Significance
Antibodies directed against microbial polysaccharides are a critical component of protective immune responses and vaccines. We used nanoparticles coexpressing pneumococcal capsular polysaccharides and a cell wall lipid antigen analog to model NKT–B-cell interactions. Our study demonstrated CD1d-restricted cognate interactions, isotype switch, affinity maturation, and long-term memory, despite the apparent failure of NKT cells to differentiate into follicular helper cells. The findings demonstrate the importance of nonconventional sources of help for B cells responding to polysaccharide antigens.
Abstract
Innate-like natural killer T (NKT) cells critically enhance cell and humoral immunity against infections through recognition of conserved microbial lipid antigens presented by CD1d-expressing antigen-presenting cells, and provision of CD40L and cytokine signals. Whereas NKT cells efficiently licensed dendritic cells to prime potent effector and memory T cells, studies based on model antigens such as alphagalactosylceramide-nitrophenyl conjugates concluded that help to B cells was associated with NKT follicular helper differentiation, but limited to short-term responses without induction of memory. We revisited this surprising conclusion in the context of the extracellular encapsulated pathogen Streptococcus pneumoniae, where recognition of lipid and capsular polysaccharide antigens by NKT cells and B cells, respectively, provide critical host protection. Using liposomal nanoparticles displaying synthetic lipid and polysaccharide antigens to elicit pure and direct NKT–B-cell interactions in vivo, we observed intense and prolonged antibody responses with isotype switch, affinity maturation, and long-lasting B-cell memory, despite modest or absent NKT follicular helper differentiation. Furthermore, conditional ablation of Cd1d demonstrated a requirement for a two-step process involving first cognate interactions with dendritic cells, for NKT cell activation, and then with B cells, for induction of isotype switch and memory. Thus, NKT help to B cells represents both a major arm of antimicrobial defense and a promising target for B-cell vaccines.
The microbial organism Streptococcus pneumoniae is a ubiquitous extracellular pathogen and a leading agent of pneumonia and meningitis causing 8–12% of all deaths in children aged <6 y worldwide (1). The protective role of antibodies is highlighted by the severity of infection in splenectomized or agammaglobulinemic patients and by passive transfer experiments (reviewed in ref. 2). Natural, circulating IgM antibodies to the phosphorylcholine (PC) residues decorating teichoic acid can provide a first barrier to infection (3), but the main protection is provided by antibodies elicited upon infection or nasopharyngeal carriage (4, 5). These antibodies target the capsular polysaccharides (PSs), leading to opsonization and complement fixation. Variations of these capsular PSs, which impact the ecological niche and the fitness of the microbial organism, define common serotypes recognized by highly specific antibodies.
Purified capsular PSs are T-independent type II antigens, which typically elicit short-lived IgM responses without germinal center formation, affinity maturation, isotype switch, or memory (reviewed in ref. 6). However, T-dependent IgG isotypes are commonly detected in patients with a history of infection by Pneumococcus or with nasopharyngeal carriage of this organism (4, 5), suggesting that PS-specific B cells can receive some form of cognate help, although the source of this help has remained unclear (reviewed in ref. 7). Capsular PSs are noncovalently associated with proteins and lipids in the underlying bacterial cell wall, implying that B cells carrying capsular PS-specific antibodies might internalize these potential T-cell antigens along with the PS antigens to recruit a form of “intermolecular” help, after endosomal digestion and loading onto MHC class II and CD1d molecules, respectively.
Lipid antigens are particularly relevant in the case of S. pneumoniae, because the pneumococcal cell wall contains α-glucosyldiacylglycerol (αGlcDAG) (8–10), a glycolipid that is structurally and functionally similar to the prototypical natural killer T (NKT) antigen alphagalactosylceramide (αGalCer). NKT-deficient mice lacking CD1d or the T-cell receptor (TCR) gene segment Jα18 exhibited diminished clearance of Pneumococcus and increased mortality after intratracheal administration of the pathogen, highlighting the importance of NKT cells in the context of live infection (8, 10, 11).
NKT cells can provide direct cognate help to antimicrobial B cells, as shown during infection of mice with Sphingomonas, a bacterium that carries agonist lipid antigens in its cell wall. Indeed, in mice reconstituted with a mixture of Igh allotype marked CD1d−/− and CD1d+/+ bone marrow cells, production of IgG2a antibodies against the PDC-E2 microbial protein was preferentially (although not exclusively) elicited from the CD1d-expressing B cells (12). PDC-E2 is a membrane-associated bacterial protein that is not covalently linked to the membrane lipid antigen, suggesting intermolecular help.
Several studies using model antigens have also established that NKT cells could directly or indirectly help B cells mount antibody responses (13–23). Indirect help was channeled through the enhanced priming of conventional CD4 T cells by NKT cell-licensed dendritic cells (DCs), with subsequent MHC class II-restricted help to B cells responding to the same protein antigen (18, 23). Direct help, involving cognate NKT–B-cell conjugate formation, has been studied in mice immunized with alphagalactosylceramide-nitrophenyl (αGalCer-NP), a conjugate of the NKT ligand αGalCer covalently linked to the B-cell antigen NP, or in MHC II-deficient or -nonresponder mice immunized with protein antigens associated with αGalCer (20–23). These studies suggested that NKT cells could induce the transcription factor Bcl6 and acquire a typical follicular helper program, entering B-cell follicles in a chemokine CXCR5-dependent manner to form long-lived conjugates with antigen-specific B cells, promoting both germinal center formation and extrafollicular foci and enhancing B-cell proliferation and antibody production. Surprisingly, despite the abundant CD40L and cytokine signals that NKT cells can supply, the B-cell response was of short duration, without generation of long-lasting plasma cells or induction of B-cell memory (“fast but does not last”), suggesting that NKT cell help was intrinsically limited compared with conventional CD4 T-cell help (21–24).
Here, instead of using NP conjugates, we have modeled NKT–B-cell interactions in the physiologically relevant context of the microbial pathogen, S. pneumoniae, and its major lipid and PS antigens. To avoid interferences by the multiple streptococcal components known to elicit or subvert immune responses and to preserve the particulate nature and the architectural relationship between the lipid and PS antigens, we engineered liposomal nanoparticles containing synthetic forms of the NKT and B-cell antigens, with the lipid inserted in the membrane bilayer and the PS displayed at the outer surface through a diacylglycerol anchor. In addition, we used mice carrying a conditional allele of Cd1d (25) to identify the antigen-presenting cells (APCs) required for NKT cell activation and for B-cell responses. The results demonstrate that, after initial interactions with CD1d-expressing DCs, NKT cells provided cognate help to B cells to promote antibody responses. In contrast with previous reports, and despite the absence of covalent linkage between the NKT and B-cell antigens, the antibody response was very long lasting, exhibited affinity maturation, and showed long-term memory. Furthermore, this strong cognate to B cells occurred despite minimal signs of NKT follicular helper (NKTfh) differentiation, suggesting a mostly extrafollicular response. Thus, the results considerably expand the significance and the scope of NKT cell help to B cells, particularly in the context of T-independent polyvalent microbial antigens. They also raise a simple and effective alternative to the conjugate PS vaccines currently used worldwide to protect children against pneumococcal infections.
Results
Antibody Response to Liposomal Nanoparticles Carrying Lipid and Capsular PS Antigens.
To study the interplay between NKT cells and B cells in a relevant microbial context, while eliminating potential confounding factors associated with the whole microbial organism, we engineered liposomal nanoparticles that mimicked the natural display of NKT and B-cell antigens by S. pneumoniae. The tetrasaccharide unit repeat of the capsular PS (serotype 14) (26) was linked to a diacylglycerol group (compound PBS150) (Fig. 1) for insertion into liposomal membranes along with the NKT lipid ligand PBS57, a modified α-galactosylceramide. Fig. 2A shows the primary and secondary responses elicited after two sequential injections of antigenic liposomes at days 0 and 39. IgM antibodies against the PS were observed in the first week, followed by IgG3 in the second week, consistent with the T-independent nature of these isotypes. “Help-dependent” isotypes IgG1 and IgG2c began to appear after 2 wk. All of the isotypes achieved very high titers, peaking 1 wk after the second injection at up to 103- to 104-fold above preimmunization levels. These circulating antibodies persisted for an extended period, as substantial titers were still observed 7 mo after the last injection, suggesting the presence of long-lived antibody-secreting cells. The antibodies elicited by the liposomes did not react against lipid components and, consistent with previous studies in mice and humans, were specific for the pneumococcal PS serotype 14 (Fig. 2B). Furthermore, omission of the NKT ligand PBS57 from liposomes partially decreased IgM titers and prevented the IgG1 antibody switch, essentially causing reversion to a pattern of T-independent B-cell response (Fig. 2C). Altogether, these results indicated that the presence of NKT ligands in PS-presenting liposomal particles enabled the generation of elevated and prolonged titers of IgM and IgG antibodies to pneumococcal PS.
Fig. 1.
DAG-anchored pneumococcal capsular polysaccharide. The tetrasaccharide repeat of S. pneumoniae capsular polysaccharide serotype 14 (Upper) was linked to a diacylglycerol as shown (Lower, compound PBS150) for insertion into liposomal membranes.
Fig. 2.
Anti-PS antibody response elicited by pneumococcal liposomes. (A) Kinetics of serum anti-PS14 antibody isotypes after immunization of B6 mice at indicated time points (arrows) with pneumococcal liposomes carrying 4 μg PS14 and 1 μg NKT lipid. Titers are shown as arbitrary units per milliliter by reference to a standard hyperimmune serum. Results represent two independent experiments with 10 mice each. (B, Left) FACS analysis of serum IgG1 antibodies binding to glass beads coated with PS14-carrying liposomes compared with empty liposomes and liposomes carrying the NKT ligand PBS57. (Right) ELISA analysis of serum antibodies against purified pneumococcal polysaccharide in individual naïve or immunized mice. (C) Serum IgM and IgG1 antibodies against PS14 measured 20 wk after immunization with liposomes carrying both PBS57 and PBS150, or PBS150 alone.
Cognate CD1d-Restricted NKT–B-Cell Interactions.
To test whether direct CD1d-restricted interactions with B cells were required for NKT cells to promote antibody responses, we immunized Cd1dfl/fl (flanked by lox site) Cd19-cre mice (lacking CD1d expression on B cells) and their littermate controls. Fig. 3A shows that genetic ablation of CD1d in B cells not only abrogated the switched antibody response, as shown with IgG1 isotypes, but also decreased the titers of IgM by >10-fold, essentially reverting the pattern to that of a T-independent type II response. Notably, the failure to enhance IgM levels and to switch isotypes occurred despite the activation and cytokine secretion by NKT cells, which presumably recognized their lipid ligand on DCs and macrophages. Indeed, Cd1dfl/fl Cd19-cre mice exhibited a normal IL-4 and IFN-γ cytokine burst as measured in the serum at 2 h and 24 h (Fig. 3B). Staining of B cells confirmed that substantial depletion of CD1d was achieved in Cd1dfl/fl Cd19-cre mice, although modest residual amounts could be detected by comparison with CD1d KO mice (Fig. 3C). These results demonstrated that cognate CD1d-restricted interactions between NKT cells and B cells, rather than bystander exposure to cytokines, were essential for potentiation of the B-cell response and induction of isotype switch.
Fig. 3.
Cognate NKT cell interactions with both DC and B cells are required for isotype-switched anti-PS antibody response. (A) Serum anti-PS antibodies after immunization of Cd1dfl/flCd19-cre (CD19ΔΔ), Cd1dfl/flCd11c-cre (CD11cΔΔ), and littermate controls (LM) with pneumococcal liposomes carrying 4 μg PS14 and 1 μg NKT lipid at indicated times (arrows). (B) Early serum IL-4 (2 h) and IFN-γ (24 h) after pneumococcal liposome injection in Cd1d conditional mutants and their littermate controls. Results are representative of two independent experiments with four to seven mice per group. (C) FACS analysis of surface expression of CD1d by CD19-gated splenocytes of wild type (LM), CD1d KO (KO), and CD19ΔΔ mice.
Requirement for NKT–DC Interactions.
The above results did not address whether CD1d expression by B cells was sufficient for the isotype-switched response. Indeed, prior studies have established that initial interaction of T cells or NKT cells with antigen-presenting DCs was required for acquisition of a follicular helper program and migration into B-cell follicles (reviewed in ref. 27). Using Cd1dfl/fl Cd11c-cre mice, which lack CD1d expression on DCs and on some CD11cintermediate (CD11cint) macrophage subsets such as F4/80 splenic macrophages, we found that the IgM response was decreased and the isotype-switched IgG1 antibodies were abrogated (Fig. 3A). In these mice, the amount of cytokines released in the serum by NKT cells was partially decreased, as expected (Fig. 3B). These results support a two-step process whereby NKT cells must be primed by DCs or macrophages before interacting with B cells.
NKT Cell Activation.
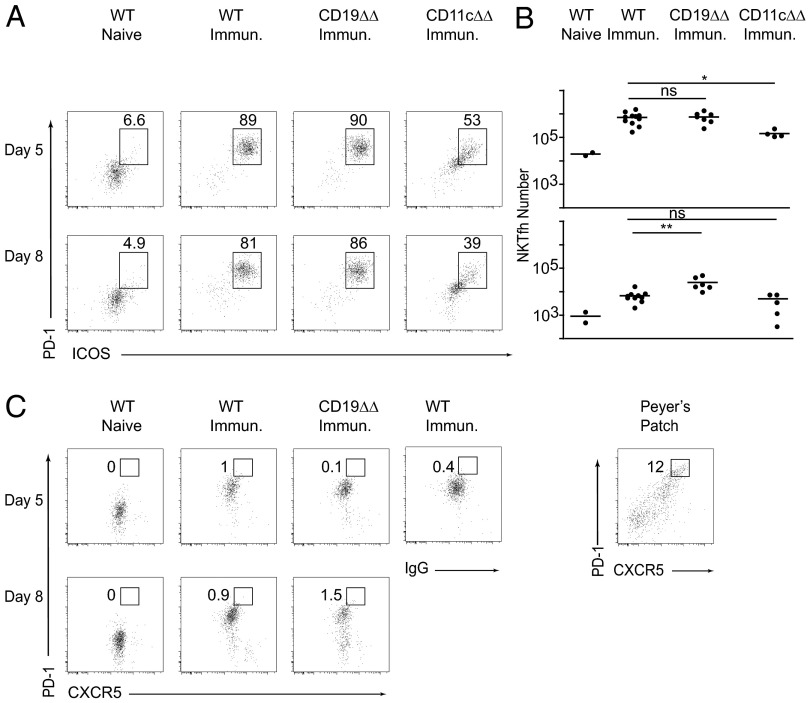
NKT cells are found in the T-cell zone as well as in the red pulp and possibly also the marginal zone of the spleen, but they are conspicuously absent from B-cell follicles (28–30). Previous studies indicated that NKT cells could acquire NKTfh differentiation upon activation by their lipid ligand and subsequently moved to B-cell follicles where they engaged in interactions with B cells (21, 22). We confirmed that NKT cells activation readily occurred after injection of liposomes carrying PS and lipid antigens, including up-regulation of PD-1 and inducible cell costimulator (ICOS), but failed to observe a significant population of PD-1highCXCR5high (PD-1hiCXCR5hi) “germinal center T follicular helper (GC-Tfh)-like” NKT cells, at least compared with reference staining of Peyer’s patch T cells, which are spontaneously enriched in GC-Tfh cells (Fig. 4 A–C). Furthermore, we demonstrated that this activation depended on CD1d expression by CD11c-expressing DCs or macrophages, because it was massively decreased in Cd1dfl/fl Cd11c-cre mice. In Cd1dfl/fl Cd19-cre mice lacking CD1d on B cells, however, NKT activation was readily observed with a kinetics and persistence similar to that of littermate controls. This persistence suggested that cognate interactions with B cells were neither required for the induction nor the maintenance of NKT cell activation, although it might also be associated with residual CD1d protein on the surface of Cd1dfl/fl Cd19-cre B cells.
Fig. 4.
The NKT follicular helper response requires CD1d expression by DCs but not B cells. (A and B) FACS staining of splenic NKT cells after pneumococcal liposome immunization of Cd1d conditional mutants and their littermate (LM) controls, as indicated. Results are shown as percentages for representative mice (Left) and absolute numbers for each individual mouse (Right) of ICOShiPD-1hi cells among gated CD1d-αGC tetramer+ NKT cells at day 5 (Upper) and day 8 (Lower). Results are representative of three independent experiments with five to seven mice per group. (C) Same analysis as in A for CXCR5 and PD-1. Staining controls used to set the PD-1hiCXCR5hi GC-Tfh gate included WT immunized NKT cells stained with IgG isotype instead of anti-CXCR5, as indicated, and Peyer’s patch CD4 T cells (rightmost dot plot), which are naturally enriched in GC-Tfh cells.
Affinity Maturation.
To test whether NKT cells also promoted affinity maturation of anti-PS antibodies, we compared the binding of IgG3 and IgG1 antibodies to liposomes carrying high or low levels of PS-DAG, respectively, 20% and 0.5% of liposomal lipids. Fig. 5 shows that IgG3 and IgG1 antibodies produced early in the response bound poorly to PS-0.5% compared with PS-20%. However, late antibodies after boost bound nearly as well to PS-0.5% as to PS-20% in several individual mice, demonstrating affinity maturation. Thus, NKT cell help can lead to affinity maturation of antibodies to capsular PS.
Fig. 5.
Affinity maturation of the NKT cell-helped anti-PS antibody response. (Left) FACS analysis of serum IgG3 and IgG1 antibody (1/200 dilution) binding to liposomes carrying low (0.5%) or high (20%) density PS in the early and late (after boost) response of a representative mouse showing affinity maturation. (Right) Ratios of reciprocal antibody titers to PS-20% over PS-0.5% for individual mice in the early and late response, as indicated. Results are representative of two independent experiments with five to seven mice each.
Memory Formation.
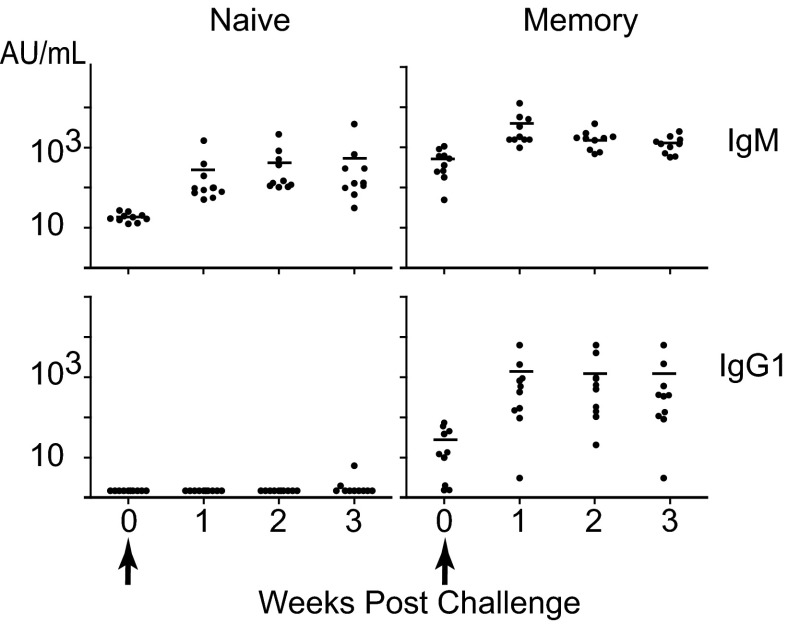
The hallmark of T-dependent help to B-cell responses is the formation of long-term B-cell memory. Fig. 6 shows that, when challenged 8 mo after the initial primary and secondary immunizations, the average titer of IgG1 antibodies was increased by ∼100-fold within 7 d, a much faster and greater response than naïve littermates exposed to the challenge. Thus, NKT cell help induces potent long-term B-cell memory.
Fig. 6.
Cognate NKT cell help promotes long-term B-cell memory. Mice immunized twice with pneumococcal liposomes (as in Fig. 1) and unimmunized, naïve littermates were reinjected 8 mo later with pneumococcal liposomes. The plots show individual titers of PS-specific IgM and IgG1 after challenge. Note that preimmunized mice had detectable residual PS-specific IgM and IgG1 antibodies before challenge. Results are representative of two independent experiments with a total of 20 mice.
Discussion
Our studies of the B-cell response to microbial PS antigens stand in contrast with previous studies using model antigens such as αGalCer-NP, which concluded that NKT cells readily acquired follicular helper differentiation based on CXCR5 expression but were intrinsically inefficient at promoting isotype switch, affinity maturation, long-term antibody production, and memory (21–24). These results were surprising because the same studies suggested that NKT cells readily acquired a SLAM-associated protein-dependent, Bcl6-driven follicular helper program, engaged in prolonged interactions with antigen-specific B cells, and promoted germinal centers and extrafollicular foci. In addition, NKT cells are well known to provide strong CD40 and cytokine-mediated signals, for example to DCs (31, 32). Our results differ in two important respects. We observed very little induction of PD-1hiCXCR5hi GC-Tfh-like NKT cells (<1–2%), suggesting a mostly extra-GC response, yet NKT cell-mediated cognate help to B cells was potent, comparable to a typical follicular helper response. The different outcome in our study may be related to one of several differences between the experimental systems used. These include the distinct chemical nature of the B-cell antigens, i.e., NP vs. PS antigens, and their different mode of association with NKT ligands as covalent conjugates, simple mixtures, or liposomes. In particular, it is notable that αGalCer-NP conjugates expressed only one NP group per lipid molecule and might not induce a level of B-cell receptor (BCR) cross-linking comparable to that observed with PS liposomes. Indeed, αGalCer-NP did not induce anti-nitroiodophenol (NIP) IgM antibodies in the absence of NKT cells or CD1d in vivo (19), whereas, in our system, substantial IgM responses could be observed even in the absence of CD1d, as is typical of T-independent type II antigens. BCR cross-linking is essential for efficient internalization of antigen and transport to endosomal compartments for CD1d loading and presentation, as shown in the response to nanoparticle-bound hen egg lysozyme (HEL) and αGalCer (20). With this particulate antigen, isotype switch was detected but the studies were not conducted beyond day 14 and the potential contribution of HEL-specific T cells elicited in the presence of αGalCer could not be eliminated. The distinct outcomes may also be influenced by changes in lipid uptake and presentation (33) or by distinct targeting of B-cell subsets such as B1-B or CD1dhigh marginal zone B cells (30, 34–36). In any case, our results radically alter the previous conclusion that NKT cells were “timid” helpers and instead emphasize their superior efficiency at eliciting affinity-matured and isotype-switched antibodies, as well as long-term B-cell memory against clinically relevant microbial capsular PS. Further studies are needed, however, to determine the location of NKT–B-cell interactions and the origin of the B cells involved in the anti-PS response. Although it remains to be seen whether natural pneumococcal NKT ligands can also promote antibody responses, our results also have important potential implications on host–pathogen interactions and microbial strategies of immune evasions, as subtle changes in microbial lipids can have drastic consequences on CD1d-mediated presentation and activation of NKT cells (9, 37, 38). Furthermore, as liposomal nanoparticles are a well-established pharmaceutical method of antigen delivery in humans (39, 40), our study highlights the untapped potential of NKT cell help for B-cell vaccines, especially against T-independent antigens such as pneumococcal capsular PS.
Materials and Methods
Mice.
C57BL/6.Cd19-cre (B6.129P2(C)-Cd19tm1(cre)Cgn) and C57BL/6 mice were from The Jackson Laboratory. C57BL/6.Cd11c-cre (C57BL/6J-Tg(Itgax-cre,-EGFP)4097Ach/J) mice were obtained from Alexander Chervonsky at the University of Chicago. C57BL/6.Cd1dfl/fl mice were generated in our laboratory and were crossed to Cd19-cre and Cd11c-cre to obtain Cd19 Δ/Δ and Cd11c Δ/Δ mice (25). Littermate controls included a mixture of cre−/−, cre+/− Cd1dfl/+, or cre+/− Cd1d+/+ littermates. All animal experiments were conducted according to protocols approved by the Institutional Animal Care and Use Committee of the University of Chicago.
Flow Cytometry.
Splenic lymphocytes were isolated by mincing and passing through a 70-μm nylon cell strainer (Falcon). Fluorochrome-labeled monoclonal antibodies against mouse CD3ε, PD-1, ICOS, CD1d, and CD19 were purchased from eBioscience or Biolegend. CXCR5 was detected using purified anti-CXCR5 (BD Pharmingen), followed by biotinylated anti-rat IgG (Jackson ImmunoResearch Laboratories) and APC-labeled streptavidin (Invitrogen). In CXCR5/PD-1 double staining experiments, cells were incubated with 100 μg/mL of unconjugated rat IgG2a-kappa (BD Pharmingen) after CXCR5 staining and before addition of the rat anti-mouse PD-1 antibody, to saturate free rat IgG binding sites in the biotinylated anti-rat anti-IgG antibody used to reveal anti-CXCR5. CD1d-PBS57 tetramers were obtained from the National Institute of Allergy and Infectious Diseases Tetramer Core Facility. Samples were analyzed on an LSR II or, after staining with CD1d-PBS57 tetramer and CD3ε, were sorted on a FACS Aria (BD Biosciences).
Lipid and Polysaccharide Antigens.
The 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) was from AvantiPolar Lipids (850375C) and cholesterol from Sigma (47127-U). PBS57 was produced as described previously (41–43). For synthesis of PBS150, the diacylthioglycerol–tetrasaccharide conjugate was prepared via amide bond formation using the corresponding activated ester and amine. All of the lipids were dissolved in chloroform and stored at −20 °C at concentrations of 5–25 mg/mL.
Liposomes.
For immunizations, a mixture containing DOPC, cholesterol, PBS150, and PBS57 (35/40/20/5 molar ratio) was dried under a stream of nitrogen to remove chloroform, then hydrated and subjected to freeze/thaw cycles to produce multilamellar vesicles, and finally sized by extrusion through a polycarbonate filter of 400-nm pore size (Whatman; 80028) in the Avanti miniextruder (AvantiPolar Lipids; 610000). The quality and size of these “pneumococcal” liposomes was monitored by cryoelectron microscopy. Liposomes were used immediately or within 1 wk of storage at 4 °C.
Anti-Polysaccharide Antibody Assay.
DOPC/cholesterol liposomes containing 20% (molar ratio) PS were adsorbed onto 3- to 10-μM glass beads (Polysciences Inc.; 07666) and incubated with serial dilutions of immune or naïve sera before adding FITC-conjugated isotype-specific goat antibodies from Southern Biotech (anti-IgM, 1020–02; IgG1, 1070–02; IgG2c, 1079–02; and IgG3, 1100–02) and measuring mean fluorescence intensity by flow cytometry. Arbitrary units (AU) per milliliter were determined by reference to a standard hyperimmune serum pool, with the following reciprocal end-point titer equivalences for 1 AU/mL: IgM, 16; IgG1, 32; IgG2c, 64; and IgG3, 8). For estimation of affinity maturation, sera were tested against glass beads coated with liposomes containing 20% PBS150 (PS-20%) or 0.5% PBS150 (PS-0.5%) and the ratio of reciprocal titers was calculated as an estimate of affinity. Alternatively, anti-PS antibodies were measured by ELISA in microplates coated with 10 μg purified pneumococcal capsular PS serotype 14 or serotype 3 (ATCC).
Immunization.
Mice were injected intramuscularly in the thigh with 20 μL of liposome solution containing the equivalent of 1 μg NKT lipid PBS57 and 4 μg polysaccharide PBS150.
Statistical Analysis.
Different groups were compared using two tailed t test or Mann–Whitney test. *P < 0.05, **P < 0.01.
Acknowledgments
We thank members of the laboratories of A.B., L.T., and P.B.S. for help and advice and Dr. Clifford Snapper for discussions. This work was supported by National Institutes of Health Grant P01 AI053725 (to A.B., L.T., and P.B.S.), Major State Basic Research Development Program of China (973 Program 2012CB825806), National Natural Sciences Foundation of China 31021061 and 31271430, Fundamental Research Funds for Central Universities and NNCAS-2011-5 (to L.B.), and the University of Chicago Digestive Diseases Research Core Center (P30 DK42086). A.B. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.O’Brien KL, et al. Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 2.AlonsoDeVelasco E, Verheul AF, Verhoef J, Snippe H. Streptococcus pneumoniae: Virulence factors, pathogenesis, and vaccines. Microbiol Rev. 1995;59(4):591–603. doi: 10.1128/mr.59.4.591-603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briles DE, et al. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981;153(3):694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberger DM, et al. Epidemiologic evidence for serotype-specific acquired immunity to pneumococcal carriage. J Infect Dis. 2008;197(11):1511–1518. doi: 10.1086/587941. [DOI] [PubMed] [Google Scholar]
- 5.Goldblatt D, et al. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: A longitudinal household study. J Infect Dis. 2005;192(3):387–393. doi: 10.1086/431524. [DOI] [PubMed] [Google Scholar]
- 6.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 7.Snapper CM. Mechanisms underlying in vivo polysaccharide-specific immunoglobulin responses to intact extracellular bacteria. Ann N Y Acad Sci. 2012;1253:92–101. doi: 10.1111/j.1749-6632.2011.06329.x. [DOI] [PubMed] [Google Scholar]
- 8.Brigl M, et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011;208(6):1163–1177. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girardi E, et al. Unique interplay between sugar and lipid in determining the antigenic potency of bacterial antigens for NKT cells. PLoS Biol. 2011;9(11):e1001189. doi: 10.1371/journal.pbio.1001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinjo Y, et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol. 2011;12(10):966–974. doi: 10.1038/ni.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawakami K, et al. Critical role of Valpha14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol. 2003;33(12):3322–3330. doi: 10.1002/eji.200324254. [DOI] [PubMed] [Google Scholar]
- 12.Mattner J, et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3(5):304–315. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galli G, et al. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J Exp Med. 2003;197(8):1051–1057. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang GA, Devera TS, Lang ML. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood. 2008;111(4):2158–2162. doi: 10.1182/blood-2007-10-117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang GA, Exley MA, Lang ML. The CD1d-binding glycolipid alpha-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner. Immunology. 2006;119(1):116–125. doi: 10.1111/j.1365-2567.2006.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galli G, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA. 2007;104(10):3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devera TS, Shah HB, Lang GA, Lang ML. Glycolipid-activated NKT cells support the induction of persistent plasma cell responses and antibody titers. Eur J Immunol. 2008;38(4):1001–1011. doi: 10.1002/eji.200738000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonti E, et al. NKT-cell help to B lymphocytes can occur independently of cognate interaction. Blood. 2009;113(2):370–376. doi: 10.1182/blood-2008-06-166249. [DOI] [PubMed] [Google Scholar]
- 19.Leadbetter EA, et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA. 2008;105(24):8339–8344. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barral P, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci USA. 2008;105(24):8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang PP, et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol. 2012;13(1):35–43. doi: 10.1038/ni.2166. [DOI] [PubMed] [Google Scholar]
- 22.King IL, et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol. 2012;13(1):44–50. doi: 10.1038/ni.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tonti E, et al. Follicular helper NKT cells induce limited B cell responses and germinal center formation in the absence of CD4(+) T cell help. J Immunol. 2012;188(7):3217–3222. doi: 10.4049/jimmunol.1103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehuen A, Fazilleau N. Innate iNKT cell help to B cells: Fast but does not last. Nat Immunol. 2012;13(1):11–13. doi: 10.1038/ni.2186. [DOI] [PubMed] [Google Scholar]
- 25.Bai L, et al. Distinct antigen-presenting cells explain the cytokine bias of α-galactosylceramide variants in vivo. J Immunol. 2012;188(7):3053–3061. doi: 10.4049/jimmunol.1102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safari D, et al. Identification of the smallest structure capable of evoking opsonophagocytic antibodies against Streptococcus pneumoniae type 14. Infect Immun. 2008;76(10):4615–4623. doi: 10.1128/IAI.00472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity. 2011;35(5):671–680. doi: 10.1016/j.immuni.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Thomas SY, et al. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J Exp Med. 2011;208(6):1179–1188. doi: 10.1084/jem.20102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barral P, Sánchez-Niño MD, van Rooijen N, Cerundolo V, Batista FD. The location of splenic NKT cells favours their rapid activation by blood-borne antigen. EMBO J. 2012;31(10):2378–2390. doi: 10.1038/emboj.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muppidi JR, et al. Cannabinoid receptor 2 positions and retains marginal zone B cells within the splenic marginal zone. J Exp Med. 2011;208(10):1941–1948. doi: 10.1084/jem.20111083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199(12):1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujii S, et al. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci USA. 2006;103(30):11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venkataswamy MM, Porcelli SA. Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Semin Immunol. 2010;22(2):68–78. doi: 10.1016/j.smim.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23(1):7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Rubtsov A, et al. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity. 2005;23(5):527–538. doi: 10.1016/j.immuni.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Kang YS, et al. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc Natl Acad Sci USA. 2004;101(1):215–220. doi: 10.1073/pnas.0307124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long X, et al. Synthesis and evaluation of stimulatory properties of Sphingomonadaceae glycolipids. Nat Chem Biol. 2007;3(9):559–564. doi: 10.1038/nchembio.2007.19. [DOI] [PubMed] [Google Scholar]
- 38.Kinjo Y, et al. Natural Sphingomonas glycolipids vary greatly in their ability to activate natural killer T cells. Chem Biol. 2008;15(7):654–664. doi: 10.1016/j.chembiol.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 40.Moon JJ, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10(3):243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goff RD, et al. Effects of lipid chain lengths in alpha-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126(42):13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, et al. A modified alpha-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006;312(1–2):34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Savage PB, Teyton L, Bendelac A. Glycolipids for natural killer T cells. Chem Soc Rev. 2006;35(9):771–779. doi: 10.1039/b510638a. [DOI] [PubMed] [Google Scholar]