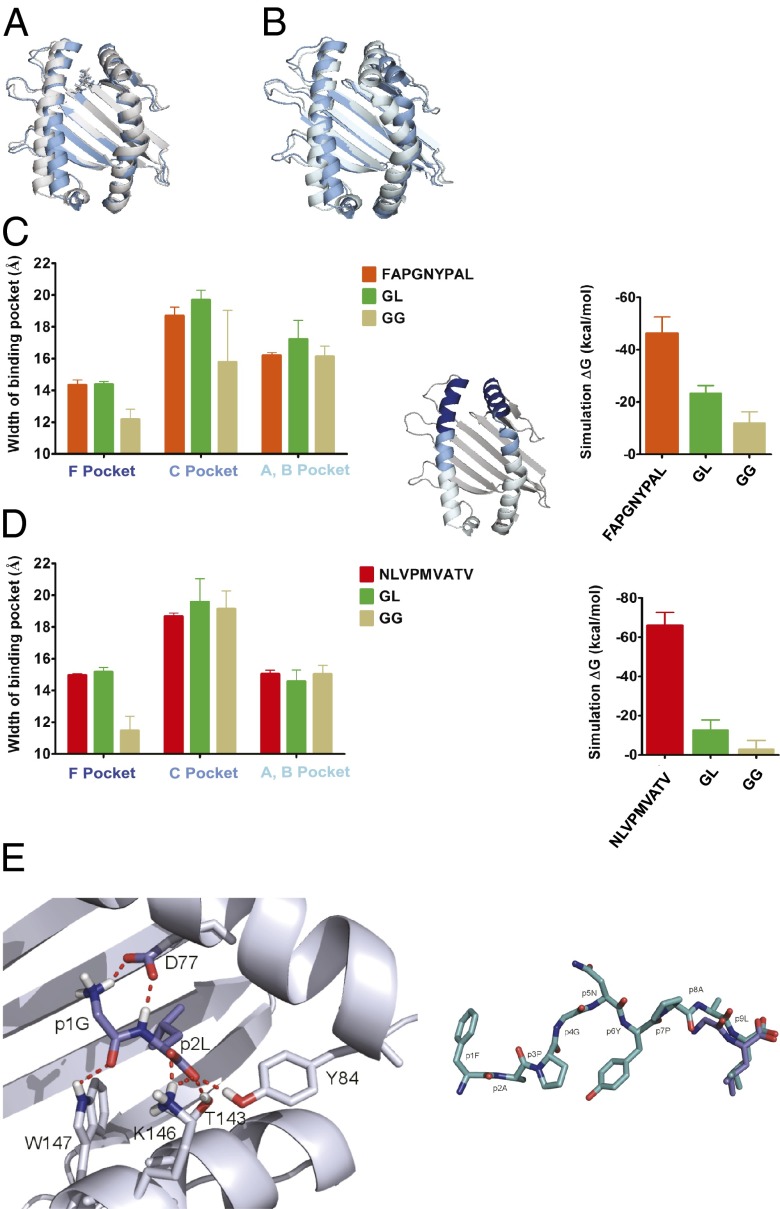
Fig. 4.
Binding of GL into the F pocket area. (A and B) GL keeps the Kb binding pocket in shape. Snapshot from a 20-ns MD simulation after 16 ns with GL (A, gray) and GG (B, light blue; dipeptide dissociated after 1.2 ns) placed in the F pocket, overlaid with the starting structure (dark blue; the same in both simulations). (C and D) GL serves the as a placeholder for the full-length peptide. (Left) Average widths (shown as distances of the centers of mass of the α-carbons of opposing helical segments ± SD; n = 3) of the F pocket region (residues 74–85 vs. 138–149; dark blue in the small structure), the C pocket region (residues 67–73 vs. 150–156; cyan), and the A/B pocket region (residues 50–66 vs. 157–174; light blue) for Kb (C) and A2 (D) from the simulations with FAPGNYPAL/NLVPMVATV, GL, and GG as indicated. (Right) Theoretical binding energies for high-affinity peptides, GL, and GG derived from 5-ns MD simulations. (E, Left) Close-up view of GL (blue) bound to the F pocket region of Kb from a snapshot during the MD simulation. (E, Right) Comparison of the structures of FAPGNYPAL bound to Kb (cyan; crystal structure) and GL bound to the F pocket region of Kb (blue; MD simulation).