Abstract
Background
Psoriasis is a hyper-proliferative disease of the skin in which immunological mechanisms play a direct pathogenetic role. There have been limited studies of natural killer (NK) cells in psoriasis.
Objectives
To examine the phenotype of NK cells in skin biopsies and peripheral blood mononuclear cells from patients with psoriasis and healthy controls.
Methods
CD56+CD16− and CD56+CD16+ NK cells were isolated from lesional skin, unaffected skin and PBMC of psoriasis patients, and normal skin and PBMC from healthy controls. The expression of CD57, NKG2A, and NKG2C was assessed by flow cytometry.
Results
NK cells in psoriasis skin lesions were skewed in their expression of CD57, a marker of NK cell maturity, with CD57 expression significantly reduced and NKG2A expression increased on NK cells in lesional and unaffected skin compared to controls.
Conclusions
These data suggest that in this patient cohort NK cells could be isolated from psoriasis lesions and exhibit an immature phenotype.
Keywords: psoriasis, natural killer cells, CD57, immunosenescence
Background
Psoriasis is a chronic inflammatory disease of the skin with significant morbidity (1). Whether NK cells are involved in the immunopathogenesis of psoriasis remains controversial, although genetic and immunological analyses support a role (2-4). NK cells function without prior sensitization and could initiate plaque development (5, 6).
NK cells express a heterogeneous repertoire of receptors that regulate their effector function (7, 8). Although NK cell function (cytotoxicity and cytokine secretion) is related to their stage of development, both immature CD56brightCD16neg and mature CD56dimCD16+ NK cell subpopulations secrete IFNγ, critical in psoriasis pathogenesis (6).
Expression of CD57 identifies terminally differentiated T cells (9). CD57 is also expressed on highly mature cells within the CD56dimCD16+ NK cell compartment and might provide a marker of “memory” NK cells (10-12). NK cell differentiation is described as a progression from a less mature phenotype displaying high CD56 expression together with NKG2A but lacking CD16, to a more differentiated phenotype expressing CD16 and CD57 with a decreasing ability to replicate in vitro(10-12).
Questions Addressed
We investigated the distribution of CD57+ NK cells in lesional skin, non-lesional skin and peripheral blood of psoriasis patients compared to controls. We also investigated the expression of the inhibitory receptor NKG2A and the activating receptor NKG2C on NK cells in the skin and blood of patients and controls.
Experimental Design
Peripheral blood mononuclear cells (PBMCs) and skin biopsies were collected from 12 patients with psoriasis and PBMC alone were collected from 10 healthy controls.
The inclusion criteria and protocols are described in Supplemental Materials and Methods.
Results
Nine males and 3 females were included, median age 48 years (range 21 to 84). Five subjects had plaque and 7 subjects had guttate psoriasis. The majority of subjects had mild disease (n = 8), 3 had moderate disease, and 1 had severe disease. Matched skin and PBMC samples were available from 9 out of 12 psoriasis subjects. Median age of healthy controls was 45 years.
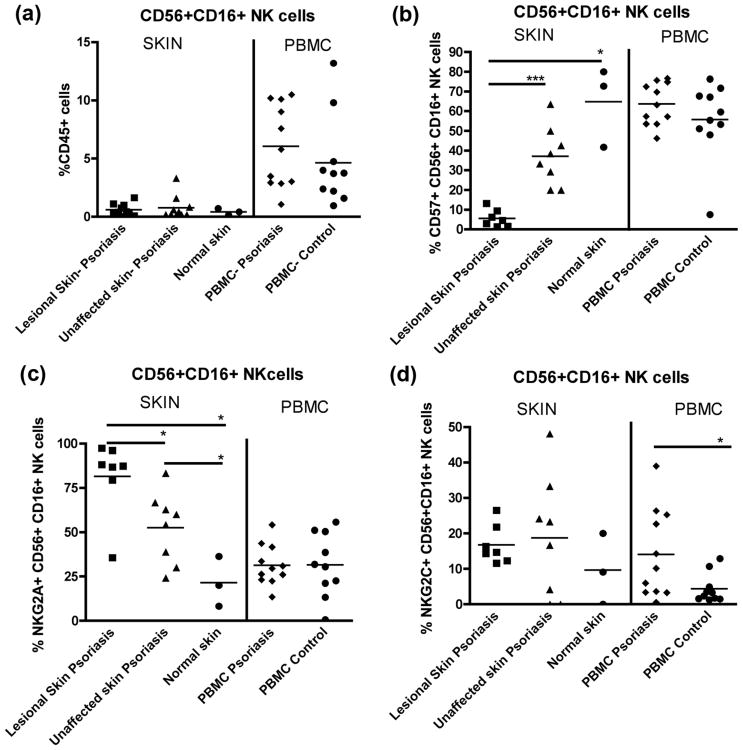
Both lesional and non-lesional skin of psoriasis patients had similar frequencies of CD56+CD16+ NK cells; however, in the blood patients had a trend toward an increased frequency of CD56+CD16+ NK cells compared to controls (Fig. 1a). The frequency of CD57+ NK cells in lesional skin (less than 10%) was significantly lower than in non-lesional skin (∼ 40%) (Fig. 1b).
Figure 1.
(a) Frequency of CD56+CD16+ NK cells in lesional skin (n=7) and unaffected skin (n=8) of psoriasis patients and skin from healthy controls (n=3), and in the blood of psoriasis patients (n=11) and healthy controls (n=10). (b) CD57+CD56+CD16+ NK cells in skin and the blood. (c) NKG2A expression on CD56+CD16+ NK cells in skin and the blood. (d) NKG2C expression on CD56+CD16+ NK cells from skin and PBMC. * = p<0.05, *** = p<0.001.
An assessment of NKG2A and NKG2C expression on skin CD56+CD16+ NK cells revealed a significant expansion of NKG2A+ NK cells in skin of patients (Fig. 1c). The frequency of NKG2A+ NK cells in PBMC was similar between patients and controls, while patients had a significantly greater frequency of circulating NKG2C+ NK cells compared to controls (Fig. 1d).
Co-expression of CD57, NKG2A, and NKG2C on skin NK cells was different between patients and controls (Supplemental Fig. 1). NK cells from the skin of patients preferentially expressed NKG2A, particularly in lesional skin. In contrast, NK cells in the skin of healthy donors contained CD57+ NK cells that were NKG2A− and NKG2C−. These data suggest that NK cells in the skin of this cohort of patients are less mature.
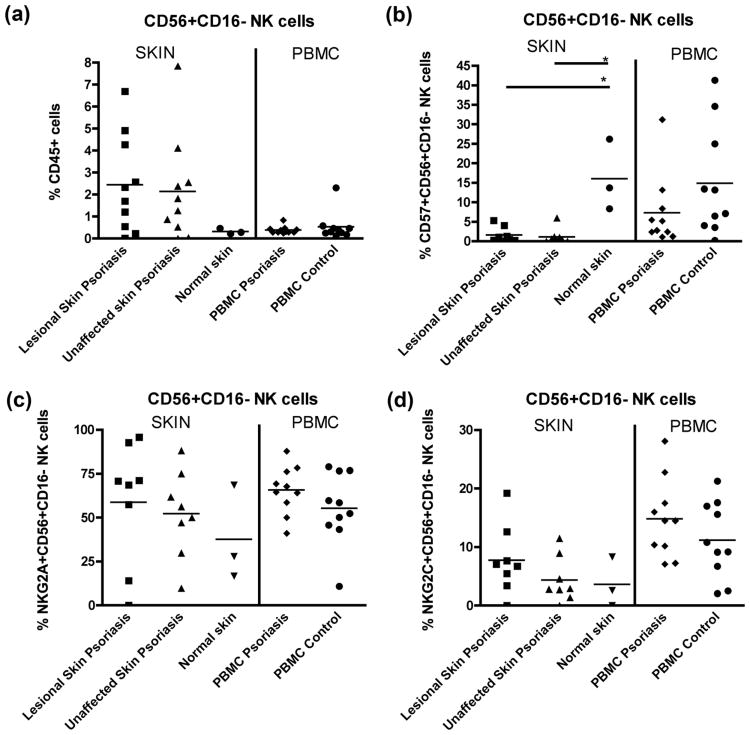
There was a trend toward an increased frequency of CD56+CD16− NK cells in psoriasis lesional and unaffected skin compared to control skin (Fig. 2a). CD57 expression on CD56+CD16− NK cells was significantly reduced in lesional and non-lesional skin of patients compared to controls (Fig. 2b). A trend of greater NKG2A expression in lesional skin compared to unaffected skin and control skin was observed (Fig. 2c), with lack of difference for NKG2C expression in the skin or blood of patients versus controls (Fig. 2d).
Figure 2.
(a) Frequency of CD56+CD16− NK cells in lesional skin and unaffected skin of psoriasis patients and skin from healthy controls and in the blood of psoriasis patients and healthy controls. (b) CD57+CD56+CD16− NK cells in skin and the blood. (c) NKG2A expression on CD56+CD16− NK cells in skin and the blood. (d) NKG2C expression on CD56+CD16− NK cells from skin and PBMC. * = p<0.05.
Comparison between plaque and guttate psoriasis for all measured parameters demonstrated no difference, although numbers for each group were small (Supplemental Figs. 2 and 3).
Conclusions
In this study, we found variations in NK cell distribution in blood and tissue of psoriasis patients compared to healthy controls.
We observed a substantially lower percentage of CD57+CD56+CD16+ NK cells in lesional skin compared to control skin. CD57−CD56+CD16+ correspond to activated cells with a higher turnover and degranulation ability (13). They are highly responsive to IL-2 stimulation, which could determine their preferential migration to the skin (14). CD57−CD56+CD16+ NK cells produce higher amounts of IFNγ following cytokine stimulation compared to CD57+CD56+CD16+ NK cells, and could contribute to the inflammatory environment (11, 13).
We observed a significant difference in the proportion of NKG2A+ cells in lesional skin. NKG2A is inducible by IL-12, which is likely to be highly expressed in lesions. The acquisition of functional capacities by NK cells, specifically IFNγ production, is correlated with NKG2A expression on CD56+CD16+ NK cells (15).
The difference observed in the proportion of circulating NKG2C+ NK cells could be due to differences in CMV serostatus, as NK cells expressing NKG2C are preferentially expanded in CMV seropositive individuals (16).
Together, these data suggest that NK cells from this group of psoriasis patients harbor a less differentiated phenotype. Future studies are needed to determine the functional significance of these less differentiated NK cells in psoriasis lesions, as this study included only a small number of patients with heterogenous characteristics.
Supplementary Material
Supplemental Figure 1: Co-expression of CD57, NKG2A, and NKG2C on CD56+CD16+ NK cells in lesional and unaffected skin of psoriasis patients and normal skin of healthy controls. Both pie charts and bar graphs are shown. Comparisons are made between psoriasis groups (lesional and unaffected skin) in relation to the control group (normal skin). Significant differences (p<0.05) are represented by a (+). A significant difference between groups was noted in CD57 and NKG2A expression alone and in NKG2A and NKG2C co-expression. + = p<0.05.
Supplemental Figure 2: Lack of difference between plaque and guttate psoriasis for the expression of markers on CD56+CD16+ NK cells. (a) CD57. (b) NKG2A. (c) NKG2C.
Supplemental Figure 3: Lack of difference between plaque and guttate psoriasis for the expression of markers on CD56+CD16− NK cells: (a) CD57. (b) NKG2A. (c) NKG2C.
Acknowledgments
The authors would like to acknowledge the generosity and scientific curiosity of patients and their families for blood and tissue donations.
This work was supported in part by the NIH (grants AI64520), FAPESP (04/15856-9/Kallas and 2010/05845-0/Kallas and Nixon), and the Peter and Shelagh Godsoe Family Foundation through the AIDS Research Institute at UCSF. E.L.H. was an American Academy of Neurology Clinical Research Training Fellowship recipient. J.M.M.: supported in part by the NIH (grant 5T32HL007185). W.L: supported in part by grants from the National Institute of Musculoskeletal and Skin Diseases (5K08AR057763) and the International AIDS Society in collaboration with NIH-funded Centers for AIDS Research, U.S. National Institutes of Health, and UW Institute of Translational Health Sciences. L.L.L. is an American Cancer Society Professor and supported by NIH grant AI068129.
Abbreviations used
- IFN-γ
Interferon-gamma
- NK
Natural Killer Cell
- PBMC
peripheral blood mononuclear cells
Footnotes
Conflict of Interest Statement: No conflicts of interest to disclose.
Author's contributions: MB, EH, VY: performed experiments
MB, EH, JM, LL, WL, EK, DN: designed study
DC: provided samples
MB, EH, JM: analyzed data
MB, PK, JM, LL, EK, WL, DN: wrote paper
WL, PU, KL, TM: patient recruitment/ critical revision
References
- 1.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009 Jul 30;361(5):496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 2.Tobin AM, Lynch L, Kirby B, O'Farrelly C. Natural killer cells in psoriasis. J Innate Immun. 2011;3(4):403–10. doi: 10.1159/000328011. [DOI] [PubMed] [Google Scholar]
- 3.Dunphy S, Gardiner CM. NK cells and psoriasis. J Biomed Biotechnol. 2011;2011:248317. doi: 10.1155/2011/248317. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki Y, Hamamoto Y, Ogasawara Y, Ishikawa K, Yoshikawa Y, Sasazuki T, et al. Genetic polymorphisms of killer cell immunoglobulin-like receptors are associated with susceptibility to psoriasis vulgaris. J Invest Dermatol. 2004 May;122(5):1133–6. doi: 10.1111/j.0022-202X.2004.22517.x. [DOI] [PubMed] [Google Scholar]
- 5.Cameron AL, Kirby B, Griffiths CE. Circulating natural killer cells in psoriasis. Br J Dermatol. 2003 Jul;149(1):160–4. doi: 10.1046/j.1365-2133.2003.05319.x. [DOI] [PubMed] [Google Scholar]
- 6.Ottaviani C, Nasorri F, Bedini C, de Pita O, Girolomoni G, Cavani A. CD56brightCD16(-) NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. 2006 Jan;36(1):118–28. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- 7.Sun JC, Lanier LL. Versatility in NK cell memory. Immunol Cell Biol. 2011 Mar;89(3):327–9. doi: 10.1038/icb.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. 2010 Sep 17;142(6):847–56. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003 Apr 1;101(7):2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 10.Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010 Nov 11;116(19):3853–64. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010 Nov 11;116(19):3865–74. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, et al. Expansion of a unique CD57+NKG2Chi Natural Killer cell subset during acute human cytomegalovirus infection. PNAS. 2011 doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong HS, Eberhard JM, Keudel P, Bollmann BA, Ballmaier M, Bhatnagar N, et al. HIV infection is associated with a preferential decline in less-differentiated CD56dim CD16+ NK cells. J Virol. 2010 Jan;84(2):1183–8. doi: 10.1128/JVI.01675-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caligiuri MA, Zmuidzinas A, Manley TJ, Levine H, Smith KA, Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990 May 1;171(5):1509–26. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beziat V, Descours B, Parizot C, Debre P, Vieillard V. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLoS One. 2010;5(8):e11966. doi: 10.1371/journal.pone.0011966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, et al. Expansion of a unique CD57NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011 Sep 6;108(36):14725–32. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Co-expression of CD57, NKG2A, and NKG2C on CD56+CD16+ NK cells in lesional and unaffected skin of psoriasis patients and normal skin of healthy controls. Both pie charts and bar graphs are shown. Comparisons are made between psoriasis groups (lesional and unaffected skin) in relation to the control group (normal skin). Significant differences (p<0.05) are represented by a (+). A significant difference between groups was noted in CD57 and NKG2A expression alone and in NKG2A and NKG2C co-expression. + = p<0.05.
Supplemental Figure 2: Lack of difference between plaque and guttate psoriasis for the expression of markers on CD56+CD16+ NK cells. (a) CD57. (b) NKG2A. (c) NKG2C.
Supplemental Figure 3: Lack of difference between plaque and guttate psoriasis for the expression of markers on CD56+CD16− NK cells: (a) CD57. (b) NKG2A. (c) NKG2C.