Abstract
Oleanolic acid (OA) is a triterpenoids that exists widely in plants. OA is effective in protecting against hepatotoxicants. Whereas a low dose of OA is hepatoprotective, higher doses and longer-term use of OA produce liver injury. This study characterized OA-induced liver injury in mice. Adult C57BL/6 mice were given OA at doses of 0, 22.5, 45, 90, and 135 mg/kg, s.c., daily for 5 days, and liver injury was observed at doses of 90 mg/kg and above, as evidenced by increases in serum activities of alanine aminotransferase and alkaline phosphatase, increases in serum total bilirubin, as well as by liver histopathology. OA-induced cholestatic liver injury was further evidenced by marked increases of both unconjugated and conjugated bile acids (BAs) in serum. Gene and protein expression analysis suggested that livers of OA-treated mice had adaptive responses to prevent BA accumulation by suppressing BA biosynthetic enzyme genes (Cyp7a1, 8b1, 27a1, and 7b1); lowering BA uptake transporters (Ntcp and Oatp1b2); and increasing a BA efflux transporter (Ostβ). OA increased the expression of Nrf2 and its target gene, Nqo1, but decreased the expression of AhR, CAR and PPARα along with their target genes, Cyp1a2, Cyp2b10 and Cyp4a10. OA had minimal effects on PXR and Cyp3a11. Taken together, the present study characterized OA-induced liver injury, which is associated with altered BA homeostasis, and alerts its toxicity potential.
Keywords: Oleanolic acid, cholestasis, serum bile acids, bile acid synthesis genes, bile acid transporters, nuclear receptors
Introduction
Oleanolic acid (OA) is a triterpenoid that exists widely in fruits, vegetables and medicinal herbs. For example, olive fruit and leaf contain high amounts of OA (Guinda et al., 2010). OA is rich in apple skin, papaya fruit, persimmon fruit, and loquat (Liu, 1995; Laszczyk, 2009). OA is also found in soybeans (Zhang and Popovich, 2009), filamentous fungi, and Sedium aizoon (Laszczyk, 2009). Ginseng, Thunder-God-Vine, rose hip powder, and many medicinal herbs contain OA as a natural product (Liu, 1995; Saaby et al., 2011). The concentrations of OA can be as high as 1–3% as in Ginseng, papaya fruit, olive fruit, apple skin, and dark plums (Pollier and Goossens, 2012).
OA is used as a dietary supplement and an over-the-counter Chinese medicine for treatment of liver disorders, inflammatory diseases, type-II diabetes, as well as anticancer therapeutics (Liu, 2005; Petronelli et al., 2009; Laszczyk, 2009; Pollier and Goossens, 2012). We described the protective effects of OA against the hepatotoxic effects of a diverse group of chemicals in mice (Liu et al., 1993a, 1993b, 1994, 1995). Later, OA was shown as an activator of Nrf2 (Liu et al., 2008; Reisman et al., 2009), which is a transcription factor activating the transcription of various genes to protect against oxidative stress and nucleophiles (Wu et al., 2012). However, whereas a low dose of OA is hepatoprotective, higher doses and longer-term use of OA could produce liver injury (Liu, 2005; Sato et al., 2007; Lu et al., 2013).
Some herbal products may potentially benefit people with liver diseases by possessing antioxidant, antifibrotic, immunomodulatory, or antiviral activities. However, “hepatoprotective” herbs such as rhubarb, have been documented as having both therapeutic and toxic effects to the liver, leading to a complex situation of distinguishing benefits from risks (Wang et al., 2011). OA and its derivatives have been shown to produce both hepatoprotective and hepatotoxic effects in cultured rat hepatocytes (Kinjo et al., 1999). Although OA produces hepatotoxicity in cell cultures, its ability to produce hepatotoxicity in vivo, the difference in dose to be hepatoprotective vs hepatotoxic is not known, nor characterization of the liver injury in animals.
OA and OA analogues are used in traditional medicines, alone or in combination with other drugs, in the treatment of liver diseases and other disorders (Pollier and Goossens, 2012). Recently, an OA derivative (Bardoxolone methyl) was halted in phase-3 clinical trials due to safety concerns, and analogues of bardoxolone induced liver injury after 3-months of administration to rats (Zoja et al., 2013). Indeed, “beneficial” herbal products can produce toxicity when not used appropriately (Seeff, 2007; Wang et al., 2011). Our initial studies to examine the hepatoprotective effects of OA used outbred CF1 mice (Liu et al. 1993a, 1993b, 1994; 1995), but later we noted that C57BL/6 mice were more susceptible to OA-induced liver injury. This study was therefore initiated to examine the hepatotoxic potential of OA, using the more common C57BL/6 mouse strain.
Materials and Methods
Chemicals
Oleanolic acid (OA) was obtained from Guiyang Pharmaceutical Company (Guiyang, China), with a purity of 98%, as described previously (Liu et al., 1994). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Animals and treatments
Adult female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were housed in an AAALAC (the American Animal Association of Laboratory Animal Care) accredited animal facility at the University of Kansas Medical Center, and they had free access to Teklad Rodent Diet #8604 (Harlan Laboratories, Madison, WI) and tap water. Mice (n = 5/group) were given OA (22.5–135 mg/kg, dissolved in sterile saline with 2% Tween-80) daily for 5 consecutive days. Controls received the same volume of vehicle. Twenty-four hrs after the last dose, mice were anesthetized with pentobarbital (50 mg/kg, ip), blood and livers were collected for analysis. All animal experimental procedures followed AAALAC guidelines, and were approved by the Animal Use and Care Committee at the University of Kansas Medical Center.
Serum biochemistry
Blood was placed on ice for 60 min and centrifuged (8000 g, 10 min) to prepare serum. Serum samples were analyzed by standard enzymatic assays using commercial kits for alanine aminotransferase (Infinity™ ALT, Thermo Scientific, Middletown, VA), lactate dehydrogenase (Infinity™ LDH), alkaline phosphatase (ALP, Poinite Scientific, Canton, MI), direct and total bilirubin (Poinite Scientific, Canton, MI), and total bile acids (Poinite Scientific) according to the manufacturer's protocols.
Serum BA extraction and quantification
Serum BAs were extracted as described previously (Zhang et al., 2012), and quantified using ultra performance liquid chromatography – tandem mass spectrometry (UPLC/MS/MS) (Zhang and Klaassen, 2010). BA stock solutions were diluted with 50% methanol and spiked with internal standards to construct standard curves between 5 and 20,000 ng/ml with a correlation coefficient for all BAs above 0.99. The limit of detection (signal/noise ratio = 3) for the various BAs was in the range of 5–10 ng/ml, which equals 0.01–0.02 nmol/ml.
Histopathology
Liver samples were fixed in 10% formalin, and subjected to standard histological procedures and paraffin embedding. Liver sections (5 µm in thickness) were stained with hematoxylin and eosin and evaluated for histopathological lesions.
RNA isolation
Total RNA was isolated using RNA-Bee reagent (Tel-Test Inc., Friendswood, TX, USA) according to the manufacturer’s instructions. RNA quality was determined by the 260/280 ratio (>1.8) and by formaldehyde-agarose gel electrophoresis for visualization of 18S and 28S rRNA bands after ethidium bromide staining. Total RNA was diluted with diethyl pyrocarbonate-treated deionized water to a concentration of 200 ng/µL.
Real-time RT-PCR analysis
Total RNA was reverse transcribed with Multiscript reverse transcriptase using a High Capacity RT kit from Applied Biosystems (Applied Biosystems, Foster City, CA). Primers were designed with Primer3 software (version 4), and listed in Supplemental Table 1. The Power SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) was used for real-time RT-PCR analysis. Differences in gene expression between groups were calculated using cycle threshold (ΔΔCt) values, which were normalized with G3pdh and β-actin, and expressed as relative transcript levels, setting controls as 100%.
Western blot analysis
Western-blot analysis of selected proteins was performed as described (Cheng et al., 2007; Reisman et al., 2009). Briefly, liver protein was extracted with T-PER tissue protein extraction kit (Thermo Scientific, Rockford, IL) with freshly prepared proteinase inhibitors (Sigma, St. Louis, MO). Protein concentrations were determined using a BCA protein assay according to the manufacturer's instructions (Thermo Scientific, Rockford, IL). Approximately 40 µg of cytosolic protein were used for immunoblotting proteins of interest. The primary antibodies used in this study include Nrf2 (sc-30915) and Cyp8b1 (sc-101387) were from Santa Cruz Biotechnology (Santa Cruz, CA); Nqo1 (Ab2346), Cyp7a1 (Ab79847), and β-actin (Ab8227) from Abcam (Cambridge, MA); Ntcp and Bsep were kindly provided by Dr. Bruno Stieger, University of Zurich, Switzerland), (Cheng et al., 2007), and others routinely used in our laboratory (Tanaka et al., 2009; Reisman et al., 2009). Secondary antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Protein-antibody complexes were detected using an enhanced chemiluminescent kit (Thermo Scientific, Rockford, IL) and exposed to HyBlot CL autoradiography film (Denville Scientific Inc., Metuchen, NJ).
Statistical analysis
All data were analyzed using a one-way ANOVA, followed by Duncan's multiple range post hoc test. Significance was set at p < 0.05. Bars represent means ± SEM.
Results
OA induced hepatotoxicity in a dose-dependent manner
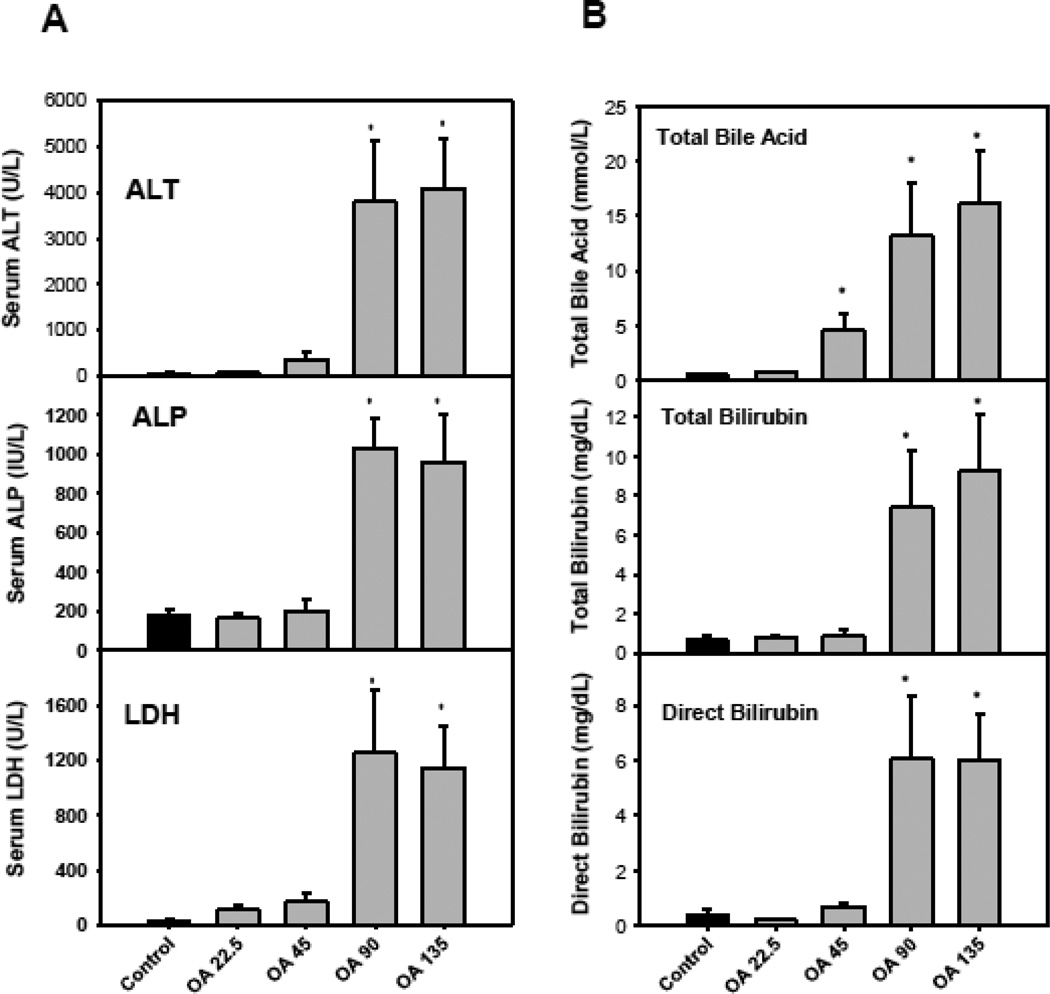
Mice were given OA (22.5–135 mg/kg, dissolved in 2% Tween-80 saline, s.c), daily for 5 consecutive days. All mice survived the study, but body weight loss (up to 20%) was evident with doses of 90 mg/kg or higher (data not shown). On day 6, blood was collected and serum biochemistry was determined. OA produced a dosedependent increase in serum activities of alanine aminotransferase (ALT), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) (Fig. 1A), indicative of hepatocellular damage and cell death. Fig. 1B illustrates that OA at doses of 45 mg/kg or higher increased serum concentrations of total bile acids, and at 90 mg/kg or higher increased total bilirubin and direct bilirubin, all indicative of liver injury.
Figure 1.
Serum biochemistry. C57BL/6 mice were given s.c injections of oleanolic acid (OA) at doses of 22.5, 45, 90, and 135 mg/kg, daily for 5 days. Twenty-four hr after the last dose, blood was collected to determine changes in serum biochemistry as detailed in Methods. Data are mean ± SEM (n = 5). *Significantly different from control p < 0.05.
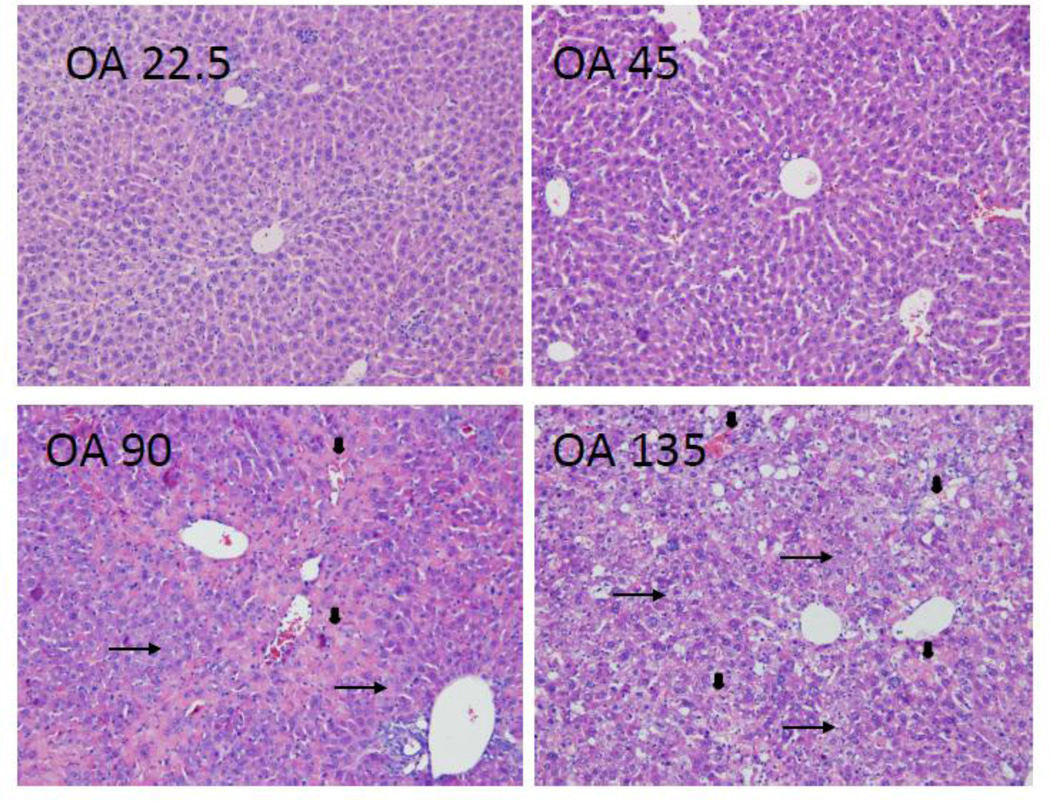
Liver pathology in mice after OA treatment was in good agreement with serum biochemistry. Generally, OA treatment at 22.5 and 45 mg/kg had little effect on liver morphology. At 90 mg/kg of OA, foci of liver degeneration could be seen, but liver morphology was mainly normal. Although bile droplets and bile-duct blockage were not evident, feathery-like degeneration was widespread at OA doses of 90 mg/kg and above (arrows). Feathery-like degeneration is a well-known characteristic of cholestasis, as bile acids act as detergents to produce feathery-like morphology (Li and Crawford, 2004). Hepatocellular death (arrowheads) also occurred. At the highest dose of 135 mg/kg, widespread feathery degeneration and severe hepatocellular death were evident (Fig. 2). Taken together, OA produced cholestasis injury was observed at doses of 90 mg/kg and above in C57/BL mice.
Figure 2.
Representative liver histopathology. C57BL/6 mice were given s.c injections of oleanolic acid (OA) at doses of 22.5, 45, 90, and 135 mg/kg, daily for 5 days. Twenty-four hr after the last dose, livers were fixed in neutral formalin and underwent the standard histology procedures. Slides were stained H&E. Arrows indicate featherylike degeneration and arrowheads indicate hepatocellular cell death. Magnitude (x 200).
OA increased the concentrations of both unconjugated and conjugated BAs in serum
Because OA increased serum total bilirubin and total BA concentrations, we quantified individual BAs to further investigate the effect of OA on BA homeostasis using a newly developed ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method (Zhang and Klaassen, 2010). Figure 3 illustrates individual BA changes following OA treatment. The BAs that increased in serum include unconjugated cholic acid (CA, 36.2-fold), chenodeoxycholic acid (CDCA, 2.4-fold), deoxycholic acid (DCA, 120%), α-muricholic acid (αMCA, 4.1-fold) and β-muricholic acid (βMCA, 31.4-fold), and taurine conjugated bile acids such as taurocholic acid (TCA, 351-fold), taurochenodeoxycholic acid (TCDCA, 20.1-fold), taurodeoxycholic acid (TDCA, 4.4-fold), tauroursodeoxycholic acid (TUDCA, 6.8-fold), and tauro-α/β-muricholic acid (Tα/βMCA, 609-fold). Therefore, OA treatment resulted in serum accumulation of both unconjugated and conjugated BAs in mice. The bile acid concentrations were not further increased with the highest dose, possibly due to saturation of serum bile acids or liver toxicity.
Figure 3.
Serum bile acid concentrations. C57BL/6 mice were given s.c injections of oleanolic acid (OA) at doses of 22.5, 45, 90, and 135 mg/kg, daily for 5 days. Twentyfour hr after the last dose, blood was collected and serum concentrations of bile acids and their metabolites were determined by UPLC-MS/MS and normalized with the internal standards. Data are mean ± SEM (n = 5). *Significantly different from control p < 0.05.
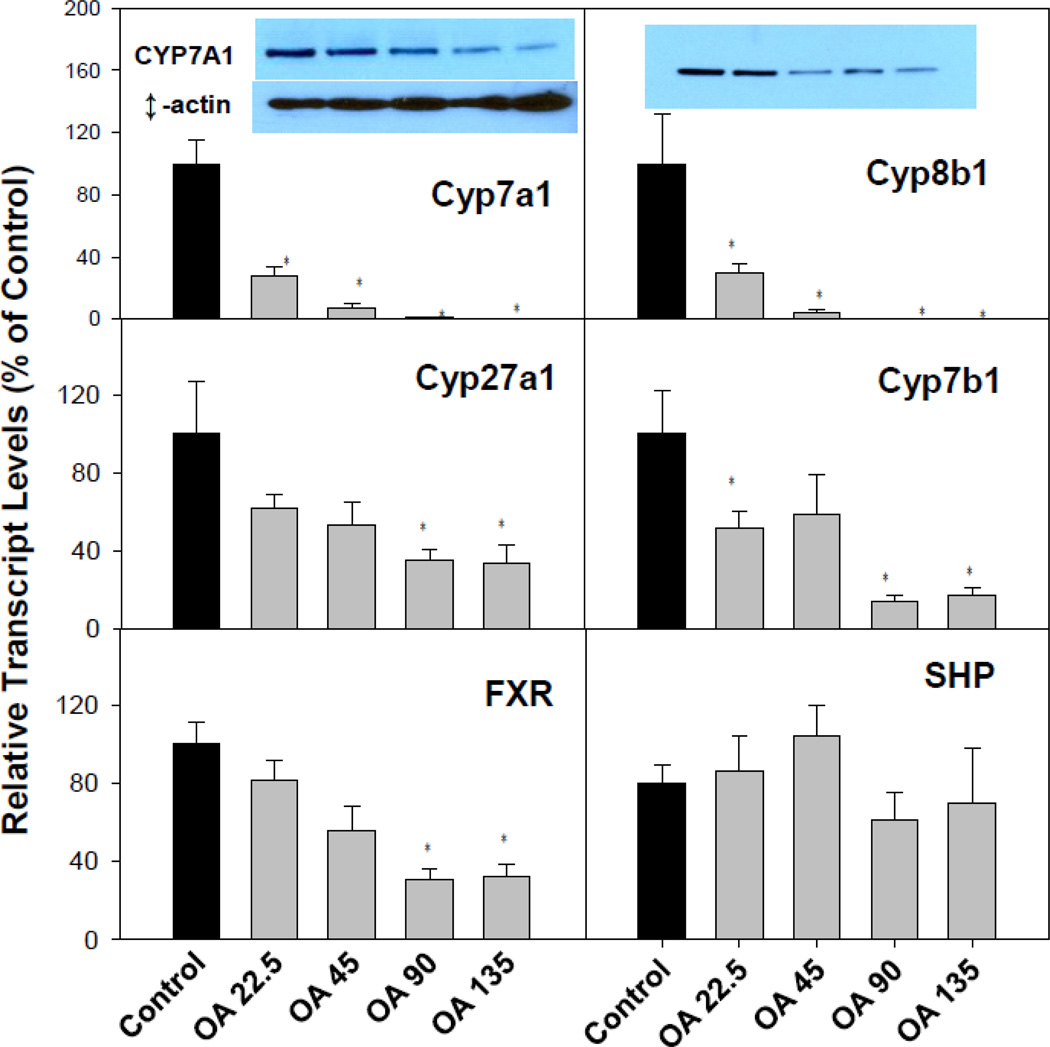
OA inhibited BA synthesis in livers of mice
Fig 4 illustrates the effects of OA on the expression of bile acid synthesis genes. The classic bile acid synthetic pathway genes, Cyp7a1 and Cyp8b1, were suppressed over 95%, and the alterative bile acid synthesis pathway genes Cyp27a1 (34% of control) and Cyp7b1 (15% of control) were also suppressed (Fig. 4). The farnesoid X receptor (FXR) plays a central role in feedback repression of the BA biosynthesis rate-limiting enzyme Cyp7a1 through FXR-SHP signaling in liver (Chiang, 2009). The mRNA of the nuclear receptor FXR was decreased at higher doses, but SHP was less affected. This suggests that the decrease of Cyp7a1 is not solely regulated via the FXR-SHP pathway. Western-blot analysis confirmed the decreased CYP7A1 and CYP8B1 protein (Fig. 4, top panel inserts).
Figure 4.
Expression of bile acid synthesis genes. C57BL/6 mice were given s.c injections of oleanolic acid (OA) at doses of 22.5, 45, 90, and 135 mg/kg, daily for 5 days. Twenty-four hr after the last dose, Livers were collected to isolate total RNA. The expression of hepatic transport genes were determined by real-time RT-PCR as detailed in the Methods. Data are mean ± SEM (n = 5). *Significantly different from control p < 0.05. Western-blot analysis of CYP7A1, CYP8B1 and β-actin are inserted into corresponding mRNA figures.
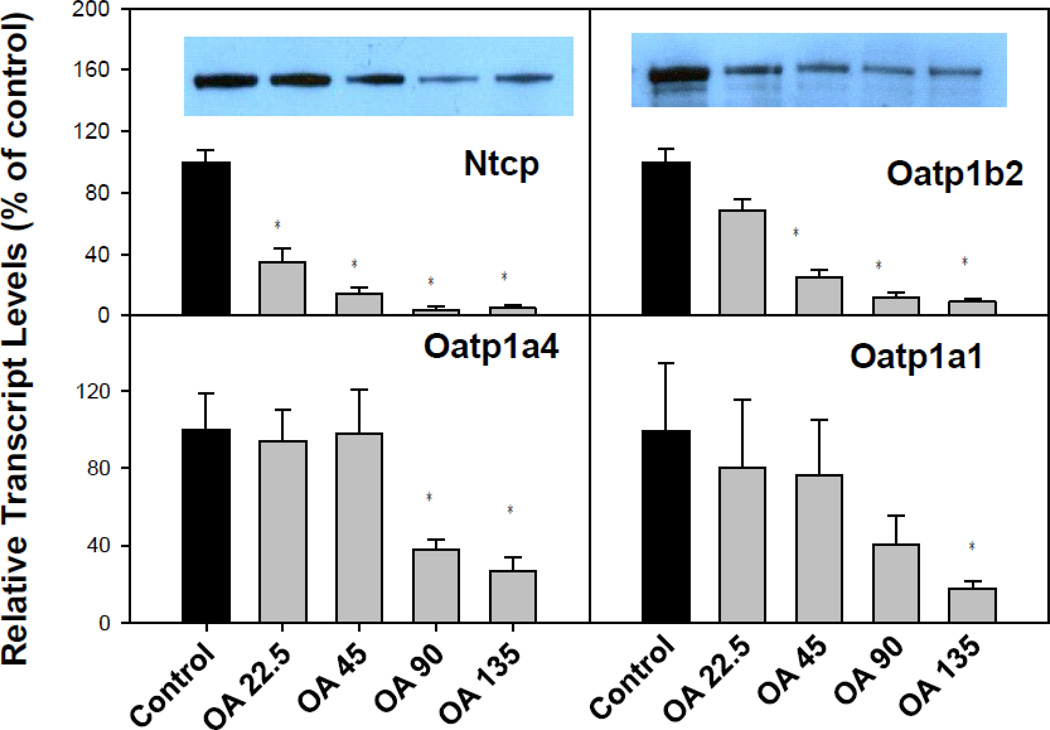
OA inhibited BA uptake transporters in livers of mice
To investigate whether OA-induced BA accumulation in serum was due to decreased ability of the liver to clear BAs from the serum, we quantified the mRNA of major liver transporters (Fig. 5). In liver, the BA uptake transporters on the basolateral membranes of hepatocytes include the liver specific BA transporter Na+-taurocholate co-transporting polypeptide (Ntcp) for conjugated BAs, and organic anion transporting peptide (Oatp)1b2 for unconjugated bile acids (Csanaky et al., 2011; Klaassen and Aleksunes, 2010). OA suppressed markedly the mRNA of Ntcp and Oatp1b2 in a dose-dependent manner. In addition, OA also suppressed the mRNA of other major hepatic uptake transporters, such as Oatp1a1 and Oatp1a4. Western-blot analysis (Fig. 5, top panel inserts) indicate that OA decreased the BA uptake transporters, Ntcp and Oatp1b2 protein, in a dose-dependent fashion.
Figure 5.
Expression of bile acid uptake genes. C57BL/6 mice were given s.c injections of oleanolic acid (OA) at doses of 22.5, 45, 90, and 135 mg/kg, daily for 5 days. Twenty-four hr after the last dose, Livers were collected to isolate total RNA. The expression of hepatic transport genes were determined by real-time RT-PCR as detailed in the Methods. Data are mean ± SEM (n = 5). *Significantly different from control p < 0.05. Western-blot analysis of NTCP and OATP1B2 are inserted into corresponding mRNA figures (β-actin is shown in Figure 4).
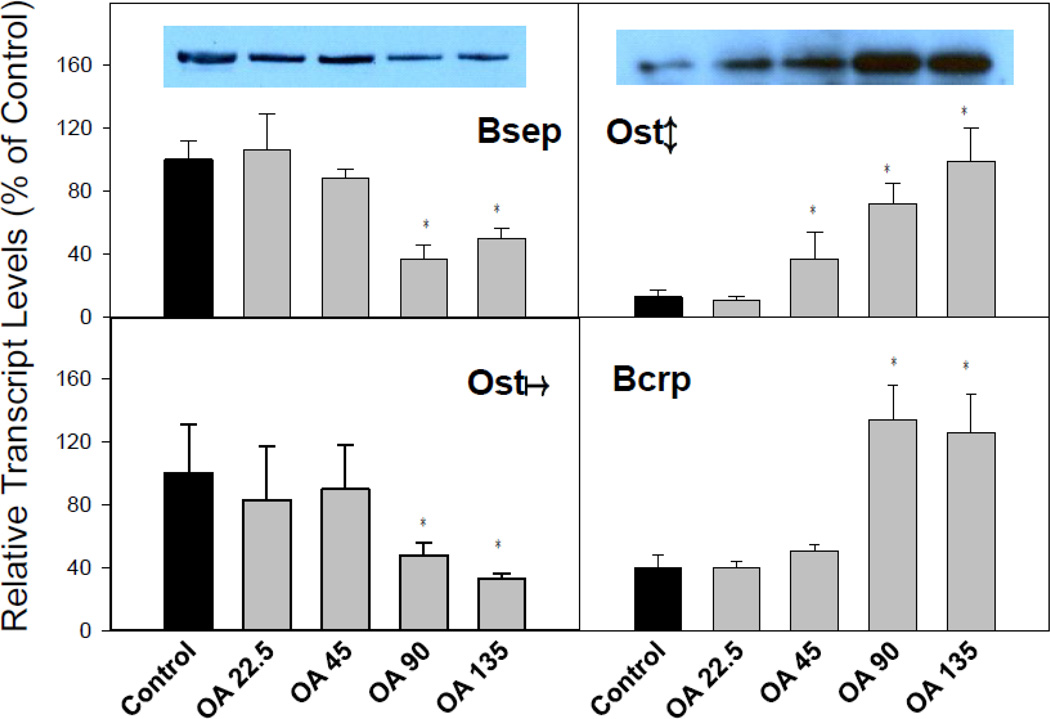
Effects of OA on BA efflux transporters in livers of mice
Canalicular transporters that are responsible for the biliary excretion of bile acids and chemicals include the bile salt export pump (Bsep), breast cancer resistant protein (Bcrp), and organic solute transporter Ostα and Oatβ. OA treatment decreased the mRNA of Bsep, but increased Bcrp. Interestingly, while OA decreased Ostα, it dose-dependently increased Ostβ (Fig. 6). Western-blot analysis (Fig. 6, top panel inserts) confirmed that OA decreased the efflux transporters Bsep, but increased BA efflux transporter Ostβ, consistent with RT-PCR analysis. Taken together, OA treatment markedly inhibited liver uptake transporters, and increased the BA efflux transporter Ostβ.
Figure 6.
Expression of bile acid efflux genes. C57BL/6 mice were given s.c injections of oleanolic acid (OA) at doses of 22.5, 45, 90, and 135 mg/kg, daily for 5 days. Twenty-four hr after the last dose, Livers were collected to isolate total RNA. The expression of liver metabolic genes was determined by real-time RT-PCR as detailed in the Methods. Data are mean ± SEM (n = 5). *Significantly different from control p < 0.05. Western-blot analysis of BSEP and OST-β are inserted into corresponding mRNA figures (β-actin is shown in Figure 4).
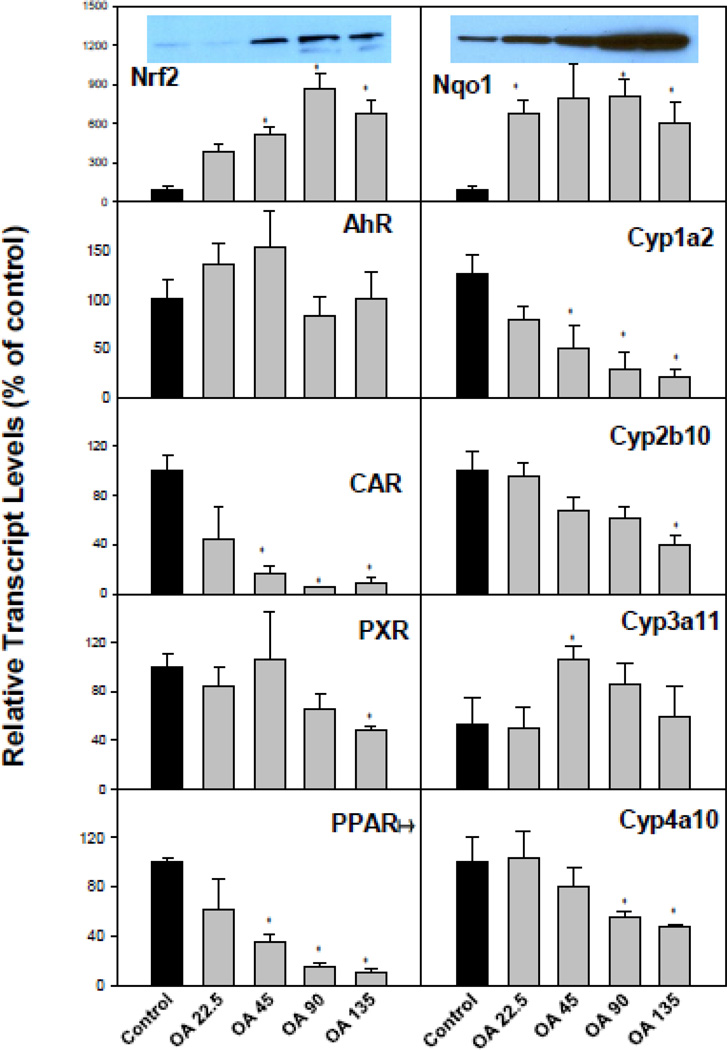
OA treatment resulted in dysfunction of liver protective mechanisms against cholestasis
Figure 7 illustrates the effects of OA on major drug processing genes and corresponding nuclear receptors in liver. OA is a known activator of Nrf2 (Liu et al., 2008; Reisman et al., 2009), and as a result, the expression of Nrf2 and its target gene NAD(P)H:quinone oxidoreductase 1 (Nqo1) were increased. Many drug processing genes in the liver are regulated by nuclear receptor transcription factors, such as the aryl hydrocarbon receptor (AhR), constitutive androstane receptor (CAR), pregnane X receptor (PXR), and peroxisome proliferator-activated receptor (PPAR) (Klaassen and Aleksunes, 2010). These nuclear receptors also regulate protective mechanisms against cholestasis (Wagner et al., 2010; Fiorucci et al., 2012). There was no significant change in the expression of AhR, but a marked decrease in AhR-regulated Cyp1a2; there was a significant decrease in CAR, but a mild decrease in CAR-regulated Cyp2b10; the effect of OA on PXR and PXR-regulated Cyp3a11 was not appreciable; and a dose-dependent decrease in PPARα and PPARα-regulated Cyp4a10 was also evident (Fig. 7). It is noteworthy that CAR and PPARα decreased more dramatically, but the expression of their target genesCyp2b10 and Cyp4a10 did not decline proportionally, a phenomenon needs further investigation. The dysfunction of these nuclear receptor networks could worsen the OA-induced cholestasis. Western-blot analysis (Fig. 7, top panel inserts) showed that OA increased Nrf2 and Nrf2-targeted proteins Nqo1 in a dose-dependent manner, consistent with real-time PCR results.
Figure 7.
Expression of hepatic drug metabolism genes. C57BL/6 mice were given s.c injections of oleanolic acid (OA) at doses of 22.5, 45, 90, and 135 mg/kg, daily for 5 days. Twenty-four hr after the last dose, Livers were collected to isolate total RNA. The expression of major hepatic nuclear receptors and their targeted genes were determined by real-time RT-PCR as detailed in the Methods. Data are mean ± SEM (n = 5). *Significantly different from control p < 0.05. Western-blot analysis of NRF2 and NQO1 are inserted into corresponding mRNA figures (β-actin is shown in Figure 4).
Discussion
We reported on the hepatoprotective effects of OA about two decades ago (Liu et al., 1993a, 1993b, 1994, 1995), and noticed mild cholestasis at higher doses, but did not investigate this phenomenon. It should be mentioned that the original studies were performed in outbred CF-1 mice, which are relatively resistant to the hepatotoxic effects of OA (administered at 100–200 mg/kg, s.c.) (Liu et al., 1993a, 1993b, 1994, 1995). However, in C57BL/6 mice, OA produces cholestasis when fed in the diet at a dose of 100 mg/kg/d for 7 days (Sato et al., 2007), and at a subcutaneous dose of 90 mg/kg in the present study. In comparison, OA at doses of 22.5 mg/kg, s.c. is hepatoprotective against the panel of hepatoxicants in C57BL/6 mice (data not shown), consistent with our prior publications. The margin of the safety of OA is quite narrow, and the ratio of toxic dose (TD) over effective dose (ED) is about 4 (the toxic dose of 90 mg/kg vs the effective hepatoprotective dose of 22.5 mg/kg, sc) in C57BL/6 mice.
Herbal hepatotoxicity is increasingly recognized as a major toxicity associated with herbal medicines (Seeff et al., 2007; Licata et al., 2013), slimming aids (Chitturi and Farrel, 2008), and single herbs such as Greater Celandine (Teschke et al., 2011), and Kava (Teschke et al., 2012). It is also known that hepatotoxicity can be produced by pure phytochemicals such as pyrrolizidine alkaloids (Li et al. 2011), pulgenone (Seeff, 2007), and OA-type triterpenoids (Sharma et al., 1999; Sato et al., 2007; Miljiewicz and Heathcote, 2011). Hepatotoxicity from herb-drug interactions also occurs, such as the combination of Panax notoginseng with Imatinib (Bilgi et al., 2008) and Teucrium chamaedrys with pentobarbital (Seeff, 2007). As an “over-the-counter” drug in China, OA is used alone or in combination with other therapeutics, at oral doses of 50–80 mg, three to four times per day (200–240 mg/person/day) for months (Xi et al., 2009). Analogues of the OA-type triterpenoids CDDO-Me (Bardoxolone methyl), were found to worsen diabetic nephropathy and produce adverse effects including liver injury after 3-month administration to rats (Zoja et al., 2013). Thus, liver function should be monitored when OA is used on a longer-term basis in the treatment of human diseases.
Cholestasis results from disruption of hepatic BA homeostasis, and can be produced by administration of α-naphthylisothiocyanate (ANIT) (intrahepatic cholestasis) (Tanaka et al., 2009) or by bile duct ligation (extrahepatic cholestasis) (Copple et al., 2010). Mechanisms of cholestatic liver injury include bile acid-induced apoptosis, bile acid-induced aberrant cell signaling and the inflammatory responses in the pathophysiology (Copple et al., 2010). In the present study, feathery hepatocyte degradation is apparent at OA doses 90 mg/kg and above. Although bile droplets and bile-duct blockage are not evident, the feathery-like degeneration can be easily seen. Feathery-like degeneration is a characteristic pathology of cholestatic liver injury as the accumulated bile acids act as detergents to make feathery-like changes in cytoplasm of hepatocytes (Li and Crawford, 2004). Increase in serum ALP implies the damage to biliary duct, and increases in serum bilirubin and BAs further support that cholestasis is a major characteristic of OA-induced liver injury. The present study focused on alteration of BA homeostasis in OA toxicity.
We first examined the expression of genes encoding BA synthesis. The “classic” BA synthesis pathway (Cyp7a1 and Cyp8b1) was decreased almost totally, while the “alternative pathway” (Cyp27a1 and Cyp7b1) was suppressed about 65%. The BA transcription factor FXR was suppressed 50% at the high dose of OA, and the expression of its target gene SHP was decreased 20%. OA was shown to be a selective FXR inducer in cell cultures (Liu and Wong, 2010), however, in the present studies, OA did not increase FXR in mice. The decrease in the BA synthesis ratelimiting enzyme Cyp7a1 does not appear to be solely mediated through the FXR-SHP pathway, other mechanisms, such as the FXR-Tgr5 pathway in the intestine, might be involved. The suppression of BA synthesis genes could be envisioned as an adaptive mechanism to reduce BA accumulation in the liver.
The expression of BA uptake transporters was investigated. The Ntcp transporter for conjugated BA and Oatp1b2 for unconjugated BA (Csanaky et al., 2011; Klaassen and Aleksunes, 2010) were suppressed by OA, contributing to an increase in serum bile acid concentrations. Oatp1b2 also plays an important role in hepatocellular uptake of xenobiotics (Lu et al., 2008). OA and ursolic acid (an OA-type triterpenoids) have been shown to inhibit OATP1B1-mediated transport of estradiol-17β-glucuronide and OATP1B3-mediated transport of estrone-3-sulfate into Chinese hamster ovary cells stably transfected with human OATP1B1 and OATP1B3 (homologues to mouse Oatp1b2) (Roth et al., 2011, Klaassen and Aleksunes, 2010), suggesting that OA-mediated inhibition of Oatp1b2 could affect not only BA uptake but also xenobiotic uptake. In addition, hepatic Oatp1a1 and Oatp1a4, also important for hepatic uptake of BA and xenobiotics (Klaassen and Aleksunes, 2010; Csanaky et al., 2011), were suppressed. Thus, inhibition of hepatic BA uptake transporters could be a mechanism for the increase in serum BA concentrations, as the liver failed to take BAs back into the hepatocytes for metabolism.
The primary transporter responsible for bile salt excretion into bile is Bsep (Abcb11), a member of the ATP-binding cassette (ABC) superfamily located at the bile canalicular apical domain of hepatocytes (Klaassen and Aleksunes, 2010). Bsep was not increased in present study, but Bcrp efflux pump increased (Fig. 6). The Ostα/Ostβ transporter is thought to be partially responsible for the efflux of BAs in the liver back into the blood (Ballatori et al., 2005). In FXR-null mice with bile duct ligation, the mRNA expression of Ostα and Ostβ did not increase, suggesting that induction of Ostα/Ostβ by BAs is FXR dependent (Boyer et al., 2006). OA decreased mRNA expression of Ostα but increased Ostβ. The increase in Ostβ was also confirmed at the protein levels. The increases in Ostβ and Bcrp might aid in transporting accumulated BAs out of hepatocytes.
The nuclear receptor networks are known to protect against cholestatic liver injury (Wagner et al., 2010; Fiorucci et al., 2012), and their disruption could compromise the protective mechanisms during OA-induced cholestasis. In the present study, OA does not affect the expression of AhR, but decreased the expression of the AhR-regulated gene Cyp1a2; OA suppressed CAR and CAR-targeted gene Cyp2b10; OA had no appreciable effects on PXR, and at the hepatoprotective doses (50–100 µmoles/kg), increased the expression of Cyp3a11, similar to our prior observations (Liu et al., 1995). OA also dose-dependent decreased PPARα and PPARα-regulated Cyp4a10. Overall, the nuclear receptor networks were disrupted by OA, compromising the protection against cholestasis.
OA is a known activator of Nrf2 (Reisman et al., 2009). Consistent with Nrf2 activation, the expression of the Nrf2 targeted gene/protein Nqo1 was markedly increased. Nrf2 is a major determinant of bile acid homeostasis in liver and intestine by regulating bile acid synthetic enzymes (Weerachayaphorn et al., 2012), and bile acid efflux during cholestasis (Maher et al., 2008). While Nrf2-overexpressing Keap1-KD mice were resistant to lithocholic acid-induced cholestasis (Okada et al., 2010), Nrf2-null mice were not more sensitive to BDL-induced cholestatic liver injury (Tanaka et al., 2009), and even seemed to be more sensitive to BDL-induced hepatotoxicity than wild-type mice (Weerachayaphorn et al., 2012), suggesting that Nrf2 activation does not protect against OA-induced liver injury. The role of Nrf2 in OA cholestasis is currently under investigation using the Nrf2 “gene-dose” model (Wu et al., 2012).
In summary, the present study clearly demonstrates that OA in a dose-dependent manner produces hepatotoxicity in C57BL/6 mice, indicating the hepatotoxic potential of a hepatoprotective compound. The OA-induced cholestatic liver injury appears to be due, at least in part, to disruption of bile acid hemostasis.
Supplementary Material
Highlights.
Oleanolic acid is a triterpenoids with many beneficial effects. However, higher dose and long-term use can produce liver injury.
Oleanolic acid induced liver injury is characterized by cholestasis, as evidenced by serum biochemistry, histopathology, and serum bile acid concentrations.
Oleanolic acid alters bile acid homeostasis, including bile acid synthesis, transport, and corresponding nuclear receptors.
Acknowledgments
This work was supported by National Institute of Health (NIH) grants DK-081461 and ES-019487, as well as by a Chinese National Science Foundation grant 81160415.
Abbreviations
- OA
oleanolic acid
- ALT
alanine aminotransferase
- UPLC-MS/MS
ultra performance liquid chromatography – tandem mass spectrometry
- Ntcp
Na+-taurocholate co-transporting ploypeptide
- Oatp1b2
organic anion-transporting polypeptide 1b2
- Bsep
bile salt export pump
- Nrf2
Nuclear factor erythroid-derived 2- like 2
- Nqo1
NAD(P)H:quinone oxidoreductase 1 (Nqo1)
- AhR
Aryl hydrocarbon Receptor
- CAR
Constitutive androstane receptor
- PXR
Pregnane X receptor
- PPAR
Peroxisome proliferator-activated receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that there are no conflicts of interest.
Author Contributions:
Conceived and designed the experiments: Jie Liu, Curtis D. Klaassen
Performed the experiments: Yuan-Fu Lu, Youcai Zhang, Kai Connie Wu, Jie Liu
Analyzed the data: Yuan-Fu Lu, Youcai Zhang, Jie Liu
Histopathology analysis: Professor Fang Fan
Contributed reagents/materials/analysis tools: Youcai Zhang, Kai Connie Wu,
Wrote the manuscript: Jie Liu, Curtis D. Klaassen, Yuan-Fu Lu, Youcai Zhang, Kai Connie Wu
Part of this work was presented previously in poster form: Liu J, Lu Y-F, Zhang Y, Wu KC and Klaassen CD (2013) Oleanolic acid produced cholestatic liver injury in mice Toxicologist, 132: A1087.
Reference
- Ballatori N, Li N, Fang F, Boyer JL, Christian WV, Hammond C. OST alpha-OST beta: a key membrane transporter of bile acids and conjugated steroids. Front. Biosci. 2009;14:2829–2844. doi: 10.2741/3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgi N, Bell K, Ananthakrishnan AN, Atallah E. Imatinib and Panax ginseng: a potential interaction resulting in liver toxicity. Ann. Pharmacother. 2010;44:926–928. doi: 10.1345/aph.1M715. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G1124–G1130. doi: 10.1152/ajpgi.00539.2005. [DOI] [PubMed] [Google Scholar]
- Cheng X, Buckley D, Klaassen CD. Regulation of hepatic bile acid transporters Ntcp and Bsep expression. Biochem. Pharmacol. 2007;74:1665–1676. doi: 10.1016/j.bcp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JY. Bile acids: regulation of synthesis. J. Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitturi S, Farrell GC. Hepatotoxic slimming aids and other herbal hepatotoxins. J. Gastroenterol. Hepatol. 2008;23:366–373. doi: 10.1111/j.1440-1746.2008.05310.x. [DOI] [PubMed] [Google Scholar]
- Copple BL, Jaeschke H, Klaassen CD. Oxidative stress and the pathogenesis of cholestasis. Semin. Liver Dis. 2010;30:195–204. doi: 10.1055/s-0030-1253228. [DOI] [PubMed] [Google Scholar]
- Csanaky IL, Lu H, Zhang Y, Ogura K, Choudhuri S, Klaassen CD. Organic anion-transporting polypeptide 1b2 (Oatp1b2) is important for the hepatic uptake of unconjugated bile acids: Studies in Oatp1b2-null mice. Hepatology. 2011;53:272–281. doi: 10.1002/hep.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci S, Zampella A, Distrutti E. Development of FXR, PXR and CAR agonists and antagonists for treatment of liver disorders. Curr. Top. Med. Chem. 2012;12:605–624. doi: 10.2174/156802612799436678. [DOI] [PubMed] [Google Scholar]
- Guinda A, Rada M, Delgado T, Gutiérrez-Adánez P, Castellano JM. Pentacyclic triterpenoids from olive fruit and leaf. J. Agric. Food Chem. 2010;58:9685–9691. doi: 10.1021/jf102039t. [DOI] [PubMed] [Google Scholar]
- Kinjo J, Okawa M, Udayama M, Sohno Y, Hirakawa T, Shii Y, Nohara T. Hepatoprotective and hepatotoxic actions of oleanolic acid-type triterpenoidal glucuronides on rat primary hepatocyte cultures. Chem. Pharm. Bull. 1999;47:290–292. doi: 10.1248/cpb.47.290. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol. Rev. 2010;62:1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszczyk MN. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009;75:1549–1560. doi: 10.1055/s-0029-1186102. [DOI] [PubMed] [Google Scholar]
- Li MK, Crawford JM. The pathology of cholestasis. Semin. Liver Dis. 2004;24:21–42. doi: 10.1055/s-2004-823099. [DOI] [PubMed] [Google Scholar]
- Li N, Xia Q, Ruan J, Fu PP, Lin G. Hepatotoxicity and tumorigenicity induced by metabolic activation of pyrrolizidine alkaloids in herbs. Curr. Drug Metab. 2011;12:823–834. doi: 10.2174/138920011797470119. [DOI] [PubMed] [Google Scholar]
- Licata A, Macaluso FS, Craxì A. Herbal hepatotoxicity: a hidden epidemic. Intern. Emerg. Med. 2013;8:13–22. doi: 10.1007/s11739-012-0777-x. [DOI] [PubMed] [Google Scholar]
- Liu J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Madhu C, Klaassen CD. Protective effects of oleanolic acid on acetaminophen-induced hepatotoxicity in mice. J. Pharmacol. Exp. Ther. 1993a;266:1607–1613. [PubMed] [Google Scholar]
- Liu Y, Kreppel H, Liu J, Choudhuri S, Klaassen CD. Oleanolic acid protects against cadmium hepatotoxicity by inducing metallothionein. J. Pharmacol. Exp. Ther. 1993b;266:400–406. [PubMed] [Google Scholar]
- Liu J, Liu Y, Mao Q, Klaassen CD. The effects of 10 triterpenoid compounds on experimental liver injury in mice. Fundam. Appl. Toxicol. 1994;22:34–40. doi: 10.1006/faat.1994.1005. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Klaassen CD. Protective effect of oleanolic acid against chemical-induced acute necrotic liver injury in mice. Zhongguo Yao Li Xue Bao. 1995;16:97–102. [PubMed] [Google Scholar]
- Liu J, Wu Q, Lu YF, Pi J. New insights into generalized hepatoprotective effects of oleanolic acid: key roles of metallothionein and Nrf2 induction. Biochem. Pharmacol. 2008;76:922–928. doi: 10.1016/j.bcp.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Liu W, Wong C. Oleanolic acid is a selective farnesoid X receptor modulator. Phytother. Res. 2010;24:369–373. doi: 10.1002/ptr.2948. [DOI] [PubMed] [Google Scholar]
- Lu H, Choudhuri S, Ogura K, Csanaky IL, Lei X, Cheng X, Song PZ, Klaassen CD. Characterization of organic anion transporting polypeptide 1b2-null mice: essential role in hepatic uptake/toxicity of phalloidin and microcystin-LR. Toxicol. Sci. 2008;103:35–45. doi: 10.1093/toxsci/kfn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YF, Wan XL, Xu Y, Liu J. Repeated oral administration of oleanolic acid produces cholestatic liver injury in mice. Molecules. 2013;18:3060–3071. doi: 10.3390/molecules18033060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher JM, Aleksunes LM, Dieter MZ, Tanaka Y, Peters JM, Manautou JE, Klaassen CD. Nrf2- and PPAR alpha-mediated regulation of hepatic Mrp transporters after exposure to perfluorooctanoic acid and perfluorodecanoic acid. Toxicol. Sci. 2008;106:319–328. doi: 10.1093/toxsci/kfn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkiewicz P, Heathcote J. Cholestasis induced by Chinese herbal remedy Xia- Ku-Hua-Tan-Pian. Liver Int. 2011;31:746–747. doi: 10.1111/j.1478-3231.2011.02506.x. [DOI] [PubMed] [Google Scholar]
- Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, Ohtsuki M, Goto N, Sugimoto H, Utsunomiya H, et al. Nrf2 counteracts cholestatic liver injury via stimulation of hepatic defense systems. Biochem. Biophys. Res. Commun. 2010;389:431–436. doi: 10.1016/j.bbrc.2009.08.156. [DOI] [PubMed] [Google Scholar]
- Petronelli A, Pannitteri G, Testa U. Triterpenoids as new promising anticancer drugs. Anticancer Drugs. 2009;20:880–892. doi: 10.1097/CAD.0b013e328330fd90. [DOI] [PubMed] [Google Scholar]
- Pollier J, Goossens A. Oleanolic acid. Phytochemistry. 2012;77:10–15. doi: 10.1016/j.phytochem.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Reisman SA, Aleksunes LM, Klaassen CD. Oleanolic acid activates Nrf2 and protects from acetaminophen hepatotoxicity via Nrf2-dependent and Nrf2- independent processes. Biochem. Pharmacol. 2009;77:1273–1282. doi: 10.1016/j.bcp.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M, Araya JJ, Timmermann BN, Hagenbuch B. Isolation of modulators of the liver-specific organic anion-transporting polypeptides (OATPs) 1B1 and 1B3 from Rollinia emarginata Schlecht (Annonaceae) J. Pharmacol. Exp. Ther. 2011;339:624–632. doi: 10.1124/jpet.111.184564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaby L, Jäger AK, Moesby L, Hansen EW, Christensen SB. Isolation of immunomodulatory triterpene acids from a standardized rose hip powder (Rosa canina L.) Phytother. Res. 2011;25:195–201. doi: 10.1002/ptr.3241. [DOI] [PubMed] [Google Scholar]
- Sato H, Genet C, Strehle A, Thomas C, Lobstein A, Wagner A, Mioskowski C, Auwerx J, Saladin R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 2007;362:793–798. doi: 10.1016/j.bbrc.2007.06.130. [DOI] [PubMed] [Google Scholar]
- Seeff LB. Herbal hepatotoxicity. Clin. Liver Dis. 2007;11:577–596. doi: 10.1016/j.cld.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Sharma S, Sharma OP, Dawra RK, Bhat TK. Disposition of lantadene A, the pentacyclic triterpenoid hepatotoxin, orally administered to guinea pigs. Toxicol. Lett. 1999;105:59–66. doi: 10.1016/s0378-4274(98)00382-8. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Aleksunes LM, Cui YJ, Klaassen CD. ANIT-induced intrahepatic cholestasis alters hepatobiliary transporter expression via Nrf2-dependent and independent signaling. Toxicol. Sci. 2009;108:247–257. doi: 10.1093/toxsci/kfp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschke R, Glass X, Schulze J. Herbal hepatotoxicity by Greater Celandine (Chelidonium majus): causality assessment of 22 spontaneous reports. Regul. Toxicol. Pharmacol. 2011;61:282–291. doi: 10.1016/j.yrtph.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Teschke R, Sarris J, Schweitzer I. Kava hepatotoxicity in traditional and modern use.The presumed Pacific kava paradox hypothesis revisited. BrJClin. Pharmacol. 2012;73:170–174. doi: 10.1111/j.1365-2125.2011.04070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Zollner G, Trauner M. Nuclear receptor regulation of the adaptive response of bile acid transporters in cholestasis. Semin. Liver Dis. 2010;30:160–177. doi: 10.1055/s-0030-1253225. [DOI] [PubMed] [Google Scholar]
- Wang JB, Zhao HP, Zhao YL, Jin C, Liu DJ, Kong WJ, Fang F, Zhang L, Wang HJ, Xiao XH. Hepatotoxicity or hepatoprotection? Pattern recognition for the paradoxical effect of the Chinese herb Rheum palmatum L. in treating rat liver injury. PLoS One. 2011;6:e24498. doi: 10.1371/journal.pone.0024498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerachayaphorn J, Mennone A, Soroka CJ, Harry K, Hagey LR, Kensler TW, Boyer JL. Nuclear factor-E2-related factor 2 is a major determinant of bile acid homeostasis in the liver and intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G925–G936. doi: 10.1152/ajpgi.00263.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KC, Liu JJ, Klaassen CD. Nrf2 activation prevents cadmium-induced acute liver injury. Toxicol. Appl. Pharmacol. 2012;263:14–20. doi: 10.1016/j.taap.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Xi J, Tang H, Zheng Y. Oral dosage forms of oleanolic acid and their pharmacokinetics. Chin J New Drugs. 2009;18:507–515. [Google Scholar]
- Zhang W, Popovich DG. Chemical and biological characterization of oleanane triterpenoids from soy. Molecules. 2009;14:2959–2975. doi: 10.3390/molecules14082959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Klaassen CD. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. J. Lipid Res. 2010;51:3230–3240. doi: 10.1194/jlr.M007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hong JY, Rockwell CE, Copple BL, Jaeschke H, Klaassen CD. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2012;32:58–69. doi: 10.1111/j.1478-3231.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoja C, Corna D, Nava V, Locatelli M, Abbate M, Gaspari F, Carrara F, Sangalli F, Remuzzi G, Benigni A. Analogues of bardoxolone methyl worsen diabetic nephropathy in rats with additional adverse effects. AmJPhysiol. Renal Physiol. 2013;304:F808–F819. doi: 10.1152/ajprenal.00376.2012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.