-directed depletion through CRL4DCAF1. Here we identify the endoribonuclease Dicer as a target of HIV-1 Vpr-directed proteasomal degradation through CRL4DCAF1. We show that HIV-1 Vpr inhibits short hairpin RNA function as expected upon reduction of Dicer levels. Dicer inhibits HIV-1 replication in T cells. We demonstrate that Dicer also restricts HIV-1 replication in human monocyte-derived macrophages (MDM) and that reducing Dicer expression in MDMs enhances HIV-1 infection in a Vpr-dependent manner. Our results support a model in which Vpr complexes with human Dicer to boost its interaction with the CRL4DCAF1 ubiquitin ligase complex and its subsequent degradation.
Keywords: Vpr, HIV-1, ubiquitin ligase, CRL4, Dicer, DCAF1, Cullin4, miRNA, suppressor of silencing (SRS)
Introduction
Plants and insects were first found to employ RNA silencing, a nucleic acid-dependent immune response, to combat viral infections. Subsequent work demonstrated that mammals also exploit RNA silencing as a part of their anti-viral armamentarium (Andersson et al., 2005; de Vries and Berkhout, 2008; Lecellier et al., 2005; Lu and Cullen, 2004; Wang et al., 2006). To achieve RNA silencing, double-stranded RNAs are processed in mammalian cells by the nuclear endoribonuclease Drosha and exported into the cytosol. There they are further processed by the endoribonuclease Dicer into shorter 21–23 nucleotide duplexes. These are loaded into the RNA-induced silencing complex (RISC) to confer specificity to complementary sequences within messenger RNA. Assembly with RISC results in either destruction or translational inhibition of the targeted mRNA.
RNA silencing could restrict virus replication at least three ways: (1) by direct cleavage of viral RNA which could both destroy the RNA and process it to specifically target other viral or cellular RNA, (2) by cellular miRNA-mediated suppression of transcript expression or interference with viral processes that employ RNA, or (3) by use of cellular miRNAs to thwart expression of cellular proteins required for viral replication. Since most viral infections do not succumb to these restrictions, many viruses may have evolved strategies to evade or defeat restrictions imposed through RNA silencing. Indeed, plant and animal viruses that infect invertebrates encode suppressors of silencing (SRS). The mechanisms that the SRS factors use to inhibit RNA silencing are not well understood. SRS activity is encoded by a variety of mammalian viruses and include Ebola virus VP35 protein (de Vries and Berkhout, 2008), Adenovirus VA RNA I and RNA II (Andersson et al., 2005; Lu and Cullen, 2004), primate foamy virus type 1 Tas protein (Lecellier et al., 2005) and hepatitis C core protein (Wang et al., 2006). This indicates that battles between miRNA restriction and viral SRSs are taking place in organisms ranging from plants to mammals.
HIV-1 was among the first mammalian viruses shown to possess SRS activity. Yeung and colleagues demonstrated that HIV-1 hinders miRNA expression in HeLa cells (Yeung et al., 2005). In the presence of HIV-1-specific siRNAs, HIV-1 adopted mutations to break the complementarity (Boden et al., 2003; Das et al., 2004; Jacque et al., 2002). Further, HIV-1 encodes a double-stranded RNA trans-activator response (TAR) loop that binds to, and may sequester TARBP, a Dicer co-factor that functions as part of the RISC complex (Bennasser et al., 2006). Other work attributes partial SRS activity to Tat (Bennasser et al., 2005; Haasnoot et al., 2007; Qian et al., 2009; Schnettler et al., 2009), however the results of another study conflict with these findings (Lin and Cullen, 2007). Finally, experimental evidence that miRNAs restrict HIV-1 in otherwise permissive cells suggests that this virus must encode SRS factors. This evidence demonstrates that cellular miRNAs target HIV-1 genes (Ahluwalia et al., 2008; Hariharan et al., 2005; Huang et al., 2007; Omoto et al., 2004; Yeung et al., 2009) or inhibit host factors that enable HIV replication (Sung and Rice, 2009; Triboulet et al., 2007). Further, HIV encodes dsRNA regions that could be targeted by the RNA silencing machinery directly like those found in primate foamy retrovirus and vesicular stomatitis virus (Lecellier et al., 2005). Regardless of how miRNAs restrict HIV-1, it is clear that HIV-1 has evolved to overcome miRNA restriction to allow effective replication.
Host cells maintain mechanisms aside from miRNA to inhibit HIV replication and HIV encodes several specialized proteins to counter them. Some viral defenses act through host cell ubiquitin ligases. Vif, for example, associates with an elonginBC-Cul5 ubiquitin ligase complex to trigger destruction of the host antiviral factor APOBEC3G (Conticello et al., 2003; Kobayashi et al., 2005; Marin et al., 2003; Mehle et al., 2004; Sheehy et al., 2003; Stopak et al., 2003; Yu et al., 2003). In the absence of APOBEC3G degradation, this cytidine deaminase is incorporated into viral particles and deaminates viral cytosines to form uracils during reverse transcription. APOBEC3G hinders reverse transcription, but the exact mechanism has not been determined (reviewed in (Wissing et al., 2010)). Vpu, another specialized HIV-1 protein, partners with the SCFβTrCP ubiquitin ligase complex to facilitate virus release by keeping the cellular protein tetherin/BST-2 from the cell surface (Goffinet et al., 2009; Gupta et al., 2009; Mangeat et al., 2009). HIV-2/SIV protein Vpx supports virus replication by depleting cellular SAMHD1 through the CRL4 ubiquitin ligase complex (Hrecka et al., 2011; Laguette et al., 2011). SAMHD1 a deoxynucleoside triphosphate triphosphohydrolase, acts to hinder retroviral infection by depleting dNTP stores in non-dividing cells (Goldstone et al., 2011; Hofmann et al., 2012). While HIV-1 Vpr does not deplete SAMHD1, it aids macrophage infection albeit less efficiently than Vpx (Balliet et al., 1994; Connor et al., 1995; Heinzinger et al., 1994). HIV-1 Vpr also acts through the CRL4DCAF1 ubiquitin ligase complex to trigger G2 cell cycle arrest, to deplete cellular UNG2, and to boost expression of NKG2D cell surface ligand on infected cells. Vpr-mediated enhancement of macrophage infectivity was originally attributed to Vpr-mediated transport of the viral pre-integration complex (PIC) into the nucleus of non-dividing cells (Nie et al., 1998; Popov et al., 1998; Subbramanian et al., 1998). Other findings however show that Vpr is not required for nuclear transport of the PIC (Riviere et al.; Yamashita and Emerman, 2004). Based on the association of Vpr with the CRL4 ubiquitin ligase complex we hypothesized that Vpr, like Vif, Vpu and Vpx commandeers a host ubiquitin ligase complex to relieve a host cell restriction to infection.
UNG2 and SMUG1 are the only substrates identified for HIV-1 Vpr-mediated protein degradation through the CRL4 ubiquitin ligase complex. Neither of these enzymes has been shown to impact Vpr-mediated G2 cell cycle arrest (Selig et al., 1997). The role of UNG2 in HIV infection remains unclear. Early work showed that Vpr brings UNG2 into HIV-1 virions (Chen et al., 2004; Mansky et al., 2000). More recent work showed that Vpr triggers degradation of UNG2 through the CRL4 complex to protect the viral genome against UNG2-mediated processing of APOBEC3G-mediated damage (Schrofelbauer et al., 2005). Fenard et al. however demonstrated that UNG2 suppresses transcription from the HIV-1 LTR (Fenard et al., 2009) while Langevin and colleagues showed that Vpr suppresses UNG2 gene transcription (Langevin et al., 2009). Further, UNG2, in association with integrase, was shown to be vital for HIV propagation (Priet et al., 2005). Interestingly Jones et al. linked the requirement for UNG2 to co-receptor usage (Jones et al., 2010) while other work showed that UNG2 has little or no effect on HIV replication (Kaiser and Emerman, 2006; Mbisa et al., 2007). In contrast, Weil et al. showed that UNG2 triggers degradation of uracil-containing HIV-1 cDNA and prevents its integration (Weil et al., 2013). Finally, Vpr-elicited expression of NK-cell ligands (Pham et al., 2011; Ward et al., 2009) requires both UNG2 and Vpr (Norman et al., 2011).
While working to discover targets for CRL4 action in the presence of HIV-1 Vpr, we identified human Dicer as a cellular Vpr partner. We discovered that it is a substrate for Vpr-mediated degradation through the CRL4DCAF1 ubiquitin ligase complex. Probing the impact of the interaction between Dicer and Vpr on HIV-1 replication in MDMs, we found that HIV infectivity increased in these cells upon depletion of Dicer. The increase in infectivity was greater for virus lacking Vpr than for wild-type virus, indicating that Vpr acts to suppress RNA silencing in MDMs.
Results
HIV-1 Vpr interacts with human Dicer
UNG2 and SMUG1 are the only known targets for Vpr-mediated proteasomal degradation (Schrofelbauer et al., 2005). Targeting of each requires the CRL4 ubiquitin ligase complex and both share a tryptophan-X-X-phenylalanine (WXXF) motif that is required for UNG2 assembly with Vpr (BouHamdan et al., 1998). We initiated work to determine whether other cellular proteins are also targets of Vpr-enhanced proteasomal degradation and whether the WXXF motif is required for Vpr-mediated targeting.
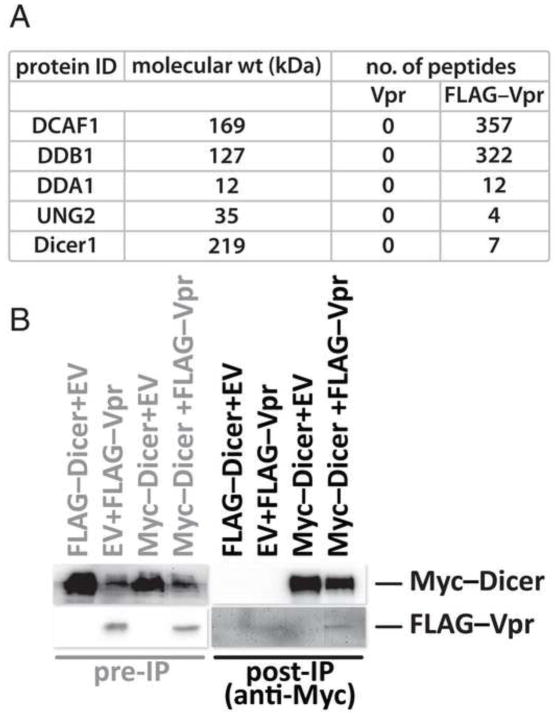
To discover candidates for Vpr-mediated ubiquitination, we first identified proteins that co-isolate with Vpr. FLAG-epitope-tagged HIV-1 Vpr was over-expressed in HEK293T cells from a transfected expression vector. FLAG–Vpr was isolated from cleared lysates with FLAG-specific antibodies conjugated to agarose beads. Bound proteins were eluted by competition with FLAG peptide. Analysis of the complete eluate by mass spectrometry identified known cellular partners of Vpr, including components of the CRL4DCAF1 ubiquitin ligase complex and UNG2. Our results also, for the first time, revealed Dicer as a cellular Vpr partner (Figure 1A). None of these proteins were detected in our negative control, an eluate prepared in parallel, originating from cells transfected with untagged Vpr.
Figure 1. HIV-1 Vpr assembles with human Dicer.
HEK293T cells were transfected with expression vector for untagged Vpr or FLAG-epitope tagged Vpr. FLAG-specific antibody bound to beads was used to isolate proteins from the cell lysates. Proteins were eluted from the beads by competition with FLAG peptide and identified by mass spectroscopy. Proteins isolated from lysates expressing untagged Vpr served to identify non-specifically isolated proteins. The number of peptides identified in the negative control (untagged Vpr) and the experimental (FLAG–Vpr) samples are shown (A). Lysates from HEK293T cells co-transfected with the indicated expression vectors (EV designates empty expression vector) were immunoblotted directly or after immunoprecipitation of Myc–Dicer with Myc-specific antibody. Samples were resolved by SDS-PAGE and blotted for Dicer or HIV-1 FLAG–Vpr with FLAG-specific antibody (B).
To confirm that human Dicer assembles in a complex with HIV-1 Vpr, we performed co-immunoprecipitation assays to determine whether Vpr could be co-isolated with Dicer from lysates of cells transfected with expression vectors for both. Immunoprecipitation of Myc-tagged Dicer resulted in the co-purification of HIV-1 Vpr (Figure 1B). Thus, we confirmed that Dicer and HIV-1 Vpr can assemble in the same protein complex.
In our co-immunoprecipitation experiments, we consistently observed lower Dicer levels in lysates from cells co-expressing Vpr (Figure 1B). Consequently, less Dicer was immunoprecipitated in the presence of Vpr than in its absence. Vpr is, of course, known to engage the CRL4 ubiquitin ligase complex but the substrates of this complex relevant to HIV infection remain unknown. These observations led us to hypothesize that expression of Vpr reduced Dicer levels by targeting this protein for degradation through the ubiquitin pathway.
Expression of HIV-1 Vpr triggers depletion of human Dicer both alone and in the context of an infection
To test our hypothesis that expression of HIV-1 Vpr triggers degradation of Dicer, we measured Dicer protein levels in the absence and presence of Vpr. We saw a substantial decrease in the level of Myc-tagged Dicer in the presence of Vpr (Figure 2A), consistent with our previous observations in the blots of the pre-IP lysates (Figure 1B, left). In both sets of experiments we over-expressed Dicer. In order to rule out the possibility that Vpr-mediated depletion targets only over- or exogenously-expressed proteins, we measured the effect of Vpr expression on endogenous Dicer levels. Immunoblotting lysates of mock- or Vpr-transfected cells with Dicer-specific antibody revealed a substantial decrease in endogenous Dicer levels in the presence of Vpr (Figure 2B). Finally, in order to determine whether Vpr expressed in the context of an infection is sufficient to cause depletion of endogenous Dicer, we infected cultures of the SupT1 T cell line and probed cell lysates for Dicer. Here too we saw depletion of endogenous Dicer (Figure 2C). As a control we also probed for UNG2, an established target of HIV-1 Vpr. Like Dicer, UNG2 was depleted only in cultures infected with HIV-1 encoding Vpr. The capacity of HIV-1 Vpr to reduce cellular Dicer levels was not shared by the Vpr and Vpx proteins of HIV-2. Infection of SupT1 or HEK293T cells with HIV-2 or HIV-2 lacking Vpr and/or Vpx did not change Dicer levels whereas SAMHD1 was markedly depleted in HEK293T cells infected with HIV-2 encoding Vpx (Figure 2 D and E). From this data we conclude that Dicer depletion is specific for HIV-1 Vpr like SAMHD1 depletion is for HIV-2 Vpx.
Figure 2. Expression of HIV-1 Vpr triggers depletion of human Dicer both alone and in the context of an infection.

Lysates of HEK293T cells co-transfected with the indicated expression vectors were immunoblotted for Dicer and β-actin. Dicer protein levels, relative to the β-actin protein signal, were calculated based on densitometric analysis of four separate experiments. A representative immunoblot is shown (A). Please note that in this and all other figures, error bars represent ± SD of the mean. Lysates of HEK293T cells transfected with either empty vector or HIV-1 FLAG–Vpr expression vector were immunoblotted for endogenous Dicer using antibody specific for Dicer. A representative immunoblot is shown, n=4 (B). Cultures of SupT1 cells were mock infected or infected with HIV-1 (pNL4-3 env(−) nef(−) gfp(+)) or HIV-1 with deleted Vpr sequences (pNL4-3 env(−) vpr(−) nef(−) gfp(+)) at a MOI=2. Cells were harvested 48 hours after infection for immunoblotting and analysis by flow cytometry. The levels of Dicer, UNG2, p24, Vpr, and α-tubulin were assessed by western blot (left). The percentage of infected cells was determined by GFP expression as assessed by flow cytometry (right). The data is representative of n=2 (C). Cultures of SupT1 cells or HEK293T cells were mock infected or infected with HIV-2 or HIV-2 lacking Vpr or Vpx at equivalent MOIs. Cells were harvested 48 hours after infection for immunoblotting. The levels of Dicer, UNG2, SAMHD1, p27, and α-tubulin were assessed by western blot (D and E).
In summary, our observations show that levels of Dicer, exogenous or endogenous in origin, are significantly decreased in the presence of Vpr. Further, we observed that expression of Vpr in the absence of other viral proteins is sufficient to trigger Dicer depletion, and that the quantities of Vpr present in an infection are sufficient to mediate this process as well. Based on the established interaction between Vpr and the CRL4DCAF1 ubiquitin ligase complex, we hypothesized that Vpr recruits Dicer to CRL4DCAF1 for ubiquitination and subsequent proteasomal destruction.
Vpr reduces cellular Dicer levels after translation
Vpr modulates transcription from numerous promoters (Agostini et al., 1996; Amini et al., 2004; Chowdhury et al., 2003; Cohen et al., 1990; Cullen, 1991; Fenard et al., 2009; Forget et al., 1998; Gummuluru and Emerman, 1999; Kino et al., 2005; Kino et al., 2002; Roux et al., 2000; Sawaya et al., 1998; Sherman et al., 2000; Wang et al., 1995; Zhu et al., 2003). Langevin et al. even demonstrated that Vpr expression specifically down-modulates transcription from the UNG2 promoter (Langevin et al., 2009). We therefore tested whether Vpr acts to reduce Dicer levels by decreasing transcription. Real-time PCR analyses demonstrated that Dicer mRNA levels do not differ significantly in cells expressing Vpr as compared to those expressing vector alone (Figure 3A). This finding indicates that Vpr does not act as a modulator of Dicer mRNA production.
Figure 3. Vpr reduces Dicer levels after protein translation in a manner that is not linked to the cell cycle.
HEK293T cells were transfected with either empty expression vector or expression vector for HIV-1 FLAG–Vpr. Dicer mRNA levels were measured by real-time PCR and were normalized to β-actin mRNA. Quantification was calculated using the Pfaffl method and the relative mean was calculated from three experiments (A). HEK293T cells, transfected with either empty expression vector or expression vector for HIV-1 FLAG–Vpr, were treated with 1μM of proteasome inhibitor, epoxomicin, or DMSO. Lysates were prepared as described in Materials and Methods and immunoblotted for exogenous Myc-Dicer expression. A representative immunoblot is shown, n=3 (B). HEK293T cells were treated with 25 μg/ml cycloheximide and harvested at the time points indicated. Lysates were prepared and immunoblotted for endogenous Dicer and α-tubulin expression (C). HEK293T cells were transfected with 1 μg of expression vector for FLAG–Dicer and 3 μg of empty expression vector or expression vector for the Vpr mutant indicated. Cells were harvested 48 hours after transfection and lysates were probed for Dicer, Vpr, or tubulin, as indicated. The table summarizes the phenotypes of Vpr mutants used (D).
In order to determine whether Vpr-mediated Dicer depletion proceeds through proteasomal degradation, we treated cells with the irreversible proteasome inhibitor epoxomicin prior to lysis. Inhibition of proteasomal function restored Dicer levels in the presence of Vpr, suggesting that Vpr decreases Dicer via a proteasome-dependent pathway (Figure 3B).
We established that expression of HIV-1 Vpr reduces cellular Dicer levels in a proteasome-dependent manner, but did not significantly impact levels of Dicer mRNA. In order to confirm that HIV-1 Vpr impacts Dicer after translation, we measured the stability of Dicer protein in the presence or absence of HIV-1 Vpr. Cells were transfected with either an expression vector for FLAG-tagged Vpr or an empty expression vector. Twenty-four hours later, the cells were seeded into separate plates. Forty-eight hours after transfection, the cultures were treated with the translation inhibitor cycloheximide and then harvested 1, 2.5, 8 and 24 hours later. An untreated control was also harvested for each transfection (T=0). In Figure 3C, we show that the rate of Dicer depletion is greatly enhanced in the presence of Vpr (T1/2≈2.5 hours) compared to our vector control (T1/2>24 hours). Together with the previous experiments these data show that Vpr targets Dicer for depletion after protein translation by dramatically shortening its half life.
Vpr-mediated Dicer depletion is not dependent on G2 cell cycle arrest
Since the levels of some proteins are regulated in a cell cycle-dependent manner, we tested whether the decrease in Dicer levels is directly linked to Vpr-mediated G2 arrest. We transfected HEK293T cells with an expression vector for FLAG-epitope-tagged Dicer together with empty expression vector or expression vector encoding wild-type Vpr or well-characterized Vpr mutants (Figure 3D). Among the Vpr species, wild-type Vpr, Vpr W54R and Vpr R90K caused depletion of Dicer while Vpr R80A produced partial depletion and Vpr 64LQQAA68 caused no depletion. Wild-type, Vpr W54R and Vpr 64LQQAA68 cause G2 arrest while Vpr R80A and Vpr R90K do not (Schrofelbauer et al., 2005; Selig et al., 1997; Sherman et al., 2000). Thus, both arresting and non-arresting mutants can trigger depletion or fail to do so, demonstrating that Dicer-depletion is not dependent on the cell cycle. Additionally, the Vpr W54R mutant, which does not bind the WXXF-motif of UNG2 and therefore fails to deplete UNG2 (BouHamdan et al., 1998; Schrofelbauer et al., 2005), causes Dicer depletion. This, together with the observation that Dicer has no WXXF motifs indicates that Vpr is not strictly dependent on this motif for the recruitment of target proteins.
In summary, Vpr expression causes depletion of cellular Dicer levels after translation by decreasing Dicer half-life. This phenotype was not amplified by changes in Dicer transcription. The levels of Dicer could be at least partially restored, in the time-frame tested, by adding proteasome inhibitor to the culture media. Finally, the mechanism responsible for accelerated Dicer turnover does not depend on the ability of Vpr to cause G2 cell cycle arrest.
Vpr-mediated depletion of Dicer depends on the CRL4DCAF1 ubiquitin ligase complex
Vpr-directed G2 cell cycle arrest, depletion of UNG2, and expression of NK-cell ligands have all been linked to the assembly of Vpr with the CRL4DCAF1 ubiquitin ligase complex. We therefore tested whether Vpr is required for the recruitment of Dicer to the CRL4 complex. We expressed, in cultures of HEK293T cells, FLAG epitope tagged Cul4A alone, together with Myc-Dicer or with both Myc-Dicer and HA epitope tagged Vpr. We then isolated FLAG–Cul4A and probed for the presence of Dicer and Vpr among the co-isolated proteins. In order to minimize HIV-1 Vpr-mediated depletion of Dicer in these experiments, we harvested cells at 18 hours post-transfection, to capture the proteins as they assembled but before extensive degradation was observed. In the absence of FLAG-Cul4A neither Dicer nor Vpr was isolated. Small quantities of Dicer were co-isolated with FLAG-Cul4A alone, but these were increased in the presence of HIV-1 Vpr (Figure 4A). We have previously shown that UNG2 assembles with and is turned over through the CRL4DCAF1 complex in the absence of Vpr but that Vpr enhances this interaction. This observation prompted us to investigate whether Dicer is also a substrate for the CRL4DCAF1 complex in the absence of Vpr. To test this, we depleted HEK293T cells of Cul4A or DCAF1 by siRNA transfection. A non-targeting siRNA was used as control. Upon depletion of either Cul4A or DCAF1, the steady state levels of endogenous UNG2 increased whereas those of Dicer remained constant (Figure 4B).
Figure 4. HIV-1 Vpr-mediated depletion of Dicer relies on the CRL4DCAF1 ubiquitin ligase complex.

HEK293T cells were co-transfected with Myc-Dicer, FLAG-Cul4A and HA-Vpr. FLAG-Cul4A was immunoprecipitated with anti-FLAG beads. Bound proteins were eluted and immunoblotted for Myc-Dicer and HA-Vpr. Myc-Dicer was detected using Dicer specific antibody. FLAG-Cul4A and HA-Vpr were detected using antibodies specific for their respective tags (A). HEK293T cells were transfected with either non-targeting siRNA or siRNA specific for Cul4A or for DCAF1. The levels of Dicer, UNG2, DCAF1, Cul4A, and α-tubulin were assessed by immunoblotting with protein-specific antibodies (B). HEK293T cells were transfected with either non-targeting siRNA or siRNA specific for Cul4 (C), DCAF1 (D), or DDB1 (E). Lysates were prepared and corresponding blots were immunoblotted for endogenous Dicer, Flag–Vpr and α-tubulin. Representative immunoblots are shown.
Having demonstrated that Vpr enhances the assembly of Dicer with the CRL4 complex and that the depletion of CRL4 complex components in the absence of Vpr does not alter steady-state Dicer levels, we determined whether we could protect Dicer from Vpr mediated degradation by interfering with CRL4 function. To ascertain whether the CRL4DCAF1 complex is important for Vpr-directed depletion of Dicer, we individually depleted three of its components and probed the impact on Dicer degradation. We transfected HEK293T cells with siRNA specific for Cul4, DCAF1, or DDB1 and then used western blotting to determine whether the lack of specific CRL4 components interfered with Vpr-directed Dicer degradation (Figures 4C–E, respectively). Treatment with siRNA specific for the ubiquitin ligase components, but not with non-targeting siRNA, restricted the capacity of HIV-1 Vpr to cause Dicer depletion.
Taken together, these data support a model in which Vpr assembles with Dicer and the CRL4DCAF1 complex, and that this complex is required for Vpr-mediated Dicer degradation. Importantly, Dicer is only the third protein target identified for Vpr-directed proteasomal degradation through this ubiquitin ligase complex and Dicer is the first target identified that lacks a WXXF motif.
Vpr inhibits shRNA function
Dicer processes double stranded RNAs to products that ultimately lend specificity to RNA interference. Vpr-directed depletion of Dicer should thus impede processing of double stranded RNAs. We therefore investigated the functional consequence of Vpr expression on Dicer activity. Using a luciferase reporter assay, we tested whether Vpr suppresses the silencing function of firefly luciferase-specific shRNA. We transfected HeLa cells with a firefly luciferase expression plasmid together with either a shRNA designed to target firefly luciferase or a scrambled, non-targeting shRNA as well as a plasmid expressing renilla luciferase to control for transfection efficiency and cell viability. In the absence of Vpr, the luciferase-directed shRNA reduced luciferase activity by approximately 80% relative to the scrambled control shRNA (Figure 5A). When we co-expressed Vpr, the efficacy of the luciferase-specific shRNA was significantly decreased, resulting in increased luciferase expression relative to the vector control (shLuc/Vector versus shLuc/Vpr in Figure 5A).
Figure 5. HIV-1 Vpr suppresses shRNA function.

HeLa cells were co-transfected with firefly and renilla luciferase reporter plasmids as well as with either scrambled or firefly luciferase-specific shRNA expression vectors and the indicated HIV-1 Vpr expression plasmids. Firefly luciferase activity was measured and normalized against the internal control, renilla luciferase activity. These data were normalized to the level of luciferase activity in cells transfected with scrambled shRNA, which was set at 100%. Means were calculated from at least three experiments performed in duplicate (A). HEK293T cells co-transfected with Myc–Dicer expression vector along with either empty vector, or expression vector for wild-type HIV-1 FLAG–Vpr or for HIV-1 FLAG–Vpr Q65R. Lysates were prepared and immunoblotted for expression of Myc–Dicer, FLAG–Vpr or α-tubulin as indicated. A representative immunoblot is shown, n=4 (B).
The Vpr Q65R mutant was significantly impaired in its capacity to cause Dicer depletion (Figure 5B), likely due to its impaired interaction with DCAF1 (Le Rouzic et al., 2007). Vpr Q65R showed significantly less repression of Dicer activity than did wild-type HIV-1 Vpr, paralleling the observation that it is less effective at promoting Dicer depletion. Overall, our results are consistent with a Vpr-specific abrogation of shRNA-mediated silencing. These data show that Vpr can act as a HIV-1-encoded suppressor of RNA silencing (SRS).
Reduction of Dicer levels enhances macrophage infection in a Vpr-dependent manner
Vpr enhances HIV-1 infectivity in macrophages and Dicer impairs HIV infectivity in CD4+ T cells. We therefore investigated how Dicer depletion impacts HIV-1 infection of macrophages and whether this effect is Vpr-dependent.
We transfected primary human monocyte-derived macrophages (MDM) with either an siRNA that specifically targets human Dicer or a non-targeting control siRNA. Dicer-specific siRNA reduced Dicer protein levels to approximately 20% of those found in cultures treated with the non-targeting siRNA (Figure 6A). We used these cultures to test whether Dicer hinders HIV-1 in MDMs in the context of a spreading infection. For these experiments we used vpr(+) or vpr(−) forms of NL81A, a HIV-1 which encodes envelope sequences from the macrophage-tropic BaL strain in place of the V1–V3 loops of the NL4-3 envelope. The viruses also contained a GFP gene in place of 5′ nef sequences. Images of the infected cells were recorded 19 days after infection. Depletion of Dicer increased infectivity of HIV-1 vpr(−) to a much greater extent (Figure 6B panels c and d) than the infectivity of Vpr-encoding HIV (Figure 6B panels a and b). Depletion of Dicer however also enhanced infectivity of Vpr-encoding HIV-1 suggesting that Vpr may not completely deplete Dicer (Figure 6B panels a and b).
Figure 6. Reduction of Dicer expression in human MDMs enhances HIV-1 infectivity and production in a Vpr-dependent manner.
Primary human MDM cultures were transfected with either non-targeting siRNA or siRNA specific for Dicer. 48 hours after transfection, lysates were prepared and immunoblotted for endogenous Dicer and tubulin. A representative immunoblot is shown, n=2 (A). Primary human MDM cultures were transfected with either non-targeting siRNA or siRNA specific for Dicer and infected 24 hours after transfection at equivalent MOI with either macrophage-tropic HIV-1 GFP or HIV-1 GFP vpr(−). Infectivity was visualized 19 days after infection by phase and fluorescence microscopy. Images are shown from a representative experiment. The experiment was performed with primary cells from at least three different donors and equivalent results were obtained (B). Supernatants from MDM cultures infected in (B) were harvested at days 7, 10 and 14 post-infection and were analyzed for p24 Gag production by p24 antigen-capture ELISA (C). p24 Gag production data were normalized to the levels of p24 in MDM cultures transfected with non-targeting siRNA to obtain the fold enhancement of viral production by Dicer siRNA. Means were calculated from p24 analysis of two separate experiments (D). Primary human MDM cultures were transfected with either non-targeting siRNA or siRNA specific for Dicer. These were infected 24 hours after transfection at an equivalent MOI with either VSV-G pseudotyped single cycle HIV-1 GFP or HIV-1 GFP vpr(−). Cells were harvested five days after infection and analyzed by flow cytometry. These data were normalized to the value of cells transfected with non-targeting siRNA, which was set to 100%. Means were calculated from five separate experiments using primary cells from five different donors (E).
We further tested the impact of Vpr-mediated Dicer depletion on infection by measuring the quantity of p24 produced by cells during spreading infections (Figure 6C). Here, depletion of Dicer increased the levels of p24 for an infection with HIV-1 vpr(−) to levels comparable to those of an infection with Vpr-expressing HIV-1. In this analysis siRNA-mediated depletion of Dicer also increased the p24 output of infections with Vpr-expressing HIV-1. However, comparison of the ratios of p24 produced by cultures transfected with siRNA specific for Dicer with those transfected with the non-targeting siRNA control demonstrates that depletion of Dicer by siRNA had a much greater impact on HIV-1 vpr(−) virus production than on Vpr-expressing HIV-1 virus production (Figure 6D). This suggests that the enhancement mediated by reduction of Dicer abundance is Vpr-dependent. Our observations underscore that the effects of Vpr or siRNA-mediated Dicer depletion on HIV-1 infectivity appear to determine whether a viable infection can be established.
In order to ascertain whether HIV is inhibited by Dicer before GFP expression from the provirus, we performed single-round infections in the presence or absence of Dicer and determined the number of infected cells using flow cytometry. Two days after transfection with either Dicer-specific or non-targeting siRNA, we infected the primary human MDMs with VSV-G pseudotyped, env(−) HIV-1 that either encodes or fails to encode Vpr. Both of these viruses have a green fluorescent protein (GFP) reporter gene in place of nef. Five days after infection, we harvested the cells and used flow cytometry to determine the fraction of GFP-expressing cells as a measure of infection. We found that transfection with Dicer-specific siRNA significantly enhanced infectivity of vpr(−) HIV-1 but not that of HIV-1 capable of expressing wild-type Vpr (Figure 6E).
Consistent with our data from the spreading infections, depletion of Dicer very reproducibly enhanced single-round infection with vpr(−) HIV-1 in primary human MDMs, albeit modestly. This suggests that the anti-viral function of Dicer can act on the virus on its way to establishing a functional provirus in the absence of Vpr, but leaves open the possibility that Dicer impacts HIV-1 at other phases of the infection cycle. The observation that depletion of Dicer does not enhance single-round infection with wild-type virus however suggests that Vpr action is already maximal during this phase of infection. Our ability to enhance the infectivity of wild-type virus in spreading infections by depleting Dicer suggests that Vpr may be less efficient at protecting the virus in another phase of replication such as during virus production.
Discussion
In this work we showed for the first time that HIV-1 Vpr assembles with Dicer. The primary functional consequence of this interaction is depletion of Dicer through the CRL4DCAF1 ubiquitin ligase complex. Indeed we showed that Dicer, Vpr and Cul4 all assembled into the same protein complex. We further showed that Vpr-mediated depletion of Dicer interferes with shRNA function. Importantly, we found that siRNA-mediated depletion of Dicer boosts HIV-1 infectivity in primary human macrophage cultures and that this enhancement is significantly more pronounced in infections with vpr(−) virus than with wild-type virus. This of course suggests that the Vpr associated with the wild-type virus alleviates the need for Dicer inhibition that is vital for infection with vpr(−) virus. Of note, Coley et al. previously showed that Vpr prevents expression of Dicer upon differentiation of monocytes (Coley et al., 2010). Our work offers an explanation for this finding. Rather than inhibiting Dicer production, Vpr triggers its elimination.
Our observations are also consistent with previous reports showing enhancement of HIV-1 infection after Dicer depletion in peripheral blood mononuclear cells and the cell lines, Jurkat and HEK293T (Nathans et al., 2009; Triboulet et al., 2007). Importantly, these earlier studies did not link the Dicer restriction directly with a viral protein while our data provides evidence that reducing Dicer expression offers a greater advantage to the virus in the absence of Vpr than in its presence. This indicates that Vpr counteracts the Dicer-dependent restriction. Viral protein-directed depletion of RNA silencing pathway components has not been described. Other mechanisms for SRS function rely on viral protein binding to either RNA or protein targets. The tomato bushy stunt virus protein p19, targets double-stranded RNA with 2-nucleotide 3′ overhangs to prevent their assembly into the RISC complex (Scholthof et al., 1995; Silhavy et al., 2002). The flock house virus B2 protein more generally targets double stranded RNA to prevent its processing by Dicer (Li et al., 2002). The cucumber mosaic virus 2b protein interacts with and inhibits Argonaute in the RISC complex (Zhang et al., 2006). Thus, a variety of mechanisms are emerging for SRS function and curiously none appear to be specific for a particular RNA sequence.
Dicer may not be the only factor that Vpr blocks to aid HIV-1 infection in macrophages. Infection with Vpr-expressing HIV was more efficient than with vpr(−) HIV-1, even when Dicer was depleted with siRNA. It is likely that depletion of Dicer resulted in a smaller improvement of wild-type virus infectivity because virus-encoded Vpr could deplete at least a portion of cellular Dicer. Further, while Dicer depletion aided single-round infection with vpr(−) HIV, we cannot rule out that Vpr-mediated Dicer depletion can also boost virus production. Since viral RNA is a key component for both incoming and outgoing virus, it is conceivable that Dicer function could impact both. Further it is also possible that if Dicer wasn’t sufficiently depleted in the cultures to which the corresponding siRNA was applied, that the wild-type virus had the advantage of carrying Vpr to neutralize remaining Dicer.
Vpr directs depletion of both UNG2 and SMUG1 proteins, both of which encode WXXF motifs. The WXXF motif on UNG2 is critical for its assembly with Vpr (BouHamdan et al., 1998). The WXXF motif on SMUG1 has not been tested but may perform a similar function in assembly with Vpr. Addition of this motif to integrase, allowed its assembly with Vpr (Kulkosky et al., 1999). Dicer, the third target for Vpr-mediated destruction by CRL4-DCAF1, does not have a WXXF motif. Further, Dicer was depleted efficiently by the Vpr W54R mutant which fails to bind UNG2 or to deplete UNG2 (Schrofelbauer et al., 2007). Dicer therefore is the first protein to be identified that is susceptible to Vpr-mediated depletion by CRL4-DCAF1in the absence of the WXXF motif. This observation suggests that Vpr can flag a range of proteins for destruction rather than one specific target.
Previous work showed that Tat can partially suppress Dicer activity although the significance of this finding hasn’t been fully resolved. Future experiments comparing the SRS activity of Tat to that of Vpr will be necessary to determine their respective contributions to alleviating miRNA restriction, especially in macrophages.
HIV-1 Vpr paralog HIV-2 Vpx does not deplete Dicer. Vpx enhances macrophage infection albeit more dramatically than HIV-1 Vpr (Sharova et al., 2008; Srivastava et al., 2008) by combating antiviral protein SAMHD1 through the CRL4DCAF1 complex. SAMHD1 (Hrecka et al., 2011) also acts as a restriction that can be overcome by Vpx in monocyte-derived dendritic cells (Laguette et al., 2011).
While work presented here is focused on the role of the Dicer interaction with HIV-1 Vpr and its effect on macrophage infection efficiency, this interaction may also impact other Vpr functions. For example, recent work demonstrated that Vpr expression, triggering the DNA damage response, up-regulates ULBP-1 and ULBP-2 ligands in infected cells to enhance natural killer cell-mediated lysis (Richard et al.; Ward et al., 2009). Interestingly, decreasing Dicer expression can also trigger a DNA damage response to up-regulate NKGD2 ligands albeit not ULBP-1 or ULBP-2 (Wu et al., 2011). The factors that determine which NKGD2 ligands are upregulated in response to DNA damage are not clear. Of note, macrophages may not trigger the Dicer-mediated DNA damage response because Vpr does not activate ATR in these cells (Zimmerman et al., 2006). Future experiments examining whether Dicer degradation and Vpr-mediated up-regulation of ULBP-1 and ULBP-2 are linked could reveal yet another Vpr function impacted by its interaction with Dicer.
In summary, accumulating evidence has established that the miRNA pathway can restrict HIV replication in mammalian cells. Here we identified Vpr as an HIV-encoded protein that possesses SRS activity and determined its mechanism for defeating the miRNA restriction, specifically triggering destruction of Dicer. Further, this work identifies a novel substrate of Vpr-mediated degradation via the CRL4DCAF1 complex. Future studies to identify miRNAs that are impacted by Vpr expression will offer new insights into the mechanism of this evolutionarily conserved antiviral response.
Materials and Methods
Ethics statement
All primary human monocyte samples were from de-identified donors at the University of Nebraska Medical Center, Omaha, NE. Our protocol for the use of primary human monocytes was approved by the Albany Medical College Committee on Research Involving Human Subjects and granted a category 4 exemption from consent procedures based on the anonymous nature of the samples.
Proviral clones and expression plasmids
Macrophage-tropic proviral clones, pNL81A GFP and pNL81A vpr(−) GFP, were constructed by inserting a BamHI-XhoI fragment containing eGFP from pNL4-3 GFP env(−) (kindly provided by Dr. Dana Gabuzda, Dana-Farber Cancer Institute, Boston, MA) into pNL81A or pNL81A vpr(−) that had been digested with BamHI and XhoI. The Nef open reading frame was thus replaced with the eGFP gene. pNL81A and its vpr-deficient counterpart were previously described (Eckstein et al., 2001; Toohey et al., 1995). FLAG/HA–Dicer was purchased from Addgene (Addgene plasmid #19881, provided by Dr. Thomas Tuschl, Rockefeller University, New York NY). 5′ Myc–Dicer pcDNA3.1 was kindly provided by Dr. Patrick Provost (Université Laval, Quebec, Canada). The expression vector for codon-optimized FLAG-epitope tagged Vpr, pcDNA3.1(−) huFLAG–Vpr was previously described (Wen et al., 2007). The Vpr mutants tested in Figure 3 were similarly generated or derived by PCR-based mutagenesis of the original codon-optimized clones. The proviruses used in single-round infections, pNL4-3GFP env(−) and pNL4-3GFP env(−)vpr(−), were kindly provided by Dr. Vicente Planelles (University of Utah, Salt Lake City, UT). pGL-AN nef(−)GFP, pGL-St nef(−)GFP and pGL-Ec nef(−)GFP originated as pGL-AN, pGL-St and pGL-Ec, a kind gift of Dr. Mikako Fujita, but have GFP inserted in place of nef sequences upstream of the 3′ LTR.
Cell culture
HEK293T and HeLa cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 2 mM glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin.
SupT1 cells were maintained in RPMI medium supplemented with 10% fetal calf serum, 2 mM glutamine, 100 units/ml penicillin and 100ug/ml streptomycin.
Human monocytes were obtained from healthy donors at the University of Nebraska Medical Center, Omaha, NE. Elutriated monocytes were differentiated for 7 days in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% human serum, and recombinant human macrophage colony-stimulating factor (rhM-CSF, Cell Sciences, Canton, MA). Cultures were maintained by replacing one half of the cell culture medium with fresh medium every 2–3 days. After 7 days in differentiation media, MDMs were maintained in DMEM supplemented with 10% human serum without rhM-CSF.
Immunoprecipitation and Tandem Mass Spectroscopy Analysis
4 × 106 HEK293T cells were seeded into each of 10, 10 cm plates. Each culture was transfected, using calcium phosphate, with 20 μg of pcDNA3.1(−)HIV-1huVpr or pcDNA3.1(−)HIV-1FLAG–huVpr. Forty-eight hours after transfection the cells were lysed with 1 ml cold RIPA buffer (25 mM Tris-HCl pH 8.0, 250 mM NaCl, 10% (v/v) glycerol, 1% (v/v) IGEPAL CA-630, 0.25% (w/v) deoxycholic acid, 2 mM EDTA, 1 mM NaF, 50 mM β–glycerophosphate and Complete™ protease inhibitor cocktail (Roche)). The lysates were cleared twice by centrifugation at 10,000 × g for 10 min at 4°C. The supernatants were incubated with 50 μl of anti-FLAG M2 agarose resin (Sigma-Aldrich) for 2 h at 4°C with constant rotation. The resin with the bound proteins was washed three times with lysis buffer. The remaining bound proteins were eluted by competition with 50 μl of 200 mg/ml FLAG peptide (Sigma-Aldrich) at room temperature for 10 min. The eluted proteins were identified using tandem mass spectroscopy (NextGen Sciences, Ann Arbor, Michigan).
Immunoprecipitation and immunoblotting
HEK293T cells were transfected using calcium phosphate. Cells were lysed in ELB buffer (50mM HEPES pH 7.3, 400mM NaCl, 0.2% NP-40, 5mM EDTA, 0.5mM DTT) for 15 minutes and then centrifuged for 10 minutes to pellet debris. Clarified supernatants were either stored as a total cellular lysates or mixed with 50 μl of antibody-conjugated beads and incubated overnight at 4°C followed by competitive elution with peptide corresponding to the epitope tag. Immunoprecipitates or total cell lysates were resolved by SDS/PAGE and transferred to PVDF membranes (Millipore). The following primary antibodies were used for immunoblotting: c-myc-specific monoclonal antibody 9E10 (Stratagene), FLAG-specific monoclonal antibody M2 (Sigma-Aldrich), HA-specific monoclonal antibody 12CA5 (Roche Applied Science), Dicer-specific polyclonal anti-serum (Cell Signaling), Tubulin-specific monoclonal antibody N356 (Amersham) and β-actin-specific monoclonal antibody (Sigma-Aldrich). The primary antibodies were detected with corresponding horseradish peroxidase-conjugated secondary antibodies. These were visualized using Immobilon Western horseradish peroxidase substrate (Millipore).
Protein levels are reported as relative densitometric intensity measured by NIH Image J software. Each protein level was corrected against the corresponding loading control. Means and standard deviation of means were calculated from at least three separate experiments.
Proteasome Inhibition
HEK293T cells were treated with 1 μM epoxomicin (Sigma-Aldrich) or an equivalent volume of DMSO for 5–7 hours at 37°C.
Cycloheximide Time Course
10 cm plates of HEK293T cells were transfected with either empty vector or with an expression vector for FLAG-epitope-tagged HIV-1 Vpr. Twenty-four hours later, the cells were replated into 60 mm dishes. Forty eight hours later, pre-warmed media containing 25 μg/ml cycloheximide was added to all cells at time = 0. Cells were harvested at the indicated time-points and immediately frozen at −20°C. Once all samples were harvested, cells were lysed in ELB buffer (50mM HEPES pH 7.3, 400mM NaCl, 0.2% NP-40, 5mM EDTA, 0.5mM DTT) for 10 min on ice. Cell debris was pelleted by centrifugation at 14K rpm for 10min. Supernatants were mixed with 2×Laemmli buffer and boiled to ensure complete denaturation and lysis. Samples were resolved by SDS/PAGE and transferred to PVDF membranes (Millipore) for immunoblotting.
Real-Time PCR
Cytoplasmic RNA was isolated from transfected HEK293T cells using the RNeasy Mini Kit according to manufacturer’s instructions (Qiagen, Inc., Valencia, CA). DNase-treated RNA was quantified by Nanodrop™ spectrophotometer, and 1 μg of RNA was reverse transcribed using the iScript cDNA synthesis kit, following manufacturer’s instructions (BioRad, Hercules, CA). Dicer and β-actin mRNA was amplified from 20 ng of cDNA with an Applied Biosystems 7500 real-time PCR cycler, using SYBR green reagent (Bio-Rad). The sequence of Dicer- and β-actin-specific primers is shown below. These were used at 250 nM or 400 nM concentrations respectively. Standard curves were established to determine the amplification efficiency of each primer set. The efficiency for Dicer was 93.8% and that for β-actin was 93%.
Dicer forward: 5′–CATGGATAGTGGGATGTCAC–3′ (Chendrimada et al., 2005)
Dicer reverse: 5′–CTACTTCCACAGTGACTCTG–3′ (Chendrimada et al., 2005)
β-actin forward: 5′–AAAGACCTGTACGCCAACAC–3′ (Chendrimada et al., 2005)
β-actin reverse: 5′–GTCATACTCCTGCTTGCTGAT–3′ (Chendrimada et al., 2005)
The Pfaffl method was used to quantify mRNA levels, and Dicer expression was normalized to β-actin expression.
Short Hairpin RNA and siRNA
The firefly luciferase specific and scrambled luciferase shRNA vector was constructed using the GeneClip™ U1 Hairpin Cloning System. The oligonucleotides corresponding to the sequences shown below were synthesized and annealed with complementary oligonucleotides, as specified in the manufacturer’s instructions, and ligated into the expression vector provided. The integrity and identity of the inserts was confirmed by DNA sequencing.
Luciferase-specific shRNA: 5′–TCTCAAGTGTTGTTCCATTCCATAAGTTCTCTATGGAATGGAACAACACTTCT–3′
Scrambled luciferase shRNA: 5′–TCTCGATTTTAGCCGTACTTCGTAAGTTCTCTACGAAGTACGGCTAAAATCCT–3′
The following siRNAs were purchased from Dharmacon:
Non-targeting control siRNA (CAT# D-001210-02-20)
Dicer: 5′–UGCUUGAAGCAGCUCUGGA(dTdT)–3′
DCAF1: 5′–AUAUGGCCGUUUCCGUAAA(dTdT)–3′
DDB1: 5′–CCCAGUUUCUGCAGAAUGAATA(dTdT)–3′
Cul4 (mix): 5′–CGGCUUCAGCUUUGAGGAGAUA(dTdT)–3′
and 5′–AGGGACUACAUGGAAAGAGAUA(dTdT)–3′
Dicer Activity Reporter Assay
HeLa cells were seeded in 12-well plates and transfected using FuGene HD (Roche), in triplicate, with 2.5 ng EF-1 renilla luciferase plasmid, 25 ng of pGL3 control plasmid (Promega), 375 ng of scrambled or luciferase-specific shRNA and either 100 ng of vector or expression plasmid encoding HIV-1 Vpr or HIV-1 VprQ65R. Two days after transfection, the cells were harvested in 1x Renilla lysis buffer (Promega). Lysates were mixed with either Firefly luciferase assay buffer or Renilla luciferase assay buffer (Promega) and luciferase activity was measured using a plate reader luminometer. All samples were normalized to Renilla luciferase activity. Data is expressed as luciferase activity in luciferase-specific shRNA transfected cells relative to scrambled shRNA transfected cells.
Virus Preparation and Infection
HEK293T cells were transfected with pNL81A HIV-1GFP, pNL81A HIV-1vpr(−)GFP, pGL-ANnef(−)GFP, pGL-Stnef(−)GFP or pGL-Ecnef(−)GFP using calcium phosphate or FuGENE HD. Viruses for single round infections were made by co-transfecting HEK293T cells with viral expression vector and an expression plasmid for vesicular stomatitis virus G-protein. Viruses for both spreading and single-round infections were harvested 48 hours after transfection. Supernatants were centrifuged at 800 × g for 5 minutes to sediment cellular debris. Clarified supernatants were concentrated with centrifugal filter columns (Amicon Ultra 100K from Millipore) using the manufacturer’s protocol. Concentrated virus was stored at −80°C. Viruses were titered on the GHOST X4/R5 indicator cell line (kindly provided by the NIH AIDS Research and Reference Reagent Program).
Prior to infections, MDMs that had been differentiated for 7 days were transfected with either non-targeting or Dicer-specific siRNA using Lipofectamine 2000 (Invitrogen). 48 hours after transfection, cells were infected at an equivalent multiplicity with vpr(+) and vpr(−) viruses. After 5–7 hours, cells were washed twice with PBS and incubated in fresh DMEM supplemented with 10% human AB serum.
SupT1 cells were incubated with VSV-G pseudotyped vpr(+) and vpr(−) viruses at a multiplicity of infection of two, as measured by titration on GHOST indicator cells. After 2 hours of incubation, cells were washed with PBS and resuspended in RPMI media. Forty-eight hours post infection, flow analysis was performed on a small fraction of the cells to analyze infectivity. The rest of the cells were harvested and lysed. The lysates were separated by SDS/PAGE, and immunoblotted as previously described.
Single round MDM infections were analyzed, using flow cytometry, five days after infection. Infected cells were released from their substrate by incubating cultures for 30 minutes in a 1:1 solution of 10 mM EDTA in PBS (pH 7.4) and RPMI containing 20% fetal bovine serum. Cells were fixed for 30 minutes at room temperature in 2% formaldehyde and washed twice in PBS. 30,000 events were recorded on a LSRII flow cytometer (BD Biosciences) and data was analyzed using FlowJo v7.5 software (Tree Star, Inc., Ashland, OR).
Spreading MDM infections were monitored by fluorescence microscopy using a Zeiss Axio Observer.Z1 microscope. GFP-expressing cells were visualized using 488 nm excitation with a FITC emission filter set (Zeiss). Images shown were recorded at 19 days after infection
Virus production was monitored by measuring viral p24gag protein in cell-free supernatants harvested from cultures at the specified time-points. HIV-1 p24 ELISA Assay Kits were used as per the manufacturer’s instructions (AIDS Vaccine Program, National Cancer Institute (NCI) at Frederick, MD, USA).
Statistical Analysis
All analysis was performed using the unpaired Student t-test with a two-tailed distribution.
Acknowledgments
We thank Dr. Karen M. Duus (Albany Medical College, Albany, NY) for producing virus for some of the infectivity experiments and Ashanti Lewis for technical support. This work was supported by a grant to C.N. from the National Institutes of Health (R01AI073178).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agostini I, Navarro JM, Rey F, Bouhamdan M, Spire B, Vigne R, Sire J. The human immunodeficiency virus type 1 Vpr transactivator: cooperation with promoter-bound activator domains and binding to TFIIB. J Mol Biol. 1996;261:599–606. doi: 10.1006/jmbi.1996.0485. [DOI] [PubMed] [Google Scholar]
- Ahluwalia JK, Khan SZ, Soni K, Rawat P, Gupta A, Hariharan M, Scaria V, Lalwani M, Pillai B, Mitra D, Brahmachari SK. Human cellular microRNA hsa-miR-29a interferes with viral nef protein expression and HIV-1 replication. Retrovirology. 2008;5:117. doi: 10.1186/1742-4690-5-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini S, Saunders M, Kelley K, Khalili K, Sawaya BE. Interplay between HIV-1 Vpr and Sp1 modulates p21(WAF1) gene expression in human astrocytes. J Biol Chem. 2004;279:46046–46056. doi: 10.1074/jbc.M403792200. [DOI] [PubMed] [Google Scholar]
- Andersson MG, Haasnoot PC, Xu N, Berenjian S, Berkhout B, Akusjarvi G. Suppression of RNA interference by adenovirus virus-associated RNA. J Virol. 2005;79:9556–9565. doi: 10.1128/JVI.79.15.9556-9565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balliet JW, Kolson DL, Eiger G, Kim FM, McGann KA, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–631. doi: 10.1006/viro.1994.1225. [DOI] [PubMed] [Google Scholar]
- Bennasser Y, Le SY, Benkirane M, Jeang KT. Evidence that HIV-1 encodes an siRNA and a suppressor of RNA silencing. Immunity. 2005;22:607–619. doi: 10.1016/j.immuni.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Bennasser Y, Yeung ML, Jeang KT. HIV-1 TAR RNA subverts RNA interference in transfected cells through sequestration of TAR RNA-binding protein, TRBP. J Biol Chem. 2006;281:27674–27678. doi: 10.1074/jbc.C600072200. [DOI] [PubMed] [Google Scholar]
- Boden D, Pusch O, Lee F, Tucker L, Ramratnam B. Human immunodeficiency virus type 1 escape from RNA interference. J Virol. 2003;77:11531–11535. doi: 10.1128/JVI.77.21.11531-11535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BouHamdan M, Xue Y, Baudat Y, Hu B, Sire J, Pomerantz RJ, Duan LX. Diversity of HIV-1 Vpr interactions involves usage of the WXXF motif of host cell proteins. J Biol Chem. 1998;273:8009–8016. doi: 10.1074/jbc.273.14.8009. [DOI] [PubMed] [Google Scholar]
- Chen R, Le Rouzic E, Kearney JA, Mansky LM, Benichou S. Vpr-mediated incorporation of UNG2 into HIV-1 particles is required to modulate the virus mutation rate and for replication in macrophages. J Biol Chem. 2004;279:28419–28425. doi: 10.1074/jbc.M403875200. [DOI] [PubMed] [Google Scholar]
- Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury IH, Wang XF, Landau NR, Robb ML, Polonis VR, Birx DL, Kim JH. HIV-1 Vpr activates cell cycle inhibitor p21/Waf1/Cip1: a potential mechanism of G2/M cell cycle arrest. Virology. 2003;305:371–377. doi: 10.1006/viro.2002.1777. [DOI] [PubMed] [Google Scholar]
- Cohen EA, Terwilliger EF, Jalinoos Y, Proulx J, Sodroski JG, Haseltine WA. Identification of HIV-1 vpr product and function. J Acquir Immune Defic Syndr. 1990;3:11–18. [PubMed] [Google Scholar]
- Coley W, Van Duyne R, Carpio L, Guendel I, Kehn-Hall K, Chevalier S, Narayanan A, Luu T, Lee N, Klase Z, Kashanchi F. Absence of DICER in monocytes and its regulation by HIV-1. J Biol Chem. 2010;285:31930–31943. doi: 10.1074/jbc.M110.101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- Conticello SG, Harris RS, Neuberger MS. The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G. Curr Biol. 2003;13:2009–2013. doi: 10.1016/j.cub.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Cullen BR. Regulation of HIV-1 gene expression. Faseb J. 1991;5:2361–2368. doi: 10.1096/fasebj.5.10.1712325. [DOI] [PubMed] [Google Scholar]
- Das AT, Brummelkamp TR, Westerhout EM, Vink M, Madiredjo M, Bernards R, Berkhout B. Human immunodeficiency virus type 1 escapes from RNA interference-mediated inhibition. J Virol. 2004;78:2601–2605. doi: 10.1128/JVI.78.5.2601-2605.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries W, Berkhout B. RNAi suppressors encoded by pathogenic human viruses. Int J Biochem Cell Biol. 2008;40:2007–2012. doi: 10.1016/j.biocel.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Eckstein DA, Sherman MP, Penn ML, Chin PS, de Noronha CM, Greene WC, Goldsmith MA. HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4+ T cells. J Exp Med. 2001;194:1407–1419. doi: 10.1084/jem.194.10.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenard D, Houzet L, Bernard E, Tupin A, Brun S, Mougel M, Devaux C, Chazal N, Briant L. Uracil DNA Glycosylase 2 negatively regulates HIV-1 LTR transcription. Nucleic Acids Res. 2009;37:6008–6018. doi: 10.1093/nar/gkp673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget J, Yao XJ, Mercier J, Cohen EA. Human immunodeficiency virus type 1 vpr protein transactivation function: mechanism and identification of domains involved. J Mol Biol. 1998;284:915–923. doi: 10.1006/jmbi.1998.2206. [DOI] [PubMed] [Google Scholar]
- Goffinet C, Allespach I, Homann S, Tervo HM, Habermann A, Rupp D, Oberbremer L, Kern C, Tibroni N, Welsch S, Krijnse-Locker J, Banting G, Krausslich HG, Fackler OT, Keppler OT. HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell host & microbe. 2009;5:285–297. doi: 10.1016/j.chom.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HC, Rice GI, Christodoulou E, Walker PA, Kelly G, Haire LF, Yap MW, de Carvalho LP, Stoye JP, Crow YJ, Taylor IA, Webb M. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature. 2011 doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- Gummuluru S, Emerman M. Cell cycle- and Vpr-mediated regulation of human immunodeficiency virus type 1 expression in primary and transformed T-cell lines. J Virol. 1999;73:5422–5430. doi: 10.1128/jvi.73.7.5422-5430.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Hue S, Schaller T, Verschoor E, Pillay D, Towers GJ. Mutation of a single residue renders human tetherin resistant to HIV-1 Vpu-mediated depletion. PLoS Pathog. 2009;5:e1000443. doi: 10.1371/journal.ppat.1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasnoot J, de Vries W, Geutjes EJ, Prins M, de Haan P, Berkhout B. The Ebola virus VP35 protein is a suppressor of RNA silencing. PLoS Pathog. 2007;3:e86. doi: 10.1371/journal.ppat.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan M, Scaria V, Pillai B, Brahmachari SK. Targets for human encoded microRNAs in HIV genes. Biochem Biophys Res Commun. 2005;337:1214–1218. doi: 10.1016/j.bbrc.2005.09.183. [DOI] [PubMed] [Google Scholar]
- Heinzinger NK, Bukinsky MI, Haggerty SA, Ragland AM, Kewalramani V, Lee MA, Gendelman HE, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci U S A. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H, Logue EC, Bloch N, Daddacha W, Polsky SB, Schultz ML, Kim B, Landau NR. The Vpx Lentiviral Accessory Protein Targets SAMHD1 for Degradation in the Nucleus. J Virol. 2012;86:12552–12560. doi: 10.1128/JVI.01657-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wang F, Argyris E, Chen K, Liang Z, Tian H, Huang W, Squires K, Verlinghieri G, Zhang H. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med. 2007;13:1241–1247. doi: 10.1038/nm1639. [DOI] [PubMed] [Google Scholar]
- Jacque JM, Triques K, Stevenson M. Modulation of HIV-1 replication by RNA interference. Nature. 2002;418:435–438. doi: 10.1038/nature00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Roche M, Gantier MP, Begum NA, Honjo T, Caradonna S, Williams BR, Mak J. X4 and R5 HIV-1 have distinct post-entry requirements for uracil DNA glycosylase during infection of primary cells. J Biol Chem. 2010;285:18603–18614. doi: 10.1074/jbc.M109.090126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser SM, Emerman M. Uracil DNA glycosylase is dispensable for human immunodeficiency virus type 1 replication and does not contribute to the antiviral effects of the cytidine deaminase Apobec3G. J Virol. 2006;80:875–882. doi: 10.1128/JVI.80.2.875-882.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, De Martino MU, Charmandari E, Ichijo T, Outas T, Chrousos GP. HIV-1 accessory protein Vpr inhibits the effect of insulin on the Foxo subfamily of forkhead transcription factors by interfering with their binding to 14-3-3 proteins: potential clinical implications regarding the insulin resistance of HIV-1 infected patients. Diabetes. 2005;54:23–31. doi: 10.2337/diabetes.54.1.23. [DOI] [PubMed] [Google Scholar]
- Kino T, Gragerov A, Slobodskaya O, Tsopanomichalou M, Chrousos GP, Pavlakis GN. Human immunodeficiency virus type 1 (HIV-1) accessory protein Vpr induces transcription of the HIV-1 and glucocorticoid-responsive promoters by binding directly to p300/CBP coactivators. J Virol. 2002;76:9724–9734. doi: 10.1128/JVI.76.19.9724-9734.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Takaori-Kondo A, Miyauchi Y, Iwai K, Uchiyama T. Ubiquitination of APOBEC3G by an HIV-1 Vif-Cullin5-Elongin B-Elongin C complex is essential for Vif function. J Biol Chem. 2005;280:18573–18578. doi: 10.1074/jbc.C500082200. [DOI] [PubMed] [Google Scholar]
- Kulkosky J, BouHamdan M, Geist A, Pomerantz RJ. A novel Vpr peptide interactor fused to integrase (IN) restores integration activity to IN-defective HIV-1 virions. Virology. 1999;255:77–85. doi: 10.1006/viro.1998.9544. [DOI] [PubMed] [Google Scholar]
- Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin C, Maidou-Peindara P, Aas PA, Jacquot G, Otterlei M, Slupphaug G, Benichou S. Human immunodeficiency virus type 1 Vpr modulates cellular expression of UNG2 via a negative transcriptional effect. J Virol. 2009;83:10256–10263. doi: 10.1128/JVI.02654-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rouzic E, Belaidouni N, Estrabaud E, Morel M, Rain JC, Transy C, Margottin-Goguet F. HIV1 Vpr arrests the cell cycle by recruiting DCAF1/VprBP, a receptor of the Cul4-DDB1 ubiquitin ligase. Cell cycle (Georgetown, Tex. 2007;6:182–188. doi: 10.4161/cc.6.2.3732. [DOI] [PubMed] [Google Scholar]
- Lecellier CH, Dunoyer P, Arar K, Lehmann-Che J, Eyquem S, Himber C, Saib A, Voinnet O. A cellular microRNA mediates antiviral defense in human cells. Science. 2005;308:557–560. doi: 10.1126/science.1108784. [DOI] [PubMed] [Google Scholar]
- Li H, Li WX, Ding SW. Induction and suppression of RNA silencing by an animal virus. Science. 2002;296:1319–1321. doi: 10.1126/science.1070948. [DOI] [PubMed] [Google Scholar]
- Lin J, Cullen BR. Analysis of the interaction of primate retroviruses with the human RNA interference machinery. J Virol. 2007;81:12218–12226. doi: 10.1128/JVI.01390-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Cullen BR. Adenovirus VA1 noncoding RNA can inhibit small interfering RNA and MicroRNA biogenesis. J Virol. 2004;78:12868–12876. doi: 10.1128/JVI.78.23.12868-12876.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangeat B, Gers-Huber G, Lehmann M, Zufferey M, Luban J, Piguet V. HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog. 2009;5:e1000574. doi: 10.1371/journal.ppat.1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansky LM, Preveral S, Selig L, Benarous R, Benichou S. The interaction of vpr with uracil DNA glycosylase modulates the human immunodeficiency virus type 1 In vivo mutation rate. J Virol. 2000;74:7039–7047. doi: 10.1128/jvi.74.15.7039-7047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin M, Rose KM, Kozak SL, Kabat D. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat Med. 2003;9:1398–1403. doi: 10.1038/nm946. [DOI] [PubMed] [Google Scholar]
- Mbisa JL, Barr R, Thomas JA, Vandegraaff N, Dorweiler IJ, Svarovskaia ES, Brown WL, Mansky LM, Gorelick RJ, Harris RS, Engelman A, Pathak VK. Human immunodeficiency virus type 1 cDNAs produced in the presence of APOBEC3G exhibit defects in plus-strand DNA transfer and integration. J Virol. 2007;81:7099–7110. doi: 10.1128/JVI.00272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehle A, Strack B, Ancuta P, Zhang C, McPike M, Gabuzda D. Vif overcomes the innate antiviral activity of APOBEC3G by promoting its degradation in the ubiquitin-proteasome pathway. J Biol Chem. 2004;279:7792–7798. doi: 10.1074/jbc.M313093200. [DOI] [PubMed] [Google Scholar]
- Nathans R, Chu CY, Serquina AK, Lu CC, Cao H, Rana TM. Cellular microRNA and P bodies modulate host-HIV-1 interactions. Molecular cell. 2009;34:696–709. doi: 10.1016/j.molcel.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Bergeron D, Subbramanian RA, Yao XJ, Checroune F, Rougeau N, Cohen EA. The putative alpha helix 2 of human immunodeficiency virus type 1 Vpr contains a determinant which is responsible for the nuclear translocation of proviral DNA in growth-arrested cells. J Virol. 1998;72:4104–4115. doi: 10.1128/jvi.72.5.4104-4115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JM, Mashiba M, McNamara LA, Onafuwa-Nuga A, Chiari-Fort E, Shen W, Collins KL. The antiviral factor APOBEC3G enhances the recognition of HIV-infected primary T cells by natural killer cells. Nature immunology. 2011;12:975–983. doi: 10.1038/ni.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto S, Ito M, Tsutsumi Y, Ichikawa Y, Okuyama H, Brisibe EA, Saksena NK, Fujii YR. HIV-1 nef suppression by virally encoded microRNA. Retrovirology. 2004;1:44. doi: 10.1186/1742-4690-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TN, Richard J, Gerard FC, Power C, Cohen EA. Modulation of NKG2D-Mediated Cytotoxic Functions of Natural Killer Cells by Viral Protein R (Vpr) from HIV-1 Primary Isolates. J Virol. 2011 doi: 10.1128/JVI.05835-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov S, Rexach M, Zybarth G, Reiling N, Lee MA, Ratner L, Lane CM, Moore MS, Blobel G, Bukrinsky M. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. Embo J. 1998;17:909–917. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priet S, Gros N, Navarro JM, Boretto J, Canard B, Querat G, Sire J. HIV-1-associated uracil DNA glycosylase activity controls dUTP misincorporation in viral DNA and is essential to the HIV-1 life cycle. Molecular cell. 2005;17:479–490. doi: 10.1016/j.molcel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Qian S, Zhong X, Yu L, Ding B, de Haan P, Boris-Lawrie K. HIV-1 Tat RNA silencing suppressor activity is conserved across kingdoms and counteracts translational repression of HIV-1. Proc Natl Acad Sci U S A. 2009;106:605–610. doi: 10.1073/pnas.0806822106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard J, Sindhu S, Pham TN, Belzile JP, Cohen EA. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood. 2010;115:1354–1363. doi: 10.1182/blood-2009-08-237370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere L, Darlix JL, Cimarelli A. Analysis of the viral elements required in the nuclear import of HIV-1 DNA. J Virol. 2010;84:729–739. doi: 10.1128/JVI.01952-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux P, Alfieri C, Hrimech M, Cohen EA, Tanner JE. Activation of transcription factors NF-kappaB and NF-IL-6 by human immunodeficiency virus type 1 protein R (Vpr) induces interleukin-8 expression. J Virol. 2000;74:4658–4665. doi: 10.1128/jvi.74.10.4658-4665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya BE, Khalili K, Mercer WE, Denisova L, Amini S. Cooperative actions of HIV-1 Vpr and p53 modulate viral gene transcription. J Biol Chem. 1998;273:20052–20057. doi: 10.1074/jbc.273.32.20052. [DOI] [PubMed] [Google Scholar]
- Schnettler E, de Vries W, Hemmes H, Haasnoot J, Kormelink R, Goldbach R, Berkhout B. The NS3 protein of rice hoja blanca virus complements the RNAi suppressor function of HIV-1 Tat. EMBO Rep. 2009;10:258–263. doi: 10.1038/embor.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof KB, Scholthof HB, Jackson AO. The effect of defective interfering RNAs on the accumulation of tomato bushy stunt virus proteins and implications for disease attenuation. Virology. 1995;211:324–328. doi: 10.1006/viro.1995.1410. [DOI] [PubMed] [Google Scholar]
- Schrofelbauer B, Hakata Y, Landau NR. HIV-1 Vpr function is mediated by interaction with the damage-specific DNA-binding protein DDB1. Proc Natl Acad Sci U S A. 2007;104:4130–4135. doi: 10.1073/pnas.0610167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrofelbauer B, Yu Q, Zeitlin SG, Landau NR. Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J Virol. 2005;79:10978–10987. doi: 10.1128/JVI.79.17.10978-10987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig L, Benichou S, Rogel ME, Wu LI, Vodicka MA, Sire J, Benarous R, Emerman M. Uracil DNA glycosylase specifically interacts with Vpr of both human immunodeficiency virus type 1 and simian immunodeficiency virus of sooty mangabeys, but binding does not correlate with cell cycle arrest. J Virol. 1997;71:4842–4846. doi: 10.1128/jvi.71.6.4842-4846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharova N, Wu Y, Zhu X, Stranska R, Kaushik R, Sharkey M, Stevenson M. Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction. PLoS Pathog. 2008;4:e1000057. doi: 10.1371/journal.ppat.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med. 2003;9:1404–1407. doi: 10.1038/nm945. [DOI] [PubMed] [Google Scholar]
- Sherman MP, de Noronha CM, Pearce D, Greene WC. Human immunodeficiency virus type 1 Vpr contains two leucine-rich helices that mediate glucocorticoid receptor coactivation independently of its effects on G(2) cell cycle arrest. J Virol. 2000;74:8159–8165. doi: 10.1128/jvi.74.17.8159-8165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy D, Molnar A, Lucioli A, Szittya G, Hornyik C, Tavazza M, Burgyan J. A viral protein suppresses RNA silencing and binds silencing-generated, 21- to 25-nucleotide double-stranded RNAs. Embo J. 2002;21:3070–3080. doi: 10.1093/emboj/cdf312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Swanson SK, Manel N, Florens L, Washburn MP, Skowronski J. Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection. PLoS Pathog. 2008;4:e1000059. doi: 10.1371/journal.ppat.1000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopak K, de Noronha C, Yonemoto W, Greene WC. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Molecular cell. 2003;12:591–601. doi: 10.1016/s1097-2765(03)00353-8. [DOI] [PubMed] [Google Scholar]
- Subbramanian RA, Yao XJ, Dilhuydy H, Rougeau N, Bergeron D, Robitaille Y, Cohen EA. Human immunodeficiency virus type 1 Vpr localization: nuclear transport of a viral protein modulated by a putative amphipathic helical structure and its relevance to biological activity. J Mol Biol. 1998;278:13–30. doi: 10.1006/jmbi.1998.1685. [DOI] [PubMed] [Google Scholar]
- Sung TL, Rice AP. miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS Pathog. 2009;5:e1000263. doi: 10.1371/journal.ppat.1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toohey K, Wehrly K, Nishio J, Perryman S, Chesebro B. Human immunodeficiency virus envelope V1 and V2 regions influence replication efficiency in macrophages by affecting virus spread. Virology. 1995;213:70–79. doi: 10.1006/viro.1995.1547. [DOI] [PubMed] [Google Scholar]
- Triboulet R, Mari B, Lin YL, Chable-Bessia C, Bennasser Y, Lebrigand K, Cardinaud B, Maurin T, Barbry P, Baillat V, Reynes J, Corbeau P, Jeang KT, Benkirane M. Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science. 2007;315:1579–1582. doi: 10.1126/science.1136319. [DOI] [PubMed] [Google Scholar]
- Wang L, Mukherjee S, Jia F, Narayan O, Zhao LJ. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and trans-activation of viral long terminal repeat. J Biol Chem. 1995;270:25564–25569. doi: 10.1074/jbc.270.43.25564. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kato N, Jazag A, Dharel N, Otsuka M, Taniguchi H, Kawabe T, Omata M. Hepatitis C virus core protein is a potent inhibitor of RNA silencing-based antiviral response. Gastroenterology. 2006;130:883–892. doi: 10.1053/j.gastro.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Ward J, Davis Z, DeHart J, Zimmerman E, Bosque A, Brunetta E, Mavilio D, Planelles V, Barker E. HIV-1 Vpr triggers natural killer cell-mediated lysis of infected cells through activation of the ATR-mediated DNA damage response. PLoS Pathog. 2009;5:e1000613. doi: 10.1371/journal.ppat.1000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil AF, Ghosh D, Zhou Y, Seiple L, McMahon MA, Spivak AM, Siliciano RF, Stivers JT. Uracil DNA glycosylase initiates degradation of HIV-1 cDNA containing misincorporated dUTP and prevents viral integration. Proc Natl Acad Sci U S A. 2013;110:E448–457. doi: 10.1073/pnas.1219702110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Duus KM, Friedrich TD, de Noronha CM. The HIV1 protein Vpr acts to promote G2 cell cycle arrest by engaging a DDB1 and Cullin4A-containing ubiquitin ligase complex using VprBP/DCAF1 as an adaptor. J Biol Chem. 2007;282:27046–27057. doi: 10.1074/jbc.M703955200. [DOI] [PubMed] [Google Scholar]
- Wissing S, Galloway NL, Greene WC. HIV-1 Vif versus the APOBEC3 cytidine deaminases: an intracellular duel between pathogen and host restriction factors. Mol Aspects Med. 2010;31:383–397. doi: 10.1016/j.mam.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JF, Shen W, Liu NZ, Zeng GL, Yang M, Zuo GQ, Gan XN, Ren H, Tang KF. Down-regulation of Dicer in hepatocellular carcinoma. Med Oncol. 2011;28:804–809. doi: 10.1007/s12032-010-9520-5. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Emerman M. Capsid is a dominant determinant of retrovirus infectivity in nondividing cells. J Virol. 2004;78:5670–5678. doi: 10.1128/JVI.78.11.5670-5678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung ML, Bennasser Y, Myers TG, Jiang G, Benkirane M, Jeang KT. Changes in microRNA expression profiles in HIV-1-transfected human cells. Retrovirology. 2005;2:81. doi: 10.1186/1742-4690-2-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung ML, Bennasser Y, Watashi K, Le SY, Houzet L, Jeang KT. Pyrosequencing of small non-coding RNAs in HIV-1-infected cells: evidence for the processing of a viral-cellular double-stranded RNA hybrid. Nucleic Acids Res. 2009;37:6575–6586. doi: 10.1093/nar/gkp707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P, Yu XF. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yuan YR, Pei Y, Lin SS, Tuschl T, Patel DJ, Chua NH. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes & development. 2006;20:3255–3268. doi: 10.1101/gad.1495506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Roshal M, Li F, Blackett J, Planelles V. Upregulation of survivin by HIV-1 Vpr. Apoptosis. 2003;8:71–79. doi: 10.1023/a:1021653119934. [DOI] [PubMed] [Google Scholar]
- Zimmerman ES, Sherman MP, Blackett JL, Neidleman JA, Kreis C, Mundt P, Williams SA, Warmerdam M, Kahn J, Hecht FM, Grant RM, de Noronha CM, Weyrich AS, Greene WC, Planelles V. Human immunodeficiency virus type 1 Vpr induces DNA replication stress in vitro and in vivo. J Virol. 2006;80:10407–10418. doi: 10.1128/JVI.01212-06. [DOI] [PMC free article] [PubMed] [Google Scholar]