Abstract
Objective
Atherosclerosis-prone regions of arteries are characterized by complex flow patterns where the magnitude of shear stress is low and direction rapidly changes, termed disturbed flow. How endothelial cells sense flow direction and how it impacts inflammatory effects of disturbed flow are unknown. We therefore aimed to understand how endothelial cells respond to changes in flow direction.
Approach and Results
Utilizing a recently developed flow system capable of changing flow direction to any angle, we show that responses of aligned endothelial cells are determined by flow direction relative to their morphological and cytoskeletal axis. Activation of the atheroprotective eNOS pathway is maximal at 180° and undetectable at 90°, while activation of pro-inflammatory NF-κB is maximal at 90° and undetectable at 180°. Similar effects were observed in randomly oriented cells in naïve monolayers subjected to onset of shear. Cells aligned on micro-patterned substrates subjected to oscillatory flow were also examined. In this system, parallel flow preferentially activated eNOS and production of nitric oxide, whereas perpendicular flow preferentially activated reactive oxygen production and NF-κB.
Conclusions
These data show that the angle between flow and the cell axis, defined by their shape and cytoskeleton, determines endothelial cell responses. The data also strongly suggest that the inability of cells to align in low and oscillatory flow is a key determinant of the resultant inflammatory activation.
Keywords: Flow direction, flow shear stress, mechanotransduction, hemodynamics, atherosclerosis
Introduction
Fluid shear stress from blood flow plays a key role in vascular physiology and pathology through its effects on vascular endothelial cell (EC) function 1-5. Atherosclerotic lesions occur preferentially at regions of flow disturbance, i.e. bifurcations, branch points and regions of high curvature 6,7. These regions are characterized by lower shear stress magnitude and complex changes in flow direction during the cardiac cycle. Bypass grafting and stenting can also introduce regions of disturbed flow, which correlate strongly with neointimal hyperplasia and atherosclerotic lesions 8-11; indeed, there has been considerable effort to reduce flow disturbance from these interventions 11-13. In vitro studies on ECs have demonstrated both pro-inflammatory effects of disturbed flow and suppressive effects of high steady or pulsatile laminar flow 1,14. These results have led to the concept that flow patterns critically influence the initiation of atherosclerosis, in-stent restenosis, and bypass graft failure 3-5.
Atherosclerosis-prone regions in vivo also strongly correlate with failure of the ECs to elongate and align15-17. Alignment has been proposed to be a mechanism by which cells adapt to flow and down-regulate inflammatory pathways,14,18 but there are few data to directly support this notion. Recently, it was shown that cell alignment itself decreases inflammatory signaling in ECs even in the absence of flow. 19 However, a causal connection to flow has not been established.
Many aspects of fluid shear stress such as magnitude,20,21 and temporal and spatial gradients 22-25 have been studied for their effects on vascular ECs, but the effects of flow direction are poorly understood. Most previous studies focused of the effects of flow reversal, due in part to the absence of in vitro systems that provide well defined changes in flow direction other than 180° 26. Flow reversal on shear stress-aligned endothelial changed the expression levels of multiple growth and inflammatory genes, including PDGF and NOS3 27. Nitric oxide is reduced in reverse flow compared to a forward flow with same magnitude in ex vivo porcine arteries 28. Flow reversal on pre-aligned cells affects microvascular permeability 29 and cell-cell junction inclination30.
However, realistic in vivo local shear stresses at regions of disturbed flow can be multi-directional due to complex flow patterns such as time-varying vortices and helical flows 31-37. For example, shear stress at the side walls of the proximal internal carotid artery change direction sharply during systole over a range of 70°.33 Formation of aneurysms following repair of aortic coarctation and the distal anastomotic intimal hyperplasia of vascular bypass grafts also correlate strongly with shear stresses with significant off-axis components. 31,34 Efforts to study the effects of off-axis flow (angles other than 0° and 180°) included computational analysis of the effects of perpendicular flow (90°) on subcellular stress distribution in aligned cells38 and endothelial cell morphology39. The effects of biaxial oscillatory shear stress on cell morphology have also been studied40. However, effects of flow direction on signaling pathways have not been examined.
Most in vitro flow systems use oscillatory shear along one axis to model the more complex atherogenic flows found in vivo.26,41 Some systems have modeled complex, time varying in vivo flow magnitude along one axis.42,43 We therefore developed and validated a novel flow system that can change the direction of shear by any angle.26 In the present work, we utilized this system to address how ECs respond to changes in the direction of flow. When ECs were pre-aligned with flow and then subjected to a single change of flow direction, activation of NF-κB, eNOS and Akt had distinct directional requirements. Aligning cells on micro-patterned fibronectin or analysis of randomly oriented cells in a monolayer indicated that the angle between flow direction and the cell axis, defined by cell shape and F-actin, dictates these flow responses. The data therefore identify a central role for cell alignment in the response to shear stress.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Responses of flow–aligned endothelial cells to flow direction
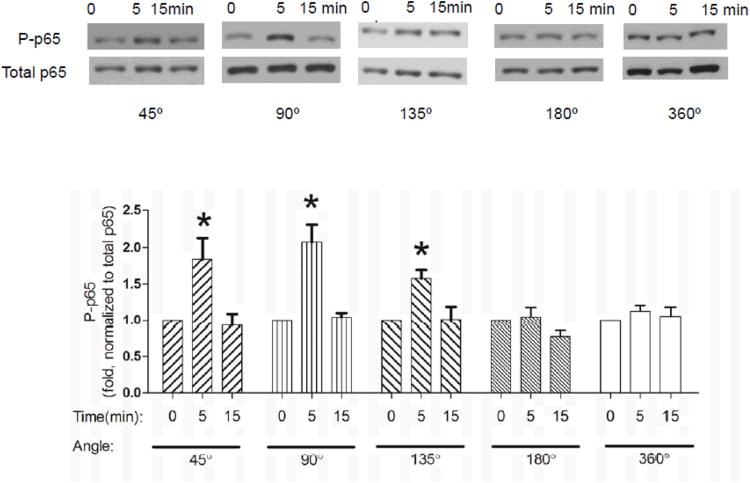
Disturbed flow often involves complex changes in the direction of flow. To investigate how these might affect EC function, we subjected aligned BAECs to a single change in flow direction. We examined four critical pathways, NF-κB (p65), which is implicated in inflammation; eNOS, which has been implicated in flow-dependent vasodilation and suppression of inflammation 44; Akt and AMP kinase (AMPK) which have important roles in shear stress induced cellular responses45,46. Following alignment of BAECs under 24h of laminar flow, flow direction was changed to angles between 45° and 180°. A 360° rotation served as a control for the effects of the rotation itself. We found that phosphorylation of eNOS and Akt were induced only by a change in shear to high angles and was maximal at 180°, whereas a 45° or 90° change in flow direction had no effect (Fig. 1). Similar results for eNOS and Akt are consistent with the known role of Akt upstream of eNOS S1177 47. By contrast, NF-κB activation, assessed by phosphorylation of p65 on Ser 536, was activated by lower angles, being maximal at 90°, but was unchanged following flow reversal (i.e., 180°; Fig. 2). A 360° rotation had no effect on any of these pathways, indicating that the brief rotation itself did not stimulate these pathways. AMPK activation was negligible under all of these conditions (data not shown).
Figure 1.
Activation of eNOS and Akt after changing flow direction.
Cells aligned in flow for 24 h were subject to a change in direction by 45°, 90°, 135° and 180° and 360°. At the indicated times, cells were harvested and phospho-eNOS (ser1179) (A) and phospho-Akt (ser473) (B) were assayed by Western blotting. Values are means ± SEM, n=4; *P<0.05.
Figure 2.
Activation of NF-kB after changing flow direction.
Flow-aligned cells were subject to a change in flow direction as in Figure 1. Phospho-p65 was assayed by Western blotting. Values are means ± SEM, n=4; *P<0.05.
Short-term shear without alignment
To determine whether these anisotropic responses require cell alignment, BAECs were pre-conditioned under flow for only 2 hours. At this time, NF-kB, eNOS and Akt activity had decreased to levels well below their peaks at 30-60 min but cells were not yet substantially aligned. Under these conditions, changing flow direction did not elicit significant changes in eNOS, p65 or Akt phosphorylation (Fig. 3). These results show that cell alignment is a prerequisite for the anisotropic responses to changes in flow direction.
Figure 3.
Responses of non-aligned cells to change of flow direction.
Cells under flow for 2h were subject to a change in flow angle as in Figure 1. (A) Phospho-eNOS; (B) Phospho-p65; (C) Phospho-Akt. Values are means ± SEM, n=3.
Responses of elongated cells in naïve cell monolayers to flow
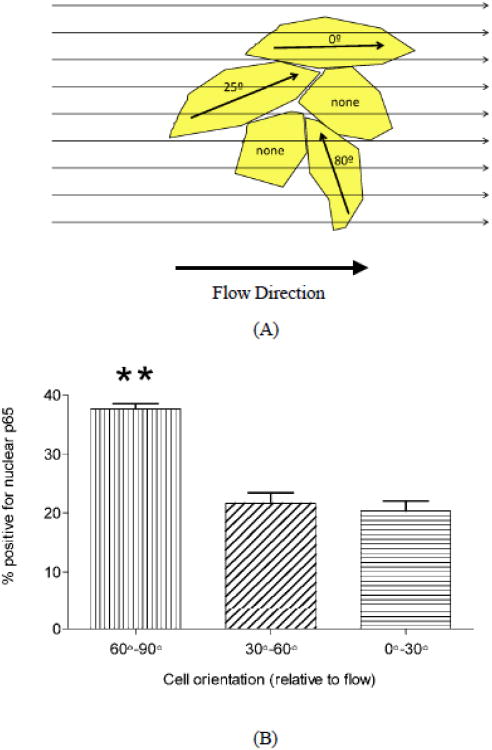
We next asked whether the polarity that determines responses to flow direction was due to the axis related to cell shape and the cytoskeleton, or by another type of polarity induced by flow. In naïve endothelial monolayers, a fraction of the cells are elongated though randomly oriented. We therefore applied onset of flow (12 dynes/cm2) to naïve monolayers for 30 min, then assessed NF-κB activation by staining cells for p65. Activation was assessed by scoring nuclear translocation as a function of cell orientation relative to the flow direction. Cell orientation was defined as was parallel (0°-30°), intermediate (30°-60°) or perpendicular (60°-90°) to the direction of flow. Cells that were perpendicular to the flow direction showed substantially higher nuclear p65 than intermediate or parallel (Fig. 4B). Thus, in cells not previously exposed to flow, the angle of the flow relative to the axis defined by cell shape is critical for NF-κB activation in individual elongated cells.
Figure 4.
Responses of naive cells to the onset of flow.
(A): Cartoon of cells in a naive cell monolayer with different angles relative to the flow direction. (B): NF-κB nuclear translocation in elongated cells that were perpendicular (60°-90°), intermediate (30°-60°) or parallel (0°-30°) to the flow direction. Shear stress of 15 dynes/cm2 was applied for 30 min. Values are means ± SEM, n=3; **P<0.001.
Responses of micropattern–aligned endothelial cells to flow direction
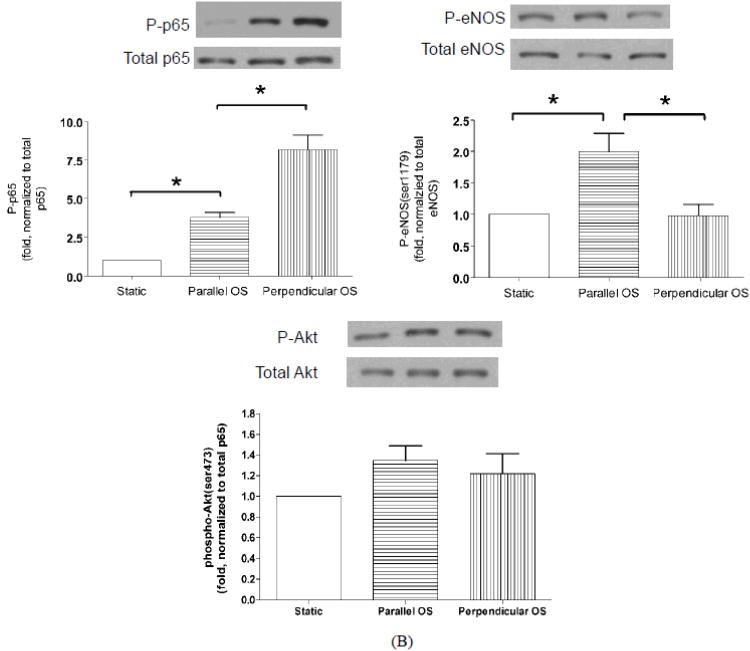
Oscillatory flow, which is commonly used to model in vivo disturbed flow, does not induce cell alignment and induces sustained activation of NF-κB 48. If cellular responses to oscillatory shear depend on the angle between flow direction and the cell axis in the same manner as for changes in flow direction or in onset of flow, failure to align might be causal for NF-κB activation in oscillatory flow. To test this hypothesis, BAECs were plated on coverslips with micropatterned fibronectin (FN) lines, which induce cell alignment in the direction of the lines without flow. Cells were then subjected to oscillatory flow (0.5±3 dynes/cm2 for 2h) parallel or perpendicular to the FN lines. NF-κB was activated to a higher extent in cells that were perpendicular to the flow direction than parallel (Fig. 5). Activation of eNOS showed the opposite behavior, with higher activation by parallel compared to perpendicular flow (Fig. 5B). Activation of Akt showed a similar trend as eNOS but did not reach statistical significance (Fig 5B). As before, AMPK activity was unchanged (data not shown).
Figure 5.
Response of micropattern-aligned cells to oscillatory flow.
Cells aligned on micropattterned fibronectin lines were analyzed by staining for p65 and visualizing nuclear translocation. (A): Images of cells without flow (left), and with 2h oscillatory flow parallel (center) or perpendicular (right) to the lines. (B): Cells were extracted and analyzed by Western blotting for phospho-p65 (left), phospho-eNOS (right) and phospho-Akt (bottom). Values are means ± SEM, n=3; *P<0.05.
The balance between nitric oxide (NO) and reactive oxygen species (ROS) is a major determinant of endothelial function vs. dysfunction and atherosclerotic progression in arteries 49,50. We therefore measured their levels in cells on the micropatterned substrates. Perpendicular oscillatory shear stress induced higher ROS and lower NO compared with parallel flow (Fig. 6). These data strongly implicate effects of flow direction on the pathways that play major roles in determining endothelial function and disease.
Figure 6.
Reactive oxygen and nitric oxide production.
Cells on micropatterned surfaces were subject to oscillatory flow for 1h. (A): DAF-FM detection of nitric oxide. (B): H2DCFDA detection of ROS. Images on the left, quantified values on the right are means ± SEM, n=3; *P<0.05.
Discussion
In vivo, shear stress at regions of disturbed flow are often multidirectional due to complex patterns such as time-varying vortices and helical flows 31-37,51. While the effects of shear stress on endothelial cells have been extensively investigated in vitro, effects of flow direction are understudied due to the limitations of commonly used in vitro systems. We therefore utilized a recently-developed flow system that enables changes in flow direction. Our results showed that eNOS and Akt were maximally activated by a 180° change in flow, essentially a single oscillation along the cell axis, whereas at this angle NF-κB activation was unchanged. By contrast, NF-κB was activated maximally by a 90° directional change in flow, which did not affect eNOS or Akt. These results lead to the surprising conclusion that different flow angles stimulate activation of distinct pathways.
Passerini et al 27 reported that application of reverse flow of low magnitude decreased expression of eNOS after 6h, however, the differences in magnitude and time of analysis make it difficult to directly compare our results with theirs. Another study 28 found that reverse flow in porcine arteries stimulated NO production less well than forward flow, due to superoxide production, which decreased NO availability 28,52. However, to our knowledge, our data are the first to compare effects of forward, reverse and perpendicular flow.
Further experiments with randomly aligned, naive cells and with cells aligned on micropatterned substrates demonstrated that the cell axis that determines flow responses does not require flow but instead is determined by “conventional” cell shape. These results strongly suggest that cytoskeletal and adhesive structures provide the internal compass against which flow is measured. This idea is consistent with our previous study in which we observed that pre-aligned cells subject to a 90° rotation in flow remained aligned to each other but rotated toward the new flow direction at a constant speed 26. This mechanism of realignment can be best by a model in which cells essentially “compare” flow direction to an internal axis and activate cytoskeletal and signaling pathways accordingly. Thus, several distinct cell responses support the concept that flow direction is sensed relative to the cells' cytoskeletal axis.
It is well established that oscillatory flow activates inflammatory pathways such as NF-κB that promote atherogenesis. Our results, however, showed that activation of NF-κB by oscillatory flow in micropatterned cells was also strongly dependent on flow direction. Interestingly, low laminar shear activates NF-κB nearly as well as oscillatory flow 48. Low and oscillatory flow patterns are also distinct from high laminar shear in that they fail to induce alignment. These results suggest that an important reason why low and oscillatory flow are atherogenic is because cells fail to align in the flow direction, so that many cells experience flow at high angles relative to their long axes. These effects govern whether flow stimulates activation of eNOS and production of NO vs. production of ROS and activation of NF-κB The balance between these two pathways is an important determinant of vascular function such that increased ROS and decreased NO leads to endothelial dysfunction and promotes atherosclerosis 53,54. These direction-dependent differential responses of endothelial cells to flow are therefore likely to be functionally important.
These results therefore provide evidence that EC alignment under laminar flow is an important atheroprotective, adaptive process. These results are also likely to be directly relevant to bypass surgery, where connecting arteries of different dimensions induces regions of disturbed flow downstream of the junction. It has been proposed that intimal hyperplasia in his regions is mainly caused by oscillatory flow55. Many studies56 (mostly computational) have attempted to optimize the local shear stress to minimize intimal hyperplasia. However, none of these studies considered off-axis flow direction as a factor, which our data suggest is critical. Thus, our findings indicate that minimizing inflammatory responses and restenosis should require minimizing off-axis flow or enhancing cell alignment in the direction of flow 57,58.
Future work will be directed toward identifying the molecular sensing mechanisms that mediate activation of distinct pathways depending on flow direction, and in investigating the effects of complex, multi-directional flow patterns that more accurately model in vivo disturbed flow.
Supplementary Material
Significance.
Regions of arteries under low and disturbed fluid shear stress are susceptible to atherosclerosis, whereas regions under high laminar flow are atherosclerosis-resistant. Susceptibility to atherosclerosis strongly correlates with poor endothelial alignment. In vitro, inflammatory flow profiles also fail to induce cell alignment. The current study demonstrates that signals stimulated by flow are determined by direction relative to the axis defined by cell shape and the cytoskeleton. Thus, flow parallel to the cell axis preferentially stimulates eNOS activation and NO production, whereas perpendicular flow preferentially stimulates reactive oxygen and NF-kB activation. Thus, the same flow activates functionally opposite pathways depending on direction. These data fundamentally alter our understanding of how flow acts upon the endothelium and provide a direct causal link between cell alignment and inflammatory activation. They also suggest new strategies for inhibiting restenosis in vascular grafts and other interventions.
Acknowledgments
The authors thank Dr. Brett Blackman for providing bovine aortic endothelial cells.
Sources of Funding: This study was supported by an American Heart Association post-doctoral fellowship #10POST4140009 to CW, NIH Ruth Kirschstein postdoctoral fellowship to BB, USPHS grant R01 EB00262 to CSC, and USPHS grant RO1 HL75092 to MAS.
Footnotes
Disclosures: None.
References
- 1.Berk B. Atheroprotective Signaling Mechanisms Activated by Steady Laminar Flow in Endothelial Cells. Circulation. 2008;117:1082–1089. doi: 10.1161/CIRCULATIONAHA.107.720730. [DOI] [PubMed] [Google Scholar]
- 2.Chatzizisis Y, Coskun A, Jonas M, Edelman E, Feldman C, Stone P. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodelingmolecular, cellular, and vascular behavior. Journal of the American College of Cardiology. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 3.Chiu J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiological reviews. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies P. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nature Clinical Practice Cardiovascular Medicine. 2008;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malek A, Alper S, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA: The Journal of the American Medical Association. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 6.Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circulation research. 1990;66:1045–1066. doi: 10.1161/01.res.66.4.1045. [DOI] [PubMed] [Google Scholar]
- 7.DeBakey M, Lawrie G, Glaeser D. Patterns of Atherosclerosis and their Surgical Significance. Annals of Surgery. 1985;201:115–131. doi: 10.1097/00000658-198502000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinstreuer C, Hyun S, Buchanan J, Longest P, Archie J, Truskey G. Hemodynamic parameters and early intimal thickening in branching blood vessels. Crit Rev Biomed Eng. 2001;29:1–64. doi: 10.1615/critrevbiomedeng.v29.i1.10. [DOI] [PubMed] [Google Scholar]
- 9.Sanmartín M, Goicolea J, García C, García J, Crespo A, Rodríguez J, Goicolea J. Influence of shear stress on in-stent restenosis: in vivo study using 3D reconstruction and computational fluid dynamics. Rev Esp Cardiol. 2006;59:21–27. [PubMed] [Google Scholar]
- 10.Kim Y, Chandran K, Bower T, Corson J. Flow dynamics across end-to-end vascular bypass graft anastomoses. Ann Biomed Eng. 1993;21:311–320. doi: 10.1007/BF02368624. [DOI] [PubMed] [Google Scholar]
- 11.Liu S. Prevention of focal intimal hyperplasia in rat vein grafts by using a tissue engineering approach. Atherosclerosis. 1998;140:365–377. doi: 10.1016/s0021-9150(98)00143-9. [DOI] [PubMed] [Google Scholar]
- 12.Kissin M, Kansal N, Pappas P, DeFouw O, Durán W, Hobson RN. Vein interposition cuffs decrease the intimal hyperplastic response of polytetrafluoroethylene bypass grafts. J Vasc Surg. 2000;31:69–83. doi: 10.1016/s0741-5214(00)70069-3. [DOI] [PubMed] [Google Scholar]
- 13.Greenwald S, Berry C. Improving vascular grafts: the importance of mechanical and haemodynamic properties. J Pathol. 2000;190:292–299. doi: 10.1002/(SICI)1096-9896(200002)190:3<292::AID-PATH528>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 14.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. AJP: Heart and Circulatory Physiology. 2006;292:H1209–H1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 15.Nerem R, Levesque M, Cornhill J. Vascular endothelial morphology as an indicator of the pattern of blood flow. J Biomech Eng. 1981;103:172–176. doi: 10.1115/1.3138275. [DOI] [PubMed] [Google Scholar]
- 16.Flaherty J, Pierce J, Ferrans V, Patel D, Tucker W, Fry D. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ Res. 1972;30:23–33. doi: 10.1161/01.res.30.1.23. [DOI] [PubMed] [Google Scholar]
- 17.Davies P. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn C, Schwartz M. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vartanian K, Berny M, McCarty O, Hanson S, Hinds M. Cytoskeletal structure regulates endothelial cell immunogenicity independent of fluid shear stress. Am J Physiol Cell Physiol. 2010;298:C333–341. doi: 10.1152/ajpcell.00340.2009. [DOI] [PubMed] [Google Scholar]
- 20.Noris M, Morigi M, Donadelli R, Aiello S, Foppolo M, Todeschini M, Orisio S, Remuzzi G, Remuzzi A. Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circ Res. 1995;76:536–543. doi: 10.1161/01.res.76.4.536. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Friedman M. The Adaptive response of vascular endothelial cells to an acute increase in shear stress magnitude. Am J Physiol Heart Circ Physiol. 2012;302:H983–991. doi: 10.1152/ajpheart.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagel T, Resnick N, Dewey CJ, Gimbrone MJ. Vascular endothelial cells respond to spatial gradients in fluid shear stress by enhanced activation of transcription factors. Arterioscler Thromb Vasc Biol. 1999;19:1825–3184. doi: 10.1161/01.atv.19.8.1825. [DOI] [PubMed] [Google Scholar]
- 23.White C, Haidekker M, Bao X, Frangos J. Temporal gradients in shear, but not spatial gradients, stimulate endothelial cell proliferation. Circulation. 2001;103:2508–2013. doi: 10.1161/01.cir.103.20.2508. [DOI] [PubMed] [Google Scholar]
- 24.LaMack J, Friedman M. Individual and combined effects of shear stress magnitude and spatial gradient on endothelial cell gene expression. Am J Physiol Heart Circ Physiol. 2007;293:H2853–2859. doi: 10.1152/ajpheart.00244.2007. [DOI] [PubMed] [Google Scholar]
- 25.Blackman B, Garcia-Cardena G, Gimbrone MAJ. A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. Journal of Biomechanical Engineering. 2002;124:397–407. doi: 10.1115/1.1486468. [DOI] [PubMed] [Google Scholar]
- 26.Wang C, Lu H, Schwartz M. A novel in vitro flow system for changing flow direction on endothelial cells. J Biomech. 2012;45:1212–1218. doi: 10.1016/j.jbiomech.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Passerini AG, Milsted A, Rittgers SE. Shear stress magnitude and directionality modulate growth factor gene expression in preconditioned vascular endothelial cells. Journal of vascular surgery. 2003;37:182–190. doi: 10.1067/mva.2003.66. [DOI] [PubMed] [Google Scholar]
- 28.Lu X, Kassab GS. Nitric oxide is significantly reduced in ex vivo porcine arteries during reverse flow because of increased superoxide production. The Journal of physiology. 2004;561:575–582. doi: 10.1113/jphysiol.2004.075218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adamson RH, Sarai RK, Altangerel A, Clark JF, Weinbaum S, Curry FE. Microvascular permeability to water is independent of shear stress, but dependent on flow direction. American journal of physiology Heart and circulatory physiology. 2013;304:H1077–1084. doi: 10.1152/ajpheart.00956.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melchior B, Frangos JA. Shear-induced endothelial cell-cell junction inclination. American journal of physiology Cell physiology. 2010;299:C621–629. doi: 10.1152/ajpcell.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frydrychowicz A, Arnold R, Hirtler D, Schlensak C, Stalder AF, Hennig J, Langer M, Markl M. Multidirectional flow analysis by cardiovascular magnetic resonance in aneurysm development following repair of aortic coarctation. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2008;10:30. doi: 10.1186/1532-429X-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frydrychowicz A, Stalder AF, Russe MF, Bock J, Bauer S, Harloff A, Berger A, Langer M, Hennig J, Markl M. Three-dimensional analysis of segmental wall shear stress in the aorta by flow-sensitive four-dimensional-MRI. Journal of magnetic resonance imaging : JMRI. 2009;30:77. doi: 10.1002/jmri.21790. [DOI] [PubMed] [Google Scholar]
- 33.Ku D, Giddens D, Zarins C, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 34.Kute SM, Vorp DA. The Effect of Proximal Artery Flow on the Hemodynamics at the Distal Anastomosis of a Vascular Bypass Graft: Computational Study. Journal of Biomechanical Engineering. 2001;123:277. doi: 10.1115/1.1374203. [DOI] [PubMed] [Google Scholar]
- 35.Stalder A, Russe MF, Frydrychowicz A, Bock J, Hennig J, Markl M. Quantitative 2D and 3D phase contrast MRI: optimized analysis of blood flow and vessel wall parameters. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2008;60:1218. doi: 10.1002/mrm.21778. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji T, Suzuki Ji, Shimamoto R, Yamazaki T, Nakajima T, Nagai R, Komatsu S, Ohtomo K, Toyo-Oka T, Omata M. Vector analysis of the wall shear rate at the human aortoiliac bifurcation using cine MR velocity mapping. AJR American journal of roentgenology. 2002;178:995. doi: 10.2214/ajr.178.4.1780995. [DOI] [PubMed] [Google Scholar]
- 37.Zhao S, Xu X, Hughes A, Thom S, Stanton A, Ariff B, Long Q. Blood flow and vessel mechanics in a physiologically realistic model of a human carotid arterial bifurcation. Journal of Biomechanics. 2000;33:975–984. doi: 10.1016/s0021-9290(00)00043-9. [DOI] [PubMed] [Google Scholar]
- 38.Barbee KA, Mundel T, Lal R, Davies PF. Subcellular distribution of shear stress at the surface of flow-aligned and nonaligned endothelial monolayers. The American journal of physiology. 1995;268:H1765–1772. doi: 10.1152/ajpheart.1995.268.4.H1765. [DOI] [PubMed] [Google Scholar]
- 39.Kataoka N, Ujita S, Sato M. Effect of flow direction on the morphological responses of cultured bovine aortic endothelial cells. Medical & biological engineering & computing. 1998;36:122–128. doi: 10.1007/BF02522869. [DOI] [PubMed] [Google Scholar]
- 40.Chakraborty A, Chakraborty S, Jala VR, Haribabu B, Sharp MK, Berson RE. Effects of biaxial oscillatory shear stress on endothelial cell proliferation and morphology. Biotechnology and bioengineering. 2012;109:695–707. doi: 10.1002/bit.24352. [DOI] [PubMed] [Google Scholar]
- 41.Frangos J, Eskin S, Mcintire L, Ives C. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 42.Bussolari S, Dewey C, Gimbrone M. Apparatus for subjecting living cells to fluid shear stress. Review of Scientific Instruments. 1982;53:1851. doi: 10.1063/1.1136909. [DOI] [PubMed] [Google Scholar]
- 43.Blackman B, Barbee K, Thibault L. In vitro cell shearing device to investigate the dynamic response of cells in a controlled hydrodynamic environment. Annals of Biomedical Engineering. 2000;28:363–372. doi: 10.1114/1.286. [DOI] [PubMed] [Google Scholar]
- 44.Wever R, Lüscher T, Cosentino F, R TJ. Atherosclerosis and the two faces of endothelial nitric oxide synthase. Circulation. 1998;97:108–112. doi: 10.1161/01.cir.97.1.108. [DOI] [PubMed] [Google Scholar]
- 45.Guo D, Chien S, Shyy JY. Regulation of endothelial cell cycle by laminar versus oscillatory flow: distinct modes of interactions of AMP-activated protein kinase and Akt pathways. Circulation research. 2007;100:564–571. doi: 10.1161/01.RES.0000259561.23876.c5. [DOI] [PubMed] [Google Scholar]
- 46.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. Journal of cell science. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 47.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 48.Mohan S, Mohan N, Sprague E. Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. American Journal of Physiology- Cell Physiology. 1997;273:C572–C578. doi: 10.1152/ajpcell.1997.273.2.C572. [DOI] [PubMed] [Google Scholar]
- 49.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circulation research. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 50.Darley-Usmar V, Wiseman H, Halliwell B. Nitric oxide and oxygen radicals: a question of balance. FEBS letters. 1995;369:131–135. doi: 10.1016/0014-5793(95)00764-z. [DOI] [PubMed] [Google Scholar]
- 51.Moore J, Ku D, Zarins C, Glagov S. Pulsatile flow visualization in the abdominal aorta under differing physiologic conditions: implications for increased susceptibility to atherosclerosis. Journal of Biomechanical Engineering. 1992;114:391. doi: 10.1115/1.2891400. [DOI] [PubMed] [Google Scholar]
- 52.Godbole AS, Lu X, Guo X, Kassab GS. NADPH oxidase has a directional response to shear stress. American journal of physiology Heart and circulatory physiology. 2009;296:H152–158. doi: 10.1152/ajpheart.01251.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovascular research. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 54.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiological reviews. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 55.Bassiouny HS, White S, Glagov S, Choi E, Giddens DP, Zarins CK. Anastomotic intimal hyperplasia: mechanical injury or flow induced. Journal of vascular surgery. 1992;15:708–716. doi: 10.1067/mva.1992.33849. discussion 716-707. [DOI] [PubMed] [Google Scholar]
- 56.Owida AA, Do H, Morsi YS. Numerical analysis of coronary artery bypass grafts: an over view. Computer methods and programs in biomedicine. 2012;108:689–705. doi: 10.1016/j.cmpb.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Huang NF, Zaitseva T, Paukshto M, Sun J, Fuller G, Cooke JP. Biomedical Engineering Society Annual Conference. Vascular Cellular Morphology on Aligned Collagen Matrices. Austin, TX: Oct 6-9, 2010. [Google Scholar]
- 58.Uttayarat P, Perets A, Li M, Pimton P, Stachelek S, Alferiev I, Composto R, Levy R, Lelkes P. Micropatterning of three-dimensional electrospun polyurethane vascular grafts. Acta Biomater. 2010;6:4229–4237. doi: 10.1016/j.actbio.2010.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.