Abstract
Scavenger receptor CD36 mediates Staphylococcus aureus phagocytosis and initiates TLR2/6-signaling. We analyzed the role of CD36 in the uptake and TLR-independent signaling of various bacteria, including Escherichia coli, Klebsiella pneumoniae, Salmonella typhimurium, S. aureus and Enterococcus faecalis. Expression of human CD36 in HeLa cells increased the uptake of both Gram-positive and Gram-negative bacteria compared with the control mock-transfected cells. Bacterial adhesion was associated with pathogen phagocytosis. Upon CD36-transfection, HEK293 cells, which demonstrate no TLR2/4 expression, acquired LPS responsiveness as assessed by IL-8 production. The cells demonstrated a marked 5- to 15-fold increase in cytokine release upon exposure to Gram-negative bacteria, while the increase was much smaller (1.5- to 3-fold) with Gram-positive bacteria and lipotechoic acid. CD36 down-regulation utilizing CD36 small interfering RNA reduced cytokine release by 40%–50% in human fibroblasts induced by both Gram-negative and Gram-positive bacteria as well as LPS. Of all MAP kinase signaling cascade inhibitors tested, only the inhibitor of JNK, a stress activated protein kinase, potently blocked E. coli/LPS-stimulated cytokine production. NF-κB inhibitors were ineffective, indicating direct TLR-independent signaling. JNK activation was confirmed by Western blot analyses of phosphorylated JKN1/2 products. Synthetic amphipathic peptides with an α-helical motif were shown to be efficient inhibitors of E. coli- and LPS-induced IL-8 secretion as well as JNK1/2 activation/phosphorylation in CD36-overexpressing cells. These results indicate that CD36 functions as a phagocytic receptor for a variety of bacteria and mediates signaling induced by Gram-negative bacteria and LPS via a JNK-mediated signaling pathway in a TLR2/4-independent manner.
Keywords: CD36, lipopolysaccharide, bacteria, phagocytosis, signal transduction
Introduction
Scavenger receptor CD36 is an 88-kDa transmembrane glycoprotein and a member of the class B scavenger receptor family. CD36 is found in macrophages, microglia, microvascular endothelium, cardiac and skeletal muscle, adipocytes and platelets (1). As a pattern recognition receptor, CD36 binds a diverse set of ligands, including oxidized low-density lipoprotein (oxLDL)3, anionic phospholipids (2), long-chain fatty acids, thrombospondin-1, fibrillar β-amyloid, and the membrane of cells undergoing apoptosis (1, 3, 4). CD36 has been implicated in a wide variety of normal and pathologic biological functions, including angiogenesis, atherosclerosis, phagocytosis, inflammation, lipid metabolism and removal of apoptotic cells (1, 3).
Recent findings provide evidence for the essential role of type B scavenger receptors in the innate immune response of the mammalian host to exogenous pathogens, in particular, to bacteria and their inflammatory compounds. Philips et al. (5) demonstrated that two members of the CD36 family, namely human Cla-1 and Cla-2 (orthologs of rodent SR-BI and SR-BII), mediate the uptake of Mycobacterium fortuitum into non-phagocytic cells, while the transfection of cells with the murine CD36 results in intracellular uptake of Escherichia coli (E. coli) and, to a lesser extent Staphylococcus aureus (S. aureus). Other findings by Stuart et al. (6) indicated that CD36 is predominantly involved in phagocytosis and cytokine production in response to the Gram-positive S. aureus and its cell component lipoteichoic acid (LTA), and only to a lesser extent to the Gram-negative E. coli and its cell wall component, lipopolysaccharides (LPS). There is considerable evidence for an important role of Toll-like receptors (TLRs) as sensors for a variety of microbial pathogens (7–9). Toll-like receptor 4 (TLR4) is considered to be the primary signaling receptor for Gram-negative bacteria and their surface cell wall component – LPS. Membrane protein CD14 (mCD14) recruits LPS to TLR4, thereby facilitating signal transduction (10). Despite this widely accepted concept, it has been recognized that, at least in mCD14-negative cells such as like endothelial and epithelial cells, the scavenger receptor pathway could be implicated in LPS uptake and clearance (11–13). Furthermore, results of recent studies have demonstrated that scavenger receptor CD36 is an essential co-receptor involved in recognition of LTA and certain diacylglycerides, leading to activation of TLR2/6 (14). By analogy with mCD14, some authors have proposed that CD36 functions as an accessory protein, required to present bacterial ligands to the TLR-mediated signaling pathways (6, 15). The C-terminal domain of CD36 was shown to be a requirement for the internalization of S. aureus and LTA as well as for the activation of TLR2/6 signaling. It has been also reported that CD36 plays a role as a TLR-independent signaling receptor, initiating a down-stream cascade upon ligand binding. CD36 receptor was found to be physically associated with three members (Fyn, Yes and Lyn proteins) of the Src family of protein tyrosine kinases (PTKs), known to be upstream GTPases involved in MAP kinase activation, in platelets, C32 melanoma and some other CD36-expressing cell lines (16,17). A CD36-mediated signaling pathway has been identified in endothelial cells, where stimulation by thrombospondin-1 results in activation of two stress-activated kinases, Jun N-terminal kinase-1 (JNK-1) and p38 mitogen-activated protein (MAP) kinases, leading to apoptosis-dependent inhibition of angiogenesis (18). Moore et al. (19) have demonstrated that binding of the fibrillar amyloid peptide, β-amyloid, to CD36 initiates a proinflammatory signaling cascade, involving a Src kinase family member, Fyn, and p44/42 MAP kinase. Recently, oxLDL has been shown to induce CD36-dependent activation of JNK1/2 MAP kinases in peritoneal macrophages (17).
In this study, we sought to elucidate the role of human CD36 (hCD36) in recognizing different Gram-negative and Gram-positive bacteria as well as their respective major cell wall constituents, LPS and LTA. We used HEK 293 and HeLa epithelial cell lines, transfected with hCD36, as model systems having little or no expression of TLR2/4 and functionally related proteins (20–22). Our results failed to reveal distinct preferences for hCD36 regarding Gram-negative or Gram-positive bacterial uptake. However, LPS appeared to be the preferred ligand for hCD36 compared to LTA. Pathogen binding to hCD36 induced pro-inflammatory downstream signaling via the JNK signaling pathway that was independent of TLR2/4 and was associated with pathogen phagocytosis.
Materials and Methods
Reagents
All media, sera, and antibiotics were obtained from Invitrogen (Carlsbad, CA). The MAPK inhibitors PD98059, SB203580, SP600125, peptide inhibitors, JNKI and JNKIII, and corresponding negative control peptides were purchased from EMD Biosciences (San Diego, CA). Inhibitors for NF kappa B and D-JNKi1 inhibitor (D-retro-inverso form of the cell-permeable JNK peptide inhibitor) were obtained from BioMol International (Plymouth Meeting, PA). All LPS preparations and S. aureus LTA were from Sigma (St. Loius, MO). Anti-CD36 monoclonal antibody FA16 was purchased from Abcam Inc. (Cambridge, MA). Synthetic amphipathic peptides were synthesized by a solid-phase procedure (23, 24). Peptide sequences were described in a previous report (25). Human high-density lipoproteins (HDL) and LDL were purchased from EMD Biosciences. Extensively oxidized LDL (oxLDL) was prepared by incubation with 5 µM CuSO4 at 37 °C for 24 h as described in (26). All cell trackers and reactive fluorescent dyes were obtained from Invitrogen.
Cell Cultures
HeLa (Tet off) cells were transfected with Fu-GENE-6 (Roche Diagnostics, Indianapolis, IN), using the expression plasmid pTRE2 (Clontech, Palo Alto, CA), encoding a human CD36 protein (pTRE2-CD36). Cells were co-transfected with pTRE2-hCD36 and pTK-Pur (Clontech), using a 1:20 ratio, and selected with 400 µg/ml puromycin. Puromycin-resistant cells were screened for the expression of the human CD36 protein utilizing mouse anti-hCD36 antibody (Abcam) in Western blotting. The HEK293 cell line was stably transfected with CD36 pIRES-hrGFP-2a plasmid (Stratagene, La Jolla, CA) followed by selecting cells with the highest green fluorescent protein expression. Normal human skin fibroblasts (ATCC, Manassas, VA), hCD36-overexpressing HeLa cells, HeLa (Tet-off) cells (Clontech) as well as human embryonic kidney cell line HEK293 (ATCC, Manassas, VA), both wild type and CD36-overexpressing, were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, 100 IU/ml penicillin, 100 µg/ml streptomycin, and 100 µg/ml G418 at 37 °C in a 5% CO2 humidified atmosphere. Rat CD36-null (CD36 (−/−)) and wild type (CD36 (+/+)) macrophages were isolated from bone marrow cells of spontaneously hypertensive Wistar-Kioto male rat (SHR/NCrl) and its control strain Wistar-Kyoto/NCrl (Charles River Laboratories), respectively. The macrophages were differentiated by growing them in RPMI 1640, 20% FCS, in the presence of 10 ng/ml of mouse macrophage colony-stimulating factor (M-CSF) and 10 ng/ml of mouse IL-4 for 7 days.
Fluor-labeled ligand uptake
Native and oxidized lipoproteins, peptides, and bacteria were conjugated with Alexa Fluor® 488, using a protein labeling kit (Invitrogen) following the vendor's instructions. LTA from S. aureus was labeled with BODIPY TMR-X, whereas S. minnesota Re 595 LPS was labeled with a BODIPY FL, SE labeling kit, both from Molecular Probes (Eugene, OR) following the manufacturer's suggested procedure with previously reported modifications (25, 27). All bacterial uptake and phagocytosis studies were performed by using DMEM containing 2 mg/ml bovine serum albumin (BSA) without antibiotics. HeLa cells were incubated with bacteria at ≈50 labeled bacteria per cultured cell. After a brief centrifugation of the plate to accelerate bacterial sedimentation, the cells were incubated at 37°C for 1 h and then washed extensively with phosphate-buffered saline (PBS), detached with Cellstripper dissociation solution (Mediatech, Herndon, VA), fixed with 4% paraformaldehyde, and analyzed by a fluorescence-activated cell sorter (FACS, model A; Hitachi). A similar protocol, excluding the centrifugation step, was used for uptake experiments with other fluoro-labeled ligands.
Confocal microscopy imaging
Confocal microscopy experiments designed to demonstrate bacterial co-localization with various intracellular compartments were conducted as reported before (5, 25, 27). Briefly, 8-well microscopy slides with cultured cells were added with Alexa 488-labeled bacteria, centrifuged (3000 × g for 5 min) to accelerate bacterial sedimentation, and further incubated for 30–60 min. After three washings with PBS to remove unattached bacteria, slides were incubated with fluorescent Lysotracker Red. After a 5- to 30-min incubation with trackers, slides were washed with PBS, fixed with 4% paraformaldehyde and sealed or viewed immediately without fixation. Visualization was achieved by using Alexa Fluor 488/594-labeled anti-mouse/rabbit IgG secondary antibodies (Invitrogen). To assess subcellular localization, images were obtained with a Zeiss 510 laser scanning confocal microscope (Zeiss, Jena, Germany), using a krypton-argon-Omnichrome laser with excitation wavelengths of 488 and 568 nm for Alexa-488 and Alexa-568 labels, respectively.
CD36 expression manipulations
For CD36 knockdown or overexpression, human skin fibroblasts were electroporated in the presence of On-Targetplus Smartpool SiRNA CD36 (Dharmacon) (5 µg/106 cells) or hCD36 coding plasmid (2 µg/106 cells), utilizing a Nucleofector II instrument and Nucleofector kit T following recommended procedures (www.amaxa.com). Cells treated with a non-targeting siRNA served as a negative control. Following the nucleofection, cells were cultured for 24 h in DMEM containing 10% FCS followed by overnight incubation in serum-free media before stimulant additions.
Detection of activated JNK1/2 by Western blot analysis
Wild type and CD36-overexpressing HEK293 cells were grown in 6-well culture plates to confluence. Before the JNK1/2 activation assay, the cells were incubated overnight in serum-free DMEM. The cells were stimulated for varying periods of time with LPS (10 ng/ml) or E. coli (2 ng/ml) at 37 °C. After stimulation, the culture medium was immediately aspirated; the cells were placed on ice and washed three times with ice-cold PBS. Afterwards, the cells were scrapped into 100 µl of lysis buffer (20 mM Tris-HCl, pH 7.5, 2 mM EDTA, 0.5% (v/v) Triton X-100, 1 mM NaF, 1 mM Na3VO4, 50 mM 2β-mercaptoethanol, 5 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, and 2% (v/v) protease inhibitor mixture set III (EMD Biosciences). After a 10-min incubation on ice, the samples were sonicated for 7–8 s and centrifuged at 12,000 rpm for 10 min at 4 °C. The cell extracts were collected and mixed with the 2× SDS sample buffer. The samples were separated on SDS-PAGE in 8% Tris-glycine pre-cast gels (Invitrogen) and then transferred to nitrocellulose membranes. The membranes were blocked with Tris-buffered saline containing 0.1% Tween 20 and 5% (w/v) nonfat dry milk and further incubated either with the primary anti-phospho JNK1/2 antibodies or with antibodies that recognize both active and inactive JNK forms (BioMol International) overnight at 4°C. Immunoreactive bands were detected with a horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence (GE Healthcare, Piscataway, NJ). Alternatively, the immunoreactive bands were detected using an alkaline phosphatase-conjugated secondary antibody and Western Blue Stabilized Substrate for Alkaline Phosphatase (Promega, Madison, MI).
Other procedures
Cytokine secretion was analyzed in culture media after a 20-h incubation utilizing a commercial enzyme-linked immunosorbent assay kits for human IL-8 (Invitrogen), rat IL-6 and rat TNF-alpha (Pierce Biotechnology). Bacterial preparations from different species were standardized according to the bacterial lysates’ protein content measured by the Bradford dye (Bio-Rad Laboratories, Hercules, CA) protein assay (28). CelLytic B Plus Kit (Sigma-Aldrich) was used for efficient bacterial lysis and protein extraction.
Results
Uptake of FITC-labeled ligands is enhanced in hCD36 transfected vs. mock transfected HeLa cells
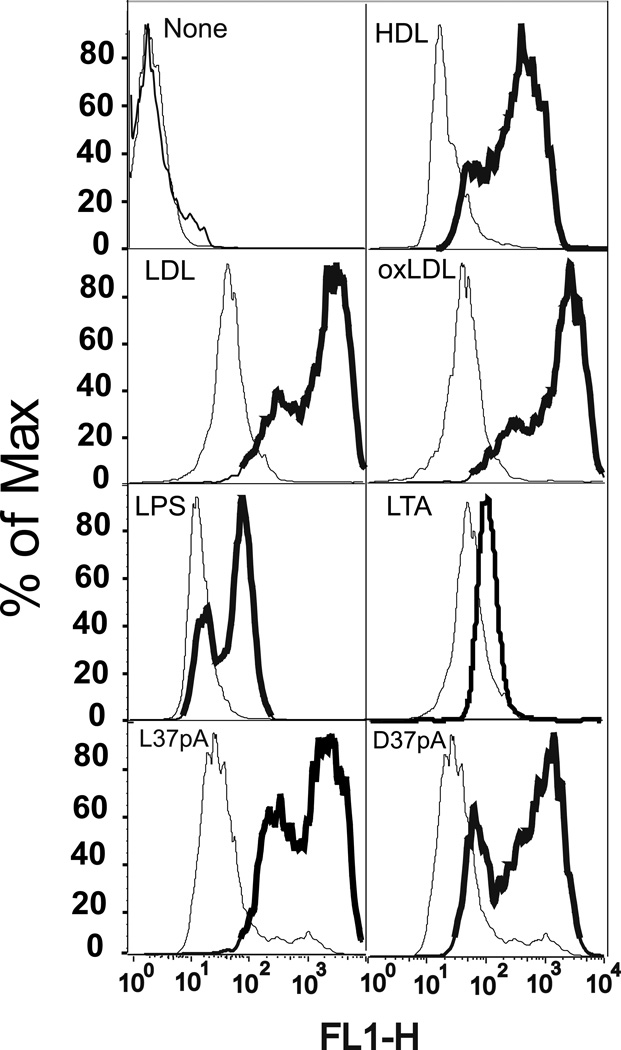
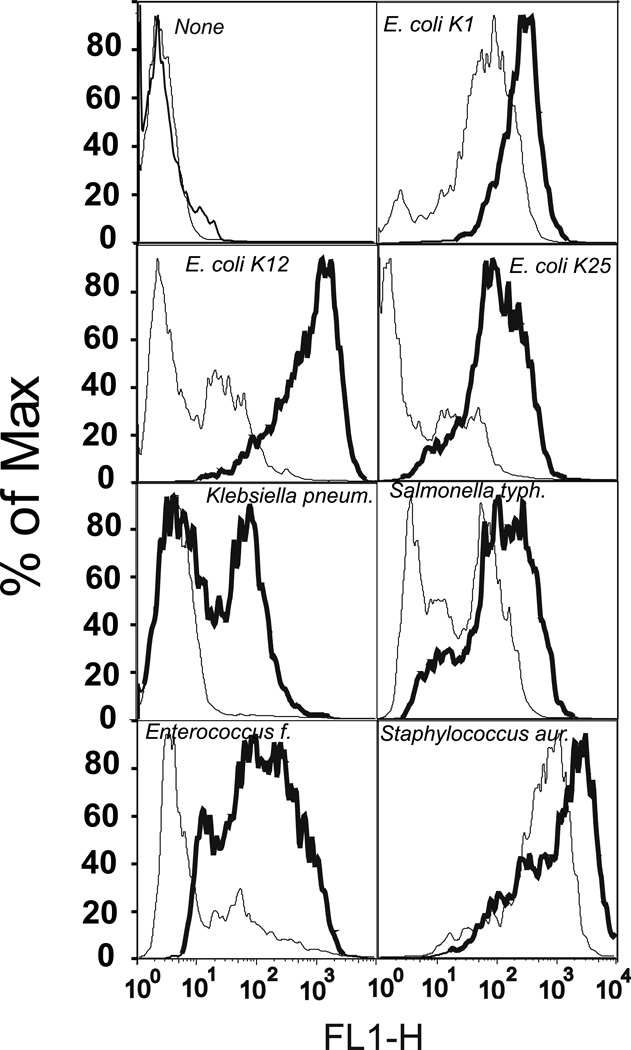
To test the functional activity of CD36 as a lipoprotein receptor, we measured cellular uptake of fluorescently labeled HDL, LDL, and oxLDL in HeLa cells transfected with hCD36 using FACS analysis. Compared to mock transfected cells, expression of hCD36 markedly increased the uptake of fluorescently labeled lipoproteins (Fig. 1). Synthetic amphipathic helical peptides have been previously shown to be the ligands for another CD36 family member – the Cla-1 (SR-BI) receptor (29). Fig. 1 shows that the uptake of Alexa 488-labeled L-37pA and D-37pA α–helical peptides was also increased in CD36-overexpressing vs. control cells. As previously reported by Stuart et al. (6), murine CD36 can act as a phagocytic receptor for Gram-positive and, to a lesser extent, Gram-negative bacteria in HEK 293 cells. According to Philips et al. (5), HEK293 cells transfected with murine CD36 are capable of taking up E. coli and to a lesser extent S. aureus. In order to see whether hCD36 has any preference for internalization of Gram-positive vs. Gram-negative bacteria, we evaluated the cellular uptake of the various Alexa 488-labeled bacteria in hCD36 transfected vs. control HeLa cells. We found the most pronounced increases in the uptake of the Gram-negative E. coli K-12 and K-25 strains as well as the Gram-positive Enterococcus faecalis (Fig. 2). Although the increases were less pronounced, internalization of two other tested Gram-negative bacteria - Klebsiella pneumoniae and Salmonella typhimurium - was also enhanced in CD36-overexpressing vs. control HeLa cells (Fig. 2). In contrast, the difference in S. aureus internalization between CD36 and mock-transfected cells was masked by a relatively high bacterial uptake in control cells, possibly due to the presence of CD36-independent mechanisms of bacterial uptake in HeLa cells. Altogether these results suggest that hCD36-mediated uptake is not selective for Gram-positive or Gram-negative bacteria, and hCD36 functions as a receptor capable of recognizing and mediating intracellular uptake of either type of bacteria.
FIGURE 1.
Uptake of fluorescently labeled ligands in CD36-overexpressing and mock-transfected HeLa cells. Cells were incubated with various Alexa 488-labeled lipoproteins, synthetic peptides or Bodipy-labeled LPS or LTA at the concentration of 10 µg/ml for 1 h at 37°C. Cell-associated fluorescence was analyzed by FACS analysis. The uptake of each ligand in mock-transfected (thin line) and CD36-overexpressing (bold line) HeLa cells is indicated on the plot area. The plots are representative of at least two separate experiments that yielded similar results.
FIGURE 2.
Uptake of Alexa 488-labeled bacteria in CD36-overexpressing and mock-transfected HeLa cells. Cells were incubated with fluorescently labeled live bacteria for 1 h at 37°C and then harvested for FACS analysis. The uptake for each bacterial species in mock-transfected (thin line) and CD36-overexpressing (bold line) HeLa cells is indicated on the plot area. The plots are representative of at least two separate experiments that yielded similar results.
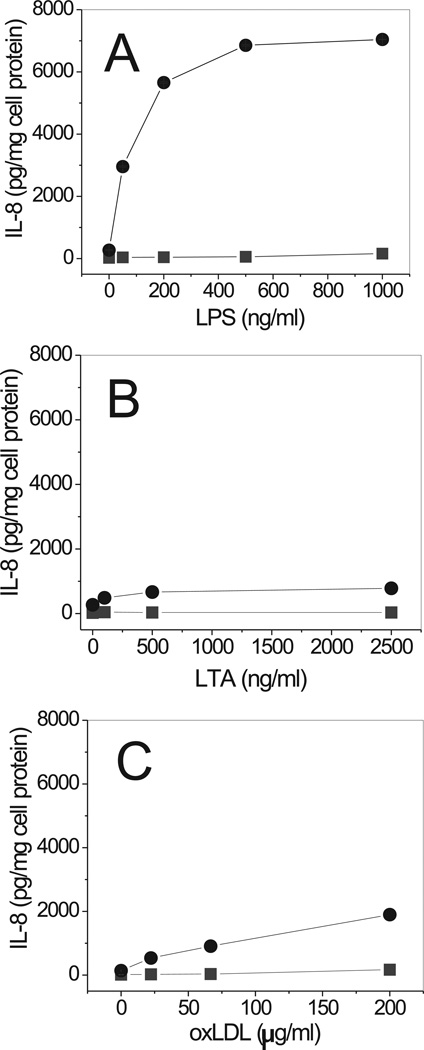
Regarding the intracellular uptake of fluorescently-labeled LPS and LTA, we observed a 5–10-fold increase in internalization of Bodipy 558/568-LPS in CD36-transfected vs. mock HeLa cells, while the levels of Bodipy-LTA uptake were comparable in the two cell types (Fig. 1).
CD36 mediates bacterial phagocytosis in CD36-overexpressing HeLa cells
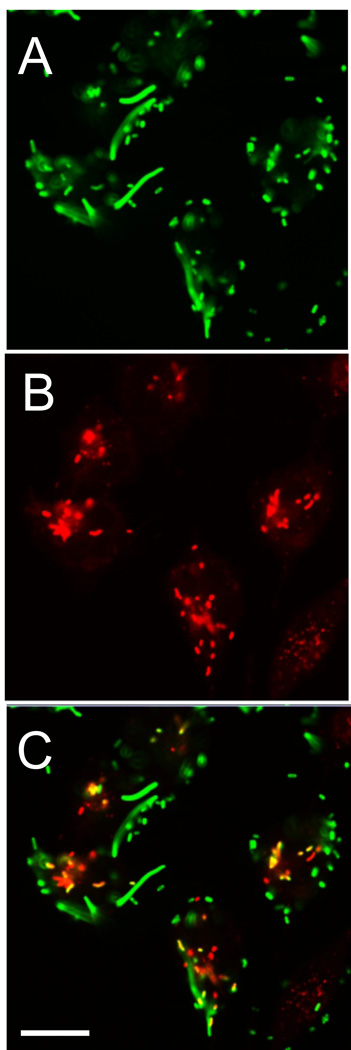
CD36-mediated bacterial uptake was further analyzed in immunofluorescence studies with confocal microscopy. hCD36-transfected HeLa cells were incubated with Alexa 488-E. coli (K-12 strain) followed by co-localization with the lysosomal marker, LysoTracker Red (Fig. 3A–C). The merged image (Fig. 3C) demonstrated co-localization of E. coli K-12 with the lysosomal compartment in hCD36-overexpressing HeLa cells. These results confirm the involvement of CD36 in bacterial phagocytosis and provide visual evidence that CD36-dependent bacterial uptake in HeLa cells is at least partially associated with lysosomal degradation of bacteria. No appreciable bacterium uptake was found in control hCD36-negative HeLa cells.
FIGURE 3.
E. coli K12 lysosomal phagocytosis in CD36-overexpressing HeLa cells. Live Alexa 488-labeled E. coli K12 were incubated with CD36-overexpressing HeLa cells for 45 min. After washing with PBS, the cells were further incubated with LysoTracker red for 15 min, fixed, and analyzed by confocal microscopy. The color images represent bacteria (A), the lysosomal compartment (B), and bacteria in lysosomes (C-overlay). Scale bars: 10 µm.
Bacterium and LPS-induced secretion of IL-8 is increased in hCD36-overexpressing HEK293 cells
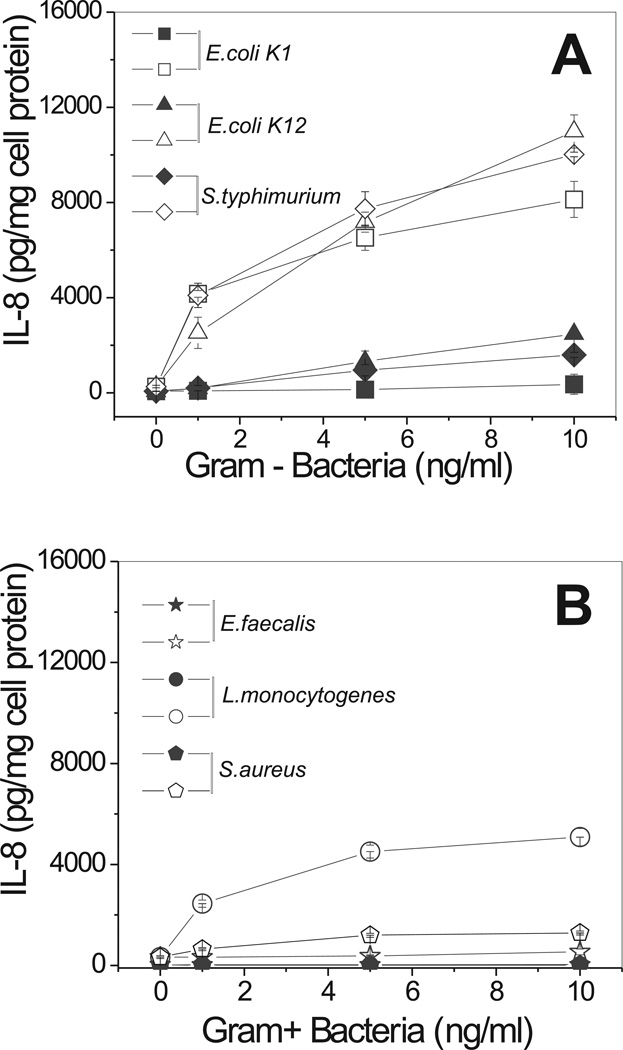
We next tested the ability of various live bacteria to induce IL-8 secretion via a CD36-dependent pathway. All Gram-negative bacteria tested induced marked (5- to 20-fold) dose-dependent increases of IL-8 secretion in CD36-transfected HEK293 cells. Control cells demonstrated low or no IL-8 secretion upon exposure to the bacteria (Fig. 4). The response of CD36-overexpressing cells to Gram-positive bacteria was either weak or non-existent, except for a robust response induced by Listeria monocytogenes. Incubation of CD36-overexpressing HEK293 cells with increasing doses of LPS, a structural component of the cell wall of Gram-negative bacteria, resulted in an even more pronounced stimulation of IL-8 secretion than the treatment with whole bacterium preparations (Fig. 5A). Control cells were unresponsive to LPS in concentrations from 1 to 100 ng/ml. While higher doses of LPS were able to induce detectable levels of IL-8 in control cells as well, the responses were 10- to 20-times lower than those in CD36-transfected cells. Various preparations of LPS, derived from wild strains of E. coli (serotype 0111:B4 and 055:B5) or S. enterica (serotype Minnesota) as well as LPS Re 595 mutant (from S. enterica serotype Minnesota) were efficient stimulators of IL-8 secretion in CD36-HEK293 cells (data not shown). Unlike LPS, LTA was a less potent stimulator of cytokine secretion in CD36-transfected cells. Only a moderate IL-8 (1.5- to 1.7-fold) increase was observed in CD36-overexpressing cells upon LTA stimulation when compared to control cells (Fig. 5B). Another well-known CD36 receptor ligand, oxLDL, was recently reported to mediate signaling via a CD36-dependent pathway (17). In our experiments, oxLDL induced IL-8 secretion in CD36-HEK293 cells in a dose-dependent manner (Fig. 5C).
FIGURE 4.
Dose-dependent IL-8 secretion induced by various Gram-negative and Gram-positive bacteria in CD36-overexpressing and mock-transfected cells. CD36-overexpressing (open symbols) and mock-transfected (closed symbols) HEK293 cells were incubated with increasing concentrations of Gram-negative (A) and Gram-positive (B) bacteria for 20 h. IL-8 levels in the supernatants were assayed in duplicates. Plots are representative of at least three separate experiments that yielded similar results.
FIGURE 5.
Dose-dependent IL-8 secretion induced by LPS, LTA and oxLDL in CD36-overexpressing and mock-transfected cells. CD36-overexpressing (circles) and mock-transfected (squares) HEK293 cells were incubated with increasing concentrations of LPS (A), LTA (B) or oxLDL (C) for 20 h. IL-8 levels were determined in duplicate samples of conditioned media. Data represent one of three separate experiments that yielded similar results.
Bacterium and LPS-induced secretion of IL-8 is decreased by CD36 siRNA knockdown in human fibroblasts
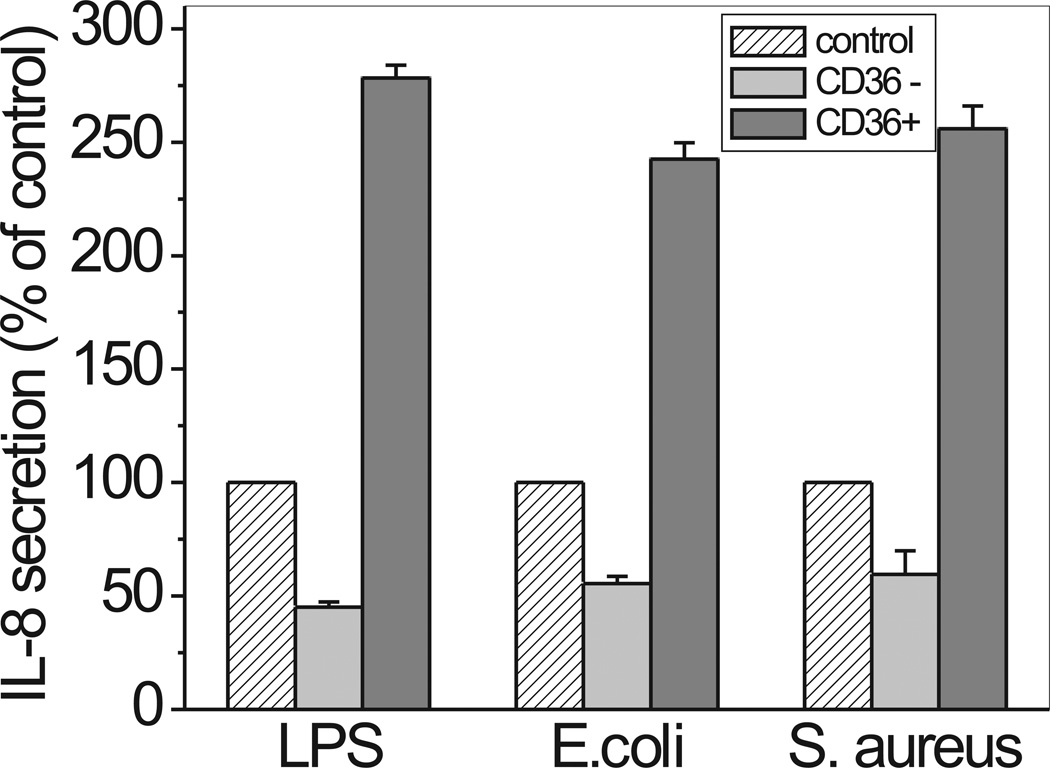
It has been previously reported that monocytes from CD36 knockout mice differentiated into macrophage demonstrate a 30–50% decreased response to LPS and Gram-negative bacteria when compared to normal cells (6). To study the role of CD36 in the cells moderately expressing TLR2/4, we manipulated CD36 expression in human fibroblasts. As seen at Fig. 6, cells treated with CD36 siRNA demonstrated 40–50% decrease in IL-8 release when stimulated with LPS and Gram-negative E. coli. A similar decrease in IL-8 secretion was observed with Gram-positive S. aureus. Nucleofection with scrambled siRNA had no effect. Importantly, CD36 overexpression was associated with a 2.5–3-fold increase in IL-8 secretion levels following cell treatment with LPS and both bacteria - E. coli and S. aureus, when compared to control cells.
FIGURE 6.
Effect of CD36 siRNA treatment and CD36 overexpression on IL-8 release in human fibroblasts. Normal human fibroblasts were electroporated in the presence of non-targeting siRNA (control), on-Target plus Smartpool CD36 siRNA (CD36−) and hCD36 DNA plasmid (CD36+), and further cultured for 24 h in DMEM containing 10% FCS. After overnight incubation in serum-free media, cells were stimulated with 100 ng/ml LPS (from S. enterica serotype minnesota) or 1 ng/ml of either E.coli K-12 or S. aureus for 20 h. IL-8 levels in the supernatants were assayed as previously described. Results are presented as percentage of IL-8 secretion levels in CD36− or CD36+ cells vs. levels observed in control cells (treated with non-targeting siRNA). Data represent the mean ± S.D. of two separate experiments, each performed in duplicates.
Bacterium-, LTA- and LPS-induced cytokine production is significantly impaired in CD36 (−/−) rat macrophages
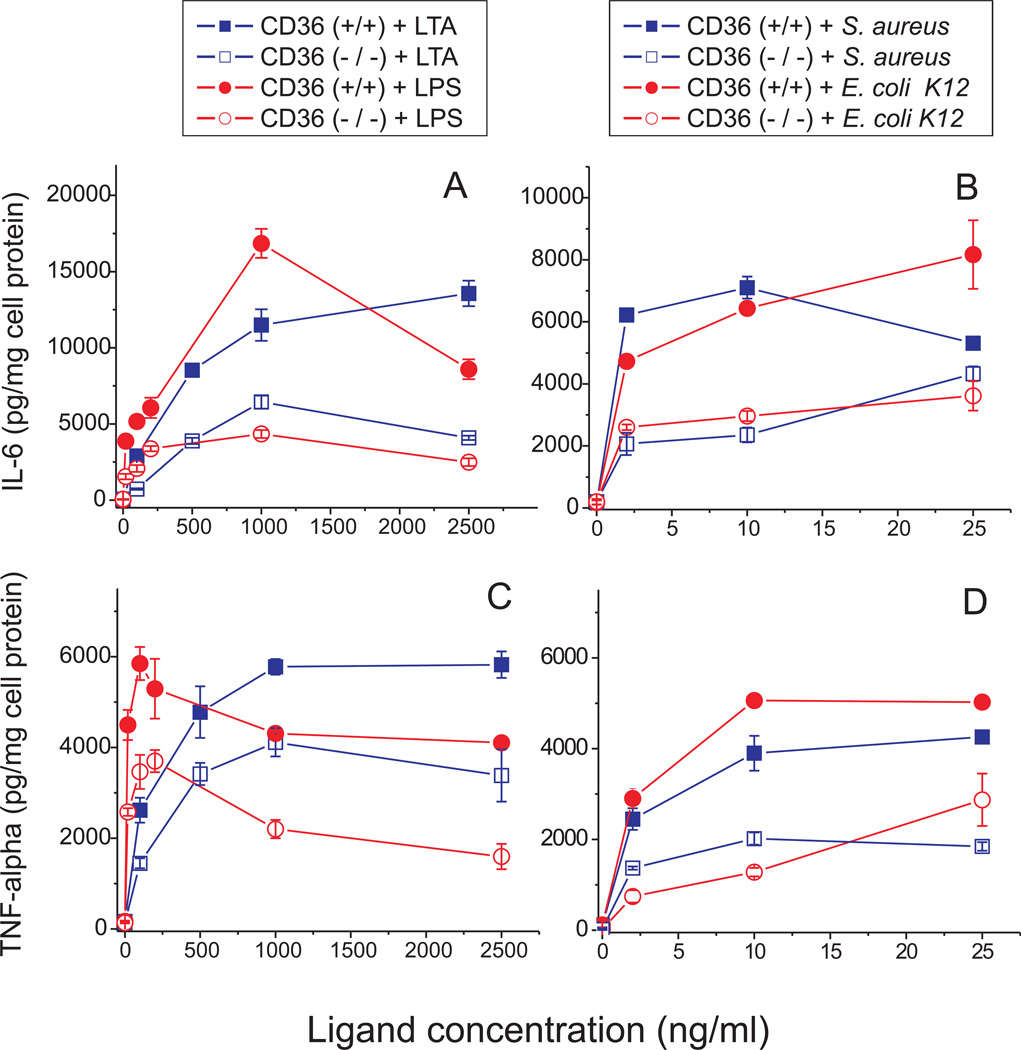
To evaluate the role of CD36 in the inflammatory response to bacteria and their derived ligands in cells with high levels of TLR2/4 expression, we measured IL-6 and TNF-α secretion in CD36 (+/+) and CD36 (−/−) rat macrophages following a 20-h cell treatment with different inflammatory stimuli. CD36 (−/−) cells demonstrated significantly decreased levels of IL-6 (60–70% reduction) and TNF-α (40–60% reduction) upon stimulation with E. coli K12, S. aureus as well as their cell wall components, LPS and LTA, when compared to CD36 (+/+) macrophages (Fig 7.). Similar levels of cytokine production impairment occurred in the absence of a functional CD36 with both Gram-negative and Gram-positive bacteria, as well as with LPS and LTA. These data indicate that CD36 plays an important role in macrophage cytokine response to both Gram-negative and Gram-positive pathogens.
FIGURE 7.
Dose-dependent LTA-, LPS-, S. aureus- and E. coli-induced secretion of IL-6 (A and B), and TNF-α (C and D) in CD36 (+/+) and CD36 (−/−) rat macrophages. Cells were exposed to increasing doses of various inflammatory stimuli for 20 h, and cytokine levels were determined in duplicate samples of cell culture supernatants. Data represent one of three separate experiments that yielded similar results.
Amphipathic α-helical peptides block LPS and E. coli (K-12)-induced IL-8 secretion in CD36-overexpressing HEK293 cells
Amphipathic α-helical peptides, that were found to be high affinity CD36 agonists in our uptake experiments, were also tested as potential inhibitors of CD36-dependent IL-8 secretion induced by LPS and bacteria. Two amphipathic α-helical motif containing peptides, L-37pA and D-37pA, demonstrated a 60–80% inhibition of cytokine secretion in CD36-overexpressing HEK293 cells challenged with LPS Re595 from mutant S. enterica or E. coli K-12 bacteria. In contrast, the L3D-37pA peptide, which contains three D-amino acid substitutions that disturb the amphipathic α-helical motif, did not affect IL-8 secretion induced by either of the two CD36 ligands (Fig. 8).
FIGURE 8.
Inhibition of LPS- and E. coli - induced IL-8 secretion by synthetic α-helical peptides in CD36-overexpressing HEK293 cells. Cells were incubated with 100 ng/ml of LPS or 1 ng/ml of E. coli K12 with or without 10 µg/ml of L-37pA, D-37pA or L3D-37pA for 20 h. IL-8 levels in the supernatants were assayed as previously described. Data represent the mean ± S.D. of three separate experiments, each performed in duplicate.
Effects of NF-κB and MAP kinase inhibitors on LPS - and E. coli (K-12) - induced cytokine secretion in CD36-overexpressing HEK293 cells and normal rat macrophages
In order to elucidate the potential mechanisms of the observed increase in IL-8 secretion induced by LPS and bacterial stimuli in CD36-overexpressing cells, we tested various pharmacological inhibitors of different signaling cascades. In particular, inhibitors of the NF-κB and MAP kinase pathways, known to be linked to cytokine production were evaluated. As seen from Table I, except for sulfasalasine with its highest concentration, none of the NF-κB inhibitors tested was capable of blocking LPS- or E. coli- induced IL-8 secretion in CD36-overexpressing HEK293 cells. These results present indirect evidence that the pro-inflammatory CD36-mediated response in this type of cells may occur independently from the TLR4 receptor, known to mediate ligand-induced signaling primarily via NF-κB activation (30). Moreover, neither SB203580, an inhibitor of p38 MAP kinase, nor PD98059, a selective inhibitor of MAP kinase kinase (MEK), was able to inhibit LPS or E. coli-induced IL-8 production in hCD36-overexpressing cells and only a weak 10–15% reduction of IL-8 levels was observed in the presence of PD98059 (Table II). IL-8 secretion in hCD36 overexpressing HEK293 cells treated with the JNK1, −2 and −3 inhibitor SP600125, or the JNKi1 inhibitor (cell-permeable peptide, consisting of a C-terminal sequence derived from the JNK-binding domain, and an N-terminal peptide containing the HIV-TAT48–57 sequence), was reduced by 50–70% compared to mock-transfected cells. These data provide indirect evidence that the CD36-dependent pro-inflammatory response induced by LPS or E. coli in HEK293 cells predominantly involves activation of the JNK signaling pathway. Current data indicates that both NF-kB and JNK pathways are required for inducible IL-8 regulation in most cell types studied (31). However, the relative contribution of these pathways to the activation of cytokine expression may significantly vary depending on the specific ligand and cell type. To estimate the potential contribution of the different signaling pathways to the LPS- and E. coli-induced IL-6, another cytokine, whose expression in many respects is regulated similar to IL-8 (32), we tested the effects of the MAP kinase and NF-κB inhibitors in differentiated rat macrophages, as a cell model with high expression of both TLR4 and CD36 receptors. Our results in Table III demonstrate that both the MEK inhibitor PD98059 and p38 MAPK inhibitor SB203589 moderately (up to 40–50%) blocked LPS and E. coli-induced IL-6 secretion, whereas JNK1/2/3 inhibitor SP600125 caused the most pronounced reduction (up to 80–90%) of cytokine production in rat macrophages challenged with either LPS or E. coli K-12. PDTC, one of two NF-kB inhibitors tested also potently (up to 60–75%) decreased LPS and E. coli- induced IL-6 secretion in rat macrophages.
Table I.
Effects of NF-kappaB inhibitors on LPS- and E.coli K-12-induced IL-8 secretion in CD36-overexpressing HEK 293 cellsa
| NF-kappaB Inhibitor b | IL-8 (ng/mg cell protein) | |
|---|---|---|
| LPS | E.coli K-12 | |
| SN50 Inhibitor Peptide | ||
| 0 µM | 5.53 ± 0.77 | 3.92 ± 0.54 |
| 5 µM | 5.74 ± 0.3 | N/D |
| 10 µM | 3.94 ± 0.11 c | 4.02 ± 0.11 |
| 25 µM | 5.68 ± 0.29 | 4.63 ± 0.44 |
| SN50 Inactive Control Peptide | ||
| 0 µM | 5.53 ± 0.77 | 3.92 ± 0.54 |
| 5 µM | 6.24 ± 0.2 | N/D |
| 10 µM | 8.36 ± 0.69 | 3.16 ± 0.46 |
| 25 µM | 9.31 ± 0.27 c | 4.5 ± 0.38 |
| Sulfasalasine | ||
| 0 µM | 5.53 ± 0.77 | 3.92 ± 0.54 |
| 0.01mM | 6.86 ± 0.53 | 4.49 ± 0.3 |
| 0.1mM | 6.44 ± 0.65 | 4.23 ± 0.21 |
| 1 mM | 4.49 ± 0.11 c | 3.71 ± 0.19 |
| PDTC (pyrrolidinedithiocarbamate) | ||
| 0 µM | 5.53 ± 0.77 | 3.92 ± 0.54 |
| 1 µM | 5.54 ± 0.24 | 3.99 ± 0.17 |
| 5 µM | 4.85 ± 0.18 | 3.87 ± 0.11 |
| 10 µM | 5.67 ± 0.25 | 4.1 ± 0.14 |
| 50 µM | 8.08 ± 0.92 | 6.06 ± 0.52 |
Data are presented as means ± S.D. of one of two separate experiments that were performed in duplicates and yielded similar results. IL-8 levels are expressed in ng per mg of cell protein.
Cells were treated with the indicated concentrations of inhibitors for 1hr prior to a 20h stimulation with LPS (100ng/ml) or E. coli K-12 (2ng/ml).
p < 0.05, IL-8 levels in the presence of inhibitor vs. control (in the absence of inhibitor), Student’s t test.
Table II.
Effects of MAP kinase inhibitors on LPS- and E.coli K-12-induced IL-8 secretion in CD36-overexpressing HEK 293 cells a
| MAPK Inhibitor b | IL-8 (ng/mg cell protein) | |
|---|---|---|
| LPS | E.coli K-12 | |
| PD 98059 (MEK inhibitor – MAP kinase kinase | ||
| 0 µM | 3.91 ± 0.35 | 4.27 ± 0.41 |
| 5 µM | 3.72 ± 0.09 | 4.28 ± 0.24 |
| 20 µM | 3.46 ± 0.13 | 3.62 ± 0.14 |
| 50 µM | 3.43 ± 0.21 | 3.86 ± 0.17 |
| SB 203589 (p38 MAPK Inhibitor) | ||
| 0 µM | 3.91 ± 0.35 | 4.27 ± 0.41 |
| 1 µM | N/D | 4.51 ± 0.19 |
| 5 µM | 4.06 ± 0.14 | 5.49 ± 0.48 |
| 25 µM | 5.21 ± 0.31 | 6.02 ± 0.41 |
| SP 600125 (JNK 1/2/3 Inhibitor) | ||
| 0 µM | 3.91 ± 0.35 | 4.27 ± 0.41 |
| 5 µM | 2.07 ± 0.14 c | 2.09 ± 0.08 c |
| 20 µM | 1.81 ± 0.16 c | 1.59 ± 0.21 c |
| 50 µM | N/D | 1.36 ± 0.49 c |
| JNK Inhibitor JNKi-III | ||
| 0 µM | 3.91 ± 0.35 | 4.27 ± 0.41 |
| 5 µM | 3.27 ± 0.15 | 2.99 ± 0.17 c |
| 25 µM | 1.66 ± 0.12 c | 1.41 ± 0.15 c |
| Negative Control for JNK Inhibitor JNKi-III | ||
| 0 µM | 3.91 ± 0.35 | 4.27 ± 0.41 |
| 5 µM | 3.62 ± 0.21 | 4.33 ± 0.23 |
| 25 µM | 3.26 ± 0.36 | 3.61 ± 0.39 |
Data are presented as means ± S.D. of one of two separate experiments that were performed in duplicates and yielded similar results.
Cells were treated with the indicated concentrations of inhibitors for 1hr prior to a 20h stimulation with LPS (100ng/ml) or E. coli K-12 (2ng/ml).
p < 0.05, IL-8 levels in the presence of inhibitor vs. control (in the absence of inhibitor), Student’s t test.
Table III.
Effects of MAP kinases’ and NF-kB inhibitors on LPS- and E.coli K-12-induced IL-6 secretion in normal rat macrophagesa
| Inhibitor b | IL-6 (ng/mg cell protein) | |
|---|---|---|
| LPS | E. coli K-12 | |
| PD 98059 (MEK inhibitor – MAP kinase kinase) | ||
| 0 µM | 10.01 ± 0.54 | 15.9 ± 1.22 |
| 5 µM | 6.26 ± 0.36 c | 11.1 ± 2.47 |
| 20 µM | 5.5 ± 0.037 c | 10.6 ± 1.04 |
| 50 µM | 5.67 ± 0.42 c | 8.27 ± 0.95 c |
| SB 203589 (p38 MAPK Inhibitor) | ||
| 0 µM | 10.01 ± 0.54 | 15.9 ± 1.22 |
| 1 µM | 8.34 ± 0.061 | 9.01 ± 0.1 c |
| 5 µM | 7.72 ± 1.02 | 7.44 ± 0.55 c |
| 25 µM | 6.12 ± 0.025 c | 6.55 ± 0.06 c |
| SP 600125 (JNK 1/2/3 Inhibitor) | ||
| 0 µM | 10.01 ± 0.54 | 15.9 ± 1.22 |
| 1 µM | 6.51 ± 0.72 c | 9.53 ± 0.93 c |
| 5 µM | 4.05 ± 0.98 c | 5.02 ± 0.24 c |
| 10 µM | 3.98 ± 0.92 c | 3.14 ± 0.05 c |
| 25 µM | 2.71 ± 0.37 c | 2.81 ± 0.07 c |
| Sulfasalasine | ||
| 0 µM | 10.01 ± 0.54 | 15.9 ± 1.22 |
| 0.01mM | 10.26 ± 0.3 | 11.69 ± 0.45 |
| 0.1mM | 8.92 ± 1.65 | 11.34 ± 0.91 |
| 1 mM | 7.87 ± 0.64 | 9.69 ± 1.04 |
| PDTC (pyrrolidinedithiocarbamate) | ||
| 0 µM | 10.01 ± 0.54 | 15.9 ± 1.22 |
| 1 µM | 8.76 ± 0.27 | 11.71 ± 0.11 |
| 5 µM | 8.59 ± 0.72 | 10.17 ± 0.44 c |
| 10 µM | 7.83 ± 0.25 c | 9.21 ± 1.01 c |
| 25 µM | 5.84 ± 0.48 c | 7.81 ± 0.27 c |
| 50 µM | 3.52 ± 0.34 c | 4.14 ± 0.09 c |
Data are presented as means ± S.D. of one of three separate experiments that were performed in duplicates and yielded similar results. IL-6 levels are expressed in ng per mg of cell protein.
Cells were treated with the indicated concentrations of inhibitors for 1hr prior to a 20h stimulation with LPS (100ng/ml) or E. coli K-12 (2ng/ml).
p < 0.05, IL-8 levels in the presence of inhibitor vs. control (in the absence of inhibitor), Student’s t test.
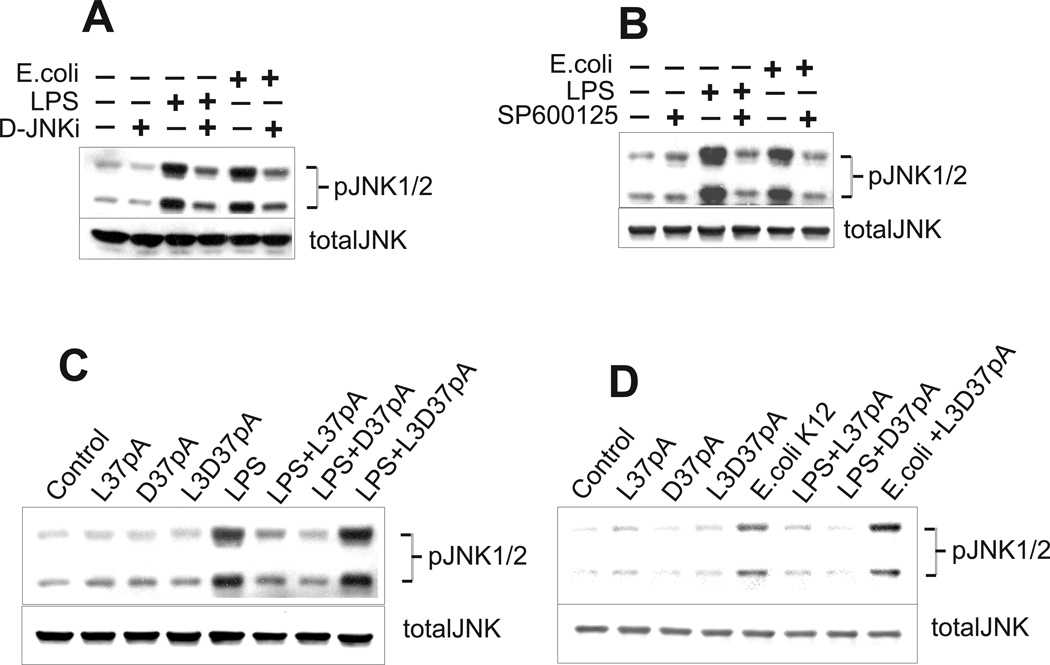
JNK1/2 inhibitors and amphipathic α-helical peptides block LPS and E. coli K-12-induced JNK1/2 activation assessed by immunoblotting
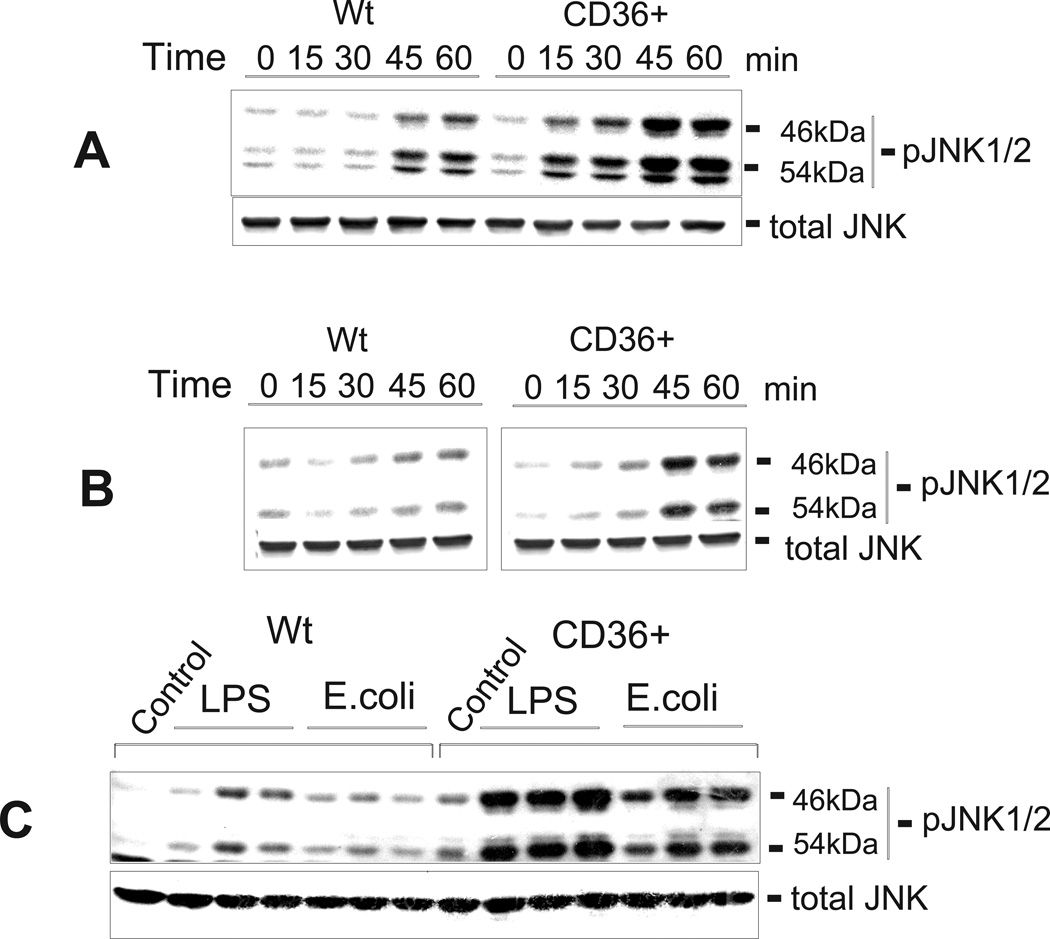
In order to confirm the role of CD36 in LPS and E. coli-induced JNK activation, we compared the levels of JNK1/2 phosphorylation in control and hCD36-overexpressing HEK293 cells by immunoblotting. Fig. 9 shows that, while JNK1/2 was transiently phosphorylated in both cell types, CD36-overexpressing cells demonstrated 3- to 8-times higher levels of JNK phosphorylation vs. control cells following treatment with increasing doses of LPS or E. coli (Fig. 8C) as well as within 1-h of treatment with either 100 ng/ml LPS (Fig. 9A) or 2 ng/ml of E. coli (Fig. 9B). A 1-h treatment of CD36-overexpressing cells with JNK inhibitors, D-JNK peptide inhibitor (Fig. 10A) or SP600125 (Fig. 10B), prior to a 45-min incubation of cells with LPS or E. coli, reduced JNK1/2 phosphorylation by 65–80 %.
FIGURE 9.
Time- and dose-dependent LPS- and E. coli- induced JNK1/2 activation in CD36-overexpressing and mock-transfected HEK293 cells. Following an overnight incubation in serum-free medium, the cells were stimulated with 100 ng/ml of LPS (A) or 1 ng/ml of E. coli K12 (B) for varying time intervals, or, alternatively, the cells were stimulated with 10, 100 or 1000 ng/ml of LPS or 1, 5 or 10 ng/ml of E. coli K12 for 45 min (C). Phosphorylated forms of JNK1/2 were detected by Western blotting using specific anti-JNK1/2 (pThr183/pTyr185) antibodies. The non-phosphorylated form of JNK was assayed simultaneously in the same samples.
FIGURE 10.
Specific JNK inhibitors and synthetic amphipathic helical peptides block CD36-mediated phosphorylation of JNK1/2 induced by LPS and E. coli K12. CD36-overexpressing HEK293 cells were treated with either 5 µM of D-retro-inverso form of the cell permeable JNK peptide inhibitor (D-JNKi1) (A) or 10 µM of SP600125, selective JNK1,−2,−3 inhibitor (B) for 2 h prior to a 45-min stimulation with LPS (100 ng/ml) or E. coli K12 (1 ng/ml). Alternatively, cells were pre-incubated for 1 h with 10 µg/ml of either peptide – L-37pA, D-37pA or 3D-37pA, followed by a 45-min incubation with 100 ng/ml of LPS (C) or 1 ng/ml of E. coli K12 (D). Data represent one of three separate experiments that yielded similar results.
To provide further evidence of CD36-dependence of LPS- and bacterium-induced JNK phosphorylation, we tested the ability of amphipathic α-helical peptides, previously shown to be CD36 receptor agonists, to alter JNK1/2 activation induced by LPS and E. coli. Both α-helical peptides, L-37pA and D-37pA, efficiently decreased the levels of JNK1/2 phosphorylation induced by LPS and E. coli in CD36-overexpressing cells, while the L3D-37pA peptide with a disturbed amphipathic α-helical motif was ineffective (Figs. 10, C and D).
Discussion
Previous reports have indicated that CD36 is an important component of the innate immune system recognizing microbial pathogens and their cell wall lipids. Philips et al. (5) demonstrated enhanced E. coli uptake and, to a lesser extent, S. aureus uptake by human HEK293 cells transfected with murine CD36. In contrast, results obtained by Stuart et al. (6) suggested that murine CD36 preferentially recognizes S. aureus and its wall component LTA, whereas CD14 plays a critical role in host cytokine responses to E. coli (33). Furthermore, there are still conflicting data regarding the mechanisms of CD36-mediated signal transduction and TLRs-CD36 interplay.
To address these questions, we used human cell based-models in which hCD36 was overexpressed in HeLa and HEK293 cell lines. Additionally, these cell lines were selected because of their negligible or nonexistent level of the primary bacterial pathogen signaling receptors, TLR2/4, allowing dissection of a direct CD36-mediated signaling pathway from those associated with CD36/TLRs crosstalk. The results of our flow cytometric analyses demonstrated that uptake of various Gram-negative and Gram-positive bacteria was markedly enhanced in hCD36-overexpressing vs. mock-transfected HeLa cells. These results did not establish a preferential uptake of bacterium species by hCD36. However, they did indicate that hCD36 functions as a pattern recognition receptor and mediates the uptake of various bacterial pathogens. Our findings also demonstrated markedly increased uptake of fluorescently labeled LPS in hCD36-overexpressing cells when compared to mock-transfected cells. The uptake of Bodipy-LTA demonstrated comparably high background in both mock-transfected and CD36 expressing HeLa cells, suggesting potential involvement of CD36-independent mechanism(s) for LTA uptake in epithelial cells. It is also important that in HEK293 epithelial cells with negligible expression of TLRs, LTA was found to be a poor ligand in terms of cytokine release induction (Fig 5B). This observation is in contrast to phagocytes, which express both - TLRs and CD36 and significantly respond to LTA (6). In agreement with an earlier reported observation that transfection of HEK293 epithelial cells with the murine CD36 results in the 2–3–fold increase of binding and internalization of both S. aureus and E. coli (6), our comparative analyses of bacterial uptake also did not reveal distinct differences between Gram-positive and Gram-negative pathogens, suggesting that something other than LTA structural components may be involved with Gram-positive bacteria uptake. Further, the results of our flow cytometry studies demonstrate for the first time that L-37pA and D-37pA, two amphipathic α-helical peptides, act as potent agonists for the CD36 receptor.
Utilizing human and mouse based models, CD36 was found to be capable of both preferentially binding Gram-positive bacterial ligands for presentation to TLR signaling pathways and internalizing circulating bacteria for subsequent clearance (6). While there is experimental evidence against TLRs functioning as phagocytic receptors (33, 34), CD36 appears to be directly engaged in bacterial phagocytosis. Our confocal imaging experiments with hCD36-dependent bacterial uptake revealed intracellular co-localization of E. coli K12 with LysoTracker Red, a lysosomal tracker, as well as with an antibody against LAMP-2, a marker of late endosomes/lysosomes, (data not shown). These results provide additional evidence that CD36 mediates bacterial clearance by sequestering bacteria within lysosomes (6).
Previous reports suggested that CD36 functions as an accessory protein, or co-receptor, sensing and clustering TLR2 ligands and, in particular LTA, to facilitate TLR2-mediated NF-kB activation (6, 14, 15). In this work, we examined the hypothesis that CD36 might activate signaling through TLR-independent pathway(s). We studied HEK293 cells that have low if any TLR2 or TLR4 expression (20, 22) as well as low levels of CD36 expression (data not shown). CD36-overexpressing HEK293 cells demonstrated high responsiveness to various Gram-negative bacterial strains and different LPS preparations as manifested by markedly elevated IL-8 secretion. The IL-8 secretion response of these cells was, however, distinctly weaker to Gram-positive bacteria and was absent with LTA. In wild type HEK293 cells we observed little or no IL-8 response towards a variety of bacteria, LPS or LTA, due to low if any expression of TLR2 or TLR4 (20, 22). These findings indicate that, in contrast to the LTA response, which requires functional TLR2 expression, LPS and Gram-negative bacteria can induce direct CD36 signaling independent of TLR activity.
The role of CD36 in TLR independent signaling was also confirmed by CD36 siRNA experiments where TLR2/4 positive fibroblasts demonstrated a reduced response towards LPS, E. coli and S. aureus when compared to control cells treated with non-targeting siRNA. In contrast, CD36 overexpression in human fibroblasts resulted in a 2–3 fold increase in cytokine release induced by both bacteria and LPS. Observing cytokine release changes with both up and down regulation of CD36 strongly supports its role in signaling.
Since a previous study was performed on HEK293 cells transfected with murine CD36 (6), we can only suggest that the species difference of human CD36 used in our study accounts for the differences in the receptor’s response towards LPS, LTA and microbial ligands. Although it is known that human, murine and rat CD36 share > 90% sequence homology at the amino acid level, it has been reported that differences observed in the binding phenotype of human and rodent CD36, namely their affinity to Plasmodium falciparum-infected erythrocytes, can be attributed to a single amino acid residue (35). The authors of that work have presented evidence that the site-directed mutagenesis of the histidine at position 242 of hCD36 to tyrosine (found in rodent CD36) confers the binding phenotype of rodent CD36 onto hCD36. Our data allow for speculating that minor differences in the sequence of human and murine CD36 binding motifs as well as in their C-terminal sequences responsible for downstream signaling, may account for CD36 species-specific receptor activities. Further evidence of a CD36 species-dependent role in the inflammatory cell response towards bacteria, and their cell wall structural components comes from our study performed on CD36-deficient and normal rat macrophages. Upon the challenge with any of the inflammatory agents, E. coli, S. aureus, LPS or LTA, CD36 (−/−) cells demonstrated a profound (40–70%) reduction in IL-6 and TNF-α production as compared to the wild type macrophages with normal levels of functional CD36. These data indicate that even in macrophages, cells highly expressing TLR2/TLR4, CD36 significantly contributes to the cell inflammatory responses towards various bacterial pathogens and their cell wall components.
Stuart et al. (6) recently demonstrated that the internalization of S. aureus is critically dependent on murine CD36 and, in particular, on its C-terminal cytoplasmic domain, and CD36 plays a crucial role in TLR2-mediated signal transduction. In contrast, data presented by other authors (14, 15) do not support the involvement of CD36 in signal transduction and consider it as a co-receptor for diacylglyceride recognition, presenting associated ligand to the TLR2/6 heterodimer. According to these authors, CD36, which lacks a signaling domain, acts merely as a facilitator, analogous to CD14, concentrating LPS for transduction through TLR4. At the same time, there is growing experimental evidence that strongly supports the function of CD36 as an independent signaling receptor capable of triggering intracellular signaling pathways upon ligand association. For instance, CD36 is known to form an extracellular loop containing ligand−binding domains, and its NH2- and COOH-terminal cytoplasmic tails are palmitoylated, localizing this receptor to lipid rafts (36). These domains are rich in signaling proteins, and CD36 has been shown to interact with 3 Src kinase family members (Fyn, Yes, and Lyn proteins) that reside within lipid rafts to engage downstream signaling cascades, including p38 and p44/42 MAP kinase pathways (4). As demonstrated by Moore et al. (19) fibrillar β-amyloid induces CD36 association with the Src phosphotyrosine kinase (PTK) Lyn and activates a signaling cascade involving another Src kinase family member, Fyn, and p44/42 MAP kinase. Another CD36 ligand, thrombospondin-1 (TSP-1), has been shown to induce activation of JNK through engagement of CD36 since the activation was blocked by anti-CD36 antibodies and stimulated by short anti-angiogenic peptides derived from TSP-1 that act exclusively via CD36 (18). Finally, a recent study by Rahaman et al. (17) demonstrated that CD36 is associated with a signaling complex containing Lyn and MEKK2, and directly mediated oxLDL-induced activation of JNK1 and JNK2.
To test the hypothesis that CD36 can directly mediate signaling, we analyzed the effects of various signaling inhibitors on CD36-associated IL-8 secretion using HEK293 cells that have little or no expression of TLR2/4. These experiments revealed that CD36-dependent IL-8 release induced by either LPS or bacteria was inhibited 50–70% by JNK1/2 blockers. No or only weak inhibitory effects (15–20%) were observed with various NF-kB inhibitors (Table I), MEK or p38 MAP kinase inhibitors, suggesting a direct JNK activation. Western blotting results confirmed that, upon challenge with E. coli K12 or LPS, CD36-overexpressing cells responded by markedly enhanced JNK1/2 phosphorylation when compared to control cells. Importantly, JNK1/2 inhibitors dose-dependently reduced CD36-associated JNK phosphorylation. Thus, our results demonstrate that enhanced LPS- and E. coli- induced IL-8 secretion in HEK 293 cells is mainly due to the up regulation of JNK kinase activity via the upstream activator of MEK4-JNK-c-Jun signaling pathway. The transcription of the IL-8 gene requires activation of several transcription factors, with NF-kB and activator protein (AP-1), to be the most prominent inducers of IL-8 in most cells (31, 37). The phosphorylation of c-Jun by the c-Jun N-terminal kinase (JNK) is a primary determinant of the AP-1 activity. The involvement of both NF-kB and JNK-mediated signaling pathways in regulating of IL-8 expression in HEK293 cells has been demonstrated previously for IL-1 and TNF-a (38). In contrast to findings obtained in several other cell types, a recent report of Venza et al. (39) demonstrated that the sequential activation of JNK and c-Jun alone followed by the AP-1 binding is responsible for increased IL-8 transcription in S. aureus challenged conjunctival epithelial cells; at the same time no NF-kB or p38 MAPK activation was detected. The results of our studies performed on the normal rat macrophages provide additional support for our observation regarding the major contribution of JNK signaling for CD36-dependent LPS and E. coli- induced cytokine production. Of all signaling inhibitors tested, SP600125, a selective JNK inhibitor, resulted in the greatest decrease of IL-6 (Table III) and TNF-α (data not shown) secretion in macrophages stimulated with LPS or E. coli. In contrast to HEK293 cells, LPS/E. coli- induced cytokine production in macrophages was also significantly decreased in presence of NF-kB inhibitor, suggesting important contributions from both JNK and NF-kB signaling pathways to the inflammatory response of phagocytic cells.
Another important finding of our study was that L-37pA and D-37pA, two amphipathic α-helical peptides, known as apoA-I mimics and human Cla-1/2 (rodent SR-BI/II) agonists, appeared to be both CD36 ligands and efficient inhibitors of CD36-mediated E. coli- and LPS- induced JNK1/2 activation and IL-8 production. This new observation may shed a light on the mechanisms of the anti-atherogenic effects of apoA-I mimetic amphipathic helical peptide drugs (40). This is also consistent with an earlier report that CD36 knockout mice are less prone to atherosclerosis (41), as well as with recent data revealing an anti-atherosclerotic effect of another novel CD36 ligand, synthetic hexapeptide, a non-active analog of the growth hormone-releasing peptide (42). Additionally, newly emerging experimental and epidemiological evidence for an important role of various infectious agents in promoting inflammation and accelerating atherosclerosis (43–48) indicate that CD36 could contribute to atherogenesis by aggravating bacterium-induced pro-inflammatory processes.
In conclusion, in addition to a previously revealed CD36 role as a TLR2/6 co-receptor required to present bacterial ligands to the TLR- mediated signaling pathways (6, 14, 15), our study demonstrates that CD36 can function as an independent pattern recognition receptor for various bacterial species and bacterial cell wall compounds and is capable to directly mediate both bacterial phagocytosis and initiation of intracellular signaling pathways with subsequent pro-inflammatory cytokine release. These events could presumably occur independently of TLRs in epithelial cells, and apparently are involved in both TLR-dependent and independent innate immune responses in phagocytic cells. Our findings implicating hCD36 in bacterium- and LPS-induced signaling support its important role in the innate immune response and provide further insights into the molecular mechanisms underlying Gram-negative sepsis. Finally, demonstration of CD36 as a receptor for synthetic amphipathic helical peptides, that appeared to be potent inhibitors of LPS- and bacterium-induced pro-inflammatory responses, raises the possibility that CD36 could be a potential therapeutic target in devising novel septic shock treatments.
Acknowledgements
We thank Dr. J. Philip McCoy, Jr., Director of the Flow Cytometry Core Facility, NHLBI, National Institutes of Health, for conducting cell sorting. We are also grateful to Dr. Daniela Malide and Dr. Christian A. Comb, NHLBI Light Microscopy Core Facility, National Institutes of Health, for their expert and generous help with confocal microscopy analyses. We also thank Dr. Dong Fu and Dr. Irwin Arias, Unit of Polarity, Cell Biology and Metabolism Program, NIHCD, National Institutes of Health, for providing Wistar-Kioto SHR/NCrl rats for our study.
Footnotes
Disclosures
The authors have no financial conflicts of interest.
This research was supported by the Intramural Research Program of the Clinical Center, National Institute of Diabetes and Digestive and Kidney Disease and the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations used in this paper: hCD36, human CD36; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LTA, lipoteichoic acid; MEK, MAP kinase kinase; oxLDL, oxidized LDL.
References
- 1.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J. Clin. Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rigotti A, Acton SL, Krieger M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J. Biol. Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- 3.Hirano K, Kuwasako T, Nakagawa-Toyama Y, Janabi M, Yamashita S, Matsuzawa Y. Pathophysiology of human genetic CD36 deficiency. Trends Cardiovasc. Med. 2003;13:136–141. doi: 10.1016/s1050-1738(03)00026-4. [DOI] [PubMed] [Google Scholar]
- 4.Medeiros LA, Khan T, El Khoury JB, Pham CL, Hatters DM, Howlett GJ, Lopez R, O'Brien KD, Moore KJ. Fibrillar amyloid protein present in atheroma activates CD36 signal transduction. J. Biol. Chem. 2004;279:10643–10648. doi: 10.1074/jbc.M311735200. [DOI] [PubMed] [Google Scholar]
- 5.Philips JA, Rubin EJ, Perrimon N. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science. 2005;309:1251–1253. doi: 10.1126/science.1116006. [DOI] [PubMed] [Google Scholar]
- 6.Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennesy EJ, Ezekowitz RAB, Moore KJ. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J. Cell Biol. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peiser L, de Winther MPJ, Makepeace K, Hollinshead M, Coull P, Plested J, Kodama T, Moxon ER, Gordon S. The class A macrophage scavenger receptor is a major pattern recognition receptor for Neisseria meningitidis which is independent of lipopolysaccharide and not required for secretory responses. Infect. Immun. 2002;70:5346–5354. doi: 10.1128/IAI.70.10.5346-5354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda K, Kaisho T, Akira S. Toll-like receptors. Ann. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 9.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mavkman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat. Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 10.Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001;7:167–202. [PubMed] [Google Scholar]
- 11.Hampton RY, Golenbock DT, Penman M, Krieger M, Raetz CR. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991;352:342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- 12.Shnyra A, Lindberg AA. Scavenger receptor pathway for lipopolysaccharide binding to Kupffer and endothelial liver cells in vitro. Infect. Immun. 1995;63:865–873. doi: 10.1128/iai.63.3.865-873.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vishnyakova TG, Bocharov AV, Baranova IN, Chen Z, Remaley AT, Csako G, Eggerman TL, Patterson AP. Binding and internalization of lipopolysaccharide by Cla-1, a human orthologue of rodent scavenger receptor B1. J. Biol. Chem. 2003;278:22771–22780. doi: 10.1074/jbc.M211032200. [DOI] [PubMed] [Google Scholar]
- 14.Triantafilou M, Gamper FGJ, Haston RM, Mouratis MA, Morath S, Hartung T, Triantafilou K. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J. Biol. Chem. 2006;281:31002–31011. doi: 10.1074/jbc.M602794200. [DOI] [PubMed] [Google Scholar]
- 15.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zaringer U U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 16.Huang MM, Bolen JB, Barnwell JW, Shattil SJ, Brugge JS. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc. Natl. Acad. Sci. U. S. A. 1991;88:7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimenez B, Volpert OV, Reiher F, Chang L, Munoz A, Karin M, Bouck N. c-Jun N-terminal kinase activation is required for the inhibition of neovascularization by thrombospondin-1. Oncogene. 2001;20:3443–3448. doi: 10.1038/sj.onc.1204464. [DOI] [PubMed] [Google Scholar]
- 19.Moore KJ, Khoury JE, Medeiros LA, Terada K, Geula C, Luster A, Freeman MW. A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J. Biol. Chem. 2002;277:47373–47379. doi: 10.1074/jbc.M208788200. [DOI] [PubMed] [Google Scholar]
- 20.Gon Y, Asai Y, Hashimoto S, Mizumura K, Jibiki I, Machino T, Ra C, Horie T. A20 inhibits toll-like receptor 2- and 4-mediated interleukin-8 synthesis in airway epithelial cells. Am. J. Resp. Cell Mol. Biol. 2004;31:330–336. doi: 10.1165/rcmb.2003-0438OC. [DOI] [PubMed] [Google Scholar]
- 21.Wyllie DH, Kiss-Toth E, Visintin A, Smith SC, Boussouf S, Segal DM, Duff GW, Dower SK. Evidence for an accessory protein function for Toll-like receptor 1 in anti-bacterial responses. J. Immunol. 2000;165:7125–7132. doi: 10.4049/jimmunol.165.12.7125. [DOI] [PubMed] [Google Scholar]
- 22.Lepper PM, Triantafilou M, Schumann C, Schneider EM, Triantafilou K. Lipopolysaccharides from Helicobacter pylori can act as antagonists for Toll-like receptor 4. Cell. Microbiol. 2005;7:519–528. doi: 10.1111/j.1462-5822.2005.00482.x. [DOI] [PubMed] [Google Scholar]
- 23.Merrifield RB. The synthesis of biologically active peptides and proteins. JAMA. 1969;210:1247–1254. [PubMed] [Google Scholar]
- 24.Fairwell T, Hospattankar AV, Brewer HB, Jr., Khan SA. Human plasma apolipoprotein C-II: total solid-phase synthesis and chemical and biological characterization. Proc. Natl. Acad. Sci. U. S. A. 1987;84:4796–4800. doi: 10.1073/pnas.84.14.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bocharov AV, Baranova IN, Vishnyakova TG, Remaley AT, Csako G, Thomas F, Patterson AP, Eggerman TL. Targeting of scavenger receptor class B type I by synthetic amphipathic alpha-helical-containing peptides blocks lipopolysaccharide (LPS) uptake and LPS-induced pro-inflammatory cytokine responses in THP-1 monocyte cells. J. Biol. Chem. 2004;279:36072–36082. doi: 10.1074/jbc.M314264200. [DOI] [PubMed] [Google Scholar]
- 26.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 27.Vishnyakova TG, Kurlander R, Bocharov AV, Baranova IN, Chen Z, Abu-Asab MS, Tsokos M, Malide D, Basso F, Remaley AT, Csako G, Eggerman TL, Patterson AP. CLA-1 and its splicing variant CLA-2 mediate bacterial adhesion and cytosolic bacterial invasion in mammalian cells. Proc. Natl. Acad. Sci. USA. 2006;45:16888–16893. doi: 10.1073/pnas.0602126103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Schulthess G, Compassi S, Werder M, Han CH, Phillips MC, Hauser H. Intestinal sterol absorption mediated by scavenger receptors is competitively inhibited by amphipathic peptides and proteins. Biochemistry. 2000;39:12623–12631. doi: 10.1021/bi0011633. [DOI] [PubMed] [Google Scholar]
- 30.Doyle SL, O’Neil LA. Toll-like receptors: from the discovery of NF-kappa B to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 2006;72:1102–1113. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- 32.Krause A, Holtmann H, Eickemeier S, Winzen R, Szamel M, Resch K, Saklatvala J, Kracht M. Stress-activated protein kinase/Jun N-terminal kinase is required for interleukin (IL)-1-induced IL-6 and IL-8 gene expression in the human epidermal carcinoma cell line KB. J. Biol. Chem. 1998;273:23681–23689. doi: 10.1074/jbc.273.37.23681. [DOI] [PubMed] [Google Scholar]
- 33.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J. Endotoxin Res. 2005;11:225–229. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- 34.Dunzendorfer S, Lee HK, Soldau K, Tobias PS. TLR4 is the signaling but not the lipopolysaccharide uptake receptor. J. Immunol. 2004;173:1166–1170. doi: 10.4049/jimmunol.173.2.1166. [DOI] [PubMed] [Google Scholar]
- 35.Serghides L, Crandall I, Hull E, Kain KC. The Plasmodium falciparum-CD36 interaction is modified by a single amino acid substitution in CD36. Blood. 1998;92:1814–1819. [PubMed] [Google Scholar]
- 36.Tao N, Wagner SJ, Lublin DM. CD36 is palmitoylated on both N- and C-terminal cytoplasmic tails. J. Biol.Chem. 1996;271:22315–22320. doi: 10.1074/jbc.271.37.22315. [DOI] [PubMed] [Google Scholar]
- 37.Roebuck KA. Regulation of interleukin-8 gene expression. J. Interferon Cytokine Res. 1999;19:429–438. doi: 10.1089/107999099313866. Review. [DOI] [PubMed] [Google Scholar]
- 38.Baud V, Liu ZG, Bennett B, Suzuki N, Xia Y, Karin M. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999;13:1297–1308. doi: 10.1101/gad.13.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venza I, Cucinotta M, Caristi S, Mancuso G, Teti D. Transcriptional Regulation of IL-8 by Staphylococcus aureus in human conjunctival cells involves activation of AP-1. Invest. Ophthalmol Vis Sci. 2007;48:270–276. doi: 10.1167/iovs.06-0081. [DOI] [PubMed] [Google Scholar]
- 40.Navab M, Anantharamaiah GM, Reddy ST, Fogelman AM. Apolipoprotein A-I mimetic peptides and their role in atherosclerosis prevention. Nat. Clin. Pract. Cardiovasc. Med. 2006;3:540–547. doi: 10.1038/ncpcardio0661. [DOI] [PubMed] [Google Scholar]
- 41.Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marleau S, Harb D, Bujold K, Avallone R, Iken K, Wang Y, Demers A, Sirois MG, Febbraio M, Silverstein RL, Tremblay A, Ong H. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. FASEB J. 2005;19:1869–1871. doi: 10.1096/fj.04-3253fje. [DOI] [PubMed] [Google Scholar]
- 43.Kuo C, Grayston JT, Campbell LA, Goo YA. Chlamydia pneumoniae (TWAR) in coronary arteries of young adults (15–34 years old) Proc. Natl. Acad. Sci. USA. 1995;92:6911–6914. doi: 10.1073/pnas.92.15.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grayston JT. Background and current knowledge of Chlamydia pneumoniae and atherosclerosis. J. Infect. Dis. 2000;181:S402–S410. doi: 10.1086/315596. [DOI] [PubMed] [Google Scholar]
- 45.Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 46.Danesh J, Wong Y, Ward M, Muir J. Chronic infection with Helicobacter pylori, Chlamydia pneumoniae, or cytomegalovirus: population based study of coronary heart disease. Heart. 1999;81:245–247. doi: 10.1136/hrt.81.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibson FC, Yumoto H, III, Takahashi Y, Chou HH, Genco CA. Innate immune signaling and Porphyromonas gingivalis-accelerated atherosclerosis. J.Dent. Res. 2006;85:106–121. doi: 10.1177/154405910608500202. [DOI] [PubMed] [Google Scholar]
- 48.Roth GA, Moser B, Roth-Walter F, Giacona MB, Harja E, Papapanou PN, Schmidt AM, Lalla E. Infection with a periodontal pathogen increases mononuclear cell adhesion to human aortic endothelial cells. Atherosclerosis. 2007;190:271–281. doi: 10.1016/j.atherosclerosis.2006.03.018. [DOI] [PubMed] [Google Scholar]