Abstract
Murine invariant natural killer T (iNKT) cells provide cognate and non-cognate help for lipid and protein-specific B cells, respectively. However, the long term B cell outcome following cognate iNKT help is currently unknown. We show that cognate iNKT cell help resulted in a B cell differentiation program characterized by extrafollicular plasmablasts, germinal center formation, affinity maturation and a robust primary IgG antibody response that was uniquely dependent on iNKT-derived interleukin 21 (IL-21). However, cognate iNKT cell help did not generate an enhanced humoral memory response. Thus, iNKT cell cognate help for lipid-specific B cells induces a unique signature which is a hybrid of classic T-dependent (TD) and T-independent type 2 (TI-2) B cell responses.
B cell responses fall into two general categories, T-dependent and T-independent. T-dependent responses require engagement of antigen through the B cell receptor (BCR) and cognate help from CD4+ T cells via major histocompatibility complex class II (MHCII)-restricted antigen presentation. B cell activation in this context results in either extrafollicular proliferation as plasmablasts or entry into germinal centers for subsequent memory or plasma cell development1. Extrafollicular plasmablasts cluster in the bridging channels and red pulp of the spleen and, although some class switch recombination may occur, these cells do not undergo affinity maturation. On the other hand, germinal center reactions occurring in the follicle involve class switch recombination, somatic hypermutation and affinity maturation, which produces higher affinity plasma and memory cells2. Both memory B cells and/or plasma cells are important for an enhanced memory response upon subsequent re-exposure to antigen.
T-independent responses by a B cell do not require any direct interaction with a T helper cell, and can be one of two subtypes- type 1 or type 2. T-independent type 1 responses result from B cell stimulation by ligands that activate without engaging the BCR, such as the Toll-like receptor (TLR) ligands lipopolysaccharide (LPS) and CpG. T-independent type 2 responses involve ligands that engage the BCR with multivalent epitopes such as polysaccharides or 4-Hydroxy-3-nitrophenylacetyl (NP)-Ficoll. Both types of T-independent ligands stimulate an innate-like response that is more transient than the T-dependent response and does not lead to an enhanced recall response. T-independent responses generally stimulate extrafollicular foci rather than germinal centers, do not generate antibodies with enhanced affinity, and produce few plasma cells and atypical memory cells1.
Well characterized T-dependent B cell responses to protein antigen depend on conventional CD4+ T cells. However, previous studies by our lab and others have described a subset of T cells, invariant natural killer T (iNKT) cells, which also provide help for B cells3, 4. Murine iNKT cells express a restricted T cell receptor repertoire comprised of the Vα14-Jα18 alpha chain paired with Vβ8.2, Vβ7 or Vβ2 TCRβ chains5. The iNKT cell invariant T cell receptor (TCR) recognizes CD1d, a β2m-associated non-polymorphic antigen presenting molecule expressed primarily on professional antigen presenting cells (APCs) such as dendritic cells (DCs), monocytes and B cells but also on other cells such as T cells and hepatocytes6, 7. The CD1 family of antigen presenting molecules is unique in that they have deep hydrophobic channels on their surface which are capable of binding and presenting lipid molecules to T cells. A number of bacterial CD1d ligands have been identified8, but the most well-studied ligand has been alpha-galactosylceramide (α−GalCer), a glycosphingolipid isolated from marine sponges that is now available in synthetic form. α−GalCer binds CD1d with high affinity and rapidly activates nearly all iNKT cells to proliferate and simultaneously secrete high amounts of TH1 and TH2-type cytokines. Like other innate-type cells, iNKT cells exist in a pre-activated state with higher expression of activation markers CD44, CD69 and CD25 on their surface, and have a reduced activation threshold compared to naïve adaptive CD4+ T cells9, 10. As such, iNKT cells can regulate and activate a myriad of different cell types (macrophages, DCs, B cells and T cells) early during infection and play an important role in defense against a number of bacterial, parasitic and autoimmune diseases8. A role for iNKT cells in production of antibodies important for defense against infection is most commonly demonstrated through comparison of live infection in intact versus CD1d- or iNKT-deficient mice. This approach has characterized a role for iNKT cells in production of anti-pathogen responses in Borellia hermsii11, 12, Streptoccocus pneumonia13, and plasmodium falciparum14 and has implicated marginal zone B cells as a likely partner for iNKT cells in the spleen3, 12, 15, 16.
Activated iNKT cells have been appreciated to have a role as both cognate and non-cognate helpers of lipid and peptide-specific B cells. Non-cognate studies have characterized an adjuvant-like effect of coadministering α−GalCer with haptenated proteins or influenza virus peptides17, 18. iNKT cell non-cognate help for protein-reactive B cells has been shown to lead to humoral memory responses, plasma cell development, affinity maturation and long term maintenance of antibody responses17, 18. While cognate iNKT help has been demonstrated for B cells3, the B cell outcome following cognate help is currently unknown. Our results show that cognate iNKT cell help for lipid antigen-specific B cells induced a robust primary IgG antibody response characterized by early extrafollicular plasmablast formation, germinal centers, antibody affinity maturation and a dependency on iNKT-derived IL-21. However, cognate iNKT cell help failed to drive classical T-dependent aspects of humoral responses, including humoral memory response and antigen-specific antibody forming cell (AFC) expansion. We propose that cognate iNKT help to B cells induces a constellation of traits that is representative of an entirely new class of B cell response: a T-dependent type 2 response.
Results
Induction of extrafollicular foci and germinal centers
To determine whether iNKT cell help for lipid and protein antigens induces similar B cell differentiation patterns, we first compared the extrafollicular plasmablast response at five days after immunization. Antigen-specific extrafollicular foci in the red pulp and bridging channels of the spleen were visualized by confocal microscopy. These splenic architectural structures were identified as clusters of cells which bound fluorescently tagged NP (NP-APC) and expressed the plasmablast marker CD138. Mice immunized with a haptenated lipid antigen, NP−α−GalCer (Supplementary Figure 1), or a haptenated protein antigen mixed with lipid adjuvant, NP-KLH + α−GalCer, developed numerous CD138+, NP-APC+ cells clustered in small groups in extrafollicular T cell areas of the spleen (Fig. 1a,b). Control immunized mice developed only a few NP-specific CD138+ foci in their red pulp or bridging channels following immunization with NP-KLH using alum as adjuvant (Fig. 1c), whereas no NP-APC+ foci developed when BSA was administered in PBS-DMSO (Fig. 1d). Flow cytometry analysis revealed that the NP-specific IgD−B220loCD138+ plasmablast B cell population had notably expanded in all immunized groups, but this population was substantially higher in the NP−α−GalCer group (Fig. 1e,f). In addition, ELISpot analysis showed that spleens from NP−α−GalCer, but not NP-KLH + α−GalCer, immunized mice contained a significantly increased number of B cells producing NP-specific IgG (Fig. 1g) despite similar increases in iNKT cell numbers (Fig. 1h). These results indicate that cognate iNKT cell help to B cells results in a robust early plasmablast expansion typical of the splenic response to T-independent antigens such as NP-Ficoll.
Figure 1. Cognate (lipid) and non-cognate (lipid plus protein) antigen stimulation of B cells induces splenic extrafollicular foci.
(a-d) 7 μm sections of OCT-embedded spleens from B1-8 Tg mice were labeled with anti-CD19 (green), anti-CD138 (red), NP-APC (blue) to identify CD138+ NP-specific plasmablasts. High resolution, knitted images of these spleen sections were captured by confocal microscope 5 days after immunization with 5 μg NP−α−GalCer (a) 100 μg NP-KLH + 5 μg α−GalCer (b), 100 μg NP-KLH + alum (c), or PBS-DMSO (d). Data are representative from 3-4mice per group in 2 independent experiments; scale bar = 100μm. (e, f) Flow cytometry analysis of splenic NP-specific IgD−, B220loCD138+ plasmablast B cells from identically immunized WT C57BL/6 mice in representative plots (e) and as a summary (f). (g) ELISPOT enumeration of NP-specific IgG secreting splenic B cells. (h) the number of TCRβ+CD1dtet+ iNKT cells from the same mice as determined by flow cytometry. Each symbol represents an individual mouse; representative (g) or pool (f, h) of 5 mice per group from 2 independent experiments; bar = mean. Unpaired two-tailed t-tests were performed for large data sets [f, i], Mann-Whitney tests were performed for small data set [h] *P≤0.05,**P≤0.001
Next, we compared germinal center formation in the spleen following lipid or lipid plus protein immunization. Twelve days into the response, immunofluorescent labeling showed that both NP−α−GalCer and NP-KLH + α−GalCer immunized B1-8 mice, in which ~5% of B cells express a BCR transgene specific for NP, developed frequent GL7+ cell clusters, a marker labeling germinal center B cells, (Fig. 2a,b) in a manner similar to NP-KLH + alum immunized mice (Fig. 2c). Specifically, there was an average of 24 GCs per spleen section in NP-KLH + α−GalCer immunized mice and 21 GCs per spleen section in NP−α−GalCer immunized mice (Fig. 2e), a notable but not significant difference. PBS-DMSO immunized mice had low background number of GCs, approximately 10 per spleen section (Fig. 2d,e). Further investigation by flow cytometry revealed that all experimental groups of immunized C57BL/6 WT mice had an increase in the number and frequency of NP-specific germinal center B cells (B220+CD95+GL7+), but were significantly more numerous in the NP-KLH + α−GalCer immunized mice compared to the NP−α−GalCer immunized mice (Fig. 2f). The number of splenic CD1d tet+ iNKT cells increased similarly, showing greater expansion following lipid plus protein, but not lipid only, immunization (Fig.2 i). These results suggest that when iNKT cells, recognizing the lipid component of NP−α−GalCer,provide cognate help to NP-specific B cells, the B cells are induced to produce germinal centers, although they were smaller than GCs derived following noncognate iNKT help. Consistent with these data, the number of NP-specific IgG-producing cells was significantly greater in both protein-immunized groups compared to lipid-immunized animals at this later time point (Fig. 2h). Of note, we also observed increases in NP-specific CD38+IgD− memory-phenotype B cells in all antigen-immunized groups at day 12 (Fig. 2g), but differences between the groups were not significant. Thus, non-cognate iNKT help seems to enhance ongoing protein-specific B cells undergoing a classical germinal center-dependent response. In contrast, cognate iNKT help recruited by a lipid-only immunization strategy induces smaller germinal centers and is unable to sustain antigen-specific B cell expansion and antibody production.
Figure 2. Cognate (lipid) and non-cognate (lipid plus protein) antigen stimulation of B cells leads to development of splenic germinal centers.
(a-d) 7 μm sections of OCT-embedded spleens from B1-8 transgenic mice were labeled with GL-7 (green), anti-CD3 (blue), and anti-CD19 (red). High resolution, knitted images of these spleen sections were captured by confocal microscope and represent duplicate sections of 4 mice/group collected 12 days after immunization with 5 μg NP−α−GalCer (a), 100 μg NP-KLH + 5 μg α−GalCer (b), 100 μg NP-KLH (c), and PBS-DMSO (d). (4mice/group, representative of 2-3 independent experiments) scale bar=500μm. Images enabled determination of total number of GL7+ GCs per spleen section (e). (f, g) Flow cytometry analysis of day 12 spleen cells from identically immunized C57BL/6 WT mice was used to quantitate CD19+CD95+GL7+ gerinal cell numbers per spleen (f), and IgD− CD38+NP-specific B cells (g). (h)ELISPOT analysis of NP-specific IgG secreting splenic B cells from WT C57BL/6 mice. (i) TCRβ+CD1dtet+ iNKT cells were also enumerated by flow cytometry. Each symbol represents an individual mouse; bar = mean. 4-5 mice per group from representative (f, h, i) or pool (e,g) of 2-3 independent experiments. Mann-Whitney test * P≤0.05,**P≤0.001.
Induction of B cell receptor affinity maturation
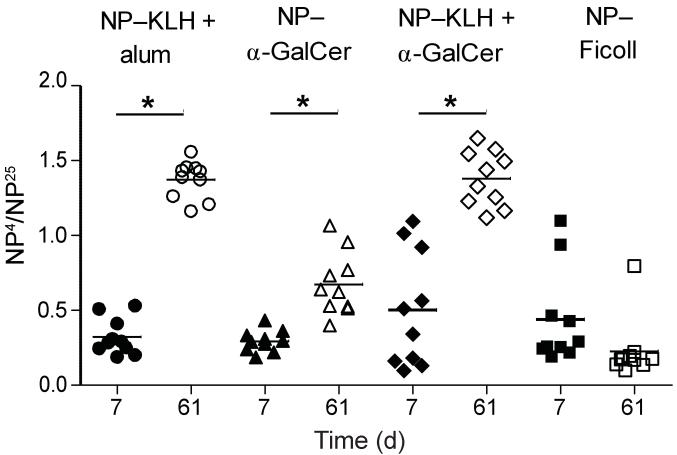
Germinal centers provide an environment for B cell maturation which enables selection for higher affinity B cell receptors19. Given that both non-cognate and cognate lipid antigens stimulate the formation of germinal centers, we next investigated antibody affinity maturation driven by both forms of lipid antigen immunization. We used a standard ELISA assay assessing the ratio of serum antibody binding to sparsely versus highly haptenated proteins20. In comparing serum from mice collected seven days after a primary immunization (day 7) to serum from the same mice collected seven days after a secondary boost (day 61), we found that both cognate and non-cognate lipid antigens induced a significant increase in antibody affinity following a boost immunization (Fig. 3). As expected, a known T-dependent antigen, NP-KLH in alum, induced antibodies with higher affinity after secondary boost, while a well-described T-independent antigen, Ficoll haptenated with 68 molecules of NP [NP68-Ficoll], failed to induce significant affinity maturation (Fig. 3). Thus, just as both forms of iNKT cell help, cognate and non-cognate, stimulate germinal centers, they also both induce B cell receptor affinity maturation.
Figure 3. Cognate and non-cognate iNKT cell help both induce antigen-specific antibody affinity maturation.
Sera was collected 7 days after primary challenge and 7 days after secondary boost (day 61) from C57BL/6 WT mice immunized with 0.5 μg NP−α−GalCer, 100 μg NP-KLH/ 0.5 μg α−GalCer, 100 μg NP-KLH + alum, or 30 μg NP68-Ficoll and tested by ELISA on plates coated with NIP5BSA vs NIP15BSA. Affinity maturation is expressed as ratio of NP4/NP25 binding as assessed by O.D. Each symbol represents an individual mouse; bar = mean. 8-10 mice per group, representative of 3 independent experiments. * P≤0.0001.
IL-21 is critical component of iNKT help for B cells
T follicular helper cells have been well described to enter the B cell follicle specifically to provide cognate help for protein-specific B cells21, 22. Interestingly, mature iNKT cells have been reported to share many of the same characteristics of traditional protein-specific TFH23 . That is, they migrate in response to the chemokine CXCL13 (via CXCR5) but not CCL21 (via CCR7)24, express ICOS (data not shown), and secrete IL-21 (ref. 25). To determine if iNKT cells provide B cell help similarly to protein-specific T follicular helper cells, we assessed the importance of IL-21and IL-21 receptor (IL-21R) signaling for cognate lipid-specific and non-cognate lipid enhanced antibody responses in this system. IL-21R-deficient mice and wild-type mice were separately immunized with lipid (NP−α−GalCer), protein (NP-KLH in alum), and protein plus lipid antigens (NP-KLH + α−GalCer). In all three cases early NP-specific IgG antibodies were reduced in IL-21R-deficient mice compared to wild-type mice (Fig. 4a). As a negative control, intraperitoneal (IP) administration of the T-independent antigen, NP-Ficoll, induced IL-21-independent IgG, with no differences between IL-21R deficient and wild-type mice. In all groups tested, there were no consistent differences in the anti-NP IgM titers between IL-21R-deficient and wild-type mice (Fig. 4b). These data suggest that IL-21 is required for antibody class switch, not merely antibody production. IL-21 expression by iNKT cells was confirmed by real-time RT-PCR at early (day 5-7) and later (day 11-13) time points after administration of 0.5 μg NP−α−GalCer (Fig. 4c).
Figure 4. IL-21 receptor signaling is required for cognate iNKT cell-mediated anti-NP antibody responses.
(a, b) C57BL/6 WT and Il21r deficient mice were immunized with 0.5 μg NP−α−GalCer, 100 μg NP-KLH + 0.5 μg α−GalCer, 100 μg NP-KLH + alum, or 30 μg NP(68)-Ficoll. Mice were bled on days 0, 7, 14 and 21 and assessed for IgG (a) and IgM (b) anti-NIP titers. (Summary of 2-5 independent experiments with 3-5 mice per group in each experiment; mean and s.e.m.) * p≤0.05 **P≤0.001 for comparisons between WT and IL-21R-KO pairs. (c) TCRβ+CD19−CD1d tetramer–positive iNKT cells were sorted by flow cytometry from Vα14 transgenic mice at 1 day, 1 week (days 5-7), and 2 weeks (days 11-13), following IP immunization with 0.5 μg NP−α−GalCer per mouse or DMSO. Real-time RT-PCR was performed to assess IL-21 mRNA expression in iNKT cells. (data shown are pooled from 2-3 experiments and include 5-11 mice per group; mean and s.e.m.) **P≤0.001
Next we asked whether iNKT cells were the critical source of IL-21. To address this question, mixed bone marrow chimeras were generated in which we selectively deleted the Il21 gene in iNKT cells. The experimental chimeras were created by reconstituting irradiated Jα18−/− hosts with a mixture of 25% Il21−/− bone marrow and 75% Jα18−/− bone marrow. Controls with IL-21-sufficient iNKT cells were created by using Jα18−/− hosts reconstituted with a mix of 25% wild-type and 75% Jα18−/− bone marrow. Immunizing these mice with NP-KLH in alum, NP-KLH + α−GalCer or the lipid NP−α−GalCer revealed that only cognate iNKT cell help depends entirely on iNKT-derived IL-21. Specifically, chimeric animals containing IL-21-deficient iNKT cells showed a reduction in NP-specific IgG at all time points examined compared to chimeras with IL-21-sufficient iNKT cells only when immunized with the cognate iNKT antigen, NP−α−GalCer (Fig. 5). Thus, non-cognate iNKT cell help elicited by NP-KLH + α−GalCer requires IL-21, but it does not need to come from iNKT cells.
Figure 5. iNKT-produced IL-21 is required for cognate lipid antigen help.
(a,b,c) Mixed bone marrow chimeras with an iNKT cell compartment capable (WT) or incapable of producing IL-21 (Il21-KO) were immunized with 0.5 μg NP−α−GalCer, 100 μg NP-KLH + 0.5 μg α−GalCer, or 100 μg NP-KLH + alum. Mice were bled on day 8 (a), day 14 (b), and day 21 (c) and assessed for anti-NIP IgG titers. (Summary of 2 independent experiments, with 3-5 mice per group in each experiment; mean and s.e.m.) * P≤0.05 for comparisons between WT and Il21 deficient pairs.
Cognate vs non-cognate humoral memory responses
Given the fact that cognate iNKT cell help results in germinal center formation and affinity-matured antibody responses, we next asked whether cognate iNKT help can generate B cell memory responses comparable to non-cognate iNKT help. To address this question, wild-type C57BL/6 mice were immunized intraperitoneally with either NP−α−GalCer or NP-KLH + α−GalCer. Using classical CD4+ T cell help as a positive control, some mice were immunized with NP-KLH in alum. Following primary immunization, animals were rested 177 days to let the initial hapten-specific antibody response wane, then boosted with a secondary challenge of lipid antigen or protein antigen in PBS. As expected, the anti-NP response following NP-KLH in alum immunization and NP-KLH + PBS (NP-KLH + alum; NP-KLH in PBS) boost showed a clear memory response (Fig. 6a). That is, the anti-NP titers following the boost were much higher than the antibody titers that resulted from the initial primary challenge. Titers following the boost were also much higher than the antibody titers in age-matched mice receiving their primary protein antigen in alum challenge on day 177 (PBS; NP-KLH + alum)(Fig. 6a). Mice that received NP−α−GalCer in PBS as a primary challenge and again as a secondary boost (NP−α−GalCer; NP−α−GalCer) developed the same antibody titer following boost as mice receiving a primary NP−α−GalCer challenge at d177(Fig. 6b). We found similar results using a shorter delay between challenges (46 rather than 177 days) and boosting via an intravenous (i.v.) route (Fig. 7a,b), a protocol more commonly used to demonstrate anti-protein memory responses. The response to lipid antigen was most similar to the anti-NP response generated by the T-independent antigen, NP-Ficoll (Fig. 7b). Similar results were obtained following challenge and boost with higher doses (5 μg/mouse) of lipid antigen, indicating that antigen availability was not a confounding factor of our studies (Supplementary Figure 2). Taken together, these data are consistent with published reports demonstrating that the humoral memory response to protein immunization is the same whether the adjuvant used is alum or the lipid α−GalCer. However, we find that responses to a haptenated lipid antigen, despite a robust primary antibody response, fail to generate a memory B cell antibody response.
Figure 6. Only non-cognate iNKT cell help induces antibody memory response after day 177 rechallenge.
(a,b) C57BL/6 WT mice were immunized IP on day 0 with PBS + alum, PBS, or 2.2 μg NP-KLH + alum (a) or 0.5 μg NP−α−GalCer (b). Mice were bled periodically up to day 166. Mice then received a secondary boost IV on day 177 with 2.2 μg NP-KLH + PBS (or IP with alum as noted), 0.5 μg NP−α−GalCer antigen, or PBS and were bled again on days 3, 7, and 14 post-boost. Legend indicates primary challenge; secondary boost. Concentration of NP-specific IgG was determined by anti-NIP5 ELISA. (One experiment with 10 mice per group; mean ± s.e.m.) * P≤0.05, **P≤0.001 as compared to PBS; NP-KLH + alum group (□). For full time-course see Supplementary Fig. 3.
Figure 7. Only non-cognate iNKT cell help induces antibody memory response after day 46 rechallenge.
(a,b) C57BL/6 WT mice were immunized IP on day 0 with 2.2 μg NP-KLH in alum, 2.2 μg NP-KLH plus 0.5 μg α−GalCer (a), 0.5 μg NP−α−GalCer, or 30 μg NP(68)-Ficoll (b) and were bled periodically up to day 45. Mice then received a secondary boost IP on day 46 with the same doses of NP-KLH (±alum), NP-KLH + α−GalCer (a), NP−α−GalCer, NP68-Ficoll or PBS (b) and were bled again on days 3, 7, 14, and 29 post-boost. Key indicates primary challenge; secondary boost. Concentration of NP-specific IgG was determined by anti-NIP5 ELISA. (One experiment with 10mice per group; mean ± s.e.m.) * P≤0.05, **P≤0.001 as compared to relevant primary immunization group (□ or ○). For full time-course see Supplementary Fig. 4.
Discussion
These studies clearly find that cognate and non-cognate iNKT cell help for B cells lead to very different B cell outcomes. We demonstrate that following both cognate and non-cognate lipid immunization, mice generate strong primary IgG anti-NP antibody responses characterized by early extrafollicular foci and later germinal centers which are dependent on IL-21 receptor signals. However, B cells receiving iNKT non-cognate help make an increased humoral memory response upon rechallenge, while those B cells receiving cognate iNKT cell help make a secondary response that is of the same magnitude as a primary response. Our results are consistent with other studies of non-cognate iNKT cell help17, 18 and support the idea that iNKT cells are memory-like innate lymphocytes capable of stimulating a rapid, robust response from the time of their initial activation.
In the context of the cognate help studies, iNKT cells may be functioning as a novel TFH population, specializing in helping lipid-specific B cells to generate germinal center responses. In response to immunization with protein antigens, IL-21 from conventional TFH cells acts directly on germinal center B cells to support plasma cell differentiation26-28. Data from our bone marrow chimera studies demonstrate that iNKT cells provide cognate lipid-specific T cell help by production of IL-21. Thus, iNKT cells are capable of functioning, in part, as iNKTFH cells. iNKT cells express many of the same surface co-stimulatory molecules as TFH cells (for example, CD40L and ICOS)29, 30, but, as we show here, differ in their ability to generate a memory B cell population.
Our imaging studies find that both the cognate iNKT antigen, NP−α−GalCer, and the non-cognate mix of NP-KLH plus α−GalCer stimulate similar antigen-specific extrafollicular foci and numbers of germinal centers. However, the B cell outcome of cognate help from an iNKT cell is notably different than the outcome following help from a conventional CD4+ T cell that is benefiting from enhanced APC function secondary to iNKT cell activation. In part, iNKT help for cognate haptenated-lipid does not entirely mimic extrafollicular foci-dominated responses to T-independent antigens, but rather reflects a more T-dependent extrafollicular foci and GC-based response. The non-cognate immunization approach using NP-KLH + α−GalCer does stimulate higher numbers of germinal center B cells, as compared to a strict lipid immunization, but results in similar numbers of total GCs. This finding is consistent with our observation that spleens from NP-KLH + α−GalCer immunized mice have relatively larger GCs suggesting, in this case, that iNKT cell activation functions more as an adjuvant than stimulating a lipid-specific response in parallel Given that NP−α−GalCer and NP-KLH + α−GalCer groups both induce affinity maturation (likely via GCs) and have similar numbers of memory phenotype CD38+ antigen-specific B cells31, it is possible that these germinal centers are present but inadequate or prematurely involute. Recently, a number of elements have been identified as critical to sustaining a T-dependent B cell germinal center response, including Dock8 expression in B cells, B:T immune synapse formation, and integrin signaling32. The quality and/or quantity of a BCR signal can be a critical aspect of GC persistence, particularly later in a germinal center response when the way in which antigen is presented, such as in the form of immune complexes, may be different33. PD-1 expression on T cells has also been shown to be important for GC B cell survival and subsequent long-lived plasma cell output without affecting affinity maturation34. Heterogeneity in memory B cell development may also impact the humoral recall response35. The contribution of these factors to iNKT-B cell interactions and their relevance to iNKT cell-induced germinal center formation remain to be determined.
Finally, iNKT cells have been proposed to localize outside of the splenic white pulp under homeostatic conditions36. Thus, how iNKT cell-B cell interactions occur in situ is not clear. One intriguing possibility supported by a recent study may be that iNKT cells preferentially interact with MZ B cells37. Marginal zone B cells are situated along the marginal sinus at the primary entry point into the spleen of blood-borne particulate antigens. This positions them to specialize in responding to T-independent type 2 antigens38. Marginal zone B cells express abundant CD1d and secrete predominantly IgM and IgG3 (ref. 39), the antibody isotypes produced in response to the pure synthetic lipid antigen, NP−α−GalCer3. As such, they may also be one of the primary B cell subpopulations to receive both cognate and non-cognate iNKT cell help16.
In conclusion, we propose lipid-specific, T-dependent type 2 as a new subcategory of B cell antigen-specific responses which is a hybrid of the other three established categories of B cell antigens. According to our assessment, the characteristics of T-dependent type 2 responses include extrafollicular foci and GC formation accompanied by affinity maturation and absent functional humoral memory responses. This category of antigen-specific responses remains to be characterized in the context of live infection, but may be playing an important role early in infection when rapid iNKT cell help could provide a unique advantage. Work in progress is investigating cognate and non-cognate lipid antigen-induced humoral immunity to live pathogens.
Methods
Mice
C57BL/6 WT, B6.SJL B1-8hi (provided by M. Nussenzweig, Rockefeller Univ., NY, NY), C57BL/6 Vα14 Tg (created by A. Bendelac at Univ. of Chicago, Chicago, IL; provided by M. Exley, Dana Farber Cancer Institute, MA) and C57BL/6 Jα18−/− mice (M. Taniguchi, RIKEN, Japan; provided by M. Exley) were housed and bred at Dana Farber Cancer Institute (Boston, MA) according to DFCI animal care and use committee standards and at the Trudeau Institute (Saranac Lake, NY) according to Trudeau ACUC standards. C57BL/6 B1-8 Ig Cre/ knock-in (generated by K. Rajewsky, Center for Blood Research, Boston, MA) were bred and housed at Yale University School of Medicine according to Yale ACUC standards. C57BL/6 Il21r-deficient mice were generated as described previously40, and Il21-deficient mice were provided by M. Rincon (University of Vermont; Burlington, VT). All live animal experimental protocols were approved by Dana Farber Cancer Institute IACUC or Trudeau Institute ACUC committees.
Flow cytometry
Single-cell suspensions were prepared from the spleen and stained with the following monoclonal antibodies for flow cytometric analysis: IgD-AF450 (11-26c.2a), B220-PE-Cy7 (RA3-6B2, Biolegend), Fas-PE (Jo2), GL7-FITC, CD38-FITC (90), CD138-PE (281-2), IgG1-Biotin (A85-1), streptavidin-allophycocyanin (APC)-Cy7 (BD) and TCRβ-Pacific Blue (H57-597, Biolegend). iNKT cells were identified using mCD1d−α−GalCer tetramers (PBS57, National Institutes of Health) conjugated to APC; Samples were acquired on a FACSCanto II and were analyzed with FlowJo software (Tree Star, Inc.).
Bone marrow chimeras
Recipient C57BL/6 Jα18−/− mice were irradiated 2 × 500 rads and allowed to rest a few hours or overnight. The recipient mice were reconstituted with 5×106 bone marrow cells. The donor bone marrow included either 75% Jα18−/− BM mixed with 25% wild-type BM or 75% Jα18−/− BM mixed with 25% Il21−/− BM. These reconstitution mixtures resulted in mice whereby only the iNKT cells were deficient in IL-21 production or all cells were normal. Reconstitution of iNKT, T and B cell lineages in the spleen and liver were confirmed by flow cytometry analysis after 7 weeks.
Antigens, Immunization, Serum collection
NP-KLH, NP-BSA, KLH, NP68-Ficoll (Biosearch Technologies Inc), N−α−GalCer, and α−GalCer (synthesized by N. Veerapen and G. Besra at Univ. of Birmingham, UK as previously described3) were administered intraperitoneally (IP) in 200 μl unless otherwise noted. Immunizations contained 2.2 μg or 100 μg protein precipitated in alum or suspended in PBS + 0.1% BSA, or 0.5-5 μg lipid antigen solubilized in ≤0.25% DMSO, then suspended in PBS with 0.1% BSA. NP-KLH in these studies contains 20 NP haptens per KLH protein, and NP−α−GalCer lipid contains a single hapten per α−GalCer molecule. In serum, the protein is most likely monomeric, while the lipid is more likely micellular or bound to a lipid binding protein, making equal comparisons of molar quantities/hapten quantities challenging. 2.2 μg NP-KLH is the molar equivalent of 0.5 μg NP−α−GalCer divided by the number of haptens on the KLH protein (20). Serum was collected retro-orbitally or sub-mandibularly and was stored at −20°C until assessment by ELISA.
ELISA
Sera was assessed for NP-specific IgG and IgM by heteroclitic NIP-specific ELISA as previously described3. Affinity was assessed by ELISA on plates simultaneously coated with NP4-BSA or NP25-BSA (expressed as a ratio of NP4/NP25 binding).
ELISPOT
Eight half-log serial dilutions of primary spleen cell suspensions from C57BL/6 mice were cultured in duplicate overnight at 37°C on NIP15-BSA coated, 1%PBS + BSA blocked, Multiscreen-HA ELISPOT plates (Millipore). Spots were detected with anti-mouse IgG-HRP (Southern Biotech) and developed with AEC staining kit (Sigma). Spots were scanned and counted on an Immunospot analyzer (CTL Analyzers LLC).
Real-time RT-PCR
TCRβ+ CD1d tetramer+ CD19− iNKT cells were isolated from Vα14 transgenic mice using a BD Influx high speed cell sorter. RNA was extracted from Trizol fixed cells using a Qiagen RNeasy mini kit according to manufacturer instructions. cDNA was produced using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) plus RNase inhibitors. Real-time RT-PCR on cDNA samples was performed using primers and probes purchased from Applied Biosystems and the TaqMan 7500 Fast System and software (Applied Biosystems). Fold expression was calculated using the ΔΔCT method and GAPDH as a reference gene.
Confocal fluorescent microscopy
7 μm sections of OCT embedded frozen spleens were labeled with anti-CD19-FITC (1D3) plus anti-FITC-Alexa 488, anti-CD138 PE (281-2), rabbit anti-CD3 (145-2C11) plus anti-rabbit Alexa 647, anti-CD19-PE, anti-GL7-FITC plus anti-FITC-Alexa488 (BD Pharmingen), and NP-APC. NP-APC was conjugated as previously reported41. Fluorescent images were taken on a Nikon TE2000-U inverted microscope with a C1 Plus Confocal System (Partners Confocal Microscopy Core) and a Zeiss Axiovert 200M fluorescence microscope (Trudeau Institute). Final stitched high resolution whole spleen confocal images were taken on a Leica TCS SP5 Confocal microscope using LAS AF 2.2.1 software (Trudeau Institute).
Statistics
GraphPad PRISM 5 software was used to perform non-parametric two-tailed t-tests for normally distributed data sets with ≥10 samples. Two tailed non-parametric Mann Whitney tests were used for smaller data sets, where normality could not be determined.
Supplementary Material
Acknowlegements
We acknowledge the NIH Tetramer Core for generously providing murine CD1d-PBS57 and murine CD1d-unloaded tetramers. We are grateful to M. Rincon (University of Vermont) for providing IL-21 deficient mice. Supported by Trudeau Institute new investigator funds (EL) and NIH (AI028973-23 MB, AI063428-06 MB, and T32 A1049823-10 IK), J. Bardrick (GSB), The Royal Society (GSB). The Wellcome Trust (084923/B/08/2 to GSB) and The Medical Research Council (GSB).
References
- 1.MacLennan IC, et al. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 2.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 3.Leadbetter EA, et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci U S A. 2008;105:8339–8344. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barral P, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci U S A. 2008;105:8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 6.Roark JH, et al. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J Immunol. 1998;160:3121–3127. [PubMed] [Google Scholar]
- 7.Mandal M, et al. Tissue distribution, regulation and intracellular localization of murine CD1 molecules. Mol Immunol. 1998;35:525–536. doi: 10.1016/s0161-5890(98)00055-8. [DOI] [PubMed] [Google Scholar]
- 8.Cohen NR, Garg S, Brenner MB. Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 9.Bendelac A, Matzinger P, Seder RA, Paul WE, Schwartz RH. Activation events during thymic selection. J Exp Med. 1992;175:731–742. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uldrich AP, et al. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar H, Belperron A, Barthold SW, Bockenstedt LK. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J Immunol. 2000;165:4797–4801. doi: 10.4049/jimmunol.165.9.4797. [DOI] [PubMed] [Google Scholar]
- 12.Belperron AA, Dailey CM, Bockenstedt LK. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J Immunol. 2005;174:5681–5686. doi: 10.4049/jimmunol.174.9.5681. [DOI] [PubMed] [Google Scholar]
- 13.Kobrynski LJ, Sousa AO, Nahmias AJ, Lee FK. Cutting edge: antibody production to pneumococcal polysaccharides requires CD1 molecules and CD8+ T cells. J Immunol. 2005;174:1787–1790. doi: 10.4049/jimmunol.174.4.1787. [DOI] [PubMed] [Google Scholar]
- 14.Schofield L, et al. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 15.Bialecki E, et al. Role of marginal zone B lymphocytes in invariant NKT cell activation. J Immunol. 2009;182:6105–6113. doi: 10.4049/jimmunol.0802273. [DOI] [PubMed] [Google Scholar]
- 16.Muppidi JR, et al. Cannabinoid receptor 2 positions and retains marginal zone B cells within the splenic marginal zone. J Exp Med. 2011 doi: 10.1084/jem.20111083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galli G, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devera TS, Shah HB, Lang GA, Lang ML. Glycolipid-activated NKT cells support the induction of persistent plasma cell responses and antibody titers. Eur J Immunol. 2008;38:1001–1011. doi: 10.1002/eji.200738000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 20.Herzenberg LA, Black SJ, Tokuhisa T. Memory B cells at successive stages of differentiation. Affinity maturation and the role of IgD receptors. J Exp Med. 1980;151:1071–1087. doi: 10.1084/jem.151.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breitfeld D, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaerli P, et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston B, Kim CH, Soler D, Emoto M, Butcher EC. Differential chemokine responses and homing patterns of murine TCR alpha beta NKT cell subsets. J Immunol. 2003;171:2960–2969. doi: 10.4049/jimmunol.171.6.2960. [DOI] [PubMed] [Google Scholar]
- 25.Coquet JM, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 26.King IL, Mohrs K, Mohrs M. A nonredundant role for IL-21 receptor signaling in plasma cell differentiation and protective type 2 immunity against gastrointestinal helminth infection. J Immunol. 2010;185:6138–6145. doi: 10.4049/jimmunol.1001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linterman MA, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zotos D, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 30.Hayakawa Y, et al. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J Immunol. 2001;166:6012–6018. doi: 10.4049/jimmunol.166.10.6012. [DOI] [PubMed] [Google Scholar]
- 31.Ridderstad A, Tarlinton DM. Kinetics of establishing the memory B cell population as revealed by CD38 expression. J Immunol. 1998;160:4688–4695. [PubMed] [Google Scholar]
- 32.Randall KL, et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol. 2009;10:1283–1291. doi: 10.1038/ni.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinuesa CG, Linterman MA, Goodnow CC, Randall KL. T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol Rev. 2010;237:72–89. doi: 10.1111/j.1600-065X.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 34.Good-Jacobson KL, et al. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204:2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stetson DB, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muppidi JR, et al. Cannabinoid receptor 2 positions and retains marginal zone B cells within the splenic marginal zone. J Exp Med. 2011;208:1941–1948. doi: 10.1084/jem.20111083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cyster JG. B cells on the front line. Nat Immunol. 2000;1:9–10. doi: 10.1038/76859. [DOI] [PubMed] [Google Scholar]
- 39.Lopes-Carvalho T, Foote J, Kearney JF. Marginal zone B cells in lymphocyte activation and regulation. Curr Opin Immunol. 2005;17:244–250. doi: 10.1016/j.coi.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Ozaki K, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 41.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.