Significance
Poor air quality from fungal growth in water-damaged, moldy buildings/residences is correlated with a negative impact on human health. The volatile organic compound 1-octen-3-ol is commonly emitted by molds and is responsible for much of the distinctive moldy odor associated with fungal colonization. Using a Drosophila model, we demonstrate via genetic, biochemical, and immunological studies that 1-octen-3-ol causes dopamine neuron degeneration through disruption of dopamine handling. These data demonstrate that 1-octen-3-ol exerts toxicity via disruption of dopamine homeostasis and may represent a naturally occurring environmental agent involved in parkinsonism. Moreover, it provides possible insights into reported movement disorders associated with human exposure to fungi and their volatile organic compounds.
Keywords: building-related illness, mold, mushroom alcohol
Abstract
Parkinson disease (PD) is the most common movement disorder and, although the exact causes are unknown, recent epidemiological and experimental studies indicate that several environmental agents may be significant risk factors. To date, these suspected environmental risk factors have been man-made chemicals. In this report, we demonstrate via genetic, biochemical, and immunological studies that the common volatile fungal semiochemical 1-octen-3-ol reduces dopamine levels and causes dopamine neuron degeneration in Drosophila melanogaster. Overexpression of the vesicular monoamine transporter (VMAT) rescued the dopamine toxicity and neurodegeneration, whereas mutations decreasing VMAT and tyrosine hydroxylase exacerbated toxicity. Furthermore, 1-octen-3-ol also inhibited uptake of dopamine in human cell lines expressing the human plasma membrane dopamine transporter (DAT) and human VMAT ortholog, VMAT2. These data demonstrate that 1-octen-3-ol exerts toxicity via disruption of dopamine homeostasis and may represent a naturally occurring environmental agent involved in parkinsonism.
Parkinson disease (PD), the most common movement disorder, is characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (1). Contributing factors include oxidative stress, mitochondrial dysfunction, disruption of dopamine handling, and protein aggregation (2). The etiology of PD remains unknown, although ∼5% of cases are linked with monogenetic inheritance and involve genetic mutations in at least six genes (SNCA, LRRK2, PARK2, PINK1, DJ-1, and ATP13A2) (3). For the remaining 95% of cases, strong epidemiological evidence associating the exposure with a variety of environmental agents, especially pesticides, has been suggested (4–6). Agents that cause formation of reactive oxygen species through mitochondrial inhibition, disruption of dopamine handling, or redox cycling, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), paraquat, and rotenone, all cause dopaminergic toxicity in animal models (7). Although interactions between genetic and environmental factors are thought to be major contributors to the etiology of PD, the disease was observed long before these synthetic chemicals were introduced. The vast majority of research has focused on synthetic chemicals, such as pesticides and industrial chemicals. Because PD has been around for centuries if not millennia, it is possible that common environmental factors contributed to the disease before the Industrial Revolution and the development and use of these man-made chemicals (6).
Among the proposed naturally occurring environmental agents for PD etiology, the role of fungi and their metabolites has never been elucidated, despite their ubiquitous presence around us. However, the presence of fungi and their metabolites has been linked with poor indoor air quality and adverse health effects (8–11). The quality of indoor air is especially important, because in the United States, people spend almost 90% of their time indoors (12). Interestingly, exposure to fungi has been linked to the presence of neurologic and neuropsychiatric signs and symptoms, including movement disorders and loss of balance and coordination (13, 14). Fungi are known to emit complex mixtures of alcohols, aldehydes, acids, ethers, esters, ketones, hydrocarbons, terpenes, and sulfur compounds and are responsible for the characteristic moldy odors related to damp indoor spaces (15).
In an attempt to develop an inexpensive, invertebrate model for studying the possible toxicological effects of fungal volatile organic compounds (VOCs) associated with indoor environments, we turned to a Drosophila model. Using this model, we reported the toxicity of a variety of fungal toxicants, including 1-octen-3-ol, trans-2-octenal, 3-octanol, 2,5-dimethylfuran, and 2-octanone, at concentrations of 2.8–14 ppm (16). Out of this screen, 1-octen-3-ol was one of the most potent agents and selectively damaged the dopamine system at high levels of exposure. Given its ubiquity in the natural and built environment and recognizing the prevalence of PD long before neurotoxic chemicals such as paraquat or MPTP were synthesized or used (17), we decided to further investigate the role of 1-octen-3-ol as a possible etiological agent for PD.
In this report, we demonstrate that exposure of Drosophila to 1-octen-3-ol vapors at lower concentrations (0.5 ppm) results in loss of dopamine neurons, accompanied by a decrease in dopamine levels. Mechanistically, 1-octen-3-ol appears to interfere with proper dopamine handling, which was reflected by an increase in the dopamine metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) and its ability to inhibit uptake of dopamine in vitro. Furthermore, we assess the role of vesicular monoamine transporter (VMAT) in 1-octen-3-ol–mediated toxicity using genetic and cell-culture assays. Taken together, these data demonstrate that low concentrations of a natural fungal volatile organic compound lead to disruption of dopamine homeostasis and also interact with genetic variants in dopamine biosynthesis to increase dopaminergic neurodegeneration.
Results
Exposure to 1-Octen-3-ol Shortens Survival Span and Affects Locomotion of Wild-Type D. melanogaster.
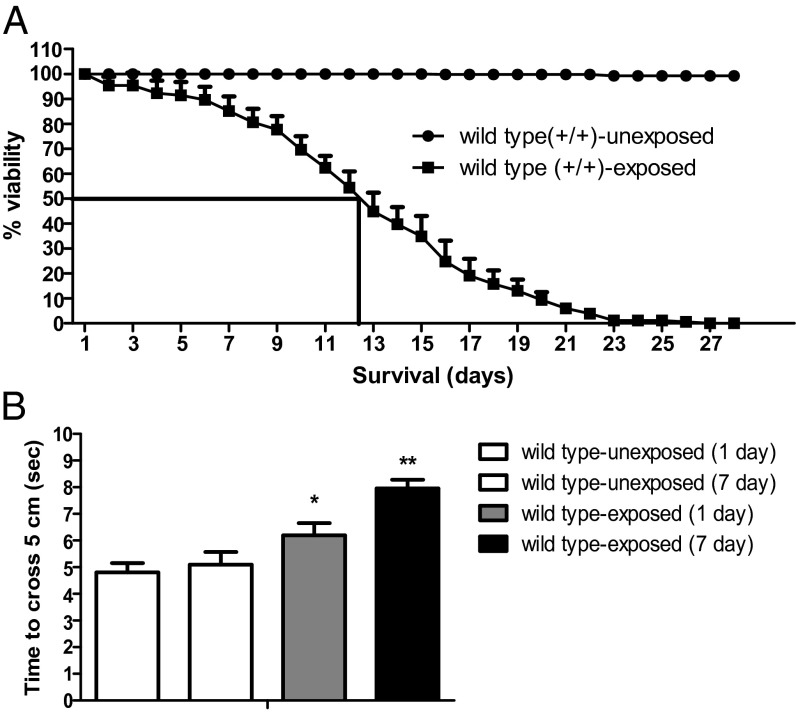
To evaluate the toxic effects of a low dose (0.5 ppm) of 1-octen-3-ol, we exposed wild-type w1118 flies to a pure form of volatile-phase 1-octen-3-ol. The time to 50% mortality of adult flies exposed to 0.5 ppm of 1-octen-3-ol was ∼16.9 d (Fig. 1A). Furthermore, exposure of wild-type flies to 0.5 ppm of 1-octen-3-ol caused locomotor defects as assessed by a negative geotaxis assay as early as 24 h, with a further decline in locomotor activity upon continuous exposure to 1-octen-3-ol until day 7 (Fig. 1B).
Fig. 1.
Common fungal VOC 1-octen-3-ol exposure truncates survival span of wild type and induces mobility defects. The data were collected daily until all of the flies exposed to 1-octen-3-ol were dead, and the percentage mortality of dead flies was calculated. (A) The 50% mortality for wild-type flies is shown (n = 125), and data are from three independent experiments. (B) Forty eight-hour-old wild-type flies were exposed to 0.5 ppm of 1-octen-3-ol, and time to cross a 5-cm distance was recorded after 1 and 7 d of continuous exposure. There was a significant difference in the locomotory activity between wild-type unexposed and exposed flies assessed with a negative geotaxis assay at days 1 and 7. Error bars represent SEM, and the significant difference between wild-type exposed and unexposed flies is indicated; *P < 0.05, **P < 0.005.
Exposure to 1-Octen-3-ol Induces Loss of Dopaminergic Neurons and Decreases Dopamine Levels in Head Extracts.
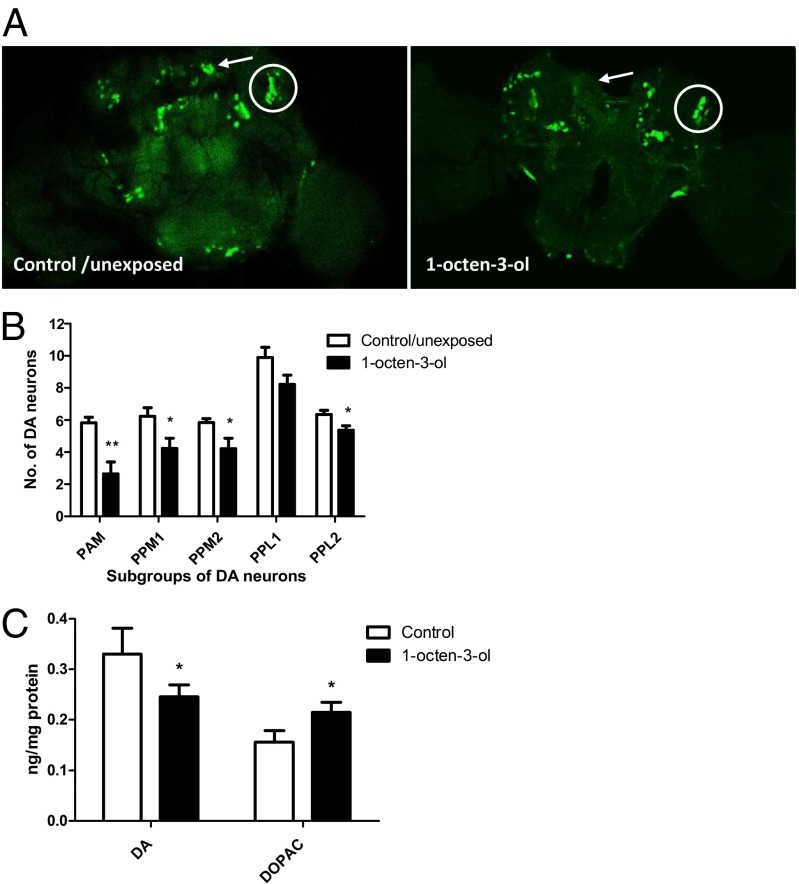
To test whether 0.5 ppm of 1-octen-3-ol causes loss of dopaminergic neurons, we used transgenic TH-GAL4; UAS-GFP adults (18). The morphology and number of posteriorly located dopaminergic neurons in these transgenic fly brains were assessed after a 24-h exposure to 0.5 ppm of 1-octen-3-ol. Exposure to 1-octen-3-ol significantly decreased the number of all of the subgroups of dopaminergic neurons, except for PPL1 (Fig. 2 A and B). We then performed HPLC to quantify the effect of 0.5 ppm of 1-octen-3-ol on levels of dopamine and its metabolites. A significant decrease in dopamine levels (28%) was observed concomitantly with an increase in DOPAC levels (40%) compared with control flies. These data suggested that a potential impairment of dopamine handling is associated with exposure to 1-octen-3-ol (Fig. 2C).
Fig. 2.
Effect of 1-octen-3-ol on dopamine pools in head extracts of Drosophila flies. (A) Exposure of TH-GAL4; UAS-GFP to 0.5 ppm of 1-octen-3-ol for 24 h led to a decrease of GFP-expressing TH neurons in adult brains. The PPM1 and PPL1 subgroups of DA neurons are indicated by an arrow and circle, respectively, in unexposed and 1-octen-3-ol–exposed adult brains. (B) Scoring for the average number of subgroups of dopaminergic neurons for control and 0.5-ppm 1-octen-3-ol–exposed TH-GAL4; UAS-GFP adult brains. Except for PPL1, there was a significant decrease in all of the subgroups of dopaminergic neurons in brains exposed to 0.5 ppm of 1-octen-3-ol (n = 10–15). (C) Exposure to 0.5 ppm of 1-octen-3-ol for 24 h caused a decrease in dopamine pools with a subsequent increase in DOPAC levels in head extracts (n = 200 for each group; data represent values from three independent experiments). Error bars represent the SE of the mean, and the significant difference between control and 1-octen-3-ol–exposed brains is indicated; *P < 0.05, **P < 0.005.
1-Octen-3-ol Exposure Inhibits Dopamine Uptake in HEK-Dopamine Transporter/VMAT Ortholog Cells via Its Action on the Dopamine Transporter.
To determine whether the increased DOPAC observed following exposure to 1-octen-3-ol was the result of impaired dopamine transport, we measured the effect of 1-octen-3-ol on [3H] dopamine (DA) uptake in human embryonic kidney (HEK) cells stably expressing human dopamine transporter (DAT) and human VMAT ortholog VMAT2. We exposed 1, 3, and 10 ppm of 1-octen-3-ol for 2 h to these cell lines and found no significant effect on DAT/VMAT activity at 1 ppm, but 3 and 10 ppm reduced the uptake to a significant extent. Exposure to 10 ppm of 1-octen-3-ol for 2 h reduced uptake by 95% in HEK-DAT cells. The experiment was repeated in HEK-DAT/VMAT2 cells that express human DAT and VMAT2 with 10 ppm, and similar results were observed (Fig. 3 A and B). Protein assays confirmed that the loss of uptake was not due to a loss of cells after exposure to 1-octen-3-ol. The near-complete inhibition of DAT prevents the assay from detecting any effect of 1-octen-3-ol on VMAT2. Additionally, methods to measure VMAT2 uptake were not compatible with the airborne exposure setup. Therefore, we took a more direct approach to assessing the putative role of VMAT in the effects of 1-octen-3-ol.
Fig. 3.
Radioactive dopamine uptake in transgenic human embryonic HEK-DAT (A) and HEK-DAT/VMAT2 cells (B). Cells were exposed to 10 ppm of 1-octen-3-ol by the airborne exposure method for 2 h. Radioactive dopamine uptake was carried out for 10 min. Uptake was significantly reduced by 95% in HEK-DAT/VMAT2 cells and 90% in HEK-DAT cells as assessed by two-way ANOVA, and the significant difference between unexposed and 1-octen-3-ol–exposed cells is indicated; ****P < 0.0001.
VMAT Mutants Are Sensitive Whereas Transgenic Strains for VMAT Are Resistant to 0.5 ppm of 1-Octen-3-ol.
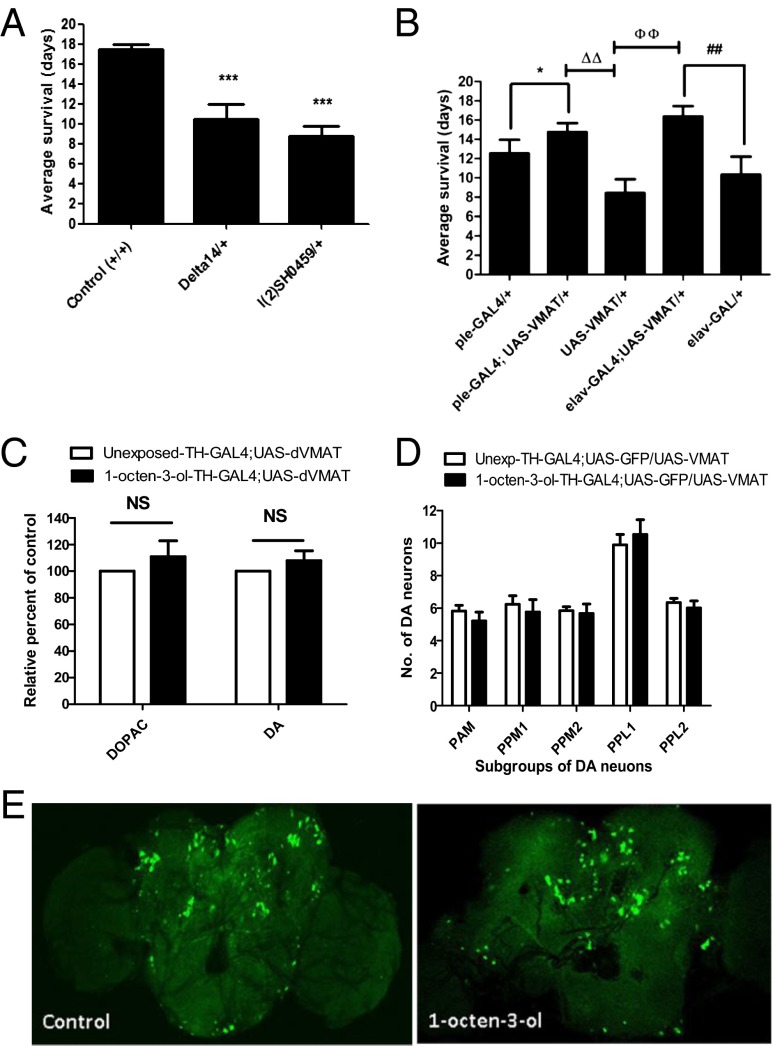
The Drosophila VMAT gene contains two splice variants, dVMAT-A and -B, where dVMAT-A is expressed in all dopaminergic, serotonergic, and octopaminergic neurons and functions similarly to mammalian VMAT2 (19, 20). To assess the potential for 1-octen-3-ol to exert dopaminergic toxicity through disruption of dopamine handling in vivo, we first exposed two loss-of-function mutant lines for dVMAT-A, l(2)SH0459/+ and Delta14/+, to 0.5 ppm of 1-octen-3-ol (21). The survival span was significantly reduced to 8 and 10 d in l(2)SH0459/+ and Delta14/+ dVMAT mutants, respectively, compared with 17 d for w1118 wild-type flies (Fig. 4A). The unexposed wild-type flies (+/+) and dVMAT mutant strains l(2)SH0459/+ and Delta14/+ used as controls for these experiments failed to show any death during this period. We then overexpressed UAS-dVMAT in dopaminergic neurons using the dopamine driver TH-GAL4. Upon exposure to 0.5 ppm of 1-octen-3-ol, average survival of overexpressed transgenic flies TH-GAL4-4; UAS-dVMAT was about 3.5 d longer than that of control flies TH-GAL4 and UAS-dVMAT. Similarly, the overexpression of UAS-dVMAT using the pan-neuronal driver elav-GAL4 led to an extension of survival duration by 4 d compared with control flies (Fig. 4B).
Fig. 4.
Effect of 1-octen-3-ol on transgenic and mutant dVMAT flies. (A) The exposure of two VMAT mutant lines, l(2)SH0459/+ and Delta14/+, to 0.5 ppm of 1-octen-3-ol led to a decrease in the survival duration compared with wild-type flies (n = 60–80). (B) The overexpression of UAS-dVMAT with TH-GAL4 and elav-GAL4 drivers improved the survival duration compared with control flies TH-GAL4/+ and UAS-dVMAT/+ (n = 80–100). (C) When the TH-GAL4;UAS-dVMAT overexpression line was exposed to 0.5ppm 1-octen-3-ol for 24 hr, dopamine and DOPAC pools in head extracts were restored to control levels (n = 160 for each group; data represent values from two independent experiments). NS, nonsignificant. (D) Scoring for the average number of subgroups of dopaminergic neurons for control and 0.5-ppm 1-octen-3-ol–exposed TH-GAL4; UAS-GFP/UAS-dVMAT adult brains. There was no significant decrease in the dopaminergic subgroups except for PPM1 for control and 0.5 ppm of 1-octen-3-ol (n = 8–12 brains for each group). (E) The exposure of 0.5 ppm of 1-octen-3-ol to TH-GAL4; UAS-GFP/UAS-dVMAT adult brains for 24 h failed to cause any detectable morphological changes in GFP-expressing TH neurons. Error bars represent the SE of the mean, and * indicates a significant difference between control and 1-octen-3-ol exposed brains where *P < 0.05 and ***P < 0.001. ##, ΔΔ, and фф represent significant differences between group of flies as shown in B and indicate P < 0.005.
HPLC analysis of dopamine and DOPAC levels in the head extracts of flies overexpressing dVMAT in dopaminergic neurons (TH-GAL4; UAS-dVMAT) exposed to 0.5 ppm of 1-octen-3-ol failed to show the alteration of DA and DOPAC levels (Fig. 4C) that was observed in wild-type flies (Fig. 2C). We further determined the protective effect of dVMAT against 1-octen-3-ol by evaluating the status of dopaminergic neurons overexpressing dVMAT. The overexpression of dVMAT prevented the 1-octen-3-ol–mediated loss of dopaminergic neurons (Fig. 4 D and E) that was seen in wild-type flies (Fig. 2 A and B).
Heterozygous tyrosine hydroxylase Mutant Flies Are Sensitive to 1-Octen-3-ol and Demonstrate Defective Locomotion Compared with Wild-Type Flies.
Considering that 1-octen-3-ol exposure led to alteration of dopamine homeostasis and dopamine neuron loss, we then investigated whether 1-octen-3-ol mediates the variation in the activity of the gene responsible for dopamine biosynthesis, tyrosine hydroxylase (TH), encoded by pale (ple) locus in Drosophila. We exposed two heterozygous loss-of-function mutant strains of the ple gene, ple2 and ple4, known to have mutations in the tyrosine hydroxylase gene and to possess low dopamine pools (22) to 0.5 ppm of 1-octen-3-ol. Heterozygous mutant strains ple2 and ple4 exhibited reduced tolerance to 0.5 ppm of 1-octen-3-ol with a 50% mortality obtained at 13.8 and 12.4 d, respectively, compared with wild-type flies exposed to the same concentration of 1-octen-3-ol. Unexposed control flies did not show any death during the observed time period (Fig. 5A).
Fig. 5.
Drosophila ple mutants demonstrated sensitivity toward 1-octen-3-ol. (A) Forty eight-hour posteclosed ple mutants ple2/+ and ple4/+ exposed to 0.5 ppm of 1-octen-3-ol showed truncation in survival span compared with age-matched wild-type flies exposed to the same concentration of 1-octen-3-ol. Data were collected daily until all of the flies exposed to 1-octen-3-ol were dead, and the percentage mortality of dead flies was calculated. The 50% mortality for wild-type flies is shown (n = 125; data are from three independent experiments). (B) Forty eight-hour-old wild-type and ple mutants ple2 and ple4 were exposed to 0.5 ppm of 1-octen-3-ol, and time to cross a 5-cm distance was recorded. There was no significant difference in the climbing capacity of ple mutants and wild-type flies upon exposure to 1-octen-3-ol for 1 d (n = 60–80 flies), but continuous exposure of 0.5 ppm of 1-octen-3-ol for 7 d led to a significant increase in time to cross a 5-cm distance by ple mutants compared with wild-type flies (n = 30–40). For both assays, two independent experiments were performed. Error bars represent SEM, and the significant difference between wild-type and ple mutants is indicated; *P < 0.05, **P < 0.005.
We also measured movement deficits in wild-type w1118, ple2, and ple4 mutant flies after 1 and 7 d of exposure to 0.5 ppm of 1-octen-3-ol. After 1 d of exposure to 0.5 ppm of 1-octen-3-ol, there was no significant difference in the average time for exposed wild-type and ple mutants to cross a 5-cm distance. However, after 7 d of continuous exposure to 1-octen-3-ol, the average time required for ple mutants was about 1.5-fold higher than for wild-type flies (Fig. 5B). In the absence of 1-octen-3-ol, both ple mutants demonstrated climbing ability similar to wild type at both time points.
Discussion
Parkinson disease is a multifactorial disease characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta and typical parkinsonian symptoms such as bradykinesia, rigidity, and resting tremor (23). There is considerable evidence that several synthetic pesticides are associated with increased risk of PD (7, 24, 25). However, PD was first observed long before synthetic pesticides and industrial solvents were produced, suggesting that natural compounds may contribute to the etiology of PD. Here we demonstrate that the fungal volatile 1-octen-3-ol damages the dopamine system and that its toxicity is exacerbated by mutations in genes involved in dopamine synthesis and packaging, suggesting it may contribute to the etiology of PD.
Salama and Arias-Carrión (6) outlined several properties that should be fulfilled in looking for suitable environmental candidates involved in PD etiology, including (i) the agent should be of natural origin; (ii) the agent should be present worldwide; and (iii) the agent should recapitulate PD pathology in experimental models. The semiochemical 1-octen-3-ol fulfills these criteria. The volatile semiochemical l-octen-3-ol is responsible for much of the characteristic odor associated with molds and mushrooms and is commonly encountered in the natural environment as well as a flavoring agent in certain commercially prepared foods. It is a product of the oxidation and cleavage of linoleic acid, functions as a powerful signal molecule in insect attraction and deterrence (26–28), and has been hypothesized to contribute to the adverse health effects seen in moldy and water-damaged buildings and houses (29). The exact concentration of any given fungal VOC in water-damaged, moldy buildings is difficult to measure because concentrations vary depending on the ventilation rate, moisture, temperature, and other parameters. However, reported concentrations of 1-octen-3-ol in moldy buildings and classrooms range from 0.2 µg/m3 (0.00004 ppm) up to 900 µg/m3 (0.16 ppm) (15, 30–32).
Currently, only limited data are available on the toxicity of 1-octen-3-ol. Kreja and Seidel (33) reported the cytotoxic and mutagenic potential of 6.4 mM (820 ppm) liquid-phase 1-octen-3-ol for A549 human lung cell cultures. The lethal concentration 50% (LC50) for the A549 human lung cell line determined with the colony-forming ability assay was 3.8 mM (486 ppm), whereas with 3(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium 50 bromide and methylene blue assays it was 3.4 mM (435 ppm) and 2.1 mM (268 ppm), respectively. More recently, we showed that 100 ppm of volatilized 1-octen-3-ol was 80 times more toxic than toluene in human embryonic stem cells (34). Human volunteers exposed to 1.9 ppm of 1-octen-3-ol for 2 h experienced irritative symptoms, along with increases in inflammatory markers, eosinophil cationic protein, myeloperoxidase, lysozyme, and albumin in the nasal secretions (35). In this report, survival duration was truncated in Drosophila exposed to 1-octen-3-ol vapor at lower concentrations (0.5 ppm). Exposed flies exhibited locomotory deficits and loss of dopamine neurons, which were accompanied by loss of dopamine and an increase in 3,4-dihydroxyphenylacetic acid (Figs. 1–3).
The increased DOPAC levels suggest that 1-octen-3-ol may interfere with the packaging of dopamine into synaptic vesicles, which increases autooxidation of dopamine and generation of oxidative damage and reactive dopamine metabolites such as 3,4-dihydroxyphenylacetaldehyde (36–38). Indeed, we previously found that an increase in reactive oxygen species is observed in head extracts from 1-octen-3-ol–exposed flies (16). Previous studies modeling Parkinson disease in Drosophila demonstrated that overexpression of the vesicular monoamine transporter promotes packaging of dopamine into vesicles, thereby lowering its cytoplasmic concentrations (19, 39). Human genetic studies have found that single nucleotide polymorphisms (SNPs) within the promoter region of VMAT2, in particular gain-of-function haplotypes, are also associated with decreased risk of PD in women (40). Furthermore, in mice, reduced VMAT2 function produces dopamine-mediated toxicity and neurodegeneration in the nigrostriatal dopamine system, and restoration of VMAT2 function may be an important intervention in the treatment of PD (41–44). Therefore, impaired vesicular packaging of dopamine may represent a common pathological mechanism of PD (45).
To determine whether the 1-octen-3-ol–mediated alterations in dopamine and DOPAC levels were due to interference with vesicular storage of dopamine, we exposed HEK cells expressing human DAT alone or DAT and VMAT2 to 1-octen-3-ol. Because the inhibition of DAT by 1-octen-3-ol prevented determination of direct effects on VMAT2, airborne exposure to 1-octen-3-ol, which is most relevant to human exposure, was not possible with our assay. Therefore, to directly assess the role of VMAT2 in the dopaminergic neurotoxicity of 1-octen-3-ol in vivo, we used a genetic approach in Drosophila.
The mechanism of dopamine uptake and packaging in synaptic vesicles and their regulated release from these vesicles is well-conserved between Drosophila and mammalian systems (46). Drosophila DAT and VMAT share high sequence similarity with mammalian DAT and VMAT2 (19, 47). The dVMAT gene has two splice variants, dVMAT-A and -B; dVMAT-A is expressed in all dopaminergic, serotonergic, and octopaminergic neurons in Drosophila (19, 20). To assess the role of dVMAT in 1-octen-3-ol–induced DA toxicity, we first exposed two heterozygous loss-of-function mutant lines of dVMAT, l(2)SH0459/+ and Delta14/+, and found decreased survival times of these strains in the presence of 1-octen-3-ol (Fig. 5A). Conversely, overexpression of dVMAT in neurons was sufficient to delay 1-octen-3-ol–mediated mortality by over 3.5 d compared with control flies (Fig. 5B). This finding is similar to those of Lawal and coworkers (39), who found that overexpression of VMAT in Drosophila protected against the toxicity of a variety of pesticides linked to PD. HPLC analysis of transgenic TH-GAL4; UAS-dVMAT flies (i.e., those where VMAT was overexpressed in DA neurons) showed restoration of DA and DOPAC levels (Fig. 5C). Moreover, no detectable decreases in the number of dopaminergic neurons or detectable morphological changes were observed between the unexposed and exposed brains of TH-GAL4; UAS-GFP/UAS-dVMAT transgenic flies (Fig. 5 D and E). Taken in concert, these data suggest that disruption of dopamine handling is a likely mechanism for the dopaminergic toxicity of 1-octen-3-ol.
The genes encoding the enzymes regulating the dopamine biosynthesis pathway of Drosophila are also well-conserved with those of mammals (48). The tyrosine hydroxylase (TH) gene is the rate-limiting step in the dopamine biosynthesis pathway and is responsible for the hydroxylation of tyrosine into 3,4-dihydroxy-l-phenylalanine, which is then converted into dopamine by dopa decarboxylase (49). Pharmacological inhibition of TH, catecholamine uptake, and α-adrenoreceptor antagonists at 1 mM concentration results in failure of viable progeny whereas lower concentration (0.1 mM) significantly delayed the development, indicating the essential function of catecholamines in Drosophila development and viability (50). Drosophila tyrosine hydroxylase is encoded by the pale locus (51), and mutations in the pale gene result in decreased dopamine pools in adult heads, even in the heterozygous state (22). Compared with wild type, ple mutants showed increased susceptibility toward various types of stress, including the dopaminergic toxicant paraquat, indicating that dopamine functions as a key element in the stress response (22, 52). Here we also found that 1-octen-3-ol was more toxic in the ple heterozygous mutant lines. Wild-type flies survived 3–6 d longer than the heterozygous mutant strains ple2 and ple4 (Fig. 5). Although these mutants failed to show any locomotory defects after 1 d of exposure to 1-octen-3-ol, there was a significant decrease in locomotory function of the mutant flies after a 7-d exposure to 1-octen-3-ol (Fig. 5B). The sensitivity of ple heterozygous mutant flies to the exposure of 1-octen-3-ol likely reflects the modulatory function of dopamine in the pathways governing survival and locomotory behavior, as seen previously with paraquat exposure (22).
In summary, these data demonstrate that 1-octen-3-ol damages the dopamine system, most likely through disruption of dopamine handling. These findings are of particular interest given recent epidemiological studies that have raised the concern of neuropsychological impairments and movement disorders in human populations exposed to moldy and water-damaged buildings (13, 14, 53, 54). Increased incidence of PD is seen in rural populations (55), where it is usually attributed to pesticide exposure. However, the prevalence of mold and mushroom in these environments may provide another plausible risk factor for the development of PD. Furthermore, 1-octen-3-ol is known to be present in human sweat (56). Being an oxidative product of linoleic acid, an essential fatty acid, its excessive endogenous production in the body may contribute to human vulnerability to developing PD. Our studies in Drosophila suggest that the common fungal VOC 1-octen-3-ol may also contribute to Parkinson disease, particularly in people who have a genetic susceptibility. Further epidemiological studies will be needed to test this hypothesis. Additionally, the toxic effects we have demonstrated for 1-octen-3-ol implies that other plant and microbial VOCs should be screened for their possible neurotoxicity.
Materials and Methods
Drosophila Strains.
All stocks were cultured on Ward’s instant Drosophila medium (blue), and all experiments were performed at 25 °C. Wild-type y1, w1118, a yellow-body and white-eyed strain that carries otherwise wild-type genes, was used for all of the experiments unless otherwise stated. The following mutant fly strains were used: for tyrosine hydroxylase, pale2/+ and pale4/+ (22); for dVMAT mutants, l(2)SH0459/+ and Delta14/+ (21). The transgenic reporter strains used were TH-GAL4; UAS-GFP, elav-GAL4; UAS-GFP, and TH-GAL4; UAS-dVMAT. Except for TH-GAL4, elav-GAL4, and dVMAT mutant and transgenic lines, which were gifts from Janis O’Donnell (University of Alabama, Tuscaloosa, AL), Venugopal Reddy (Rutgers, The State University of New Jersey, Piscataway, NJ), and David Krantz (University of California, Los Angles, CA), respectively, all mutant and transgenic strains were obtained from the Bloomington Stock Center.
Exposure of Flies to 1-Octen-3-ol.
Adult 48-h posteclosed flies were fed on 1% (wt/vol) agar media supplemented with 5% (wt/vol) sucrose in 250-mL glass flasks. The racemic form of 1-octen-3-ol (98%) was purchased from Sigma-Aldrich (O5284-25G) in liquid form. The exposure of 1-octen-3-ol was carried out using the method published in Inamdar et al. (16) with minor modifications. The flies were exposed to vapors from undiluted aqueous 1-octen-3-ol at 0.5 ppm (vol/vol) concentration.
Mobility Assay.
The mobility assays were based on negative geotaxis behavior. The assays were performed using a published protocol (16). The time taken by a single fly to cross a 5-cm distance in 8 s was recorded. Control and pale mutant flies exposed to 0.5 ppm of 1-octen-3-ol were tested at 1 and 7 d of exposure. The average value was calculated from three trials in both assays with 1 min of rest between the trials.
Confocal Microscopy.
Confocal microscopy experiments were done to monitor the effect of 1-octen-3-ol on the dopaminergic neurons in D. melanogaster using the transgenic lines TH-GAL4; UAS-eGFP and TH-GAL4; UAS-dVMAT. The brains from age-matched controls and 1-octen-3-ol–exposed flies were dissected and mounted on slides to examine the number and status of GFP-expressing dopaminergic neurons. The final image was obtained as an average of Z sections of at least 10–12 sections using a Zeiss LSM 710 confocal microscope. The number of dopaminergic neurons was scored directly under the microscope by visualizing GFP expression to acquire the quantitative data on the status of dopaminergic neurons exposed to 1-octen-3-ol.
HPLC Analysis.
One hundred heads from frozen w1118 flies were homogenized in 100 µL of 0.1 N perchloric acid and then centrifuged at 18,000 × g for 10 min at 4 °C. The pellets were kept for protein assay and the supernatants were filtered through 0.22-µm filters. Ten microliters of supernatant was injected onto an HPLC with electrochemical detection (Waters) for neurochemical analysis of DA and its metabolite DOPAC. The components were separated on a cation-exchange column (MD-150, 150 × 3.2 mm column; ESA Biosciences) using an isocratic mobile phase (MD-TM mobile phase; ESA Biosciences) containing 2.2 mM NaCl pumped at a constant flow rate of 0.5 mL/min. The compounds were quantified by electrochemical detection using a glassy carbon working electrode, 2.0-mm diameter in situ silver reference electrode (Flow Cell, 2mm GC WE, ISAAC; Waters). The pellets in the bottom of the tubes were dried overnight in an oven at 30 °C. The dried pellets were dissolved in 100 μL of 0.5 N NaOH in a sonicating water bath for 1 h at 37 °C, and then 400 µL of H2O was added to bring the final concentration of NaOH to 0.1 N. The protein concentration for each sample was determined with a bicinchoninic acid assay reagent kit (Pierce) at 562 nm with a SpectraMax microplate reader (Molecular Devices) using BSA as a standard. The data were expressed in ng/mg of protein.
Whole-Cell [3H]DA Uptake.
The human kidney transgenic cell lines HEK-DAT and HEK-DAT/VMAT2 were used. Our protocol was modified from Bernstein et al. (57). Cells were plated in Transwell inserts for 24-well plates 1 d before the experiments were performed. Cells were exposed to 1-octen-3-ol by the airborne exposure method previously described (15) for 2 h. After exposure, cells were washed with 100 µL of uptake buffer (4 mM Tris, 6.25 mM Hepes, 120 mM NaCl, 5 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, 5.6 mM d-glucose, 1.7 mM ascorbic acid, 1 µM pargyline, pH 7.4). Cells were incubated with 90 µL of uptake buffer with or without 10 µM nomifensine for 15 min. After incubation, 10 µL of uptake buffer containing [3H]DA and DA for a final concentration of 20 nM [3H]DA and 1 µM DA was added. Cells were incubated at 37 °C for 10 min. Nonspecific uptake was determined in the presence of 10 µM nomifensine. Uptake was terminated by aspirating uptake buffer and washing each well twice with 100 µL of ice-cold uptake buffer. Cells were lysed in 0.1 N NaOH and transferred to vials containing 3 mL of scintillation mixture. Radioactivity was counted using a Beckman LS6500 liquid scintillation counter.
Statistical Analysis.
All of the graphs were plotted using Prism 5 (GraphPad), and data were analyzed using a one-tail Student t test or one-way/two-way ANOVA (Dunnett’s posttest or Bonferroni posttest) wherever appropriate. We have described the details of the statistical analysis in the figure legends.
Acknowledgments
We are thankful to Richard Hung, Samantha Lee, Prakash Masurekar, Shannon Morath, and Sally Padhi for helpful discussion. This study was supported by the Rutgers University Research Fund (J.W.B.). Partial support was provided by National Institutes of Health (NIH) Grants P30ES019776 and P50NS071669 (to G.W.M.) and R01ES015991, P30ES005022, and R21NS072097 (to J.R.R.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Jellinger KA. The pathology of Parkinson’s disease. Adv Neurol. 2001;86:55–72. [PubMed] [Google Scholar]
- 2.Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson’s disease. Mov Disord. 2011;26(6):1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- 3.Kumar KR, Lohmann K, Klein C. Genetics of Parkinson disease and other movement disorders. Curr Opin Neurol. 2012;25(4):466–474. doi: 10.1097/WCO.0b013e3283547627. [DOI] [PubMed] [Google Scholar]
- 4.Hatcher JM, Pennell KD, Miller GW. Parkinson’s disease and pesticides: A toxicological perspective. Trends Pharmacol Sci. 2008;29(6):322–329. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw CA, Höglinger GU. Neurodegenerative diseases: Neurotoxins as sufficient etiologic agents? Neuromolecular Med. 2008;10(1):1–9. doi: 10.1007/s12017-007-8016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salama M, Arias-Carrión O. Natural toxins implicated in the development of Parkinson’s disease. Ther Adv Neurol Disord. 2011;4(6):361–373. doi: 10.1177/1756285611413004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon JR, Greenamyre JT. Gene-environment interactions in Parkinson’s disease: Specific evidence in humans and mammalian models. Neurobiol Dis. 2013;57:38–46. doi: 10.1016/j.nbd.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burge PS. Sick building syndrome. Occup Environ Med. 2004;61(2):185–190. doi: 10.1136/oem.2003.008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Institute of Medicine . Damp Indoor Spaces and Health. Washington, DC: Natl Acad Press; 2004. [Google Scholar]
- 10.Straus DC. Molds, mycotoxins, and sick building syndrome. Toxicol Ind Health. 2009;25(9-10):617–635. doi: 10.1177/0748233709348287. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Guidelines for Indoor Air Quality: Dampness and Mould. Germany: Druckpartner Moser; 2009. [PubMed] [Google Scholar]
- 12.Klepeis NE, et al. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11(3):231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 13.Empting LD. Neurologic and neuropsychiatric syndrome features of mold and mycotoxin exposure. Toxicol Ind Health. 2009;25(9-10):577–581. doi: 10.1177/0748233709348393. [DOI] [PubMed] [Google Scholar]
- 14.Kilburn KH. Neurobehavioral and pulmonary impairment in 105 adults with indoor exposure to molds compared to 100 exposed to chemicals. Toxicol Ind Health. 2009;25(9-10):681–692. doi: 10.1177/0748233709348390. [DOI] [PubMed] [Google Scholar]
- 15.Korpi A, Järnberg J, Pasanen AL. Microbial volatile organic compounds. Crit Rev Toxicol. 2009;39(2):139–193. doi: 10.1080/10408440802291497. [DOI] [PubMed] [Google Scholar]
- 16.Inamdar AA, Masurekar P, Bennett JW. Neurotoxicity of fungal volatile organic compounds in Drosophila melanogaster. Toxicol Sci. 2010;117(2):418–426. doi: 10.1093/toxsci/kfq222. [DOI] [PubMed] [Google Scholar]
- 17.Goetz CG. The history of Parkinson’s disease: Early clinical descriptions and neurological therapies. Cold Spring Harb Perspect Med. 2011;1(1):a008862. doi: 10.1101/cshperspect.a008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friggi-Grelin F, et al. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54(4):618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 19.Greer CL, et al. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin, and octopamine. J Neurobiol. 2005;64(3):239–258. doi: 10.1002/neu.20146. [DOI] [PubMed] [Google Scholar]
- 20.Chang HY, et al. Overexpression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Mol Psychiatry. 2006;11(1):99–113. doi: 10.1038/sj.mp.4001742. [DOI] [PubMed] [Google Scholar]
- 21.Simon AF, et al. Drosophila vesicular monoamine transporter mutants can adapt to reduced or eliminated vesicular stores of dopamine and serotonin. Genetics. 2009;181(2):525–541. doi: 10.1534/genetics.108.094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaudhuri A, et al. Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J Neurosci. 2007;27(10):2457–2467. doi: 10.1523/JNEUROSCI.4239-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas B, Beal MF. Parkinson’s disease. Hum Mol Genet. 2007;16(R2):R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 24.Uversky VN. Neurotoxicant-induced animal models of Parkinson’s disease: Understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res. 2004;318(1):225–241. doi: 10.1007/s00441-004-0937-z. [DOI] [PubMed] [Google Scholar]
- 25.Cicchetti F, Drouin-Ouellet J, Gross RE. Environmental toxins and Parkinson’s disease: What have we learned from pesticide-induced animal models? Trends Pharmacol Sci. 2009;30(9):475–483. doi: 10.1016/j.tips.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Combet E, Henderson J, Eastwood DC, Burton KS. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience. 2006;47(6):317–326. [Google Scholar]
- 27.Bennett JW, Hung R, Lee S, Padhi S. Fungal and bacterial volatile organic compounds: An overview and their role as ecological signaling agents. In: Hock B, editor. The Mycota IX Fungal Interactions. Heidelberg: Springer; 2012. pp. 373–393. [Google Scholar]
- 28.Morath SU, Hung R, Bennett JW. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol Rev. 2012;26:73–83. [Google Scholar]
- 29.Mølhave L. Volatile organic compounds and sick building syndrome. In: Lippmann M, editor. Environmental Toxicants: Human Exposures and Their Health Effects. 3rd Ed. Hoboken, NJ: John Wiley & Sons; 2008. pp. 241–256. [Google Scholar]
- 30.Strom G, West J, Wessen B, Palmgren¸ U. 1994. Quantitative analysis of microbial volatiles in damp Swedish houses. Health Implications of Fungi in Indoor Environments, Air Quality Monographs, eds Samson RA, et al. (Elsevier Science, Amsterdam), Vol 2, pp 291–305.
- 31.Kim JL, et al. Indoor molds, bacteria, microbial volatile organic compounds and plasticizers in schools—Associations with asthma and respiratory symptoms in pupils. Indoor Air. 2007;17(2):153–163. doi: 10.1111/j.1600-0668.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 32.Schleibinger H, Laussmann D, Bornehag CG, Eis D, Rueden H. Microbial volatile organic compounds in the air of moldy and mold-free indoor environments. Indoor Air. 2008;18(2):113–124. doi: 10.1111/j.1600-0668.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 33.Kreja L, Seidel HJ. On the cytotoxicity of some microbial volatile organic compounds as studied in the human lung cell line A549. Chemosphere. 2002;49(1):105–110. doi: 10.1016/s0045-6535(02)00159-5. [DOI] [PubMed] [Google Scholar]
- 34.Inamdar AA, Moore JC, Cohen RI, Bennett JW. A model to evaluate the cytotoxicity of the fungal volatile organic compound 1-octen-3-ol in human embryonic stem cells. Mycopathologia. 2012;173(1):13–20. doi: 10.1007/s11046-011-9457-z. [DOI] [PubMed] [Google Scholar]
- 35.Wålinder R, Ernstgård L, Norbäck D, Wieslander G, Johanson G. Acute effects of 1-octen-3-ol, a microbial volatile organic compound (MVOC)—An experimental study. Toxicol Lett. 2008;181(3):141–147. doi: 10.1016/j.toxlet.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Jinsmaa Y, et al. Dopamine-derived biological reactive intermediates and protein modifications: Implications for Parkinson’s disease. Chem Biol Interact. 2011;192(1-2):118–121. doi: 10.1016/j.cbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein DS, et al. Vesicular uptake blockade generates the toxic dopamine metabolite 3,4-dihydroxyphenylacetaldehyde in PC12 cells: Relevance to the pathogenesis of Parkinson’s disease. J Neurochem. 2012;123(6):932–943. doi: 10.1111/j.1471-4159.2012.07924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldstein DS, et al. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J Neurochem. 2013;126(5):591–603. doi: 10.1111/jnc.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawal HO, et al. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol Dis. 2010;40(1):102–112. doi: 10.1016/j.nbd.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brighina L, et al. Analysis of vesicular monoamine transporter 2 polymorphisms in Parkinson’s disease. Neurobiol Aging. 2013;34(6):1712.e9–1712.e13. doi: 10.1016/j.neurobiolaging.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caudle WM, et al. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27(30):8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor TN, et al. Nonmotor symptoms of Parkinson’s disease revealed in an animal model with reduced monoamine storage capacity. J Neurosci. 2009;29(25):8103–8113. doi: 10.1523/JNEUROSCI.1495-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor TN, Caudle WM, Miller GW. VMAT2-deficient mice display nigral and extranigral pathology and motor and nonmotor symptoms of Parkinson’s disease. Parkinsons Dis. 2011;2011:124165. doi: 10.4061/2011/124165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ulusoy A, Björklund T, Buck K, Kirik D. Dysregulated dopamine storage increases the vulnerability to α-synuclein in nigral neurons. Neurobiol Dis. 2012;47(3):367–377. doi: 10.1016/j.nbd.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Alter SP, Lenzi GM, Bernstein AI, Miller GW. Vesicular integrity in Parkinson’s disease. Curr Neurol Neurosci Rep. 2013;13(7):362. doi: 10.1007/s11910-013-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nichols CD. Drosophila melanogaster neurobiology, neuropharmacology, and how the fly can inform central nervous system drug discovery. Pharmacol Ther. 2006;112(3):677–700. doi: 10.1016/j.pharmthera.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Pörzgen P, Park SK, Hirsh J, Sonders MS, Amara SG. The antidepressant-sensitive dopamine transporter in Drosophila melanogaster: A primordial carrier for catecholamines. Mol Pharmacol. 2001;59(1):83–95. doi: 10.1124/mol.59.1.83. [DOI] [PubMed] [Google Scholar]
- 48.Livingstone MS, Tempel BL. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature. 1983;303(5912):67–70. doi: 10.1038/303067a0. [DOI] [PubMed] [Google Scholar]
- 49.Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- 50.Pendleton RG, Robinson N, Roychowdhury R, Rasheed A, Hillman R. Reproduction and development in Drosophila are dependent upon catecholamines. Life Sci. 1996;59(24):2083–2091. doi: 10.1016/s0024-3205(96)00562-0. [DOI] [PubMed] [Google Scholar]
- 51.Neckameyer WS, Quinn WG. Isolation and characterization of the gene for Drosophila tyrosine hydroxylase. Neuron. 1989;2(2):1167–1175. doi: 10.1016/0896-6273(89)90183-9. [DOI] [PubMed] [Google Scholar]
- 52.Neckameyer WS, Weinstein JS. Stress affects dopaminergic signaling pathways in Drosophila melanogaster. Stress. 2005;8(2):117–131. doi: 10.1080/10253890500147381. [DOI] [PubMed] [Google Scholar]
- 53.Kilburn KH. Role of molds and mycotoxins in being sick in buildings: Neurobehavioral and pulmonary impairment. Adv Appl Microbiol. 2004;55:339–359. doi: 10.1016/S0065-2164(04)55013-X. [DOI] [PubMed] [Google Scholar]
- 54.Dennis D, Robertson D, Curtis L, Black J. Fungal exposure endocrinopathy in sinusitis with growth hormone deficiency: Dennis-Robertson syndrome. Toxicol Ind Health. 2009;25(9-10):669–680. doi: 10.1177/0748233709348266. [DOI] [PubMed] [Google Scholar]
- 55.Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson’s disease: A metaanalysis. Environ Res. 2001;86(2):122–127. doi: 10.1006/enrs.2001.4264. [DOI] [PubMed] [Google Scholar]
- 56.Cork A, Park KC. Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med Vet Entomol. 1996;10(3):269–276. doi: 10.1111/j.1365-2915.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 57.Bernstein AI, Stout KA, Miller GW. A fluorescent-based assay for live cell, spatially resolved assessment of vesicular monoamine transporter 2-mediated neurotransmitter transport. J Neurosci Methods. 2012;209(2):357–366. doi: 10.1016/j.jneumeth.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]