Abstract
Hydrogen sulphide (H2S) is synthesized from L-cysteine via the action of cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS). We have earlier shown that H2S acts as a mediator of inflammation. However the mechanism remains unclear. In this study, we investigated the presence of H2S and the expression of H2S synthesizing enzymes, CSE and CBS, in isolated mouse pancreatic acini. Pancreatic acinar cells from mice were incubated with or without caerulein (10−7 M for 30 and 60 min). Caerulein increased the levels of H2S and CSE mRNA expression while CBS mRNA expression was decreased. In addition, cells pre-treated with DL-propargylglycine (PAG, 3 mM), a CSE inhibitor, reduced the formation of H2S in caerulein treated cells, suggesting that CSE may be the main enzyme involved in H2S formation in mouse acinar cells. Furthermore, substance P (SP) concentration in the acini and expression of SP gene (preprotachykinin-A, PPT-A) and neurokinin-1 receptor (NK-1R), the primary receptor for SP, are increased in secretagogue caerulein-treated acinar cells. Inhibition of endogenous production of H2S by PAG significantly suppressed SP concentration, PPT-A expression and NK1-R expression in the acini. To determine whether H2S itself provoked inflammation in acinar cells, the cells were treated with H2S donor drug, sodium hydrosulphide (NaHS), (10, 50 and 100 μM), that resulted in a significant increase in SP concentration and expression of PPT-A and NK1-R in acinar cells. These results suggest that the pro-inflammatory effect of H2S may be mediated by SP-NK-1R related pathway in mouse pancreatic acinar cells.
Keywords: cystathionine-γ-lyase, cystathionine-β-synthase, H2S, Substance P, pancreatic acinar cells
Introduction
Hydrogen sulphide (H2S) has long been known as a toxic gas [14]. However, it has emerged that H2S is also generated in mammalian tissues from the amino acids L-cysteine and homocysteine by pyri-doxal-5-phosphate-dependent enzymes such as cystathionine-β-synthase (CBS, EC4.2.1.22) and cystathionine-γ-lyase (CSE, EC4.4.1.1) [26, 28]. CBS converts homocysteine to cystathionine and hydrolyses L-cysteine to equimolar amounts of serine and H2S whereas CSE converts cystathionine to L-cysteine yielding pyruvate, NH3 and H2S [28]. Nitric oxide (NO) and carbon monoxide (CO) now including H2S are growing family of regulatory gaseous molecules involved in various physiological and pathological functions in mammalian tissues [32]. In contrast to other gaseous mediators (NO and CO [31, 33]) very little information exists on the mechanism by which H2S influences cell function.
Physiological levels of H2S in rat serum vary from 30 to 45μM [24, 27, 35] and in mouse serum, ∼23μM [10] and within this concentration range H2S induces relaxation of aorta tissues and induces transient blood pressure reduction [37]. Vascular effect of H2S may also be mediated by a direct stimulation of KATP channels with subsequent hyperpolarization of rat aortic vascular smooth muscle in mesenteric artery, aorta, and portal vein [17, 37]. Consistent with this, a reduced CSE expression/ activity and decreased H2S concentration contributes to the pathophysiology of pulmonary hypertension in rodents [34] whereas CBS deficiency leads to hyperhomocysteinemia, increased blood pressure and endothelial dysfunction [13]. In rodents, CBS deficiency induced by genetic deletion [13] or chronic treatment with DL-propargylglycine (PAG), an irreversible inhibitor of CSE [34], results in a severe hypertension and severe loss of endothelial function.
We have previously reported that caerulein-induced acute pancreatitis up-regulates pancreas CSE mRNA expression and H2S biosynthesis. Therapeutic administration of PAG reduces the severity of caerulein-induced pancreatitis and affords protection against associated lung injury in mice [10]. A similar pro-inflammatory effect of H2S has also been reported in other animal models of hindpaw edema [9], lipopolysaccharide (LPS) induced endo-toxemia [24] as well as cecal ligation and puncture (CLP) induced sepsis [36]. In these studies, tissue CSE expression is up-regulated leading to increased H2S biosynthesis whereas inhibition of H2S formation has been shown to display distinct anti-inflammatory activity [9, 24, 36]. Therefore, it is emerging that H2S is an additional and important physiological mediator in inflammation.
Acute pancreatitis is a common clinical condition, the incidence of which has been increasing over recent years [2–4, 6, 7]. It is generally believed that the earliest events in acute pancreatitis occur within acinar cells. In vitro and in vivo data [8] indicate that the earliest event and most important factor responsible for acute pancreatitis are the increase and the activation of intra-acinar digestive enzymes. After this initial insult, other components (ischemia, pancreatic glutathione deficiency, oxygen free radicals, cell death and inflammatory mediators) intervene to worsen pancreatitis. Increasing evidence indicates that neurogenic pro-inflammatory factors, such as substance P (SP), can play important roles in determining the severity of acute pancreatitis [19] both in mice and rats. SP belongs to the tachykinin family of neuropeptides, derived from the preprotachykinin (PPT-A) gene. It is a neurotransmitter and pain mediator released from both central and peripheral endings of primary afferent neurons as well as from various inflammatory cells [16, 25]. The biological actions of SP are mediated primarily by neurokinin-1 receptors (NK-1R). Previous data [1] showed that SP levels and NK-1R in pancreatic acinar cells are up-regulated during experimental pancreatitis in mice. In addition, NK-1R deletion or antagonism protects mice against pancreatitis [5, 15]. Further study showed that the antagonist treatment suppressed the increase in pancreatic and lung PPT-A mRNA expression and SP protein levels in acute pancreatitis [21].
Although recent studies have demonstrated that H2S plays a key role in inflammation, the cellular source of H2S and the mechanism by which H2S acts as an inflammatory mediator in pancreas is yet to be found. To this end we have identified H2S synthesizing enzyme activity, CSE and CBS mRNA expression in pancreatic acinar cells. The effect of H2S concentration, CBS and CSE mRNA in cholecystokinin (CCK) secretagogue caerulein-treated acinar cells has been monitored. Moreover we have investigated whether H2S as pro-inflammatory molecule is involved in SP-NK-1R related pathway in mouse pan-creatic acinar cells. We induced acinar cells with caerulein (10−7 M) and studied the effect of PAG (3 mM) an irreversible inhibitor of H2S-synthesizing enzyme CSE, on SP levels, PPT-A and NK-1R mRNA expression. We have also assessed the pro-inflammatory effect of the H2S donor, sodium hydro-sulphide (NaHS) in acinar cells.
Materials and methods
Preparation of pancreatic acini
All experiments were approved by the animal ethics committee of National University of Singapore, Singapore and carried out in accordance with the established Guiding Principles for Animal Research. Pancreatic acini were obtained from mouse pancreas by collagenase treatment as described earlier [1]. Briefly, pancreata from male Swiss albino mice (25–30 g) were removed under aseptic conditions, infused with collagenase buffer A (in mM: 140 NaCl, 4.7 KCl, 1.13 MgCl2, 1 CaCl2, 10 glucose, and 10 HEPES, pH 7.2) containing 200 IU/ml collagenase and 0.5 mg/ml soybean trypsin inhibitor, and incubated in a shaking water bath for 10 min at 37°C. The digested tissue was passed through 50 mg/ml BSA and washed twice with collagenase buffer A before further experiments. PAG was purchased from SIGMA. Caerulein was obtained from Bachem (Bubendorf, Switzerland).
Pancreatic acini were suspended in Weymouth's MB 752/1 medium (containing 0.1% BSA, 0.5 mg/ml soybean trypsin inhibitor, 25 ng/ml epidermal growth factor, and antibiotics) and treated with or without caerulein (10−7 M) at 37°C in a shaker water bath for 30 and 60 min, respectively. In some experiment cells were pretreated with PAG (2, 3, 4 and 5 mM) for 30 and 60 min before addition of caerulein and in other experiment cells were treated with NaHS (10, 50 and 100 μM) for 30 min. Cell viability was measured by trypan blue exclusion and no significant changes were observed upon treatment.
Assay of H2S synthesizing activity in pancreatic acini
H2S biosynthesis in pancreatic acini was measured essentially as described previously [30]. Frozen cells were thawed on ice and homogenized in ice-cold 100 mM potassium phosphate buffer (pH 7.4). The reaction mixture (total volume, 500 μl) contained homogenate (430 μl), L-cysteine (10 mM; 20 μl), pyridoxal 5′-phosphate (2 mM; 20 μl), and saline (30 μl). The reaction was performed in parafilmed eppendorf tubes and initiated by transferring the tubes from ice to a water bath at 37°C. In some experiments, the enzymatic reaction was stopped immediately by addition of trichloroacetic acid (10% w/v, 250 μl) to denature protein prior to addition of L-cysteine. After incubation for 30 min, zinc acetate (1% w/v, 250 μl) was added to trap evolved H2S, followed by trichloroacetic acid (10% w/v, 250 μl). Subsequently, N,N-dimethyl-p-phenylenediamine sulphate (20 μM, 133 μl) in 7.2 M HCl and FeCl3 (30 μM, 133 μl) in 1.2 M HCl were added and the absorbance of the resulting solution (670 nm) measured 15 min thereafter, using a 96-well microplate reader (Tecan Systems Inc.).The H2S concentration of each sample was calculated against a calibration curve of sodium hydrosulphide (NaHS; 3.12–250 μM) and results are expressed as nmol H2S formed mg–1 DNA [24].
Measurement of H2S in pancreatic acini
The reaction mixture contained cell homogenate (450 μl); trichloroacetic acid (10% w/v, 300 μl), zinc acetate (1% w/v, 150 μl), N,N-dimethyl-p-phenylenediamine sulphate (20 μM; 100 μl) in 7.2 M HCl and ferric chloride (30 μM; 133 μl) in 1.2 M HCl in 96-well plates. The absorbance of the resulting solution (670 nm) was measured 15 min thereafter [12]. The basis of the assay is that H2S produced in the incubate reacts with zinc acetate to form zinc sulphide which then dissolves in hydrochloride acid solution of N,N-dimethyl-p-phenylenediamine sulphate yielding, in the presence of ferric chloride, methylene blue, which is quantitated spectrophotometrically. H2S was calculated against a calibration curve of NaHS (3.125–100 μM). Results showed plasma H2S concentration in the micromolar range.
Measurement of Substance P levels
Pancreatic acinar cells were homogenized in 2 ml ice-cold assay buffer for 20 sec. The homogenates were centrifuged (13 000 g, 20 min, 4°C) and the supernatants were collected. They were adsorbed on C18 cartridge columns (Bachem) as described [21]. The adsorbed peptides were eluted with 1.5 ml 75% v/v acetonitrile. The samples were freeze-dried and reconstituted in assay buffer. Substance P content was then determined using an ELISA kit (Bachem) according to the manufacturer's instructions and expressed as ng/μg DNA [20].
RT-PCR
Total RNA from isolated pancreatic acinar cell was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol [10]. The concentration of isolated nucleic acids were determined spectrophotometrically by measuring the absorbance at 260 nm, and the integrity was verified by ethidium bromide staining of 18S and 28S rRNA bands on a denaturing agarose gel. All samples were thereafter stored at –80°C until required. RNA (1 μg) was reverse transcribed using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) at 25°C for 5 min and 42°C for 30 min, followed by 85°C for 5 min. The cDNA was used as a template for PCR amplification by iQ Supermix (Bio-Rad). The PCR primers (Table 1) for detection of CSE, CBS, PPT-A and NK-1R were synthesized by Proligo (Singapore). The reaction mixture was first subjected to 95°C for 3 min for the activation of polymerase. This was followed by an optimal cycle of amplifications (Table 1), consisting of 95°C for 30 sec optimal annealing temperature (Table 1) for 72°C for 30 sec. PCR amplification was carried out in MyCycler (Bio-Rad, Hercules, CA, USA). PCR products were analyzed on 1.5% w/v agarose gels containing 0.5 μg/ml ethidium bromide.
1.
PCR primer sequences, optimal amplification cycles, optimal annealing temperatures and product sizes
| Gene | Primer sequence | Optimal conditions | Size (bp) | ||
|---|---|---|---|---|---|
| r18S | Sense: 5_-GTAACCCGTTGAACCCCATT-3_ | 22 cycles | 150 | ||
| Antisense: 5_-CCATCCAATCGGTAGTAGCG-3′ | Annealing: 59°C | ||||
| CSE | Sense: 5_- GAC CTC AAT AGT CGG CTT CGT TTC -3_ | 22 cycles | 618 | ||
| Antisense: 5_- CAG TTC TGC GTA TGC TCC GTA ATG -3′ | Annealing: 60°C | ||||
| CBS | Sense: 5_- GAT TTG ATT CCC CCG AGT CCC AG -3_ | 22 cycles | 618 | ||
| Antisense: 5_- CAC TTA TCC ACC ACC GCC CTG TC –3′ | Annealing: 60°C | ||||
| NK1R | Sense: 5_-CTTGCCTTTTGGAACCGTGTG-3′ | 42 cycles | 501 | ||
| Antisense: 5_-CACTGTCCTCATTCTCTTGTGGG-3′ | Annealing: 59.7°C | ||||
| PPT-A | Sense: 5_-ACCTGCTCCACTCCTGCACCGCGGCCAAG-3′ | 43 cycles | 239 | ||
| Antisense: 5_-GAACTGCTGAGGCTTGGGTCTTCGGGCGAT-3′ | Annealing: 68°C | ||||
| B-actin | Sense: 5_-GGG CTG TAT TCC CCT CCA TC-3′ | 22 cycles | 552 | ||
| Antisense: 5_-GTC ACG CAC GAT TTC CCT CTC-3′ | Annealing: 61°C | ||||
Statistical analysis
Data are expressed as the mean ± standard error of the mean (S.E.M.). In all figures, vertical error bars denote the S.E.M. The absence of such error bars indicates that the S.E.M. falls within the dimensions of the data bar. The significance of differences between groups was evaluated by analysis of variance (ANOVA), with post hoc Tukey's test when comparing three or more groups. A P-value of less than 0.05 was considered statistically significant.
Results
Production of H2S by pancreatic acini in response to caerulein treatment
Pancreatic acinar cells were stimulated with caerulein (10−7 M) for 30 and 60 min at 37°C. After which the pellet was used to assess the levels of H2S. Figure 1 shows a significant increase in H2S levels following 60 min incubation with caerulein. In freshly prepared acinar cells, levels of H2S were 10.5 ± 0.8 μM. Whereas H2S levels increased from 11.2 ± 1.1 and 12.1±0.63 μM in control cells to 12.1 ± 0.65 and 25.1 ± 1.3 μM in cells incubated with caerulein for 30 and 60 min, respectively (P < 0.001 when caerulein treated cells (60 min) were compared with control cells (60 min); Fig. 1).
1.

Hydrogen sulphide (H2S) concentration in caerulein-induced mouse pancreatic acinar cells. Freshly prepared pancreatic acinar cells were incubated with or without caerulein (10−7 M for 30 and 60 min). H2S concentration was measured as described in Section ‘Materials and methods’. Results shown are the mean ± S.E.M. of at least six separate determinations. ***P < 0.001 when caerulein treated cells (60 min) were compared with control cells (60 min).
CSE and CBS mRNA expression and H2S synthesis in the pancreatic acini
Pancreatic acinar cells were found to express the gene for the H2S forming enzyme CSE and CBS, as evidenced by RT-PCR extracted from these cells (Fig. 2A and B). In addition, incubation of acinar cell homogenates with L-cysteine resulted in the formation of H2S, showing the presence of H2S synthesizing enzyme activity in the cells (Fig. 3A).
2.
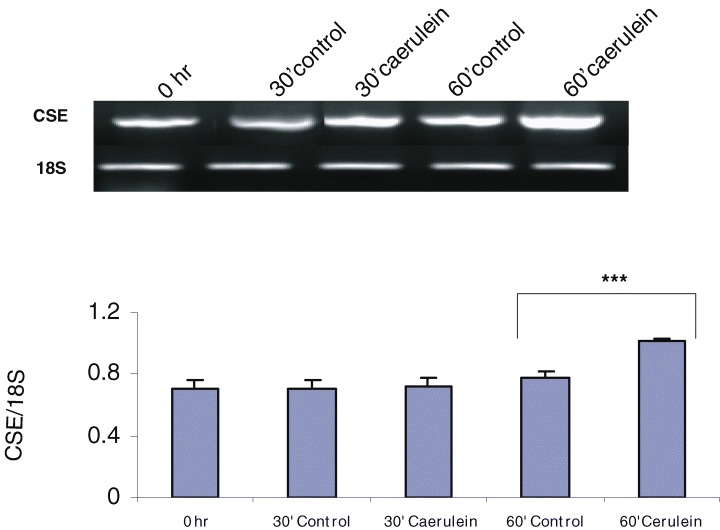
Cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS) mRNA expression in control and caerulein-treated mouse pancreatic acinar cells. (A) RT-PCR of CSE in control and caerulein (10−7 M for 30 and 60 min) treated acinar cells. The graphs represent the optical density of the bands of CSE generated from six independent experiments that were normalized with the expression of 18S. ***P < 0.001 when caerulein treated cells (60 min) were compared with control cells (60 min). (B) RT-PCR of CBS in control and caerulein treated acinar cells. The graphs represent the optical density of the bands of CBS generated from six independent experiments that were normalized with the expression of 18S. ***P < 0.001 when caerulein treated cells (60 min) were compared with control cells (0 hr).
3.

Effect of DL-propargylglycine (PAG) on H2S synthesizing activity in caerulein-treated mouse pancreatic acinar cells. (A) Freshly prepared pancreatic acinar cells were incubated for 30 and 60 min at 37°C with different concentration of PAG (2–5 mM). After which the pellet was used to detect the H2S synthesizing activity. Results shown are the mean ± S.E.M. of at least six separate determinations. ***P < 0.001 when 3 mM PAG treated cells (60 min) were compared with control cells (60 min). (B) Freshly prepared pancreatic acinar cells were pretreated with PAG (3 mM) 60 min before caerulein (10−7 M for 30 and 60 min) treatment. Pancreatic acinar cell homogenates were assessed for ability to synthesize H2S from exogenous L-cysteine. Results shown are the mean ± S.E.M. of at least six separate determinations. ***P < 0.001 when caerulein treated cells (60 min) were compared with control cells (60 min). ***P < 0.001 when caerulein treated cells (60 min) were compared with PAG pretreated cells (60 min).
To determine whether CSE and CBS mRNA expression is regulated by caerulein, we extracted total RNA from isolated acinar cells incubated for 30 and 60 min with or without caerulein. Figure 2A (P < 0.001 when caerulein treated cells (60 min) were compared with control cells (60 min)) shows a significant up-regulation of CSE mRNA expression by caerulein, whereas CBS mRNA expression was decreased following 60 min incubation with caerulein (P < 0.001 when caerulein treated cells (60 min) were compared with control cells (0 hr); Fig. 2B).
Effect of DL-propargylglycine (PAG) on H2S synthesizing activity
As CSE mRNA is up-regulated after caerulein treatment in acinar cells, we decided to investigate whether an inhibitor of CSE enzyme might exhibit anti-inflammatory activity. PAG was chosen for these experiments since it produces an irreversible inhibition of CSE [29], crosses biological membranes readily, and has been shown to cause long-lasting CSE inhibition after administration in animals [19]. Isolated pancreatic acinar cells were treated for 30 and 60 min at 37°C with different concentrations of PAG (2–5 mM). Only 60 min treatment with PAG at a dose of 3 mM showed maximum decrease in H2S production when compared with control cells (Fig. 3A). The dose of 3 mM of PAG was used to carry out subsequent experiments.
As shown in Figure 3B treatment of pancreatic acini with caerulein caused a significant increase in H2S production. Pretreatment of acini with 3 mM of PAG followed by stimulation with caerulein significantly attenuated the production of H2S when compared with caerulein treated cells. (P < 0.001). Since PAG does not block CBS activity either in vitro[30] or after administration in experimental animals in vivo[29], it is therefore likely that the observed rise in H2S in caerulein treated acinar cells may be due to enhanced CSE enzyme activity.
Effect of PAG pretreatment on substance P level and PPT-A mRNA expression in pancreatic acinar cells
Figure 4 shows the concentration of SP in the pancreatic acinar cells. As expected, hyper stimulation by caerulein increased the levels of SP in acinar cells (P < 0.001). Pretreatment of acini with 3 mM PAG followed by stimulation with caerulein significantly suppressed the SP concentration when compared with caerulein treated cells (P < 0.05). Densitometry analysis of the PCR products on agarose gel shows that the PPT-A mRNA expression increased in caerulein induced acinar cells (P < 0.001) and pretreatment with 3 mM PAG resulted in a significant decrease in PPT-A gene expression (P < 0.001; Fig. 5A).
4.

PAG treatment attenuated caerulein-induced increase in substance P (SP) level in mouse pan-creatic acinar cells.Freshly prepared pancreatic acinar cells were pre-treated with PAG (3 mM) 60 min before caerulein (10−7 M for 60 min) treatment. Substance P level was measured as described in Section ‘Materials and methods’. Results shown are the mean ± S.E.M. of at least six separate determinations. ***P < 0.001 when caerulein treated cells (60 min) were compared with control cells (60 min). *P < 0.05 when caerulein treated cells (60 min) were compared with PAG pretreated cells (60 min).
5.

PAG treatment suppressed caerulein-induced up regulation of preprotachykinin-A (PPT-A) and neurokonin-1 receptor (NK-1R) mRNA expression in mouse pancreatic acinar cells. (A) PPT-A mRNA expression in control, caerulein-treated (10−7 M for 60 min) and PAG pretreated (3 mM) (30 min before caerulein treatment) cells. The graphs represent the optical density of the bands of PPT-A generated from six independent experiments that were normalized with the expression of 18S. ***P < 0.001 between control and caerulein treated cells. (B) NK-1 receptor mRNA expression in control, caerulein-treated (10−7 M for 60 min) and PAG pretreated (3 mM) (60 min before caerulein treatment) cells. The graphs represent the optical density of the bands of NK-1 receptor generated from six independent experiments that were normalized with the expression of 18S. ***P < 0.001 between caerulein and PAG pretreated cells.
Effect of PAG treatment on NK-1R mRNA expression in pancreatic acinar cells
A significant increase in NK-1R mRNA expression was observed in caerulein-treated pancreatic acinar cells (P < 0.001; Fig. 5B). However, pretreatment with 3 mM PAG followed by stimulation with caerulein suppressed NK-1R expression when compared with caerulein treated acinar cells (P < 0.05; Fig. 5B).
Effect of H2S donor NaHS on pancreatic acinar cells
These experiments suggest that the inhibition of endogenous H2S biosynthesis results in an anti-inflammatory effect in caerulein-induced pancreatic acinar cells and thereby provides indirect evidence that H2S exerts pro-inflammatory activity in acinar cells. To investigate a possible direct role of H2S in acinar cells, we treated the cells with the H2S donor drug, NaHS (10 and 100 μM) and evaluated its effect on SP level. As shown in Fig. 6 (P < 0.01) 100 μM of NaHS significantly increased SP level in pancreatic acinar cells after 30 min treatment. In addition, PPT-A (P < 0.05; Fig 7A) and NK-1R expression were markedly increased 30 min after treatment with NaHS (P < 0.01; Fig 7B).
6.

Effect of H2S donor, sodium hydro-sulphide (NaHS), on SP level in pancreatic acinar cells. Freshly prepared pancreatic acinar cells were treated with NaHS (10, 50 and 100 μM) for 30 min. Substance P level was measured as described in Materials and Methods. Results shown are the mean ± S.E.M. of at least six separate determinations. **P < 0.01 between control and NaHS-induced cells.
7.

H2S donor, sodium hydrosulphide (NaHS), induces PPT-A and NK-1R mRNA expression in mouse pancreatic acinar cells. (A) PPT-A mRNA expression in control and NaHS-treated (10, 50 and 100 μM) cells. The graphs represent the optical density of the bands of PPT-A generated from six independent experiments that were normalized with the expression of β-actin . *P < 0.05 between control and NaHS-treated cells. (B) NK-1R mRNA expression in control and NaHS-treated (10, 50 and 100 μM) cells. The graphs represent the optical density of the bands of NK-1R generated from six independent experiments that were normalized with the expression of β-actin. ***P < 0.001 between control and NaHS-induced cells.
Discussion
H2S is increasingly recognized as a functionally relevant mediator of a number of physiological functions in mammalian tissues. It is generated endogenously during L-cysteine metabolism in many types of mammalian cells in the reaction catalyzed by cystathionine β-synthase and cystathionine γ-lyase [26,28].
We have previously reported that caerulein induced acute pancreatitis up regulates pancreas CSE mRNA expression and H2S biosynthesis. Therefore it causes a significant increase in plasma H2S concentration [10]. Blockage of H2S formation by administration of PAG, an inhibitor of CSE enzyme activity, reduced the severity of acute pancreatitis whereas exogenous H2S results in augmented MPO activity as well as histological changes in lung and pancreas [10]. A similar pro-inflammatory effect of H2S has also been reported in other animal models of inflammatory diseases, such as hindpaw edema [9], cecal ligation-induced sepsis [26] and lipopolysaccharide induced endotoxemia [24]. Based on these findings, we have proposed that H2S acts as a pro-inflammatory mediator. However, the precise mechanism by which H2S acts as an inflammatory mediator during acute pancreatitis remains unknown. Therefore, we went on to investigate the role of endogenous H2S in caerulein-treated pancreatic acinar cells.
We show here that mRNA for CSE and CBS are expressed in acinar cells (Fig. 2A and B) and the pan-creatic acinar cell homogenates convert L-cysteine to H2S ex vivo (Fig. 1). With the use of caerulein stimu-lated pancreatic acini, a well-established in vitro model for pancreatitis [23], our findings indicated an important role of H2S in pancreatic acinar cells. Stimulation of pancreatic acini with caerulein has significantly increased CSE mRNA expression (Fig. 2A). CBS mRNA expression, however reduced on caerulein treatment (Fig. 2B). The H2S synthesizing enzyme activity was also found to be elevated in the cells (Fig. 3B). It is reasonable to conclude that caerulein treatment up-regulates CSE expression and the CSE activity. The present study shows that, although both CBS and CSE are expressed by the pancreatic acinar cells, CSE may be the main enzyme involved in the H2S generation in the caerulein-induced pancreatic acinar cells. Consistent with this concept, exposure of the acinar cells to PAG, inhibits H2S formation (Fig. 3B). Inhibition of H2S synthesis using pharmacological agents acting on CSE has been shown to reduce inflammation [24] and swelling [9]. In our previous study, we have reported that therapeutic administration of PAG reduces the severity of caerulein-induced pancreatitis and affords protection against associated lung injury in mice [10]. As PAG elicits a complete and irreversible inactivation of CSE activity in vitro[18, 27], our finding suggests that CSE may be the enzyme mainly responsible for H2S formation in acinar cells.
In the present study, we observed a novel interaction between two groups of pro-inflammatory mediators – H2S and the neuropeptide substance P. Inhibition of endogenous H2S, with specific CSE inhibitor PAG resulted in attenuation in caerulein-induced SP release (Fig. 4), PPT-A and NK-1R expression (Fig. 5A and B). This suggests the pro-inflammatory effect of H2S are mediated by the release of SP. SP in turn is mediated by NK-1R in pancreatic acini.
Previously, it has been demonstrated that SP has an important role in mediating H2S-induced lung inflammation [11]. Genetic deletion of PPT-A and NK-1R reduced the severity of acute pancreatitis and associated lung injury [1, 5, 15]. We have earlier showed that NK-1R antagonist protected mice against caerulein-induced acute pancreatitis and associated lung injury (21). Further study showed that the antagonist treatment suppressed the increase in pancreatic and lung PPT-A expression and SP release in acute pancreatitis [22]. Our recent study demonstrated that H2S-induced SP release is inhibited by CP 96,345, a specific antagonist for NK-1R [11]. The above result suggests that part of the anti-inflammatory action of NK-1R antagonist may be mediated by a lowering of intracellular H2S production at sites of inflammation. Our result suggests the possibility that part of the known anti-inflammatory actions of CSE inhibitor may be mediated through an inhibition of SP-induced inflammation, although obviously other inflammatory mediators such as adhesion molecules, cytokines and nitric oxide in the interplay between H2S and SP may also be involved.
Since inhibition of endogenous H2S biosynthesis by PAG significantly reduced the SP release, we thought it of value to determine whether H2S itself is involved in SP-NK-1R-related pathway in acinar cells. For this purpose, we used the H2S donor, NaHS, to produce H2S. When mouse pancreatic acini were treated with NaHS, there was a significant increase in SP (Fig. 6), and up regulation of PPT-A and NK-1R expression in acinar cells (Fig. 7A and B), indicating that H2S by itself induces SP release and SP is mediated through the NK-1R. Our results demonstrate a novel role of H2S in SP-NK-1R-related pathway in pancreatic acinar cells.
In conclusion, we have shown that caerulein up-regulates CSE gene expression and lead to enhanced H2S biosynthesis in acinar cells. Caerulein induced inflammation is attenuated by inhibition of CSE activity by PAG. These results suggest that H2S plays a role in caerulein-induced acinar cell inflammation and inhibition of H2S synthesis may be useful in the therapy of inflammation. An extended knowledge of the biological and molecular alterations linked to H2S and acute pancreatitis will open the way for new therapies for this life-threatening condition.
Acknowledgments
This work was supported by Biomedical Research Council Grant No. R-184-000-094-305 and Office of Life Sciences Cardiovascular Biology Research Program (Grant no. R184-000-074-712).
References
- 1.Bhatia M, Saluja AK, Hofbauer B, Frossard JL, Lee HS, Castagliuolo I, Wang CC, Gerard N, Pothoulakis C, Steer ML. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci USA. 1998;95:4760–65. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117–25. doi: 10.1002/(SICI)1096-9896(200002)190:2<117::AID-PATH494>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia M, Neoptolemos JP, Slavin J. Inflammatory mediators as therapeutic targets in acute pancreatitis. Curr Opin Investig Drugs. 2001;2:496–501. [PubMed] [Google Scholar]
- 4.Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets – Inf Allergy. 2002;1:343–51. doi: 10.2174/1568010023344517. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia M, Slavin J, Cao Y, Basbaum AI, Neoptolemos JP. Preprotachykinin-A gene deletion protects mice against acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2003;284:G830–6. doi: 10.1152/ajpgi.00140.2002. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome – an update. Med Chem Rev Online. 2004;1:25–6. doi: 10.2174/1568010023344517. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Path. 2004;202:145–56. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132–44. doi: 10.1159/000085265. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia M, Sidhapuriwala J, Moochhala SM, Moore PK. Hydrogen sulfide is a mediator of carrageenan-induced hindpaw edema in the rat. Br J Pharmacol. 2005;145:141–4. doi: 10.1038/sj.bjp.0706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatia M, Wong FL, Fu D, Law HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005;19:623–5. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia M, Zhi L, Zhang H, Moore PK. Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am J Physiol Lung Mol Physiol. 2006;291:L896–904. doi: 10.1152/ajplung.00053.2006. [DOI] [PubMed] [Google Scholar]
- 12.Chunyu Z, Junbao D, Dingfang B, Hui Y, Xiuying T, Chaoshu T. The regulatory effect of hydrogen sulfide on hypoxic pulmonary hypertension in rats. Biochem Biophys Res Commun. 2003;302:810–6. doi: 10.1016/s0006-291x(03)00256-0. [DOI] [PubMed] [Google Scholar]
- 13.Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M. Endothelial dysfunction in a murine model of mild hyperhomocysteinemia. J Clin Invest. 2000;106:483–91. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gosselin RE, Smith RP, Hodge HC. Clinical toxicology of commercial products. 5. Baltimore, USA: Williams and Wilkins Ltd; 1984. [Google Scholar]
- 15.Grady EF, Yoshimi SK, Maa J, Valeroso D, Vartanian RK, Rahim S, Kim EH, Gerard C, Gerard N, Bunnett NW, Kirkwood KS. Substance P mediates inflammatory oedema in acute pancreatitis via activation of the neurokinin-1 receptor in rats and mice. Br J Pharmacol. 2000;130:505–12. doi: 10.1038/sj.bjp.0703343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haines KA, Kolasinski SL, Cronstein BN, Reibman J, Gold LI, Weissmann G. Chemoattraction of neutrophils by substance P and transforming growth factor-beta 1 is inadequately explained by current models of lipid remodeling. J Immunol. 1993;151:1491–9. [PubMed] [Google Scholar]
- 17.Hosoki R, Matsiki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1991;237:527–31. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 18.Johnston M, Jankowski D, Marcotte P, Tanaka H, Esaki N, Soda K, Walsh C. Suicide inactivation of bacterial cystathionine gamma synthase and methionine gamma-lyase during processing of L-propargyl-glycine. Biochem. 1979;18:4690–701. doi: 10.1021/bi00588a033. [DOI] [PubMed] [Google Scholar]
- 19.Kodama H, Mikasa H, Sasaki K, Awata S, Nakayama K. Unusual metabolism of sulfur-containing amino acids in rats treated with DL-propargylglycine. Arch Biochem Biophys. 1983;225:25–32. doi: 10.1016/0003-9861(83)90003-6. [DOI] [PubMed] [Google Scholar]
- 20.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure, Anal Biochem. 1980;102:344–52. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 21.Lau HY, Wong FL, Bhatia M. A key role of neurokinin 1 receptors in acute pancreatitis and associated lung injury. Biochem Biophys Res Commun. 2005;327:509–15. doi: 10.1016/j.bbrc.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 22.Lau HY, Bhatia M. Effect of CP96,345 on the expression of tachykinins and neurokinin receptors in acute pancreatitis. J Pathol. 2006;208:364–71. doi: 10.1002/path.1899. [DOI] [PubMed] [Google Scholar]
- 23.Leach SD, Modlin IM, Scheele GA, Gorelick FS. Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of chole-cystokinin. J Clin Invest. 1991;87:362–6. doi: 10.1172/JCI114995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Bhatia M, Zhu YZ, Ramnath RD, Wang ZZ, Anuar FM, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysac-charide-induced inflammation in the mouse. FASEB J. 2005;19:1196–8. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 25.Maggi CA. The effects of tachykinins on inflammatory and immune cells. Regul Pept. 1997;70:75–90. doi: 10.1016/s0167-0115(97)00029-3. [DOI] [PubMed] [Google Scholar]
- 26.Mester A, Fraser PE, Tice SV. Enzymatic desulphuration of β-mercaptopyruvate to pyruvate. J Biol Chem. 1954;206:516–75. [PubMed] [Google Scholar]
- 27.Mok YYP, Shirhan M, Cheong YP, Wang ZZ, Bhatia M, Moochhala SM, Moore PK. Role of hydrogen sulfide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulfide biosynthesis. Br J Pharmacol. 2004;143:882–9. doi: 10.1038/sj.bjp.0706014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarra P, Dello Russo C, Mancuso C, Preziosi P, Grossman A. Gaseous neuromodulators in the control of neuroendocrine stress axis. Ann NY Acad Sci USA. 2000;917:638–46. doi: 10.1111/j.1749-6632.2000.tb05429.x. [DOI] [PubMed] [Google Scholar]
- 29.Patacchini R, Santicioli P, Giuliani S, Maggi CA. Hydrogen sulfide (H2S) stimulates capsaicin-sensitive primary afferent neurons in the rat urinary bladder. Br J Pharmacol. 2004;142:31–34. doi: 10.1038/sj.bjp.0705764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stipanuk MH, Beck PW. Characterization of the enzymatic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206:267–77. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thippeswamy T, McKay JS, Quinn JP, Morris R. Nitric oxide, a biological double-faced janus-is this good or bad? Histol Histopathol. 2006;21:445–58. doi: 10.14670/HH-21.445. [DOI] [PubMed] [Google Scholar]
- 32.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–8. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 33.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 34.Yan H, Du J, Tang C. The possible role of hydrogen sulfide on the pathogenesis of spontaneous hypertension in rats. Biochem Biophys Res Commun. 2004;313:22–7. doi: 10.1016/j.bbrc.2003.11.081. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf M, Tan BKH, Hsu A, Bhatia M, Moore PK. Streptozotocin-induced diabetes in the rat is associated with enhanced tissue hydrogen sulphide biosynthesis. Biochem Biophys Res Commun. 2005;333:1146–52. doi: 10.1016/j.bbrc.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Zhi L, Moore PK, Bhatia M. The role of hydrogen sulfide in cecal ligation and puncture induced sepsis in the mouse. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1193–201. doi: 10.1152/ajplung.00489.2005. [DOI] [PubMed] [Google Scholar]
- 37.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effects of endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–16. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]


