Abstract
Myocardial infarction (MI) is the most common cause of heart failure (HF), the leading cause of death in the developed world. Oxidative stress due to excessive production of reactive oxygen species (ROS) plays a key role in the pathogenesis of cardiac remodeling leading to HF. NADPH oxidase with Nox2 as the catalytic subunit is a major source for cardiac ROS production. Nox2-NADPH expression is significantly increased in the infarcted myocardium, primarily in neutrophils, macrophages and myocytes. Moreover, mice lacking the Nox2 gene are protected from ischemic injury, implicating Nox2 as a potential therapeutic target. RNAi-mediated gene silencing holds great promise as a therapeutic owing to its high specificity and potency. However, in vivo delivery hurdles have limited its effective clinical use. Here, we demonstrate acid-degradable polyketal particles as delivery vehicles for Nox2-siRNA to the post-MI heart. In vitro, Nox2-siRNA particles are effectively taken up by macrophages and significantly knockdown Nox2 expression and activity. Following in vivo intramyocardial injection in experimental mice models of MI, Nox2-siRNA particles prevent upregulation of Nox2 and significantly recovered cardiac function. This study highlights the potential of polyketals as siRNA delivery vehicles to the MI heart and represents a viable therapeutic approach for targeting oxidative stress.
Keywords: nanoparticles, macrophages, myocardial infarction, gene expression, gene silencing
1. Introduction
Heart failure (HF) is the leading cause of death in the developed world, and myocardial infarction (MI) is the dominant cause [1]. Ischemic injury and consequent loss of viable myocardium is followed by adverse cardiac remodeling which includes progressive changes in the molecular and structural components of the myocardium [2] that eventually lead to cardiac dysfunction. An early step in this process is the inflammatory reaction that begins with the influx of neutrophils and macrophages in the infarcted area of the myocardium followed by invasion of myofibroblasts that deposit non-contractile scar tissue [3]. Production of excessive amounts of reactive oxygen species (ROS) is a key event involved in this pathogenesis [4–7]. At high levels of ROS, cells undergo oxidative stress leading to many of the injury associated changes: proinflammatory cytokine release, cardiomyocyte apoptosis, interstitial fibrosis, fibroblast proliferation and myocyte hypertrophy [8, 9]. Although surgical reperfusion, angioplasty and thrombolysis have been traditionally used to restore blood flow to the ischemic myocardium, re-introduction of coronary flow triggers further production of ROS, a phenomenon known as reperfusion injury, causing further damage to the myocardium [5, 10]. Scavenging ROS have received great attention due to their contributions to reperfusion injury as evidenced by animal studies where overexpression or delivery of antioxidants has reduced myocardial injury in the setting of ischemia and reperfusion [11–14].
Potential sources of ROS in the heart include intracellular sources such as mitochondria and uncoupled nitric oxide synthase, as well as membrane bound proteins such as the Nicotinamide adenine denucleotide phosphate (NADPH) oxidase and xanthine oxidase [15, 16]. Among these, NADPH oxidase, with Nox2 as the catalytic subunit, is a major source for cardiac superoxide levels (O2−) production [8, 9, 16–18]. After MI, NADPH oxidase expression is significantly increased in the infarcted myocardium, primarily in neutrophils, macrophages and myocytes in both animal models and human patients [8, 18–20]. Complete knockout of Nox2 prevents cardiomyocyte apoptosis and adverse remodeling following experimental MI in mice, and improves cardiac function [8]. Moreover, Nox2-NADPH oxidase is implicated in a direct functional role in the pathogenesis of Angiotensin-II induced cardiac hypertrophy [17]. In another study, Nox2−/− mice were shown to exhibit attenuated interstitial fibrosis following aortic constriction, suggesting a possible role for Nox2-NADPH on cardiac remodeling post-MI [21]. These studies highlight the therapeutic potential of targeting Nox2-NADPH in the remodeling heart in order to prevent heart failure in MI patients. Despite these results, clinical studies are lacking due to the unavailability of a Nox2-specific inhibitor. This is critical as other members of the Nox family, specifically Nox4, offer protection against cardiac remodeling and hypertrophy [22, 23]. With specific inhibitors to NADPH oxidase lacking, silencing Nox2-NADPH oxidase gene expression in macrophages, the primary responders to ischemic injury, is an exciting potential therapeutic target to improve cardiac function and prevent progression heart failure following MI.
RNA-mediated silencing (siRNA) of gene expression holds great promise as therapeutics owing to high specificity and potency. However, delivery of siRNA has been challenging and prevented the successful translation of RNAi therapeutics into the clinic [24, 25]. Renal clearance and serum RNAses considerably reduce the half-life of naked siRNA in the blood stream [25]. Additionally, siRNA is highly membrane impermeable, and even upon endocytosis, sequestration by lysosomes make delivery into the cytosol difficult [25, 26]. Transfection of macrophages is especially challenging due to the presence of potent degradative enzymes that disrupt nucleic acid integrity [27]. Chemical modifications that increase the stability of siRNA have had limited success due to often compromised RNA silencing activity and the expensive nature of these modifications [26]. Evolved delivery methods include complexing of siRNA to cationic lipids or polymers [26, 28]. However, inherent toxicity and the positively charged surface characteristics that elicit immune responses deem such vehicles undesirable for in vivo disease applications [28, 29], especially for an inflammatory disease such as post-MI cardiac dysfunction.
Here, we demonstrate use of acid-degradable polymers, polyketals [30–32], as delivery vehicles for Nox2 specific siRNA to the post-MI environment. Polyketals have controllable release kinetics and neutral degradation products and therefore cause minimal inflammatory responses [33]. The polyketal PK3 was chosen for this application due to its fast hydrolysis rate [30, 31, 34]. An endosomal disruptive molecule, chloroquine, was co-encapsulated with siRNA in order to enhance the endosomal escape of delivered siRNA [30]. Nox2 siRNA was delivered to macrophages and Nox2 expression and activity were evaluated, as well as whether this could be used as a delivery vehicle in vivo following MI.
2. Materials and Methods
2.1 PK3 synthesis
PK3 was synthesized as described in Sungmun Lee et al [30]. Briefly, the diols, cyclohexanedimethanol and 1,5-pentanediol were dissolved in distilled benzene and heated to 100°C. Recrystallized p-toluenesulfonic acid (PTSA) was dissolved (~1 mg) in ethyl acetate and added to the benzene solution to catalyze the reaction. The polymerization reaction was initiated by the addition of equimolar 2,2-diethoxypropane (DEP). Additional 2,2-dimethoxy propane (DMP) and benzene were subsequently added to the reaction to compensate for loss of volume in the form of Ethanol/methanol and the solvent benzene that had distilled off. After 48 h, the reaction was stopped with triethylamine and isolated by precipitation in cold hexanes. The solid polymer was then filtered off, rinsed in hexanes and vacuum dried prior to storage at −20°C. Polymer molecular weight/Polydispersity was determined by gel permeation chromatography.
2.2 Preparation and characterization of siRNA loaded PK3 particles
Double stranded Nox2-siRNA (mouse) (sense strand: 5'AGAGUUUGGAAGAGCAUAAUUUAGA3') and a universal negative control siRNA (siNeg) were custom made (IDT) to contain a fluorescent FITC label. Fluorescent (FI)-siNox2 or FI-siNeg was ion-paired to the cationic lipid N-[1-(2,3-Dioleoyloxy)propyl]-N,N,Ntrimethylammonium methylsulfate (DOTAP) as shown in Figure 1. Briefly, 1 mg siRNA in water and 2.2 mg of DOTAP dissolved in dichloromethane (DCM) were brought to one phase by addition of 1.05 mL of methanol. Following 15 min incubation, an additional 0.5 mL of water and DCM were added and the mixture was vortexed, and centrifuged at 750 rpm for 5 min. The siRNA:DOTAP complex in the bottom organic layer was encapsulated in PK3 via an oil/water single emulsion procedure, using DCM as the oil phase and polyvinyl alcohol (PVA) as the surfactant stabilizer [31]. Empty particles containing DOTAP not ion-paired to siRNA were also prepared to control for any effect on cells from DOTAP. Next, 1 mL of DCM containing ion paired FI-siRNA was added to 40 mg of PK3 with 1 mg of chloroquine free base. This solution was homogenized into 8 mL of 5% (w/v) PVA solution at the highest setting in the Power Gen 500 (Fisher Scientific) for 30 seconds, and sonicated at an intermediate speed (Sonic dismembrator model 100, Fisher Scientific) with 10 pulses of 1 sec duration. The emulsion was then dispersed in a 20mL 0.5% PVA solution and stirred for a period of 4–5 hours to allow the DCM to evaporate. The resulting particles were isolated by centrifugation (15000 rpm, 20min), washed three times, freeze-dried and stored in −20°C until further use. Particle size and shape were determined by scanning electron microscopy (Zeiss SEM Ultra60).
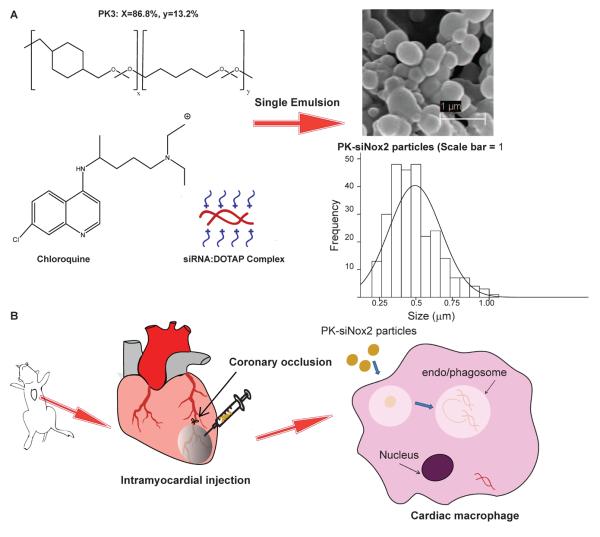
Figure 1. Schematic of PK-siNox2 particle formulation and delivery.
(A) Ion-paired siRNA:DOTAP and endosomal disruptive agent chloroquine are encapsulated into the PK3s via a single emulsion/solvent evaporation procedure generating submicron particles (500 ± 175 nm). (B) The PK3s encapsulating siRNA are intramuscularly injected into mice hearts following permanent coronary occlusion (MI) surgery. The particles are taken up my macrophages in vivo with high efficiency due to their ability to protect siRNA from serum proteins and stimulate phagocytosis and/or endocytosis. Once taken up, they degrade in the acidic environment within these compartments due to the acid-sensitive ketal linkages in PK3 (also aided by chloroquine) and escape the phagosome/endosome via a colloid osmotic mechanism to release siRNA into the cytoplasm.
2.3 Quantitation of siRNA loading efficiency of PK3 particles
FI-siNox2 particles, FI-siNeg siRNA particles and empty PK particles were prepared as described above. In order to determine loading efficiency of siRNA within PK3, 2–3mg of particles were dispersed in a 1N HCl solution and incubated for 1 hr to allow for complete hydrolysis. Afterwards, the solution was neutralized with 1N NaOH solution, and the fluorescence of the total FI-siRNA released from the particles was measured (ex/em=494/510nm) using a fluorescent plate reader (Biotek Synergy 2). A fluorescent standard curve previously constructed using the FI-siRNA was compared against the obtained fluorescent intensity blank subtracted from any background fluorescence from empty PK particles in order to calculate the loading efficiency of prepared particles.
2.4 Particle uptake by macrophages
RAW 264.7 macrophages obtained from American Type Culture Collection (ATCC number: TIB-71) were maintained at 37°C under a humidified atmosphere of 5% CO2 in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% (v/v) fetal bovine serum (FBS), supplemented with penicillin (100U/mL) and streptomycin (100mg/mL) and 2mM L-Glutamine. For flow cytometry, macrophages (1×106 cells) were incubated with PK-FI-siNox2 (1μg siRNA) for 24 hours. Cells were then washed three times with ice cold phosphate buffered saline (PBS) and scraped into tubes for flow cytometry. Untreated control cell population was used to gate population of interest and the fluorescent population was measured by flow cytometer (FACSCalibur, Becton Dickinson) using a laser for fluorescein (lex/lem = 494/510 nm) and analyzed using FlowJo (TreeStar, Inc).
In order to confirm the intracellular distribution of siRNA delivered by PK3 particles, we used laser scanning confocal microscopy (Zeiss LSM 510 META). RAW264.7 macrophages treated with PK-FI-siNox2 particles for 6 hours (1μg siRNA). Cells were then washed in PBS three times and stained with DAPI for nuclei and rhodamine-phalloidin for actin filaments.
2.5 In vitro delivery of Nox2-siRNA particles
For gene expression studies, RAW264.7 macrophages were plated in 6-well plates at a density of 1×106 cells/well in 10% FBS DMEM media and left to adhere overnight. The following day, cells were treated with PK-siNox2, PK-siNeg, or empty PK3 particles at a concentration of particles equivalent to 1 μg siRNA/well or equivalent amount of empty particles. Following 24 hours of treatment, the cells were harvested and RNA extracted using Trizol (Invitrogen) for gene expression analysis. For assessment of functional activity of Nox2-NADPH in macrophages following treatment, RAW macrophages were plated at a density of 2×105 cells/well in 24-well plates and treated with 1μg siRNA/well or equivalent amount of empty particles as above for 72 hours following which cells were subjected to analysis of O2−production.
2.6 Gene expression
Total RNA from cells was isolated using Trizol (Invitrogen) according to the manufacturer's protocol. Complementary DNA (cDNA) was synthesized using SuperScript III kit (Invitrogen). Quantitative real-time PCR (qRTPCR) was performed using Power SYBR Green (Invitrogen) master mix with Applied Biosystems StepOne Plus real time PCR system and the following primer pairs were used: Nox2, 5' GTT GGG GCT GAA TGT CTT CCT CTT T 3' (forward primer) and 5' CCA CAT ACA GGC CCC CTT CAG 3' (reverse primer); 18s rRNA, TTCCTTACCTGGTTGATCCTGCCA (forward primer) and AGCGAGCGACCAAAGGAACCATAA (reverse primer). Nox2 gene expression levels were normalized relative to the endogenous housekeeping gene 18s rRNA and expressed as a fold change compared to expression levels in untreated cells.
2.7 Detection of extracellular superoxide in vitro
To determine Nox2 activity, extracellular production of O2− following stimulation with phorbol-12-myristate 13-acetate (PMA) was measured. To determine levels of O2− following treatments in vitro, quantitative dihydroethidium (DHE) measurements were taken. DHE reacts with O2− to form a cell impermeable, characteristic fluorescent product, 2-hydroxyethidium (ex=360nm, em=567nm) that can be measured quantitatively using HPLC [35–39] and/or a fluorescent plate reader. Following 72 hours of particle treatment in 24-well plates, media was aspirated from the wells containing RAW macrophages and washed carefully with fresh cold Krebs-Hepes buffer (KHB) at a pH of 7.35. Then KHB was added to all wells and 10 μM PMA (Sigma) added to the appropriate wells. Following incubation with PMA for 10 min at 37°C, 20 μM DHE (Sigma) was added to both PMA stimulated and unstimulated wells under dark conditions and incubated for 20 minutes while obtaining kinetic readings using a fluorescent plate reader (Biotek Synergy 2), after which 100 μl of the extracellular buffer from each well was taken in 300 μl of methanol for quantitative DHE-HPLC (reverse phase-HPLC over an acetonitrile gradient, (ex=360nm and em=570 nm) analysis [38].
2.8 Myocardial infarction and particle injection
A randomized and blinded study was conducted using adult male C57BL/6 mice 8 weeks of age weighing 25g. Mice were assigned to treatment groups (n=7–10) using a random number generator and the animal surgeon was only given letter codes to identify groups. While one group was subjected to sham surgery, the other four groups received myocardial infarction. Animals were subjected to myocardial infarction surgeries as described [33, 40]. Briefly, the animals were anesthetized (1–3% isoflurane) and following tracheal intubation, the heart was exposed by separation of the ribs. Myocardial infarction was performed by ligation of the left anterior descending coronary artery. For groups receiving particle injections, immediately after coronary artery ligation, 50 μL of one of the following was injected into the cyanotic ischemic zone (3 locations) through a 30-gauge needle while the heart was beating: empty PK3 particles, PK-siNeg particles or PK-siNox2 particles. The dose of siRNA injected was 5μg/kg or the corresponding amount of empty PK3 particles. Following injection, the chests were closed and animals were allowed to recover on a heating pad until functional assessments were made at 3 days following MI surgery using echocardiography. These studies conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and all animal studies were approved by Emory University Institutional Animal Care and Use Committee.
2.9 In vivo gene expression
Animals were then sacrificed and left ventricle tissue was harvested and subjected to Trizol extraction for RNA isolation. Following reverse transcription of obtained left ventricle tissue RNA, the cDNA was subjected to qPCR for the gene expression analysis of Nox2 and the house keeping gene 18s rRNA. The copy numbers of Nox2 mRNA present in the tissue per million copies of 18s rRNA was determined using the quantitative standard curve method and results were normalized to copy number ratios from sham animals in order to minimize batch-to-batch variability in tissue processing.
2.10 Echocardiography
Anesthetized rats were subjected to echocardiography at 3 days following MI surgery. Short axis values of left ventricular end systolic (ES) and end diastolic (ED) dimension were obtained using a Vevo 770 small animal ultrasound system (Visualsonics). An average of 3 consecutive cardiac cycles was used for each measurement and was performed three times in an investigator-blinded manner. Fractional shortening was calculated as (end-diastolic diameter − end-systolic diameter)/end-diastolic diameter and expressed as a percentage.
2.11 Statistics
All statistical analyses were performed using Graphpad Prism 5 software. Quantitative results were presented as means ± SEM. Statistical comparisons were performed by one way analysis of variance (ANOVA) followed by the appropriate post-test as described in the figure legends. P values of less than 0.05 were considered significant.
3. Results
3.1 Characterization of PK-siRNA particles
The encapsulation efficiency of siRNA within the PK3 particles was evaluated following ion-pairing with DOTAP as well as the final single oil/water emulsion step. Efficiency of ion-pairing was > 98% as measured by the fluorescence reading of the remaining FI-siRNA in the aqueous phase following extraction of DOTAP:siRNA into the organic phase. After particles were generated, hydrolysis confirmed 10 μg of siRNA per 1 mg of particle, or roughly 40% encapsulation efficiency. Further, particles were analyzed by SEM and the particle diameter measurements were made and averaged using ImageJ. As the representative image and histogram in Figure 1 demonstrate, particles ranged from 200–1200 nm with an average size of 500 ± 175 nm.
3.2 Particle uptake by macrophages
Internilization of PK-siNox2 particles by macrophages was determined by flow cytometry. Macrophages treated with FI-siNox2 loaded PK3 particles (at 1 μg siRNA/well) for 24 hours demonstrated >80% uptake as shown in the representative histogram in Figure 2A. Further, confocal microscopy confirmed that the particles were not just sticking to the cell surface, but were internalized and localized within the cellular cytoplasm as shown in the representative 2D image and z-stack image (Figures 2B&C).
Figure 2. In vitro Particle uptake by macrophages.
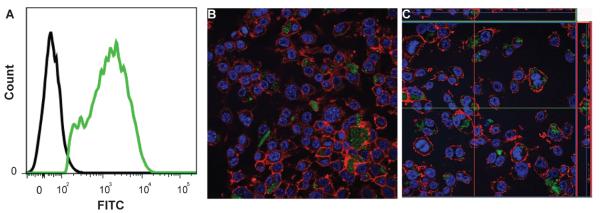
RAW macrophages were treated with FITC labeled siRNA loaded PK3 particles. (A) Following 24 hours of treatment, flow cytometry was used to determine the percentage of FITC positive cells (Green) that are indicative of percent uptake of PK-FI-siNox2 particles compared to the control cell population (Black). Following 6 hours of treatment, cells were imaged using confocal microscopy to visualize cytoplasmic localization of siRNA. A representative 2D image (B) and Z-stack image (C) confirm internalized siRNA within macrophages. FI-siNox2 (green). Nucleus (Blue, DAPI), Actin filaments (Red, Rhodamine phalloidin).
3.3 In vitro gene knockdown
To determine whether Nox2-siRNA particles knockdown Nox2 mRNA expression in vitro, we investigated the efficacy of Nox2-siRNA particles in the mouse macrophage cell line RAW 264.7. Following 24 hours of treatment, total RNA was isolated from cells and Nox2 mRNA expression levels were analyzed via qRTPCR. Results were normalized to Nox2 expression levels in untreated macrophages. Figure 3 demonstrates that PK-siNox2 particles knocked down Nox2 mRNA expression by 41.3 ± 10.0% compared to the Nox2 expression levels in the untreated macrophage group (p<0.05), empty PK3 (p<0.01) and PK-siNeg (p<0.05) treatment groups. The empty PK3 particle and PK-siNeg particle treatment groups did not show any significant difference from the untreated cells.
Figure 3. In vitro Nox2 mRNA expression.

Grouped data (mean ± SEM; n=3 per group) from 1 day following treatment of RAW macrophages. There was a significant decrease in Nox2 mRNA expression only in the PK-siNox2 treatment group compared to the untreated, empty PK3 and PK-siNeg particle treated groups. Gene expression was evaluated by qRTPCR using a ΔΔCT method, and the results were normalized to 18S expression and reported as fold changes in mRNA expression (*p<0.05, **p<0.01; One-way ANOVA followed by Newman-Keuls Multiple Comparison post-test).
3.4 In vitro functional knockdown
To determine whether gene knockdown by Nox2-siRNA particles resulted in functional changes, we treated RAW macrophages for 72 hours with similar particle formulations as in the gene expression studies and then stimulated with PMA to induce O2− production. The fluorescent DHE dye was then added in to the cells to determine kinetic activity of Nox2-mediated O2− production over a 20 minute period of time. The fluorescent intensity was expressed as fold changes in O2− production as normalized to basal O2− levels. As shown in figure 4A, the traces from all treatment groups except PK-siNox2 treatment exhibited similar levels of fold increase in O2− production while PK-siNox2 treatment showed little fluorescence over the entire 20 minute reading. These results were further confirmed by quantitative DHE-HPLC that PK-siNox2 treated macrophages demonstrated a significant reduction in Nox2 activity (p<0.05) compared to empty PK3 particle treatment.
Figure 4. In vitro Nox2-NADPH activity.
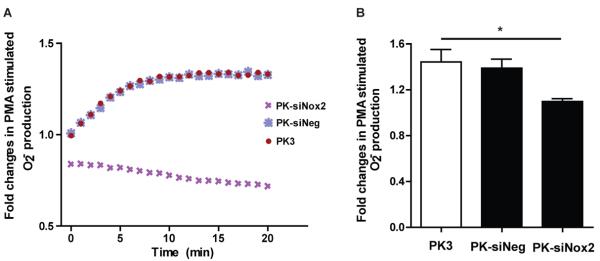
Grouped data (mean ± SEM; n=4 per group) from 3 days. (A) Kinetic measurement of PMA-stimulated O2− production from macrophages using DHE fluorescence. (B) There was a significant decrease in the production of O2− in PK-siNox2 treated macrophages compared to the empty PK3 treatment group as quantitatively measured by DHE-HPLC method (*p<0.05 vs. empty PK3; one-way ANOVA followed by Dunnett's Multiple Comparison post-test).
3.5 PK-siNox2 delivery in vivo
To determine the in vivo efficiency of siRNA delivery using polyketal particles, adult male C57BL/6 mice were randomized into 5 treatment groups. While one group was subjected to sham surgery, the other four groups received MI surgeries followed by either no injections (MI) or injections of either empty PK3 particles (MI + Empty PK3), negative control siRNA encapsulating PK3 particles (MI + PK-siNeg) or siNox2 containing PK3 particles (MI + PK-siNox2) (n≥8 for each group, N=47 total). At 3-days following injury, the expression of Nox2 at the mRNA level increased significantly (P< 0.01) in MI group by 2.50±0.30 fold compared to sham mice. The treatment groups receiving Empty PK3 or PK-siNeg particle injections following MI showed no differences in Nox2 mRNA expression compared to the group receiving MI alone. In contrast, mice receiving PK-siNox2 particles following MI demonstrated a significantly lower levels of Nox2 mRNA expression than the MI group (P<0.05) (Figure 5). In order to determine the specificity of siRNA delivery towards the Nox2 isoform, Nox4 mRNA expression levels were also analyzed. There was a trend towards an increase in Nox4 mRNA expression in all animals that received MI although it was not statistically significant. There was no observed knockdown of Nox4 mRNA expression in any treatment groups (Supplemental figure 2).
Figure 5. In vivo Nox2 mRNA expression.

Grouped data (mean ± SEM; n≥8 per group) from 3 days. There was a significant increase in Nox2 mRNA expression in animals that received an MI compared to sham. Among treatment groups, there was a significant decrease in mRNA expression only in the PK-siNox2 treated mice compared with untreated MI group. Gene expression was evaluated by qRTPCR using the quantitative standard curve method, and the results were normalized to 18S levels and reported as fold changes in copy number of mRNA levels compared to sham animals (*p<0.05; **p<0.001 vs. MI; one-way ANOVA followed by Dunnett's Multiple Comparison post-test).
3.6 Cardiac function following myocardial infarction
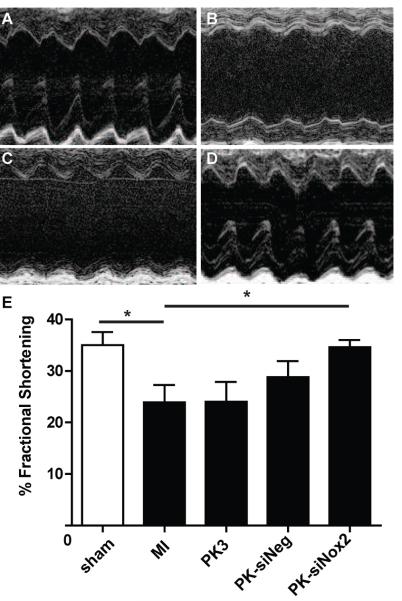
To determine the effect of sustained PK-siNox2 delivery on cardiac function following acute MI, echocardiography data was collected three days after MI surgery as described in methods. As shown in Figure 6, MI significantly reduced cardiac function (p<0.05) as measured in absolute change in fractional shortening 3 days post-injury compared to sham animals. While there was no effect of empty PK3 or PK-siNeg particles, PK-siNox2 particles significantly (p<0.05) improved function, restoring it to sham levels.
Figure 6. Echocardiographic measurement of function.
Grouped data (mean ± SEM; n≥4 per group) from 3 days. There was a significant decrease in function animals that received an MI compared to sham. Among treatment groups, there was a significant increase in function only in the PK-siNox2 treated mice compared with untreated MI group (*p<0.05; ** p<0.001 vs. MI; one-way ANOVA followed by Dunnett's Multiple Comparison post-test).
4. Discussion
Over 1.2 million new and recurring MI events occur every year and are responsible for 1 in 6 deaths in the United States. Even with improvements in clinical intervention, a significant amount of MI patients develop heart failure [1]. Heart transplant is the only definitive treatment for HF, and given the shortage of donor organs and the continuous battles with organ rejections, there is a significant need for effective cardioprotective therapies. Considerable evidence supports a role for oxidative stress due to excessive ROS such as O2− in the progression of HF [4, 5, 15]. Chronic antioxidant treatment following MI in animal models improves myocyte survival, attenuates ventricular remodeling, and leads to partial preservation of left ventricular function [11, 13]. While chronic antioxidant therapy may be feasible, the damage to the myocardium is local and oxidant signaling may be critical for homeostasis in many tissues. Therefore a localized and sustained antioxidant treatment that could be achieved by RNAi silencing during the critical earlier inflammatory stage following MI could be beneficial for the treatment of acute MI.
NADPH oxidases are major sources of O2− in the heart. [15, 16]. Originally identified in phagocytes of the innate immune system, they play a critical role in host defense by releasing a burst of O2− from molecular oxygen using NADPH as an electron donor. The prototypic NADPH oxidase of the phagocytes is a multisubunit enzyme complex consisting of a membrane-bound cytochrome b558 composed of p22phox and the catalytic subunit gp91phox (Nox2), and in association with four cytosolic regulatory subunits following activation. Over the past decade, there has been much interest in the cardiovascular NADPH oxidases [8, 9]. A family of gp91phox homologues termed Nox (Nox1–5) form the basis of distinct NADPH oxidases. Nox2 is expressed in macrophages, cardiomyocytes, fibroblasts and endothelial cells and significantly upregulated in the myocardium following MI [8, 9]. Despite the fact that there are 5 distinct Nox family members, there are no specific inhibitors for any subtype. These proteins all have different functions in many cell types and there is strong evidence suggesting that broad inhibition of NADPH oxidase activity can exacerbate ischemic injury [23]. Therefore great care is needed to ensure specific targeting in relevant cells.
In this report, we successfully encapsulated Nox2-specific siRNA into the acid sensitive polyketal PK3 particle for silencing of the Nox2 gene in cardiac macrophages and inactivation of NADPH oxidase. Polyketals (PKs) are a class of delivery vehicles formulated from a class of polymers which contain pH sensitive, hydrolyzable ketal linkages in their backbone. Unlike the predominantly polyester based delivery vehicles currently of use, PKs do not generate acidic degradation products as they degrade into acetone and diols [31]. The polymerization strategy is such that it is possible to form polymers between any diol and 2,2-dimethoxypropane affording great flexibility for generating biomaterials with properties such as variable hydrolysis and pH sensitivities suitable for a variety of applications [30–34, 40]. The properties of PKs can be easily modified to alter particle size, shape and porosity. Among these, the polyketal copolymer PK3 formed by reacting the diols cyclohexanedimethanol and 1,5-pentanediol with 2,2-diethoxypropane makes an excellent delivery vehicle for siRNA due to its faster hydrolysis at the acidic pH of the endosome. In a previous study where PK3 was used to deliver TNF-α siRNA to liver macrophages in vivo, a hydrolysis half-life of 1.8 days at pH 4.5 and 39 days at pH 7.4 was reported [30]. Additionally, PK3 is a hard material that is water insoluble and can maintain its integrity in vivo due to the high energy cost of being exposed to water, providing serum stability [30, 34]. Published studies from our laboratory demonstrate that polyketal nanoparticles are retained in the myocardium following injection and are stable at neutral pH levels [33, 40]. When engaged by macrophages, present in high quantities during MI, particles are taken up and trafficked into phagosomes/endosomes where they degrade due to the acidic environment. Although the mechanism of release of siRNA is not clearly known, it is speculated that after phagocytosis/endosomes, these particles hydrolyze and potentially cause an osmotic imbalance within these compartments, leading to release of siRNA into the cytoplasm in a timely manner.
As the schematic in Figure 1A illustrates, siRNA was loaded into PK3 particles in two steps. Firstly, the siRNA is ion paired to the cationic lipid DOTAP and the siRNA:DOTAP complex is extracted into the organic layer with greater than 95% efficiency. Chloroquine was then added to the complex and subsequently subjected to an oil-in-water single emulsion/solvent evaporation procedure to generate particles ranging from 200–1200 nm and averaging 500 nm (Figure 1A). Addition of another pH sensitive molecule such as chloroquine to these pH sensitive polymeric vehicles enhances their ability to be hydrolyzed at low pH of the endosomes/phagosomes while maintaining their integrity at the physiological pH of 7.4. Further, the weak base chloroquine gets protonated in the acidic environment of endo/phagosomal compartments, triggering swelling and destabilization of their membranes to facilitate release of siRNA into the cytoplasm [41]. Since chloroquine can inhibit acidification and maturation of endosomes, it can also protect siRNA from degradation. Additionally, complexing siRNA with a cationic lipid such as DOTAP can enhance siRNA transfection ability by increasing siRNA stability and aiding in endosomal escape [42–44]. Further, in a previous study from our laboratory, complexing with DOTAP was shown to render nanoparticles with a positive charge which enhances phagocytosis by macrophages [32]. While we did not see toxicity with DOTAP in this or prior studies, alternative cationic molecules such as spermidine could be used in the future. Additionally, by altering the homogenization speeds and/or the sonication speeds during single emulsion procedure, we were able to obtain varying size distributions (Supplemental figure 1). The mechanism of particle internalization by macrophages is dependent on particle size, and accordingly, the size range chosen for our experiments facilitates uptake by both endocytosis as well as phagocytosis [45–47]. The siRNA loading efficiency of PK3 particles was calculated to be around 40%, which amounts to about 10μg siRNA/mg of particles. This loading efficiency allowed us to conduct in vitro and in vivo studies at less than 0.1mg/mL and 1mg/mL particle concentrations respectively.
As depicted in Figure 1B, for in vivo studies, these particles were delivered into the heart via intramyocardial injection into the infarct zone of mice immediately following MI surgery. Two key requirements for the successful delivery of siRNA are cellular uptake and endosomal escape [24, 25]. We demonstrate the efficient cellular uptake of these particles in vitro by macrophages using flow cytometry following treatment of RAW 264.1 macrophages with fluorescently labeled siRNA loaded PK-FI-siNox2 particles (Figure 2A). This high transfection efficiency (>80%) in especially hard-to-transfect-macrophages demonstrates the ability of these particles to be efficiently taken up. Further, using confocal microscopy, we observe non-punctate, diffuse fluorescence inside cells (Figure 2B–C) indicating that the siRNA is not trapped in endosomes, but localized in the cytoplasm.
We then investigated whether PK3 particles could deliver functional siRNA into macrophages and inhibit Nox2 gene expression and function in vitro. Compared to untreated cells, PK-siNox2 particles demorated significant reduction in Nox2 mRNA expression while PK-siNeg and empty PK3 particles showed no effect (Figure 3). In order to confirm the corresponding impairment in function due to the knockdown of Nox2 mRNA expression, we stimulated macrophages with PMA following particle treatment. PMA is known to stimulate Nox2-specific extracellular O2− production in macrophages [48–50]. While other Nox isoforms are expressed, recent studies demonstrate that Nox4 mainly produces hydrogen peroxide and is likely an intracellular source [51]. Thus, measuring the extracellular superoxide production in macrophages upon PMA stimulation can serve as an appropriate assay for Nox2 activity [35, 37]. The kinetic traces as well as the quantitiave results from the DHE-HPLC method (Figure 4A–B) demonstrate the ability of PK-siNox2 particle tretament to significantly reduce the PMA induced O2− production by macrophages compared to empty PK3 particle treatment.
Encouraged by our in vitro functional results, we then investigated the ability of PK-siNox2 particles to knockdown Nox2 mRNA expression in vivo. At an acute 3-day time point following experimental MI, delivery of PK-siNox2 significantly reduced the Nox2 mRNA levels in LV tissue compared to MI only, as well as empty and negative control siRNA loaded PK3 particle injections (Figure 5). In most siRNA delivery studies, targeting cells such as macrophages is known to be difficult and requires high doses upwards of 2mg/kg doses at multiple time points [32, 52] while our study demonstrated gene silencing at a low dose of 5μg/kg animal. We also sought to confirm the specificity of siRNA mediated knockdown of Nox2 by determining the mRNA expression of the structurally related Nox4 isoform in these tissue samples and demonstrated that the Nox4 mRNA levels are unchanged due to PK-siNox2 treatment (Supplemental figure 2). Corresponding to the Nox2 mRNA expression knockdown, an improvement in cardiac function was observed in this treatment group compared to MI alone with no effect from controls treatments (Figure 6). These results corroborate with published studies where upregulation of Nox2-NADPH expression and derived ROS was shown to play significant role in the pathophysiology of human congestive heart failure (CHF) [20] and genetic deletion of Nox2 in transgenic mice demonstrated significantly improved cardiac function and remodeling response [8].
Increased ROS in the heart can impact cardiac remodeling and function via several mechanisms. Most ROS are key signaling molecules and are critical in maintaining cellular homeostasis. Aberrant activation of redox-sensitive signaling pathways following ischemic injury can alter gene expression and has been implicated in cardiac hypertrophy, fibrosis and remodeling leading to cardiac dysfunction [53–55]. However, this study did not investigate the mechanistic aspect for the beneficial restoration in cardiac function observed in Nox2-siRNA treated MI animals. While this study did not confirm in vivo knockdown of Nox2 mRNA expression to be primarily in macrophages, in previous studies, non-modified PK particles have not shown effective uptake by non-phagocytic cell types [30, 56]. However, while the infiltrating inflammatory cells represent a large source of O2− following infarction, Nox2 is also expressed on other cardiac cell types, and it is possible that for long-term function other cell types will need to be targeted. Our recent publication demonstrating that PKs modified to present N-acetylglucosamine are taken up by cardiomyocytes could allow for dual targeting of Nox2 in other cell types [56]. Studies to assess the efficacy of this approach are ongoing. Additionally, the chronic effects of Nox2-siRNA delivery such as its effect on fibrosis as well as long-term functional improvement will need to be studied to determine whether higher dosage or repeated injections are necessary to sustain benefits of knocking down Nox2 at the initial inflammatory stage following MI.
5. Conclusion
In this study, we have described polyketal particles as a viable system for the local delivery of siRNA to the heart following myocardial infarction at a low dose of 5μg siRNA/kg. We were able to demonstrate their intracellular localization in macrophages in vitro and the ability to knockdown Nox2-NADPH at the gene and protein levels in vitro. The delivery of Nox2-siRNA by polyketal particles was able to restore acute cardiac function following MI injury in mice. These results point to the potential of Nox2-NADPH as a therapeutic target in acute-MI and consideration of polyketal particles as siRNA delivery vehicles for future large-animal studies.
Supplementary Material
Supplemental Figure 1. Modified particle sizes depending on homegenization speeds. A) Lower magnification image of particles generated with only homegenization for 60 sec and no sonication (d=1.36 ± 0.43μm). B) Particles generated with sonication at intermediate speeds (d=0.83 ± 0.30μm). C) SEM of particles generated with sonication at higher speeds (d=0.56 ± 0.21μm). Scale bar = 2μm.
Supplemental Figure 2. In vivo Nox4 mRNA expression. Grouped data (mean ± SEM; n≥8 per group) from 3 days. There was a trend towards an increase in Nox4 mRNA expression in animals that received MI although not statistically significant. In animals that received MI and PK-siNox2 treatment, there was no observed knockdown of Nox4 mRNA expression. Gene expression was evaluated by qRTPCR using the quantitative standard curve method, and the results were normalized to 18S levels and reported as fold changes in copy number of mRNA levels compared to sham animals (One-way ANOVA followed by Dunnett's Multiple Comparison post-test).
Acknowledgements
These publications have been funded in whole or in part with the Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201000043C to MED and NM, as well as grant HL090601 to MED.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Executive Summary: Heart Disease and Stroke Statistics--2013 Update: A Report From the American Heart Association. Circulation. 2013;127(1):143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Anversa P, Li P, Zhang X, Olivetti G, Capasso JM. Ischaemic myocardial injury and ventricular remodelling. Cardiovasc Res. 1993;27(2):145–57. doi: 10.1093/cvr/27.2.145. [DOI] [PubMed] [Google Scholar]
- 3.Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res. 2005;66(1):22–32. doi: 10.1016/j.cardiores.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Bolli R. Causative role of oxyradicals in myocardial stunning: a proven hypothesis. A brief review of the evidence demonstrating a major role of reactive oxygen species in several forms of postischemic dysfunction. Basic Res Cardiol. 1998;93(3):156–62. doi: 10.1007/s003950050079. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari R, Alfieri O, Curello S, Ceconi C, Cargnoni A, Marzollo P, et al. Occurrence of oxidative stress during reperfusion of the human heart. Circulation. 1990;81(1):201–11. doi: 10.1161/01.cir.81.1.201. [DOI] [PubMed] [Google Scholar]
- 6.Bolli R, Jeroudi MO, Patel BS, DuBose CM, Lai EK, Roberts R, et al. Direct evidence that oxygen-derived free radicals contribute to postischemic myocardial dysfunction in the intact dog. Proc Natl Acad Sci U S A. 1989;86(12):4695–9. doi: 10.1073/pnas.86.12.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2003;138(4):532–43. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, et al. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51(2):319–25. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]
- 9.Cave A. Selective targeting of NADPH oxidase for cardiovascular protection. Curr Opin Pharmacol. 2009;9(2):208–13. doi: 10.1016/j.coph.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Reffelmann T, Kloner RA. The no-reflow phenomenon: A basic mechanism of myocardial ischemia and reperfusion. Basic Res Cardiol. 2006;101(5):359–72. doi: 10.1007/s00395-006-0615-2. [DOI] [PubMed] [Google Scholar]
- 11.Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, et al. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ Res. 2000;87(5):392–8. doi: 10.1161/01.res.87.5.392. [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Bolli R, Qiu Y, Tang XL, Guo Y, French BA. Gene therapy with extracellular superoxide dismutase protects conscious rabbits against myocardial infarction. Circulation. 2001;103(14):1893–8. doi: 10.1161/01.cir.103.14.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sia YT, Lapointe N, Parker TG, Tsoporis JN, Deschepper CF, Calderone A, et al. Beneficial effects of long-term use of the antioxidant probucol in heart failure in the rat. Circulation. 2002;105(21):2549–55. doi: 10.1161/01.cir.0000016721.84535.00. [DOI] [PubMed] [Google Scholar]
- 14.Wang P, Chen H, Qin H, Sankarapandi S, Becher MW, Wong PC, et al. Overexpression of human copper, zinc-superoxide dismutase (SOD1) prevents postischemic injury. Proc Natl Acad Sci U S A. 1998;95(8):4556–60. doi: 10.1073/pnas.95.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8(3):132–40. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- 16.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, et al. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8(5–6):691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 17.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105(3):293–6. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 18.Krijnen PA, Meischl C, Hack CE, Meijer CJ, Visser CA, Roos D, et al. Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. J Clin Pathol. 2003;56(3):194–9. doi: 10.1136/jcp.56.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukui T, Yoshiyama M, Hanatani A, Omura T, Yoshikawa J, Abe Y. Expression of p22-phox and gp91-phox, essential components of NADPH oxidase, increases after myocardial infarction. Biochem Biophys Res Commun. 2001;281(5):1200–6. doi: 10.1006/bbrc.2001.4493. [DOI] [PubMed] [Google Scholar]
- 20.Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, et al. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41(12):2164–71. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 21.Grieve DJ, Byrne JA, Siva A, Layland J, Johar S, Cave AC, et al. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J Am Coll Cardiol. 2006;47(4):817–26. doi: 10.1016/j.jacc.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 22.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, et al. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res. 2003;93(9):802–5. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 23.Matsushima S, Kuroda J, Ago T, Zhai P, Ikeda Y, Oka S, et al. Broad Suppression of NADPH Oxidase Activity Exacerbates Ischemia/Reperfusion Injury Through Inadvertent Downregulation of Hypoxia-inducible Factor-1alpha and Upregulation of Peroxisome Proliferator-activated Receptor-alpha. Circ Res. 2013;112(8):1135–49. doi: 10.1161/CIRCRESAHA.111.300171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6(6):443–53. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dykxhoorn DM, Lieberman J. The silent revolution: RNA interference as basic biology, research tool, and therapeutic. Annu Rev Med. 2005;56:401–23. doi: 10.1146/annurev.med.56.082103.104606. [DOI] [PubMed] [Google Scholar]
- 26.Li CX, Parker A, Menocal E, Xiang S, Borodyansky L, Fruehauf JH. Delivery of RNA interference. Cell Cycle. 2006;5(18):2103–9. doi: 10.4161/cc.5.18.3192. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Edwards JP, Mosser DM. The expression of exogenous genes in macrophages: obstacles and opportunities. Methods Mol Biol. 2009;531:123–43. doi: 10.1007/978-1-59745-396-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang G, Park K, Kim J, Kim KS, Hahn SK. Target specific intracellular delivery of siRNA/PEI-HA complex by receptor mediated endocytosis. Mol Pharm. 2009;6(3):727–37. doi: 10.1021/mp800176t. [DOI] [PubMed] [Google Scholar]
- 29.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114(1):100–9. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Yang SC, Kao CY, Pierce RH, Murthy N. Solid polymeric microparticles enhance the delivery of siRNA to macrophages in vivo. Nucleic Acids Res. 2009;37(22):e145. doi: 10.1093/nar/gkp758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang YY, Chung TS, Ng NP. Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials. 2001;22(3):231–41. doi: 10.1016/s0142-9612(00)00178-2. [DOI] [PubMed] [Google Scholar]
- 32.Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin, DMurthy N. Orally delivered thioketal nanoparticles loaded with TNF-alpha-siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater. 2010;9(11):923–8. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sy JC, Seshadri G, Yang SC, Brown M, Oh T, Dikalov S, et al. Sustained release of a p38 inhibitor from non-inflammatory microspheres inhibits cardiac dysfunction. Nat Mater. 2008;7(11):863–8. doi: 10.1038/nmat2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang SC, Bhide M, Crispe IN, Pierce RH, Murthy N. Polyketal copolymers: a new acid-sensitive delivery vehicle for treating acute inflammatory diseases. Bioconjug Chem. 2008;19(6):1164–9. doi: 10.1021/bc700442g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandes DC, Wosniak J, Jr., Pescatore LA, Bertoline MA, Liberman M, et al. Analysis of DHE-derived oxidation products by HPLC in the assessment of superoxide production and NADPH oxidase activity in vascular systems. Am J Physiol Cell Physiol. 2007;292(1):C413–22. doi: 10.1152/ajpcell.00188.2006. [DOI] [PubMed] [Google Scholar]
- 36.Peshavariya HM, Dusting GJ, Selemidis S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic Res. 2007;41(6):699–712. doi: 10.1080/10715760701297354. [DOI] [PubMed] [Google Scholar]
- 37.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3(1):8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 38.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287(4):C895–902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 39.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34(11):1359–68. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 40.Seshadri G, Sy JC, Brown M, Dikalov S, Yang SC, Murthy N, et al. The delivery of superoxide dismutase encapsulated in polyketal microparticles to rat myocardium and protection from myocardial ischemia-reperfusion injury. Biomaterials. 2010;31(6):1372–9. doi: 10.1016/j.biomaterials.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maejima Y, Kuroda J, Matsushima S, Ago T, Sadoshima J. Regulation of myocardial growth and death by NADPH oxidase. J Mol Cell Cardiol. 2011;50(3):408–16. doi: 10.1016/j.yjmcc.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akhtar S, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117(12):3623–32. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leirdal M, Sioud M. Gene silencing in mammalian cells by preformed small RNA duplexes. Biochem Biophys Res Commun. 2002;295(3):744–8. doi: 10.1016/s0006-291x(02)00736-2. [DOI] [PubMed] [Google Scholar]
- 44.Zhang S, Zhao B, Jiang H, Wang B, Ma B. Cationic lipids and polymers mediated vectors for delivery of siRNA. J Control Release. 2007;123(1):1–10. doi: 10.1016/j.jconrel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 45.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J Control Release. 2007;121(1–2):3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25(8):1815–21. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yue H, Wei W, Yue Z, Lv P, Wang L, Ma G, et al. Particle size affects the cellular response in macrophages. Euro J Pharma Sciences. 2010;41(5):650–7. doi: 10.1016/j.ejps.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 48.DeChatelet LR, Shirley PS, Johnston RB., Jr. Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood. 1976;47(4):545–54. [PubMed] [Google Scholar]
- 49.Dikalov SI, Dikalova AE, Mason RP. Noninvasive diagnostic tool for inflammation-induced oxidative stress using electron spin resonance spectroscopy and an extracellular cyclic hydroxylamine. Arch Biochem Biophys. 2002;402(2):218–26. doi: 10.1016/S0003-9861(02)00064-4. [DOI] [PubMed] [Google Scholar]
- 50.Dikalov SI, Li W, Mehranpour P, Wang SS, Zafari AM. Production of extracellular superoxide by human lymphoblast cell lines: comparison of electron spin resonance techniques and cytochrome C reduction assay. Biochem Pharmacol. 2007;73(7):972–80. doi: 10.1016/j.bcp.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58(1):32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 52.Kim SS, Ye C, Kumar P, Chiu I, Subramanya S, Wu H, et al. Targeted delivery of siRNA to macrophages for anti-inflammatory treatment. Mol Ther. 2010;18(5):993–1001. doi: 10.1038/mt.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacCarthy PA, Grieve DJ, Li JM, Dunster C, Kelly FJ, Shah AM. Impaired endothelial regulation of ventricular relaxation in cardiac hypertrophy: role of reactive oxygen species and NADPH oxidase. Circulation. 2001;104(24):2967–74. doi: 10.1161/hc4901.100382. [DOI] [PubMed] [Google Scholar]
- 54.Peng T, Lu X, Feng Q. Pivotal role of gp91phox-containing NADH oxidase in lipopolysaccharide-induced tumor necrosis factor-alpha expression and myocardial depression. Circulation. 2005;111(13):1637–44. doi: 10.1161/01.CIR.0000160366.50210.E9. [DOI] [PubMed] [Google Scholar]
- 55.Siwik DA, Tzortzis JD, Pimental DR, Chang DL, Pagano PJ, Singh K, et al. Inhibition of copper-zinc superoxide dismutase induces cell growth, hypertrophic phenotype, and apoptosis in neonatal rat cardiac myocytes in vitro. Circ Res. 1999;85(2):147–53. doi: 10.1161/01.res.85.2.147. [DOI] [PubMed] [Google Scholar]
- 56.Gray WD, Che P, Brown M, Ning X, Murthy N, Davis ME. N-acetylglucosamine conjugated to nanoparticles enhances myocyte uptake and improves delivery of a small molecule p38 inhibitor for post-infarct healing. J Cardiovasc Transl Res. 2011;4(5):631–43. doi: 10.1007/s12265-011-9292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Modified particle sizes depending on homegenization speeds. A) Lower magnification image of particles generated with only homegenization for 60 sec and no sonication (d=1.36 ± 0.43μm). B) Particles generated with sonication at intermediate speeds (d=0.83 ± 0.30μm). C) SEM of particles generated with sonication at higher speeds (d=0.56 ± 0.21μm). Scale bar = 2μm.
Supplemental Figure 2. In vivo Nox4 mRNA expression. Grouped data (mean ± SEM; n≥8 per group) from 3 days. There was a trend towards an increase in Nox4 mRNA expression in animals that received MI although not statistically significant. In animals that received MI and PK-siNox2 treatment, there was no observed knockdown of Nox4 mRNA expression. Gene expression was evaluated by qRTPCR using the quantitative standard curve method, and the results were normalized to 18S levels and reported as fold changes in copy number of mRNA levels compared to sham animals (One-way ANOVA followed by Dunnett's Multiple Comparison post-test).