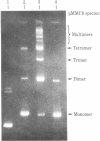
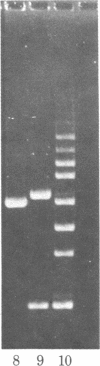
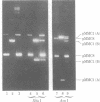
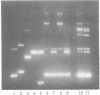
Abstract
The FLP gene of the yeast 2-microns plasmid is involved in a site-specific recombination event that results in the inversion of a set of sequences within the plasmid. This gene has been cloned and expressed in Escherichia coli. Expression of the FLP gene results in efficient recombination within the bacterial cell, which is specific for plasmids containing at least one 2-microns plasmid recombination site. This work demonstrates that (i) FLP protein is actively involved in 2-microns plasmid recombination; (ii) no other factors specific to yeast are required for the reaction; (iii) FLP protein acts efficiently in trans; (iv) FLP protein will promote site-specific insertion and deletion reactions in addition to the inversion reaction; and (v) FLP-promoted recombination is not dependent upon any DNA structural features unique to yeast chromatin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broach J. R., Guarascio V. R., Jayaram M. Recombination within the yeast plasmid 2mu circle is site-specific. Cell. 1982 May;29(1):227–234. doi: 10.1016/0092-8674(82)90107-6. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Hicks J. B. Replication and recombination functions associated with the yeast plasmid, 2 mu circle. Cell. 1980 Sep;21(2):501–508. doi: 10.1016/0092-8674(80)90487-0. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Falco S. C., Li Y., Broach J. R., Botstein D. Genetic properties of chromosomally integrated 2 mu plasmid DNA in yeast. Cell. 1982 Jun;29(2):573–584. doi: 10.1016/0092-8674(82)90173-8. [DOI] [PubMed] [Google Scholar]
- Gerbaud C., Fournier P., Blanc H., Aigle M., Heslot H., Guerineau M. High frequency of yeast transformation by plasmids carrying part or entire 2-micron yeast plasmid. Gene. 1979 Mar;5(3):233–253. doi: 10.1016/0378-1119(79)90080-5. [DOI] [PubMed] [Google Scholar]
- Hartley J. L., Donelson J. E. Nucleotide sequence of the yeast plasmid. Nature. 1980 Aug 28;286(5776):860–865. doi: 10.1038/286860a0. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Hahne S. Isolation of a condensed, intracellular form of the 2-micrometer DNA plasmid of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3727–3731. doi: 10.1073/pnas.76.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., Gellert M., Weisberg R. A., Nash H. A. Catenation and supercoiling in the products of bacteriophage lambda integrative recombination in vitro. J Mol Biol. 1980 Aug 25;141(4):485–494. doi: 10.1016/0022-2836(80)90256-9. [DOI] [PubMed] [Google Scholar]
- Nash H. A., Mizuuchi K., Enquist L. W., Weisberg R. A. Strand exchange in lambda integrative recombination: genetics, biochemistry, and models. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):417–428. doi: 10.1101/sqb.1981.045.01.056. [DOI] [PubMed] [Google Scholar]
- Nelson R. G., Fangman W. L. Nucleosome organization of the yeast 2-micrometer DNA plasmid: a eukaryotic minichromosome. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6515–6519. doi: 10.1073/pnas.76.12.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C. A vector that uses phage signals for efficient synthesis of proteins in Escherichia coli. J Mol Appl Genet. 1983;2(1):1–10. [PubMed] [Google Scholar]
- Reed R. R., Grindley N. D. Transposon-mediated site-specific recombination in vitro: DNA cleavage and protein-DNA linkage at the recombination site. Cell. 1981 Sep;25(3):721–728. doi: 10.1016/0092-8674(81)90179-3. [DOI] [PubMed] [Google Scholar]
- Reed R. R. Transposon-mediated site-specific recombination: a defined in vitro system. Cell. 1981 Sep;25(3):713–719. doi: 10.1016/0092-8674(81)90178-1. [DOI] [PubMed] [Google Scholar]
- Royer H. D., Hollenberg C. P. Saccharomyces cerevisiae 2-mum DNA. An analysis of the monomer and its multimers by electron microscopy. Mol Gen Genet. 1977 Feb 15;150(3):271–284. doi: 10.1007/BF00268126. [DOI] [PubMed] [Google Scholar]
- Simon M., Zieg J., Silverman M., Mandel G., Doolittle R. Phase variation: evolution of a controlling element. Science. 1980 Sep 19;209(4463):1370–1374. doi: 10.1126/science.6251543. [DOI] [PubMed] [Google Scholar]
- van de Putte P., Cramer S., Giphart-Gassler M. Invertible DNA determines host specificity of bacteriophage mu. Nature. 1980 Jul 17;286(5770):218–222. doi: 10.1038/286218a0. [DOI] [PubMed] [Google Scholar]