Abstract
Medulloblastoma (MB) is the most common malignant brain tumor of childhood. Current therapies are toxic and not always curative that necessitates development of targeted immunotherapy. However, little is known about immunobiology of this tumor. In this study, we show that MB cells in 9 of 20 primary tumors express CD1d, an antigen-presenting molecule for Natural Killer T cells (NKTs). Quantitative RT-PCR analysis of 61 primary tumors revealed an elevated level of CD1d mRNA expression in a molecular subgroup characterized by overactivation of Sonic Hedgehog (SHH) oncogene compared with Group 4. CD1d-positive MB cells cross-presented glycolipid antigens to activate NKT-cell cytotoxicity. Intracranial injection of NKTs resulted in regression of orthotopic MB xenografts in NOD/SCID mice. Importantly, the numbers and function of peripheral blood type-I NKTs were preserved in MB patients. Therefore, CD1d is expressed on tumor cells in a subset of MB patients and represents a novel target for immunotherapy.
1. Introduction
Medulloblastoma (MB) originates from neuronal precursors in the cerebellum and is the most common malignant brain tumor of childhood. Despite overall improvement in MB outcome in the last 30 years due to advancement in surgical techniques, radiotherapy and chemotherapy [1], many survivors suffer from debilitating long-term therapeutic toxicity, especially cognitive and endocrinal impairment caused by craniospinal irradiation at young age [2;3]. New targeted therapies are necessary to improve outcome and reduce treatment-related morbidities in children with MB.
MB has been historically classified based on clinical markers (patient age, presence of metastases at diagnosis, extent of resection) and histopathological characteristics (classic, desmoplastic/nodular, and large cell/anaplastic) [4]. However, recent advances in gene expression profiling of large number of tumors from multiple studies have provided evidence for four MB molecular subgroups (Wnt, Shh, Group 3, and Group 4) that are associated with prognosis and provide targets for therapeutic intervention [5–8]. The emerging evidence including two recent reports on MB exome sequencing [9;10] reveals further heterogeneity within these four molecular subgroups among which are genetic alterations in known oncogenic pathways that can be targeted for therapy. However, the relationship between the new molecular classification and the immunobiology of MB has not been addressed.
The identification of antigens that are selectively expressed in MB cells could lead to the development of effective immunotherapy without major side effects. A few studies examined expression of tumor-associated antigens in MB cells such as IL13Ralpha2 or HER2 that can be targeted for immunotherapy with therapeutic antibodies or T cells [11;12]. However, MB has not been evaluated as a potential target for immunotherapy with Vα24-invariant (type-I) Natural Killer T (NKT) cells [13], which have potent anti-tumor properties [14] and have been associated with good outcome in several types of cancer both in children and adults [15].
Type-I NKT cells are an evolutionary conserved sub-lineage of T cells that are characterized by the expression of an invariant TCR α-chain, Vα24-Jα18 and reactivity to self- and microbial-derived glycolipids presented by monomorphic HLA class-I-like molecule CD1d [13]. NKT cell cytotoxicity is CD1d-restricted although NKT cells have been shown to suppress growth or metastases of CD1d-negative tumors indireclty via activation of NK cells or killing of tumor-associated macrophages [15]. There are also type-II NKT cells that express a diverse TCR repertoire and react to other CD1d-bound glycolipids such as sulfatide [13;16]. In this study we investigate only type-I NKT cells for potential immunotherapy applications.
CD1d is preferentially expressed in hematopoietic cells, especially those of myelomonocytic and B-cell lineages and malignancies originating from the corresponding tissues often express CD1d [17–19]. Although the majority of non-hematopoietic solid tumors are CD1d-negative, CD1d expression by tumor cells has been reported in malignant glioma and prostate cancer [20;21]. However, neither CD1d expression nor the susceptibility to NKT-cell cytotoxcicity has been examined in MB or any other pediatric brain tumors. In this study, we analyzed CD1d expression in MB cell lines and primary tumors. Our results demonstrate that CD1d is expressed on the tumor cell surface in a subset of primary MB tumors and transcriptional analysis revealed a preferential CD1d gene expression in Shh molecular subgroup compared with Group 4. Importantly, CD1d-positive MB cell lines were highly sensitive to direct NKT cell cytotoxicity, and intracranial injection of human NKT cells resulted in regression of established orthotropic human NB xenografts in NOD/SCID mice. These findings may lead to the development of an effective NKT-cell based immunotherapy of MB.
2. Materials and Methods
2.1. Human specimens
PBMC or frozen tumor specimens from MB patients (mean age of 7.8, range 2–16 years old) were obtained at diagnosis at Texas Children’s Cancer Center, Baylor College of Medicine or Children’s Hospital Los Angeles, respectively, according to the insitution’s approved IRB protocols. Informed consent was obtained in accordance with institutional review board policies and procedures for research dealing with human specimens. PBMC of healthy donors (at least 17 years old) were isolated by gradient centrifugation from buffy coats purchased from Gulf Coast Regional Blood Center (Houston, TX). Medulloblastoma tissue arrays from 20 unidentifiable MB patients and normal human brain tissues were purchased from Cybrdi, Inc. Molecular subgroups of MB patients were identified using quantitative reverse transcription polymerase chain reaction (qRT-PCR) and a medulloblastoma gene signature derived from prior microarray studies [5;6] and a CHLA study (manuscript in preparation).
2.2. Cell lines and culture conditions
The medulloblastoma cell lines (Daoy, D283, and D341) and 293T cells were purchased from the ATCC. MED8A line was kindly provided by Dr. R. Gilbertson (St. Jude Children’s Research Hospital, Memphis). All medullobalstoma cell lines were maintained in DMEM (Invitrogen) with 10% FCS (HyClone) supplemented with 2 mM GlutaMAX-I, 1.5 g/L sodium bicarbonate, 0.1 mM nonessential amino acids, and 1 mM sodium pyruvate (Invitrogen). NKT cell lines were expanded from PBMCs of healthy volunteers as previously described with modifications [22]. Briefly, PBMCs were isolated from buffy coats by Ficoll-Hypaque gradient density centrifugation. NKTs were purified by anti-iNKT microbeads (Miltenyi Biotec). The negative PBMC fraction was irradiated (4000 Rad) and aliquoted. NKTs were stimulated with an aliquot of autologous PBMCs pulsed with αGalCer (100 ng/mL, Funakoshi Co.Ltd). rhIL-2 (200 U/ml, BDP, National Cancer Institute Frederick) was added on the second day and then every other day. NKTs were restimulated every two weeks with the remaining PBMC aliquotes. The phenotype and purity of NKTs were assessed using mAbs for CD3, Vα24-Jα18 (6B11), and CD4. NKT cell ligand 7WD8-5 was kindly provided by Dr. Moriya Tsuji (The Rockefeller University, New York, NY). βGlcCer was purchased from Avanti Polar Lipids. All lipids were dissolved in DMSO for in vitro assays and PBS buffer (5.6% sucrose, 0.75% L-histidine and 0.5% Tween20) for in vivo experiments.
2.3. RNA isolation and Real-time RT-PCR
Total RNA from cultured cells were extracted using TRIZol reagent (Invitrogen). cDNAs were synthesized by super reverse transcript kit (Invitrogen). Quantitative RT-PCR for CD1d was performed with TaqMan gene expression assay using the ABI7900 sequence Detection System (Applied Biosystems). The relative change in gene expression was calculated based on the δCt method using HPRT1 housekeeping gene as a control.
2.4. Immunohistochemistry
Human medulloblastoma tissue array slides and normal brain paraffin tissue sections were purchased from Cybrdi, Inc. H&E staining was done following a standard hematoxylin and eosin staining protocol. CD1d and CD3 expression was detected with immunohistochemistry using mouse anti-human CD1d mAb (clone NOR3.2, Abcam) and mouse anti-human CD3 mAb (clone PS-1, Vector Laboratories).
2.5. Flow cytometry
Surface CD1d expression on cell lines was assessed using an anti-CD1d-PE 42.1 mAb. NKT cells were identified after PBMC staining with anti-NKT TCR-PE 6B11 and anti-CD3-APC mAbs. Manufacture recommended fluorochrome- and isotype-matching mAbs were used for negative controls (BD Biosciences). The analysis was performed on a LSR-II four-laser flow cytometer (BD Biosciences) using BD FACDiva software v. 6.0 and FlowJo 7.2.5 (Tree Star, Inc).
2.6. Multiplex cytokine quantification assay
Cytokines released by NKT cells were assessed by CBAPlex beads on FACSArray bioanalyzer (BD biosciences) according to the manufacture’s manual and as previously described [15].
2.7. In vitro cytotoxicity assay
Daoy.ffLuc cells were pulsed overnight with a NKT cell ligand or DMSO control and plated in a 96-well black plate at 20,000 cells/well. NKT cells were added at different effector to target cell ratios. Anti-hCD1d 51.1 blocking mAb was kindly provided by Dr. S. Porcelli (Albert Einstein College of Medicine, Bronx, NY). After 4-hr co-culture, luciferin was added in each well and luminescence was quantified by a plate reader Infinite® M200 (Tecan). The number of viable Daoy.ffLuc cells in each well was calculated based on standard curve generated from serial dilutions of the target cells. NKT cell cytotoxicity was calculated using the following formula: cytotoxcicity % = (cell number in control well – cell number in assay well) * 100/cell number in control well (Daoy.ffLuc cell alone).
2.8 Orthotopic xenogenic model of medulloblastoma in NOD/SCID mice
All animal experiments were conducted on a protocol approved by the Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC). NOD/SCID mice were purchased from Jackson Lab. Intracranial injection were conducted as previously described with modifications [12]. Male 9 week to 12 week-old mice were anesthetized with rapid sequence inhalation isofluorane (Abbot Laboratories, England) followed by an intraperitoneal injection of 225–240 mg/kg Avertin® solution and then maintained on isofluorane by inhalation throughout the procedure. Mice were immobilized in a Cunningham™ Mouse/Neonatal Rat Adaptor (Stoelting, Wood Dale, IL) stereotactic apparatus fitted into an E15600 Lab Standard Stereotactic Instrument (Stoelting) and scrubbed with 1% povidone-iodine. A 10 mm skin incision was made along the midline. The tip of a 31G ½ inch needle mounted on a Hamilton syringe (Hamilton, Reno, NV) served as the reference point. A 1mm burr-hole was drilled into the skull, 1 mm anterior to and 2 mm to the right of the bregma. Daoy cells (2.5 × 105 in 2.5 μL) were injected 3 mm deep to the bregma, corresponding to the center of the right caudate nucleus over 5 minutes. The needle was left in place for 3 minutes, to avoid tumor cell extrusion, and then withdrawn over 5 minutes. Five days after tumor cell injection, animals received 2 × 106 NKT lymphocytes alone or with a ligand (αGalCer or 7DW8-5, 100 ng/) in 2.5 μL at the same coordinates. The incision was closed with 2–3 interrupted 7.0 Ethicon® sutures (Ethicon, Inc. Somerville, NJ). A subcutaneous injection of 0.03–0.1 mg/kg buprenorphine (Buprenex® RBH, Hull, England) was given for pain control.
2.9. Bioluminescence imaging
Isofluorane anesthetized animals were imaged using the IVIS® system (IVIS, Xenogen Corp) 10 minutes after 150 mg/kg D-luciferin (Xenogen) was injected intraperitoneally. The photons emitted from luciferase-expressing cells within the animal body and transmitted through the tissue were quantified using “Living Image”, a software program provided by the same manufacturer. A pseudo-color image representing light intensity (blue least intense and red most intense) was generated and superimposed over the grayscale reference image. Animals were imaged weekly. They were regularly examined for any neurological deficits, weight loss or signs of stress and euthanized according to pre-set criteria, in accordance with the Baylor College of Medicine’s Center for Comparative Medicine guidelines.
2.10. Statistical analysis
The statistical analysis was performed using GraphPad Prism™ 5.0 software (GraphPad Software) or the R project (http://www.r-project.org). Comparisons between groups were based on t-test or one-way ANOVA with the Bonferroni post-test to compare selected experimental groups according to the experimental design. All statistical tests were two-sided, and a P-value < 0.05 was considered statistically significant.
3. Results
3.1. CD1d is expressed in human MB cell lines and primary tumors
To examine CD1d expression in MB cells, we performed flow cytometry analysis of four established human MB cell lines and found that two of them (DAOY and MED8A) expressed CD1d on the cell surface (Fig. 1A). RT-PCR analysis confirmed CD1d expression in both DAOY and MED8A cell lines while two CD1d–negative cell lines (D341 and D283) did not express CD1d at the mRNA level (Fig. 1B). The immunohistochemical staining with an anti-CD1d mAb detected CD1d expression in nine of twenty primary MB tumors (Fig. 1C). Importantly, the majority if not all tumor cells in the CD1d-positive specimens expressed CD1d. In contrast, neurons and other cells within normal brain tissues including microglia were CD1d-negative (Suppl. Fig. 1). To examine whether the level of CD1d gene expression is associated with a known histological or molecular type of MB or with disease outcome, we performed qRT-PCR analysis of CD1d mRNA expression in 80 primary tumors that were histologically classified as classic (n = 48), desmoplastic (n = 17), and anaplastic/large cell (n = 15) types. Among these, the molecular subgroups of 61 tumors were determined (Shh, n = 25, Group 3, n = 19, and Group 4, n = 17) using a RT-PCR gene signature (manuscript in preparation). Wnt group represents less than 7% of MBs and was absent in our cohort. CD1d mRNA was expressed at similar levels across the histological types (Suppl. Fig. 2). Likewise, there was no association between the level of CD1d expression and the patient age or the disease outcome (data not shown). However, the molecular grouping did reveal that CD1d is expressed at a significantly higher level in the Shh subgroup compared to Group 4 (Fig. 1D, P = 0.015, t-test). Therefore, tumor cells in a subset of primary MB patients uniformly express CD1d on the cell surface while normal brain tissues are CD1d-negative, suggesting that CD1d could serve as a novel selective target for immunotherapy of MB.
Figure 1. CD1d expression in medulloblastoma cells and tissues.
(A) CD1d expression in four medulloblastoma cell lines was examined by flow cytometry: staining with isotype control (tinted) or anti-hCD1d (open). (B) CD1d mRNA expression in four medulloblastoma cell lines was analyzed by RT-PCR using GADPH as an amplification control. (C) CD1d expression in primary tumors was assessed by IHC staining. Representative sections shown are from two CD1d-positive (upper pannel) and two CD1d-negative (lower pannel) of 20 analyzed tumors. Original magnification, X10. (D) CD1d mRNA expression was analyzed in 61 primary tumors by Taqman qRT-PCR and quantified using delta Ct method relative to the expression of HPRT1. DAOY and CHLA-255 cells were used as CD1d positive and negative controls, respectively.
3.2. MB cells cross-present glycolipid antigens to NKT cells in a CD1d-restricted manner
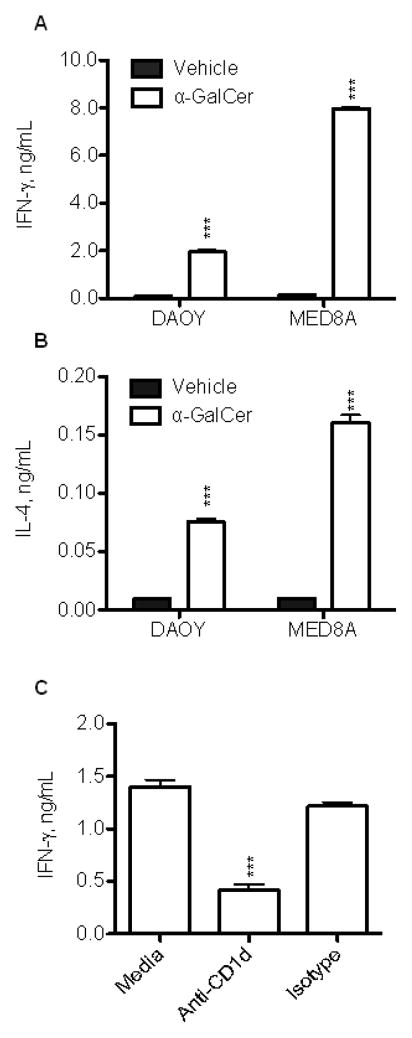
To examine whether MB cells express functional CD1d, we analyzed IFNγ and IL-4 production by NKT cells that were co-cultured with CD1d-positive MB cells in the presence or absence of a synthetic NKT ligand, αGalactosylceramide (αGalCer). Figs. 2A, B demonstrate that CD1d-positive DAOY and MED8A MB cells could effectively present αGalCer to NKT cells and induce production of IFNγ and IL-4. Pre-treatment of MB cells with anti-CD1d blocking mAb inhibited their ability to induce IFNγ in NKT cells (Fig. 2C) indicating that NKT-cell activation by MB cells is CD1d-dependent. Therefore, MB cells express CD1d without an agonistic endogenous ligand for NKT cells. However, they can effectively cross-present exogenous glycolipid antigens and activate NKT cell cytokine production in a CD1d-restricted manner.
Figure 2. NKT cell cytokine response to CD1d-positive MB cells.
Resting NKT cells (14 days after stimulation) were co-cultured with indicated CD1d+ MB cells pulsed overnight with α-GalCer (100 ng/mL) or vehicle control. Cytokines (A) IFN-γ and (B) IL-4) were measured in the culture supernatants after 24 h using CBA multiplex assay. (C) Using the same settings as above, IFN-γ production was measured in the presence of anti-hCD1d blocking mAb 51.1 or isotype control. Data are M ± SD from one of three experiments with triplicates.
3.3. NKT cells can specifically kill CD1d-positive MB cells
Next, we performed an in vitro cytotoxicity assay using luciferase-transduced DAOY cells as a target. Fig. 3A demonstrates that NKT cells were highly cytotoxic against MB cells pulsed with either αGalCer or its analogue 7DW8-5 [23]. At the concentration 100 ng/ml, αGalCer and 7WD8-5 were equally potent in inducing NKT cell cytotoxicity. However, titration experiments revealed that EC50 of 7WD8-5 was 10 times less than that of αGalCer (P < 0.001, Fig. 3B). We also tested NKT cell cytotoxicity after target cell pulsing with a recently discovered endogenous NKT cell ligand, β-D-glucopyranosylceramide (βGlcCer) [24]. NKT cells mediated potent cytotoxicity against βGlcCer-pulsed DAOY cells and this cytotoxicity was CD1d-dependent since it was inhibited by an anti-CD1d blocking mAb (Fig. 3C). Nearly identical results were obtained using another CD1d-positive MB cell line, MED8A while no cytotoxicity was observed against CD1d-negative MB lines (data not shown). Therefore, MB cells can cross-present exogenous and endogenous ligands and trigger potent CD1d-restricted NKT cell cytotoxcicty that could be exploited therapeutically.
Figure 3. NKT cell cytotoxicity against CD1d positive MB cells.

(A) DAOY/luc cells were pulsed with 100 ng/mL α-GalCer, 7WD8-5, or vehicle control overnight and co-cultured with resting NKT cells for 4 h at indicated E:T ratios. (B) DAOY/luc cells were pulsed with different concentrations of α-GalCer or 7WD8-5 overnight and co-cultured with resting NKT cells for 4 h at the fixed E:T ratio (2:1). NKT cell cytotoxicity was assessed by the loss of the target cell luminescence. Data were collected from six replicate wells per condition. (C) After pulsing overnight with β-GlcCer (2 μg/mL) or vehicle, DAOY/luc cells were blocked with anti-CD1d mAb 51.1 or isotype control for 30 min before adding NKT cells at the indicated E:T ratios. Data are M ± SD from one of three experiments with triplicates.
3.4. Peripheral blood NKT cells are preserved in MB patients
Numerical and functional deficiencies of NKT cells have been observed in patients with some types of cancer [25;26] but not with others [20;22]. FACS analysis of primary NKT cell frequency in PBMC from 10 MB patients did not reveal a significant difference compared with NKT cell frequency in PBMC of healthy volunteers (Fig. 4A). To test whether NKT cells from MB patients can be numerically expanded ex vivo, we used αGalCer-pulsed autologous PBMC and IL-2 to induce NKT cell proliferation. Fig. 4B demonstrates that NKT cells from MB patients can be ex-vivo expanded as effectively as those from healthy people. Importantly, these patient-derived NKT cells produced high levels of IFNγ (Fig. 4C) and mediated dose-dependent cytotoxicity in response to the ligand-pulsed CD1d-positive MB cells (Fig. 4D). At the same effector to target ratios, patient-derived NKT cells were slightly less cytotoxic than those derived from healthy adults. However, the majority of NKT cells in children with MB were CD4+ cells (Suppl. Fig. 3), which are less cytotoxic than CD4neg ones [27] and the latter are known to accumulate with age [28], suggesting that the observed difference in the level of NKT cell in vitro cytotoxicity is age related. Therefore, NKT cell number and functional potential are largely preserved in the peripheral blood of MB patients. These cells can be numerically expanded ex vivo and kill CD1d-positive MB cells. The lack of systemic NKT cell impairment in MB patients suggests that patient-derived NKT cells could be used for adoptive immunotherapy.
Figure 4. Normal NKT cell number and function in MB patients.
Frequency of NKT cells in freshly isolated PBMC of MB patients and healthy donors was assessed by FACS using staining for CD3 and NKT iTCRα (6B11). (B) NKT cells were expanded from PBMCs of MB patients or healthy donors in culture with α-GalCer and IL-2. (C) NKT cells expanded from PBMC of a MB patient or a healthy donor were stimulated by α-GalCer-pulsed DAOY cells and the concentration of IFNγ was measured in the culture supernatants after 24 h using CBA multiplex assay. (D) The cytotoxicity of expanded NKT cells was tested against DAOY/luc cells pulsed with 100 ng/mL α-GalCer. Data are from a representative of three experiments.
We also examined whether NKT cells are present in the MB tissues using qRT-PCR analysis of the Vα24-Jα18 invariant TCRα rearrangement in 20 primary tumors. mRNA isolated from a human NKT cell line was used as a positive control. As we have previousely reported, this method reproducibly detects one NKT cell per 10,000 tumor cells [22]. No signal for Vα24-Jα18 was detected in any of twenty tumors (data not shown). Since CD1d is recognized by both type-I and type-II NKT cells [13], all of which express CD3 and could contribute to the total T cell count, we examined the total number of tumor-infiltrating CD3+ cells in relation to CD1d expression in the same tumors. Tissue sections from 20 primary MB tumors were stained with anti-CD1d and anti-CD3 mAbs and analyzed by IHC. There was no correlation between the frequency of CD3-positive cells and the level of CD1d expression on MB cells (Suppl. Fig. 4).
3.5. Intracranial treatment with ex-vivo expanded human NKT cells has therapeutic activity in established orthotopic MB grafts in NOD/SCID mice
To test the in vivo therapeutic potential of NKT cells against MB, we used a previously described orthotopic MB model in NOD/SCID mice [12;29]. Luciferase-transduced DAOY MB cells were injected intracranially and after confirmation of tumor engraftment on day 5, mice received intracranial injections of NKT cells alone, with 7DW8-5, or equal volume of PBS (control). Tumor growth was monitored by weekly bioluminescent (BL) imaging. Fig. 5 demonstrates that NKT cells alone were able to significantly delay tumor growth compared with control (P < 0.001), and co-injection of NKT cells with 7DW8-5 resulted in even stronger anti-tumor effect and prolongation of tumor regression compared with NKT cells alone (P < 0.001) while 7DW8-5 alone had no effect (data not shown). In contrast, intravenous administration of NKT cells alone or with either 7DW8-5 or αGalCer failed to affect tumor growth. The immunohistochemical analysis of tumor tissues revealed that systemically administrated NKT cells failed to localize to the tumor site (data not shown). Therefore, the intracranial route of NKT cell administration should be considered for immunotherapy of MB patients.
Figure 5. Intracranial transfer of NKT cells is effective against established tumor in an orthotopic MB model in NOD/SCID mice.
A) DAOY/luc cells (2.5 × 105) were intracranially injected into mouse brain at the position of bragma as described in methods. NKT cells alone or with 7DW8-5 (100 ng/mouse) were injected intracranially at day 5. Shown are representative bioluminescent images at indicated time intervals. (B) Tumor progression in indicated groups as measured by BL signal intensity. Data are M ± SD from 5 mice per group, one of three experiments.
4. Discussion
Limited knowledge of the immunobiology of MB prevents development of targeted immunotherapies against this most common malignant brain tumor of childhood. The results of this study demonstrate for the first time that primary tumors in a subset of MB patients express CD1d, an antigen-presenting molecule for NKT cells. Importantly, CD1d-positive MB cells effectively cross-present exogenous and endogenous ligands to NKT cells that trigger potent NKT-cell cytotoxicity and IFNγ production. Moreover, intracranial injection of ex-vivo expanded human NKT cells has a potent therapeutic activity against MB xenografts in an orthotopic MB model.
Our analysis of four human MB cell lines and twenty primary MB tumor specimens revealed CD1d expression on tumor cell surface in nearly half of the cases. Unlike sporadic CD1d expression observed in adult gliomas [20], CD1d-positive MB tumors have uniform pattern of CD1d expression in the majority if not all tumor cells. No CD1d expression was detected in normal brain tissues suggesting that CD1d could be selectively targeted for immunotherapy of MB and other CD1d-positive brain tumors. CD1d was expressed at a significantly higher level in Shh molecular subgroup compared with Group 4 subgroup. While no cell line could match genetic signatures associated with the MB molecular subgroups, CD1d-positive cell lines DAOY [30] and MED8A [31] were derived from desmoplastic MB and have characteristics of SHH subgroup. In contrast, D341 [32] and D283 [33;34] have high levels of c-myc expression and resemble tumors in Group 3. While the difference between CD1d expression levels between Shh and Group 3 did not reach statistical significance (P = 0.15, t-test), future study with a larger sample size may determine whether CD1d downregulation is associated with a certain subtype within Group 3. Further studies are also needed to determine whether there is a causative link between CD1d expression and Shh-mediated or other oncogenic signaling pathways and how this relates to MB immune surveillance.
We found that NKT cells could kill ligand-pulsed CD1d-positive MB cells at very low effector-to-target ratios in vitro, and intracranial injection of human NKT cells to NOD/SCID mice with established MB xenografts resulted in prolonged tumor regression. While NKT cells had little in vitro cytotoxicity against MB cells in the absence of NKT ligands, in vivo treatment with NKT cells alone was quite effective, suggesting that MB cells cross-present endogenous glycolipids in the brain. Brain tissues are rich in glycosphingolilpids including βGlcCer [35], the main known endogenous ligand for type-I NKT cells. The therapeutic efficacy of NKT cells was further enhanced when they were co-administrated with a highly agonistic synthetic ligand, 7DW8-5. Tumor growth ultimately recurred in all treated animals likely due to poor persistence of human NKT cells in the mouse tissues lacking appropriate species-specific homeostatic factors. On the other hand, this limitation of the xenogenic model may also lead to an underestimation of the potential toxicity from intracranial administration of NKT cells or their ligands. Large amounts of cytokines produced by NKT cells in response to stimulation with synthetic ligands could have a dual effect contributing to both the anti-tumor efficacy and “off-target” toxicity of NKT cell immunotherapy that will need to be considered in the clinical trial design.
We found that the frequency and functional potential of peripheral blood NKT cells are preserved in MB patients to the levels similar to those in healthy adult individuals albeit with a slightly decreased cytotoxicity which is likely due to the predominance of a relatively less cytotoxic CD4+ NKT cell subset in children compared with adults [27;28]. Considering normal NKT cell frequency in peripheral blood and their abilities to expand upon antigenic stimulation, produce IFNγ and kill MB cells in aCD1d-restricted manner, we conclude that peripheral blood NKT cells are not affected by disease in MB patients. This is in contrast to the reported numerical and functional deficiencies of NKT cells in adult patients with advanced malignancies of epithelial origin [25;26] or with multiple myeloma [36]. The systemic effect of tumor growth on NKT cells appears to depend on the tumor type and the disease stage. Indeed, peripheral blood NKT cell numbers and function remained intact in adults with glioma [20] and children with neuroblastoma [22] whereas NKT cell function in patients with multiple myeloma inversely correlated with the disease stage [36]. It is quite remarkable that CD1d is expressed in both MB and glioblastoma, the most common malignant brain tumors in children and adults, respectively. Moreover, numbers and function of peripheral blood NKT cells are preserved in patients with these diverse brain tumors. This suggests that NKT cell immunotherapy could be broadly applicable in patients with brain tumors.
While NKT or T cell localization was not analyzed in CD1d-positive gliomas [36], our analysis of tumor-infiltrating CD3-positive T and NKT cells found no correlation between T/NKT cell presence and the level of CD1d expression on MB cells. Moreover, type-I NKT cells were undectable in 20 examined MB tumor using a sensitive qRT-PCR method, which readily detects NKT cell presense in primary neuroblastoma, a related pediatric neural tumor of extracranial origin [22]. These results indicate that CD1d expression is not sufficient for NKT cell localization or survival at the tumor site. The absence of a selective pressure from NKT cells during MB progression may explain the fact that CD1d-positive MB cells are very effective in presenting both exogenous and endogenous ligands to NKT cells and remain highly sensitive to NKT cell cytotoxicity. This observation cannot be simply explained by MB location in CNS since we recently demonstrated that primary MBs have varying degrees of infiltration with T cells, most of which were represented by CD8 T cells [29]. Therefore, it is possible that MB evolved to escape from NKT cell control via yet unknown mechanism, which requires a dedicated investigation.
Although systemic treatment with NKT cells was ineffective in mice with orthotopic MB xenografts, the intracranial administration of NKT cells alone or with a ligand had potent anti-tumor activity. A pilot clinical trial demonstrated that local administration of autologous NK lymphocytes after surgery was well tolerated in patients with brain tumors [37]. A recent work by our group demonstrated that MB cells often express HER2 and can be targeted for immunotherapy with T cells engineered to express HER2-specific chimeric antigen receptor (CAR) [12]. An ongoing phase-I clinical trial at our center is testing safety and potential clinical efficacy of intracranial administration of the engineered HER2-specific autologous T cells in patients with incompletely resected or unresectable HER2-positive brain tumors. We propose that NKT cell immunotherapy can be applied in a similar fashion. An advantage of using NKT cells compared with T cells is that the former does not need to be engineered with an additional specificity to treat CD1d-positive brain tumors although such engineering is possible and would further enhance and broaden NKT cell anti-tumor potential.
5. Conclusions
We demonstrate for the first time that CD1d, an antigen-presenting molecule for NKT cells is expressed on the surface of human MB cells in cell lines and primary tumor specimens. CD1d-positive MB cells effectively cross-present glycolipid antigens and can be killed by NKT cells in vitro and in vivo. These findings may lead to an effective immunotherapy of MB and other CD1d-positive brain tumors.
Supplementary Material
Highlights.
Tumor cells in a subset of patients with medulloblastoma express CD1d.
CD1d is expressed at a higher level in tumors of SHH molecular subgroup compared with Group 4.
CD1d-positive medulloblastoma cells cross-present glycolipids to NKT cells.
Treatment with NKT cells results in regression of intracranial tumors in mice.
NKT cells are preserved in peripheral blood of children with medulloblastoma.
Acknowledgments
This work was supported by grants from National Institutes of Health (RO1 CA116548), Cancer Prevention and Research Institute of Texas (RP1 100528 and RP1 110129), The Caroline Wiess Law Scholar Award (LSM); American Brain Tumor Association and Alliance for Cancer Gene Therapy (NMA); American Cancer Society, Alex’s Lemonade Stand Foundation, and St. Baldrick’s Foundation (SA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 2.Kim W, Choy W, Dye J, Nagasawa D, Safaee M, Fong B, et al. The tumor biology and molecular characteristics of medulloblastoma identifying prognostic factors associated with survival outcomes and prognosis. J Clin Neurosci. 2011;18:886–890. doi: 10.1016/j.jocn.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Sadighi Z, Vats T, Khatua S. Childhood Medulloblastoma: The Paradigm Shift in Molecular Stratification and Treatment Profile. J Child Neurol. 2012;10:1302–1307. doi: 10.1177/0883073812449690. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924–1931. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 6.Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29:1424–1430. doi: 10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123:473–484. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Northcott PA, Shih DJ, Peacock J, Garzia L, Morrissy AS, Zichner T, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488:49–56. doi: 10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pugh TJ, Weeraratne SD, Archer TC, Pomeranz Krummel DA, Auclair D, Bochicchio J, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488:106–110. doi: 10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stastny MJ, Brown CE, Ruel C, Jensen MC. Medulloblastomas expressing IL13Ralpha2 are targets for IL13-zetakine+ cytolytic T cells. J Pediatr Hematol Oncol. 2007;29:669–677. doi: 10.1097/MPH.0b013e3181468c68. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed N, Ratnayake M, Savoldo B, Perlaky L, Dotti G, Wels WS, et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007;67:5957–5964. doi: 10.1158/0008-5472.CAN-06-4309. [DOI] [PubMed] [Google Scholar]
- 13.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 14.Dhodapkar MV. Harnessing human CD1d restricted T cells for tumor immunity: progress and challenges. Front Biosci. 2009;14:796–807. doi: 10.2741/3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B, et al. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167:3114–3122. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- 16.Patel O, Pellicci DG, Gras S, Sandoval-Romero ML, Uldrich AP, Mallevaey T, et al. Recognition of CD1d-sulfatide mediated by a type II natural killer T cell antigen receptor. Nat Immunol. 2012;13:857–863. doi: 10.1038/ni.2372. [DOI] [PubMed] [Google Scholar]
- 17.Ulanova M, Tarkowski A, Porcelli SA, Hanson LA. Antigen-specific regulation of CD1 expression in humans. J Clin Immunol. 2000;20:203–211. doi: 10.1023/a:1006689514066. [DOI] [PubMed] [Google Scholar]
- 18.Metelitsa LS, Weinberg KI, Emanuel PD, Seeger RC. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003;17:1068–1077. doi: 10.1038/sj.leu.2402943. [DOI] [PubMed] [Google Scholar]
- 19.Fais F, Tenca C, Cimino G, Coletti V, Zanardi S, Bagnara D, et al. CD1d expression on B-precursor acute lymphoblastic leukemia subsets with poor prognosis. Leukemia. 2005;19:551–556. doi: 10.1038/sj.leu.2403671. [DOI] [PubMed] [Google Scholar]
- 20.Dhodapkar KM, Cirignano B, Chamian F, Zagzag D, Miller DC, Finlay JL, et al. Invariant natural killer T cells are preserved in patients with glioma and exhibit antitumor lytic activity following dendritic cell-mediated expansion. Int J Cancer. 2004;109:893–899. doi: 10.1002/ijc.20050. [DOI] [PubMed] [Google Scholar]
- 21.Nowak M, Arredouani MS, Tun-Kyi A, Schmidt-Wolf I, Sanda MG, Balk SP, et al. Defective NKT cell activation by CD1d+ TRAMP prostate tumor cells is corrected by interleukin-12 with alpha-galactosylceramide. PLoS One. 2010;5:e11311. doi: 10.1371/journal.pone.0011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metelitsa LS, Wu HW, Wang H, Yang Y, Warsi Z, Asgharzadeh S, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004;199:1213–1221. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Fujio M, Imamura M, Wu D, Vasan S, Wong CH, et al. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc Natl Acad Sci U S A. 2010;107:13010–13015. doi: 10.1073/pnas.1006662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, JP, et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12:1202–1211. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanagisawa K, Seino K, Ishikawa Y, Nozue M, Todoroki T, Fukao K. Impaired proliferative response of V alpha 24 NKT cells from cancer patients against alpha-galactosylceramide. J Immunol. 2002;168:6494–6499. doi: 10.4049/jimmunol.168.12.6494. [DOI] [PubMed] [Google Scholar]
- 26.Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 27.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baev DV, Peng XH, Song L, Barnhart JR, Crooks GM, Weinberg KI, et al. Distinct homeostatic requirements of CD4+ and CD4- subsets of Valpha24-invariant natural killer T cells in humans. Blood. 2004;104:4150–4156. doi: 10.1182/blood-2004-04-1629. [DOI] [PubMed] [Google Scholar]
- 29.Salsman VS, Chow KK, Shaffer DR, Kadikoy H, Li XN, Gerken C, et al. Crosstalk between medulloblastoma cells and endothelium triggers a strong chemotactic signal recruiting T lymphocytes to the tumor microenvironment. PLoS One. 2011;6:e20267. doi: 10.1371/journal.pone.0020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobsen PF, Jenkyn DJ, Papadimitriou JM. Establishment of a human medulloblastoma cell line and its heterotransplantation into nude mice. J Neuropathol Exp Neurol. 1985;44:472–485. doi: 10.1097/00005072-198509000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Langdon JA, Lamont JM, Scott DK, Dyer S, Prebble E, Bown N, et al. Combined genome-wide allelotyping and copy number analysis identify frequent genetic losses without copy number reduction in medulloblastoma. Genes Chromosomes Cancer. 2006;45:47–60. doi: 10.1002/gcc.20262. [DOI] [PubMed] [Google Scholar]
- 32.Friedman HS, Burger PC, Bigner SH, Trojanowski JQ, Brodeur GM, He XM, et al. Phenotypic and genotypic analysis of a human medulloblastoma cell line and transplantable xenograft (D341 Med) demonstrating amplification of c-myc. Am J Pathol. 1988;130:472–484. [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman HS, Burger PC, Bigner SH, Trojanowski JQ, Wikstrand CJ, Halperin EC, et al. Establishment and characterization of the human medulloblastoma cell line and transplantable xenograft D283 Med. J Neuropathol Exp Neurol. 1985;44:592–605. doi: 10.1097/00005072-198511000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Siu IM, Lal A, Blankenship JR, Aldosari N, Riggins GJ. c-Myc promoter activation in medulloblastoma. Cancer Res. 2003;63:4773–4776. [PubMed] [Google Scholar]
- 35.Futerman AH. Distinct roles for sphingolipids and glycosphingolipids at different stages of neuronal development. Acta Biochim Pol. 1998;45:469–478. [PubMed] [Google Scholar]
- 36.Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii SI, Dhodapkar KM, et al. A Reversible Defect in Natural Killer T Cell Function Characterizes the Progression of Premalignant to Malignant Multiple Myeloma. J Exp Med. 2003;197:1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishikawa E, Tsuboi K, Saijo K, Harada H, Takano S, Nose T, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004;24:1861–1871. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.