Review on the role of neutrophils in marginal zone B cell activation and antibody production.
Keywords: innate immunity, granulocytes, antibody production
Abstract
Neutrophils use opsonizing antibodies to enhance the clearance of intruding microbes. Recent studies indicate that splenic neutrophils also induce antibody production by providing helper signals to B cells lodged in the MZ of the spleen. Here, we discuss the B cell helper function of neutrophils in the context of growing evidence indicating that neutrophils function as sophisticated regulators of innate and adaptive immune responses.
Introduction
Neutrophils are usually viewed as terminal, short-lived, innate effector cells that remove microbes and cellular debris at sites of infection or inflammation by mediating phagocytosis and releasing proteolytic enzymes, antimicrobial proteins, and ROS. Over the last decade, a number of studies have shown that neutrophils have a lifespan longer than originally thought and mediate diverse immune functions by releasing a broad array of preformed and newly synthesized mediators, including chemokines and cytokines [1, 2]. These molecules regulate not only the mobilization and function of neutrophils but also the recruitment and activation of monocytes, DCs, NK cells, T cells, and B cells of the innate and adaptive immune systems [1, 2]. In general, neutrophils functionally interact with B cells by binding IgG and IgA, two opsonizing antibody isotypes that amplify microbial clearance by engaging powerful FcγRs and FcαRs on neutrophils [3, 4].
We found recently that human neutrophils are not only eager users but also proficient inducers of IgG and IgA, as a result of their ability to crosstalk with a unique subset of B cells lodged in the MZ of the spleen [5]. Strategically interposed between the circulatory and immune systems, MZ B cells (also known in humans as IgM memory B cells) are innate, antibody-producing lymphocytes that naturally recognize conserved microbial products and self-antigens through poorly diversified BCR (or surface Ig) molecules [6, 7]. Owing to their preactivated state and pronounced innate properties, MZ B cells rapidly mount preimmune (homeostatic) and postimmune (infection-induced) antibody responses to blood-borne antigens, including commensal antigens physiologically translocating from mucosal surfaces to the general circulation [5–8].
Our findings indicate that MZ B cells produce IgM as well as class-switched IgG and IgA antibodies after receiving helper signals from a unique subset of splenic neutrophils that are phenotypically and functionally distinct from circulating neutrophils [5]. This “mini” review will discuss the B cell helper function of splenic neutrophils in the context of recent advances on the mechanisms whereby neutrophils modulate the function of innate and adaptive immune systems.
NEUTROPHILS AS IMMUNOENHANCERS
Growing evidence shows that neutrophils enhance nonspecific innate immune responses by promoting the recruitment, activation, and maturation of monocytes, macrophages, DCs, and NK cells [2, 9]. Neutrophils also enhance specific, adaptive T and B cell responses by facilitating the differentiation of monocytes and DCs to professional APCs [2, 9]. Given the varied immunoenhancing activities of neutrophils, immunodeficient patients with quantitative or functional neutrophil disorders often develop secondary immune dysfunctions that contribute to the onset of recurrent infections [10, 11]. In general, the immunostimulating properties of neutrophils can be ascribed to their ability to produce a broad repertoire of immune mediators with pleiotropic function [1, 2].
In the initial phases of the immune response, neutrophils release the chemokines CCL3, CCL5, and CXCL10, together with the inflammatory cytokines IL-1β, IL-6 (this cytokine has been shown in mice; evidence in humans remains controversial), IL-12, and TNF, as well as a heterogeneous set of granular proteins known as alarmins [1, 12, 13]. In addition to stimulating inflammation, alarmins promote the recruitment of circulating DC precursors and stimulate their progression along a maturation program that converts them into professional APCs with T cell-stimulating capacity [1, 12, 13]. These properties are exemplified by the cationic antimicrobial peptide LL-37, an alarmin that enhances inflammatory TH1 responses by amplifying the APC activity of DCs [13, 14]. Activated neutrophils increase DC maturation further by releasing TNF, particularly in the context of a contact-dependent crosstalk involving engagement of the integrin CD11b (macrophage antigen-1) and carcinoembryonic antigen-related cell adhesion molecule 1 (or CD66), on neutrophils with the CLR DC-specific ICAM-3-grabbing nonintegrin 1 (or CD209) on DCs [15–17]. This cell-to-cell interaction enhances the conversion of DCs into T cell-stimulating APCs in the presence of TNF production by neutrophils [15, 17].
After undergoing maturation, DCs acquire APC activity and release the inflammatory cytokines IL-12 and TNF-α, which promote the differentiation of monocytes into macrophages, as well as the polarization of naïve CD4+ T cells into TH1 cells [18]. These effector CD4+ T cells activate the killing function of macrophages, NK cells, and CTLs by secreting IFN-γ [2]. Neutrophils may further enhance CTL responses after migrating to draining LNs and bone marrow in response to chemotactic signals generated by microbial intruders, including signals from CCR7 ligands [19, 20]. At these sites, antigen-transporting neutrophils not only cross-present exogenous antigens to antigen-specific CD8+ T cell precursors of CTLs [20, 21] but also release NK cell/CTL-activating cytokines, such as IFN-γ, albeit this function is still controversial in humans [9, 22].
In addition to favoring the development of NK cell precursors in the bone marrow [11, 23], neutrophils enhance NK cell activation, including IFN-γ production, by delivering contact-dependent and contact-independent signals through the adhesion molecule ICAM-1 and the cytokine IL-18, respectively [24, 25]. Neutrophils further enhance NK cell secretion of IFN-γ by triggering IL-12 production in a subset of DCs expressing slanDCs [25]. IFN-γ from NK cells further augments slanDC release of IL-12, thereby establishing a positive loop that amplifies the activation of NK cells [25]. Conversely, DC-derived IL-12 cooperates with neutrophil-derived IL-18 to increase IFN-γ production by NK cells [24]. When activated by IL-18, NK cells enhance neutrophil survival by releasing GM-CSF [26]. However, it must be noted that activated NK cells can also cause neutrophil apoptosis through a contact-dependent mechanism involving NK expression of the death-inducing molecule Fas ligand and the killer-activating receptor NKp46 [27].
Activated neutrophils further enhance immunity by recruiting TH1 cells via CXCL9 and CXCL10 [28]. Neutrophils also produce CCL2 and CCL20 that attract inflammatory TH17 cells specialized in the secretion of IL-17, a pleiotropic cytokine that promotes neutrophil recruitment and activation [28]. In addition to attracting TH1 and TH17 cells to sites of inflammation, neutrophils enhance the differentiation of these effector TH cells from naïve CD4+ T cell precursors [29]. Indeed, when exposed to inflammatory signals, some neutrophils acquire a hybrid, DC-like morphology; up-regulate the expression of APC molecules, such as MHC-II, and T cell costimulatory molecules, such as CD80 (B7.1) and CD86 (B7.2); and initiate specific T cell responses by presenting antigen to naïve CD4+ T cells and releasing TH1-inducing cytokines, such as IL-12 and IFN-γ, as well as TH17-inducing cytokines, such as IL-1β and IL-6 [29–31].
Conversely, TH17 cells enhance the differentiation of neutrophils from bone marrow myeloid precursors by eliciting stromal cell release of G-CSF via IL-17 [32]. In addition, TH17 cells augment the recruitment of neutrophils from the circulation by secreting CXCL8 [28]. Moreover, TH17 and TH1 cells augment neutrophil survival and activation by releasing GM-CSF, IFN-γ, and TNF [33]. These findings indicate that neutrophils initiate and amplify innate and adaptive immune responses by establishing bidirectional interactions with monocytes, macrophages, DCs, NK cells, and T cells through contact-dependent and contact-independent mechanisms.
Similar mechanisms are used by splenic neutrophils to activate B cells in the MZ of the spleen. Indeed, circulating neutrophils appear to acquire MZ B cell helper function after receiving contact-independent reprogramming signals via IL-10 and other STAT3-inducing cytokines produced by splenic, sinus-lining cells and macrophages [5]. Contact-independent signals are also involved in the activation of MZ B cells by neutrophils via BAFF (or B lymphocyte stimulator) and APRIL, two soluble TNF family members, structurally and functionally related to a T cell-bound, B cell-stimulating molecule, termed CD40L (or CD154) [5, 34–36].
Splenic neutrophils may further activate MZ B cells via contact-dependent mechanisms that include CD40L and NETs, which are extracellular projections that establish extensive interactions with MZ B cells [2, 5, 37]. NETs have antigen-binding and antimicrobial functions and originate from neutrophils undergoing “netosis”, a distinct form of apoptosis that causes massive chromatin decondensation [2, 37]. Recently, NET formation has been functionally linked to plasmacytoid DC production of IFN-α, a cytokine that plays a central role in autoimmune disorders [38, 39]. Given the autoreactive potential of MZ B cells and their constant exposure to foreign and autologous antigens, splenic neutrophils must deliver their MZ B cell helper activity in a highly controlled manner. Accordingly, splenic neutrophils express a host of immunoregulatory factors in addition to powerful B cell-activating factors [5].
NEUTROPHILS AS IMMUNOREGULATORS
In addition to delivering powerful immune-activating signals, neutrophils release immune regulatory signals that dampen immunity and inflammation [2]. For instance, neutrophils inhibit their own recruitment by releasing proresolving lipid mediators, such as lipoxin, resolvins, and protectins [2]. Neutrophils further accelerate the resolution of an ongoing immune response by terminating signaling from the CCR5 chemokine receptor ligands CCL3 and CCL5 through a process involving up-regulation of CCR5 expression on apoptotic neutrophils, followed by enhanced scavenging of CCL3 and CCL5 [2]. Furthermore, neutrophils attenuate the inflammatory activity of IL-1 by releasing soluble decoy IL-1Rs and IL-1R antagonist [2]. Clearance of apoptotic neutrophils by macrophages constitutes another important step in the resolution of inflammation, as neutrophil-engulfing macrophages trigger anti-inflammatory responses that lead to tissue repair [2, 40].
Neutrophils generate additional negative-feedback signals by up-regulating the expression of the IL-10R in response to microbial TLR ligands and inflammatory cytokines [41]. This process results in an increased responsiveness of neutrophils to the inhibitory activity of IL-10, an immunoregulatory cytokine produced by myeloid and lymphoid cells [41]. In mice, IL-10 is also produced by neutrophils after sensing microbes through TLRs and CLRs [42]. This pathway would allow neutrophils to attenuate the activation of innate and adaptive immune responses [42–44]. In humans, IL-10 production by neutrophils has also been reported, but more recent findings show that human neutrophils are unlikely to be a major source of IL-10, given that the IL-10 locus is inactivated in these cells [43–45]. Neutrophils further regulate T cell responses by promoting the formation of DCs that secrete the immunoregulatory cytokine TGF-β1 [46]. This pathway requires elastase, a neutrophil protease that activates proteinase-activated receptors on immune cells, including DCs [46]. Finally, recent studies have extended the regulatory activity of neutrophils to invariant NKT responses in humans and mice [47].
By delivering regulatory signals, neutrophils may tune down the magnitude of an immune response and contribute to its contraction after the clearance of intruding microbes. Neutrophils may also deliver regulatory signals to maintain a noninflammatory environment at sites continually exposed to antigen, such as the MZ of the spleen [6]. This enigmatic lymphoid structure mediates homeostatic and postimmune antibody responses to blood-borne antigens, including conserved microbial determinants that can activate MZ B cells in the absence of help from T cells [6, 7, 48]. The pathways underlying the regulation of T cells in the MZ of the spleen remain unclear, but splenic neutrophils deliver suppressor signals to T cells in addition to activating signals to MZ B cells [5]. This dual function may permit splenic neutrophils to induce homeostatic MZ B cell responses without eliciting the activation of inflammatory T cells.
NEUTROPHILS AS B CELL INDUCERS
Protective antibody responses to microbial proteins follow a slow but highly specific TD pathway that generates long-lived memory B cells and plasma cells expressing hypermutated and class-switched IgG and IgA antibodies with high affinity for antigen [48]. TD antibody production occurs through a germinal center reaction involving cognate interaction of FO B cells by DC-primed TFH cells [49]. By producing CD40L, as well as IL-21, IL-4, and IFN-γ, TFH cells stimulate FO B cells to express AID, a DNA-editing enzyme required for SHM and CSR [48–51]. SHM increases the affinity of antibodies for antigen by introducing point mutations in the variable(diversity)joining genes encoding the antigen-binding variable region of Igs, whereas CSR modulates the effector functions of B cells by replacing IgM with IgG, IgA, or IgE [50].
Neutrophils may enhance TD antibody production by collecting antigens at sites of infection or inflammation; by transporting antigen to sites involved in antibody production; by enhancing the antigen-presenting function of DCs; and by promoting the recruitment, activation, and differentiation of CD4+ T cells [2, 30, 31]. Neutrophils also release the CD40L-related molecules BAFF and APRIL [5, 36, 52, 53], which facilitate the survival of plasma cells emerging from follicular TD antibody responses [5, 36, 52–54]. BAFF and APRIL production by neutrophils and other innate cell types, such as DCs and epithelial cells, also enhances extrafollicular TI antibody responses by B cells located at mucosal sites and splenic MZ [5, 55–57].
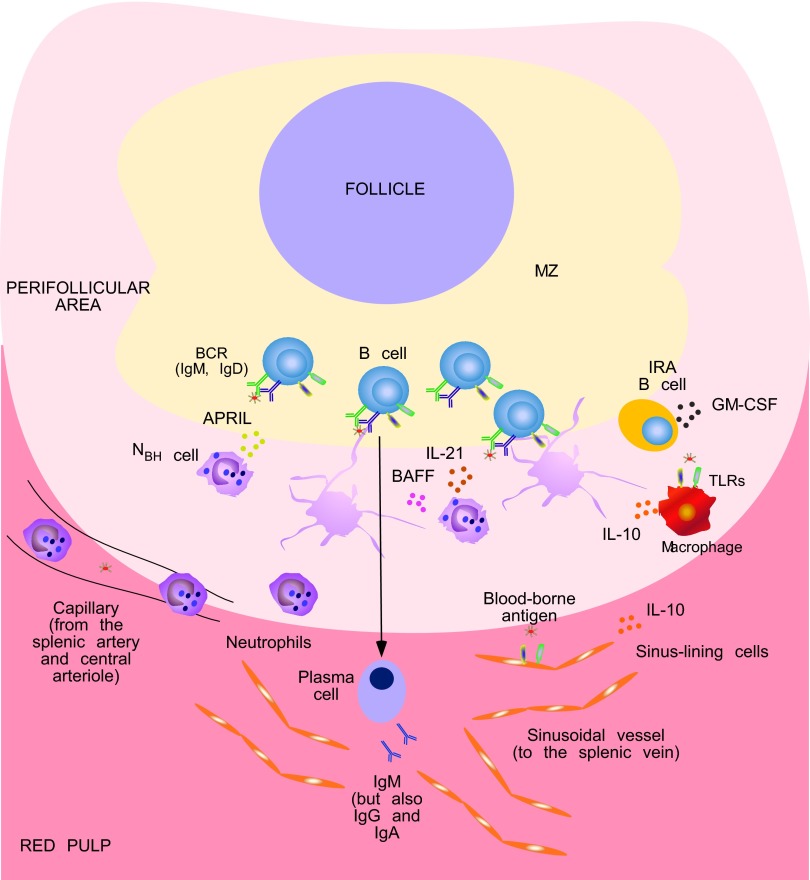
MZ B cells are naturally poised to mount IgM as well as IgG and IgA responses in a rapid TI manner, which allows MZ B cells to bridge the temporal gap (in general, 5–7 days) required for the initiation of TD antibody production by FO B cells [6, 7]. The rapid kinetics of TI antibody responses may relate to the ability of MZ B cells to use fast helper signals from DCs, macrophages, and neutrophils of the innate immune system rather than slow helper signals from TFH cells of the adaptive immune system [5, 48, 58, 59]. Neutrophils were previously thought to colonize the MZ of the spleen only in response to blood-borne infections [58, 60]. However, we found that neutrophils occupy splenic peri-MZ areas also in the absence of infection and do so through a noninflammatory pathway that begins during fetal life and accelerates after birth—a time that coincides with the colonization of mucosal surfaces by commensal bacteria [5]. Unlike circulating neutrophils, splenic neutrophils deliver powerful antibody-inducing signals to MZ B cells (Fig. 1), and thus, we have defined these cells as NBH cells [5].
Figure 1. NBH cells enhance antibody production in the MZ of the human spleen.
. Conventional circulating neutrophils may home to the perifollicular area of the human spleen by following chemotactic gradients, possibly generated by resident, sinus-lining cells and macrophages in response to blood-borne antigens, including antigens containing TLR ligands. Sinus-lining cells and macrophages also release IL-10, which contributes to the reprogramming of circulating neutrophils into splenic NBH cells. Neutrophils may receive additional reprogramming signals as well as survival and activating signals from GM-CSF produced by local IRA B cells. NBH cells induce antibody class-switching, plasma cell differentiation, and secretion of preimmune IgM, as well as IgG and IgA, by stimulating a fraction of MZ B cells via BAFF, APRIL, and IL-21. These cytokines also stimulate MZ B cell and plasma cell survival. NBH cells might further stimulate local antibody responses by making available antigenic determinants to surface BCR (B cell antigen receptor, mostly IgM but also IgD) and TLR molecules expressed on the surface of MZ B cells. Indeed, some NBH cells interact with MZ B cells by forming antigen-trapping, NET-like projections after undergoing apoptosis.
Compared with circulating neutrophils, NBH cells express an activated phenotype; secrete more B cell-stimulating factors, such as BAFF, APRIL, CD40L, and IL-21; produce more plasmablast-attracting factors, such as CXCL12; and strongly activate MZ but not FO B cells [5]. These unique features likely reflect the activation and reprogramming of NBH cells by local microenvironmental signals. Consistent with this possibility, accumulation of NBH cells coincides with postnatal exposure of splenic peri-MZ areas to microbial TLR ligands of probable mucosal origin, such as LPS and bacterial RNA [5]. In addition to activating NBH cells, these TLR ligands stimulate perifollicular sinus-lining cells to express more neutrophil-attracting chemokines, including CXCL8, which may contribute to the recruitment of NBH cells [5]. Accordingly, the congenital deficiency of molecules required for TLR signaling causes reduction of NBH cells and MZ B cells [5, 61].
Microbial TLR ligands also stimulate the reprogramming of NBH cells from conventional neutrophils by eliciting the release of noninflammatory cytokines, such as IL-10, from perifollicular sinus-lining cells and macrophages [5]. Considering that IL-10 provides regulatory signals to neutrophils [2, 41], this cytokine may be instrumental for NBH cells to operate in a noninflammatory environment. The generation and/or maintenance of NBH cells may also involve GM-CSF, a cytokine that promotes the recruitment, survival, and activation of neutrophils and whose production has been identified recently in a subset of splenic B cells termed IRA B cells [2, 62].
NBH cells induce AID expression, IgG and IgA CSR, and antibody-secreting plasmablasts by activating MZ B cells through a TI mechanism involving BAFF, APRIL, and somewhat surprisingly, IL-21 [5]. These molecules also stimulate B cell and plasma cell survival [48], raising the possibility that neutrophils sustain MZ B cell responses through multiple mechanisms in vivo. Remarkably, human NBH cells include NBH1 and NBH2 subsets that show distinct phenotypic and functional features [5]. Indeed, compared with NBH1 cells, NBH2 cells express lower levels of surface CD15 and CD16 and stimulate more effective MZ B cell responses as a result of a more pronounced release of BAFF, APRIL, and IL-21 [5]. Consistent with this, immunodeficient patients with quantitative or functional neutrophil disorders or defective BAFF, APRIL, or IL-21 signaling have fewer MZ B cells and reduced steady-state production of IgG and IgA to TI carbohydrate but not TD protein antigens [5]. Of note, antibodies to TD antigens are produced by plasma cells lodged in bone marrow niches that contain eosinophils secreting the plasma cell-survival factor APRIL [63], which suggests that there is a division of labor between neutrophils and eosinophils for the maintenance of plasma cells at distinct lymphoid sites.
The stimulation of MZ B cells by NBH cells may involve the formation of NET-like structures, which indeed appear to trap microbial products, such as bacterial RNA, and establish extensive interactions with MZ B cells [5]. By trapping blood-borne commensal antigens, possibly originating from mucosal surfaces, NETs would facilitate the provision of BCR ligands to MZ B cells under steady-state conditions [5, 8, 48, 64, 65]. NETs may also deliver TLR ligands to MZ B cells, and indeed, there is some evidence pointing to an important role of TLRs in the maturation, maintenance, activation, and function of MZ B cells [39, 66, 67]. Together with BAFF, APRIL, and IL-21, microbial and possibly autologous TLR ligands from NBH cells may contribute to the induction of AID expression and initiation of CSR and SHM in a fraction of MZ B cells under homeostatic conditions [5]. In humans, MZ B cells initiate SHM during fetal life through a TI pathway that remains active after birth [7, 48, 68]. Indeed, spleens from adult individuals contain mutated MZ B cells but few or no TFH cells and active germinal centers [7, 69, 70]. Furthermore, mutations are decreased in MZ B cells from patients with congenital neutropenia but not in patients with a congenital impairment of TD antibody production as a result of a deficiency of CD40L [5, 71, 72].
The TI nature of the helper signals provided by NBH cells is suggested further by the observation that NBH cells not only induce CSR, SHM, and antibody production in the absence of CD4+ T cells but also suppress the activation of CD4+ T cells, at least in vitro [5]. By exerting this dual B cell helper–T cell suppressor function, NBH cells may skew antibody responses to commensal blood-borne antigens toward a MZ-based TI pathway at the expense of a follicle-based TD pathway. Overall, the crosstalk between NBH cells and MZ B cells may be instrumental to generate a second line of innate antibody defense against microbial antigens that break the first line of defense provided by the mucosal barrier.
CONCLUSIONS
In contrast to the traditional view of neutrophils as unsophisticated cells of the phagocytic system, neutrophils have emerged recently as an important component of the effector and regulatory circuits that control the magnitude and quality of an immune response. By establishing bidirectional interactions with DCs, monocytes, macrophages, and T cells, neutrophils modulate innate and adaptive immune responses in health and disease states [2]. Our studies indicate that neutrophils crosstalk further with MZ B cells to enhance the generation of ready-to-use antibodies against conserved microbial antigens [48]. A possible implication of our findings is that adjuvants capable to harness the B cell helper activity of neutrophils could improve protective antibody responses to antigens recognized by MZ B cells, including poorly immunogenic TI antigens. Another implication of our studies is that the pathogenesis of recurrent infections arising in immunodeficient patients with neutrophil disorders may be more complex than anticipated originally and could involve decreased production of preimmune antibodies to specific sets of conserved microbial antigens.
ACKNOWLEDGMENTS
The authors are supported by the European Research Council 2011 Advanced Grant 20110310 (A.C.); U.S. National Institutes of Health research grants AI61093, AI057653, AI95613, AI96187, and AI07437 (A.C.); Ministerio de Ciencia e Innovación grant SAF 2011-25241 (A.C.); and Juan de la Cierva program (I.P. and G.M.).
Footnotes
- AID
- activation-induced cytidine deaminase
- APRIL
- a proliferation-inducing ligand
- BAFF
- B cell-activating factor of the TNF family
- CD40L
- CD40 ligand
- CLR
- C-type lectin receptor
- CSR
- class-switch recombination
- FO B cell
- follicular B cell
- IRA
- innate response activator
- MZ
- marginal zone
- NBH cell
- neutrophil B helper cell
- NET
- neutrophil extracellular trap
- SHM
- somatic hypermutation
- slanDC
- 6-sulfo N-acetyllactosamine DC
- TD
- T cell-dependent
- TFH cell
- T follicular helper cell
- TI
- T cell-independent
REFERENCES
- 1. Nathan C. (2006) Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 [DOI] [PubMed] [Google Scholar]
- 2. Mantovani A., Cassatella M. A., Costantini C., Jaillon S. (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531 [DOI] [PubMed] [Google Scholar]
- 3. Monteiro R. C., Van De Winkel J. G. (2003) IgA Fc receptors. Annu. Rev. Immunol. 21, 177–204 [DOI] [PubMed] [Google Scholar]
- 4. Nimmerjahn F., Ravetch J. V. (2008) Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 8, 34–47 [DOI] [PubMed] [Google Scholar]
- 5. Puga I., Cols M., Barra C. M., He B., Cassis L., Gentile M., Comerma L., Chorny A., Shan M., Xu W., et al. (2012) B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 13, 170–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pillai S., Cariappa A., Moran S. T. (2005) Marginal zone B cells. Annu. Rev. Immunol. 23, 161–196 [DOI] [PubMed] [Google Scholar]
- 7. Weill J. C., Weller S., Reynaud C. A. (2009) Human marginal zone B cells. Annu. Rev. Immunol. 27, 267–285 [DOI] [PubMed] [Google Scholar]
- 8. Clarke T. B., Davis K. M., Lysenko E. S., Zhou A. Y., Yu Y., Weiser J. N. (2010) Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 16, 228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cassatella M. A. (1999) Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 73, 369–509 [DOI] [PubMed] [Google Scholar]
- 10. Lekstrom-Himes J. A., Gallin J. I. (2000) Immunodeficiency diseases caused by defects in phagocytes. N. Engl. J. Med. 343, 1703–1714 [DOI] [PubMed] [Google Scholar]
- 11. Jaeger B. N., Donadieu J., Cognet C., Bernat C., Ordonez-Rueda D., Barlogis V., Mahlaoui N., Fenis A., Narni-Mancinelli E., Beaupain B., Bellanne-Chantelot C., Bajenoff M., Malissen B., Malissen M., Vivier E., Ugolini S. (2012) Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J. Exp. Med. 209, 565–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soehnlein O., Weber C., Lindbom L. (2009) Neutrophil granule proteins tune monocytic cell function. Trends Immunol. 30, 538–546 [DOI] [PubMed] [Google Scholar]
- 13. Yang D., de la Rosa G., Tewary P., Oppenheim J. J. (2009) Alarmins link neutrophils and dendritic cells. Trends Immunol. 30, 531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davidson D. J., Currie A. J., Reid G. S., Bowdish D. M., MacDonald K. L., Ma R. C., Hancock R. E., Speert D. P. (2004) The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 172, 1146–1156 [DOI] [PubMed] [Google Scholar]
- 15. Van Gisbergen K. P., Sanchez-Hernandez M., Geijtenbeek T. B., van Kooyk Y. (2005) Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J. Exp. Med. 201, 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bennouna S., Denkers E. Y. (2005) Microbial antigen triggers rapid mobilization of TNF-α to the surface of mouse neutrophils transforming them into inducers of high-level dendritic cell TNF-α production. J. Immunol. 174, 4845–4851 [DOI] [PubMed] [Google Scholar]
- 17. Van Gisbergen K. P., Ludwig I. S., Geijtenbeek T. B., van Kooyk Y. (2005) Interactions of DC-SIGN with Mac-1 and CEACAM1 regulate contact between dendritic cells and neutrophils. FEBS Lett. 579, 6159–6168 [DOI] [PubMed] [Google Scholar]
- 18. Banchereau J., Steinman R. M. (1998) Dendritic cells and the control of immunity. Nature 392, 245–252 [DOI] [PubMed] [Google Scholar]
- 19. Beauvillain C., Cunin P., Doni A., Scotet M., Jaillon S., Loiry M. L., Magistrelli G., Masternak K., Chevailler A., Delneste Y., Jeannin P. (2011) CCR7 is involved in the migration of neutrophils to lymph nodes. Blood 117, 1196–1204 [DOI] [PubMed] [Google Scholar]
- 20. Duffy D., Perrin H., Abadie V., Benhabiles N., Boissonnas A., Liard C., Descours B., Reboulleau D., Bonduelle O., Verrier B., Van Rooijen N., Combadiere C., Combadiere B. (2012) Neutrophils transport antigen from the dermis to the bone marrow, initiating a source of memory CD8(+) T cells. Immunity 37, 917–929 [DOI] [PubMed] [Google Scholar]
- 21. Beauvillain C., Delneste Y., Scotet M., Peres A., Gascan H., Guermonprez P., Barnaba V., Jeannin P. (2007) Neutrophils efficiently cross-prime naive T cells in vivo. Blood 110, 2965–2973 [DOI] [PubMed] [Google Scholar]
- 22. Ethuin F., Gerard B., Benna J. E., Boutten A., Gougereot-Pocidalo M. A., Jacob L., Chollet-Martin S. (2004) Human neutrophils produce interferon γ upon stimulation by interleukin-12. Lab. Invest. 84, 1363–1371 [DOI] [PubMed] [Google Scholar]
- 23. Ordonez-Rueda D., Jonsson F., Mancardi D. A., Zhao W., Malzac A., Liang Y., Bertosio E., Grenot P., Blanquet V., Sabrautzki S., de Angelis M. H., Meresse S., Duprez E., Bruhns P., Malissen B., Malissen M. (2012) A hypomorphic mutation in the Gfi1 transcriptional repressor results in a novel form of neutropenia. Eur. J. Immunol. 42, 2395–2408 [DOI] [PubMed] [Google Scholar]
- 24. Sporri R., Joller N., Hilbi H., Oxenius A. (2008) A novel role for neutrophils as critical activators of NK cells. J. Immunol. 181, 7121–7130 [DOI] [PubMed] [Google Scholar]
- 25. Costantini C., Calzetti F., Perbellini O., Micheletti A., Scarponi C., Lonardi S., Pelletier M., Schakel K., Pizzolo G., Facchetti F., Vermi W., Albanesi C., Cassatella M. A. (2011) Human neutrophils interact with both 6-sulfo LacNAc+ DC and NK cells to amplify NK-derived IFNγ: role of CD18, ICAM-1, and ICAM-3. Blood 117, 1677–1686 [DOI] [PubMed] [Google Scholar]
- 26. Costantini C., Micheletti A., Calzetti F., Perbellini O., Pizzolo G., Cassatella M. A. (2010) Neutrophil activation and survival are modulated by interaction with NK cells. Int. Immunol. 22, 827–838 [DOI] [PubMed] [Google Scholar]
- 27. Thoren F. B., Riise R. E., Ousback J., Della Chiesa M., Alsterholm M., Marcenaro E., Pesce S., Prato C., Cantoni C., Bylund J., Moretta L., Moretta A. (2012) Human NK cells induce neutrophil apoptosis via an NKp46- and Fas-dependent mechanism. J. Immunol. 188, 1668–1674 [DOI] [PubMed] [Google Scholar]
- 28. Pelletier M., Maggi L., Micheletti A., Lazzeri E., Tamassia N., Costantini C., Cosmi L., Lunardi C., Annunziato F., Romagnani S., Cassatella M. A. (2010) Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 115, 335–343 [DOI] [PubMed] [Google Scholar]
- 29. Abi Abdallah D. S., Egan C. E., Butcher B. A., Denkers E. Y. (2011) Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int. Immunol. 23, 317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geng S., Matsushima H., Okamoto T., Yao Y., Lu R., Page K., Blumenthal R. M., Ward N. L., Miyazaki T., Takashima A. (2013) Emergence, origin, and function of neutrophil-dendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood 121, 1690–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsushima H., Geng S., Lu R., Okamoto T., Yao Y., Mayuzumi N., Kotol P. F., Chojnacki B. J., Miyazaki T., Gallo R. L., Takashima A. (2013) Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood 121, 1677–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stark M. A., Huo Y., Burcin T. L., Morris M. A., Olson T. S., Ley K. (2005) Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22, 285–294 [DOI] [PubMed] [Google Scholar]
- 33. Pelletier M., Micheletti A., Cassatella M. A. (2010) Modulation of human neutrophil survival and antigen expression by activated CD4+ and CD8+ T cells. J. Leukoc. Biol. 88, 1163–1170 [DOI] [PubMed] [Google Scholar]
- 34. Litinskiy M. B., Nardelli B., Hilbert D. M., He B., Schaffer A., Casali P., Cerutti A. (2002) DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scapini P., Nardelli B., Nadali G., Calzetti F., Pizzolo G., Montecucco C., Cassatella M. A. (2003) G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J. Exp. Med. 197, 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scapini P., Carletto A., Nardelli B., Calzetti F., Roschke V., Merigo F., Tamassia N., Pieropan S., Biasi D., Sbarbati A., Sozzani S., Bambara L., Cassatella M. A. (2005) Proinflammatory mediators elicit secretion of the intracellular B-lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseases. Blood 105, 830–837 [DOI] [PubMed] [Google Scholar]
- 37. Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., Zychlinsky A. (2004) Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 [DOI] [PubMed] [Google Scholar]
- 38. Garcia-Romo G. S., Caielli S., Vega B., Connolly J., Allantaz F., Xu Z., Punaro M., Baisch J., Guiducci C., Coffman R. L., Barrat F. J., Banchereau J., Pascual V. (2011) Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lande R., Ganguly D., Facchinetti V., Frasca L., Conrad C., Gregorio J., Meller S., Chamilos G., Sebasigari R., Riccieri V., Bassett R., Amuro H., Fukuhara S., Ito T., Liu Y. J., Gilliet M. (2011) Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fox S., Leitch A. E., Duffin R., Haslett C., Rossi A. G. (2010) Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease. J. Innate Immun. 2, 216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crepaldi L., Gasperini S., Lapinet J. A., Calzetti F., Pinardi C., Liu Y., Zurawski S., de Waal Malefyt R., Moore K. W., Cassatella M. A. (2001) Up-regulation of IL-10R1 expression is required to render human neutrophils fully responsive to IL-10. J. Immunol. 167, 2312–2322 [DOI] [PubMed] [Google Scholar]
- 42. Zhang X., Majlessi L., Deriaud E., Leclerc C., Lo-Man R. (2009) Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity 31, 761–771 [DOI] [PubMed] [Google Scholar]
- 43. De Santo C., Arscott R., Booth S., Karydis I., Jones M., Asher R., Salio M., Middleton M., Cerundolo V. (2010) Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat. Immunol. 11, 1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Davey M. S., Tamassia N., Rossato M., Bazzoni F., Calzetti F., Bruderek K., Sironi M., Zimmer L., Bottazzi B., Mantovani A., Brandau S., Moser B., Eberl M., Cassatella M. A. (2011) Failure to detect production of IL-10 by activated human neutrophils. Nat. Immunol. 12, 1017–1018 [DOI] [PubMed] [Google Scholar]
- 45. Tamassia N., Zimmermann M., Castellucci M., Ostuni R., Bruderek K., Schilling B., Brandau S., Bazzoni F., Natoli G., Cassatella M. A. (2013) Cutting edge: an inactive chromatin configuration at the IL-10 locus in human neutrophils. J. Immunol. 190, 1921–1925 [DOI] [PubMed] [Google Scholar]
- 46. Maffia P. C., Zittermann S. E., Scimone M. L., Tateosian N., Amiano N., Guerrieri D., Lutzky V., Rosso D., Romeo H. E., Garcia V. E., Issekutz A. C., Chuluyan H. E. (2007) Neutrophil elastase converts human immature dendritic cells into transforming growth factor-β1-secreting cells and reduces allostimulatory ability. Am. J. Pathol. 171, 928–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wingender G., Hiss M., Engel I., Peukert K., Ley K., Haller H., Kronenberg M., von Vietinghoff S. (2012) Neutrophilic granulocytes modulate invariant NKT cell function in mice and humans. J. Immunol. 188, 3000–3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cerutti A., Cols M., Puga I. (2012) Activation of B cells by non-canonical helper signals. EMBO Rep. 13, 798–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Crotty S. (2011) Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 29, 621–663 [DOI] [PubMed] [Google Scholar]
- 50. Honjo T., Kinoshita K., Muramatsu M. (2002) Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu. Rev. Immunol. 20, 165–196 [DOI] [PubMed] [Google Scholar]
- 51. Klein U., Dalla-Favera R. (2008) Germinal centres: role in B-cell physiology and malignancy. Nat. Rev. Immunol. 8, 22–33 [DOI] [PubMed] [Google Scholar]
- 52. Huard B., McKee T., Bosshard C., Durual S., Matthes T., Myit S., Donze O., Frossard C., Chizzolini C., Favre C., Zubler R., Guyot J. P., Schneider P., Roosnek E. (2008) APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J. Clin. Invest. 118, 2887–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scapini P., Bazzoni F., Cassatella M. A. (2008) Regulation of B-cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunol. Lett. 116, 1–6 [DOI] [PubMed] [Google Scholar]
- 54. Avery D. T., Kalled S. L., Ellyard J. I., Ambrose C., Bixler S. A., Thien M., Brink R., Mackay F., Hodgkin P. D., Tangye S. G. (2003) BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J. Clin. Invest. 112, 286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. He B., Xu W., Santini P. A., Polydorides A. D., Chiu A., Estrella J., Shan M., Chadburn A., Villanacci V., Plebani A., Knowles D. M., Rescigno M., Cerutti A. (2007) Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26, 812–826 [DOI] [PubMed] [Google Scholar]
- 56. Xu W., He B., Chiu A., Chadburn A., Shan M., Buldys M., Ding A., Knowles D. M., Santini P. A., Cerutti A. (2007) Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat. Immunol. 8, 294–303 [DOI] [PubMed] [Google Scholar]
- 57. Tsuji M., Suzuki K., Kitamura H., Maruya M., Kinoshita K., Ivanov I. I., Itoh K., Littman D. R., Fagarasan S. (2008) Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity 29, 261–271 [DOI] [PubMed] [Google Scholar]
- 58. Balázs M., Martin F., Zhou T., Kearney J. F. (2002) Blood Dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 17, 341–352 [DOI] [PubMed] [Google Scholar]
- 59. Kang Y. S., Do Y., Lee H. K., Park S. H., Cheong C., Lynch R. M., Loeffler J. M., Steinman R. M., Park C. G. (2006) A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell 125, 47–58 [DOI] [PubMed] [Google Scholar]
- 60. Kesteman N., Vansanten G., Pajak B., Goyert S. M., Moser M. (2008) Injection of lipopolysaccharide induces the migration of splenic neutrophils to the T cell area of the white pulp: role of CD14 and CXC chemokines. J. Leukoc. Biol. 83, 640–647 [DOI] [PubMed] [Google Scholar]
- 61. Weller S., Bonnet M., Delagreverie H., Israel L., Chrabieh M., Maródi L., Rodriguez-Gallego C., Garty B-Z., Roifman C., Issekutz A. C., Zitnik S. E., Hoarau C., Camcioglu Y., Vasconcelos J., Rodrigo C., Arkwright P. D., Cerutti A., Meffre E., Zhang S-Y., Alcais A., Puel A., Casanova J-L., Picard C., Weill J-C., Reynaud C-A. (2012) IgM+IgD+CD27+ B cells are markedly reduced in IRAK-4-, MyD88-, and TIRAP- but not UNC-93B-deficient patients. Blood 120, 4992–5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rauch P. J., Chudnovskiy A., Robbins C. S., Weber G. F., Etzrodt M., Hilgendorf I., Tiglao E., Figueiredo J. L., Iwamoto Y., Theurl I., Gorbatov R., Waring M. T., Chicoine A. T., Mouded M., Pittet M. J., Nahrendorf M., Weissleder R., Swirski F. K. (2012) Innate response activator B cells protect against microbial sepsis. Science 335, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chu V. T., Frohlich A., Steinhauser G., Scheel T., Roch T., Fillatreau S., Lee J. J., Lohning M., Berek C. (2011) Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 12, 151–159 [DOI] [PubMed] [Google Scholar]
- 64. Kool J., De Visser H., Gerrits-Boeye M. Y., Klasen I. S., Melief M. J., Van Helden-Meeuwsen C. G., Van Lieshout L. M., Ruseler-Van Embden J. G., Van den Berg W. B., Bahr G. M., et al. (1994) Detection of intestinal flora-derived bacterial antigen complexes in splenic macrophages of rats. J. Histochem. Cytochem. 42, 1435–1441 [DOI] [PubMed] [Google Scholar]
- 65. Yeramilli V. A., Knight K. L. (2013) Development of CD27 marginal zone B cells requires GALT. Eur. J. Immunol. doi: 10.1002/eji. 201243205 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bernasconi N. L., Traggiai E., Lanzavecchia A. (2002) Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298, 2199–2202 [DOI] [PubMed] [Google Scholar]
- 67. Aranburu A., Ceccarelli S., Giorda E., Lasorella R., Ballatore G., Carsetti R. (2010) TLR ligation triggers somatic hypermutation in transitional B cells inducing the generation of IgM memory B cells. J. Immunol. 185, 7293–7301 [DOI] [PubMed] [Google Scholar]
- 68. Scheeren F. A., Nagasawa M., Weijer K., Cupedo T., Kirberg J., Legrand N., Spits H. (2008) T cell-independent development and induction of somatic hypermutation in human IgM+ IgD+ CD27+ B cells. J. Exp. Med. 205, 2033–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Weller S., Mamani-Matsuda M., Picard C., Cordier C., Lecoeuche D., Gauthier F., Weill J. C., Reynaud C. A. (2008) Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J. Exp. Med. 205, 1331–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bentebibel S. E., Schmitt N., Banchereau J., Ueno H. (2011) Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc. Natl. Acad. Sci. USA 108, E488–E497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Weller S., Faili A., Garcia C., Braun M. C., Le Deist F. F., de Saint Basile G. G., Hermine O., Fischer A., Reynaud C. A., Weill J. C. (2001) CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc. Natl. Acad. Sci. USA 98, 1166–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Berkowska M. A., Driessen G. J. A., Bikos V., Grosserichter-Wagener C., Stamatopoulos K., Cerutti A., He B., Biermann K., Lange J. F., van der Burg M., van Dongen J. J. M., van Zelm M. C. (2011) Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood 118, 2150–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]