Abstract
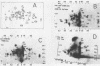
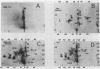
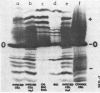
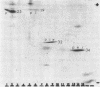
The polypeptide products formed in two cell-free protein-synthetic systems programmed with encephalomyocarditis (EMC) virus ribonucleic acid (RNA) have been compared with the virus-specific proteins found in EMC-infected cells and with the capsid proteins of the purified virion. Tryptic peptides of 35S-methioninelabeled proteins from these three sources were compared by co-chromatography and electrophoresis and by isoelectric focussing. Fifty-two methionine-containing peptides were resolved in digests of material from infected cells, of which about one-third were also clearly present in digests of the virion capsid proteins. The product formed in response to EMC RNA in cell-free systems from Krebs mouse ascites tumor cells yielded 26 to 29 such peptides. Most of these peptides were shown to behave identically with virus-specific peptides from infected cells, whereas just under half of them appeared to be identical with peptides from the virion capsid proteins. The product formed in response to EMC RNA in the L-cell cell-free system was similar, whereas six additional EMC-specific peptides were detected in mixed Krebs L-cell systems. The results indicate that the EMC RNA genome is partially translated in the mouse cell-free systems used to yield products containing both virion capsid and virus-specific noncapsid polypeptides.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awdeh Z. L., Williamson A. R., Askonas B. A. Isoelectric focusing in polyacrylamide gel and its application to immunoglobulins. Nature. 1968 Jul 6;219(5149):66–67. doi: 10.1038/219066a0. [DOI] [PubMed] [Google Scholar]
- Burness A. T. Purification of Encephalomyocarditis virus. J Gen Virol. 1969 Sep;5(2):291–303. doi: 10.1099/0022-1317-5-2-291. [DOI] [PubMed] [Google Scholar]
- Burness A. T. Ribonucleic acid content of encephalomyocarditis virus. J Gen Virol. 1970 Mar;6(3):373–380. doi: 10.1099/0022-1317-6-3-373. [DOI] [PubMed] [Google Scholar]
- FAULKNER P., MARTIN E. M., SVED S., VALENTINE R. C., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. 2. The isolation, crystallization and chemical characterization of mouse encephalomyocarditis virus. Biochem J. 1961 Sep;80:597–605. doi: 10.1042/bj0800597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- KERR I. M., MARTIN E. M., HAMILTON M. G., WORK T. S. The initiation of virus protein synthesis in Krebs ascites tumor cells infected with EMC virus. Cold Spring Harb Symp Quant Biol. 1962;27:259–269. doi: 10.1101/sqb.1962.027.001.025. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Cohen N., Work T. S. Factors controlling amino acid incorporation by ribosomes from krebs II mouse ascites-tumour cells. Biochem J. 1966 Mar;98(3):826–835. doi: 10.1042/bj0980826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Martin E. M. Virus protein synthesis in animal cell-free systems: nature of the products synthesized in resonse to ribonucleic acid of encephalomyocarditis virus. J Virol. 1971 Apr;7(4):438–447. doi: 10.1128/jvi.7.4.438-447.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn E. D., Holland J. J. Synthesis and cleavage of enterovirus polypeptides in mammalian cells. J Virol. 1970 Mar;5(3):358–367. doi: 10.1128/jvi.5.3.358-367.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN E. M., MALEC J., SVED S., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. 1. Encephalomyocarditis virus in Krebs II mouse-ascites-tumour cells. Biochem J. 1961 Sep;80:585–597. doi: 10.1042/bj0800585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M., Korner A. Mammalian cell-free protein synthesis directed by viral ribonucleic acid. Eur J Biochem. 1970 Dec;17(2):328–338. doi: 10.1111/j.1432-1033.1970.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Salaman M. R., Williamson A. R. Isoelectric focusing of proteins in the native and denatured states. Anomalous behaviour of plasma albumin. Biochem J. 1971 Mar;122(1):93–99. doi: 10.1042/bj1220093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent J. R., Vadlamudi B. P. Electrophoresis of peptides on thin layers of silica gel. Anal Biochem. 1968 Oct 24;25(1):583–587. doi: 10.1016/0003-2697(68)90137-1. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A., Mathews M. B. Translation of RNA from encephalomyocarditis virus in a mammalian cell-free system. Nature. 1970 Jan 10;225(5228):184–187. doi: 10.1038/225184a0. [DOI] [PubMed] [Google Scholar]