Summary
About a half a century has passed since dopamine was identified as a neurotransmitter, and it has been several decades since it was established that people with Parkinson’s disease receive motor symptom relief from oral levodopa. Despite the evidence that levodopa can reduce motor symptoms, there has been a developing body of literature that dopaminergic therapy can improve cognitive functions in some patients but make them worse in others. Over the past two decades, several laboratories have shown that dopaminergic medications can impair the action of intact neural structures and impair the behaviors associated with these structures. In this review we consider the evidence that has accumulated in the areas of reversal learning, motor sequence learning, and other cognitive tasks. The purported inverted-U shaped relationship between dopamine levels and performance is complex and includes many contributory factors. The regional striatal topography of nigrostriatal denervation is a critical factor as supported by multimodal neuroimaging studies. A patient's individual genotype will determine the relative baseline position on this inverted-U curve. Dopaminergic pharmacotherapy and individual gene polymorphisms can affect the mesolimbic and prefrontal cortical dopaminergic functions in a comparable inverted-U dose-response relationship. Depending on these factors, a patient can respond positively or negatively to levodopa when performing reversal learning and motor sequence learning tasks. These tasks may continue to be relevant as our society moves to increased technological demands of a digital world that requires newly learned motor sequences and adaptive behaviors to manage daily life activities.
Keywords: dopamine, ventral striatum, dorsal striatum, prefrontal cortex, learning
Introduction
A little over half a century has passed since dopamine was identified as a neurotransmitter and it was recognized that depleting monoamines in the brain through the administration of reserpine caused hunched immobility in rodents1. This finding led to the hypothesis of a dopaminergic depletion disorder as the pathophysiological basis of Parkinson’s disease. Since then, dopamine substitution using levodopa (L-3,4-dihydroxyphenylalanine) has been the most widely used pharmacotherapy for Parkinson’s disease. Despite reducing symptom manifestation, a majority of patients chronically exposed to dopamine therapy will eventually develop motor complications, including response fluctuations and drug-induced dyskinesias (abnormal involuntary movements) such as choreic and dystonic limb or truncal movements2. In addition, while restoring cognitive functions associated with dopamine depleted brain regions, dopamine substitution can also impair certain cognitive functions, such as probabilistic reversal learning, motor sequence learning and other cognitive tasks, that are associated with intact dopamine-dependent brain regions3–5. Dopamine overstimulation has been studied for over a decade since the work of Gotham and colleagues6, with papers by Swainson and colleagues7 and Cools and colleagues3 initially proposing the dopamine overdose hypothesis.
Fronto-executive cognitive deficits can be observed even in early Parkinson’s disease in the areas including executive function, learning, memory, motor inhibition, impulse control, and visuospatial processing8. These cognitive deficits may be related to mesocortical, mesolimbic, and nigrostriatal dopaminergic pathways, as well as other pathways associated with acetylcholine and norepinephrine8. Cognitive deterioration following dopaminergic therapy can occur in several tasks. In this review of the dopamine overdose hypothesis, we characterize the specific evidence supporting this hypothesis by reviewing the relevant literature in the areas of reversal learning, motor sequence learning, and other cognitive tasks. Issues related to impulsivity and reward processing are not addressed in this review, since these issues have been addressed previously9, 10. First, we discuss the physiology of the dopamine overdose hypothesis. Next, we discuss evidence for the negative effects of dopaminergic medication on probabilistic reversal learning, followed by the evidence for the negative effects of dopaminergic medication on motor sequence learning. We then discuss the evidence that other cognitive tasks can be negatively affected by levodopa when patients with Parkinson’s disease carry specific genetic polymorphisms. Finally, we discuss the clinical implications of the hypothesis as they apply to treatment and management of Parkinson’s disease patients.
Physiological Basis for the Dopamine Overdose Hypothesis
There are two major subtypes of dopaminergic neurons in the brain: the neurons of the substantia nigra pars compacta [A9 neurons]11, which give rise to the nigrostriatal pathway; and the A10 neurons of the ventral tegmental area (VTA), which give rise to the mesolimbic and mesocortical pathways that innervate parts of the limbic system and the neocortex12. The regional pattern of degeneration within the substantia nigra in patients with Parkinson’s disease appears to be specific such that a loss of pigmented neurons is greatest in the ventral lateral tier, followed by the dorsal tier, which is different to the patterns identified with healthy aging13. In vivo diffusion magnetic resonance imaging has provided evidence in support of this selective pattern of degeneration by showing that measures of structural integrity within the ventral and lateral substantia nigra are most affected in early Parkinson’s disease compared with control subjects14, 15, whereas the dorsal substantia nigra is more affected in older adults compared with young adults16.
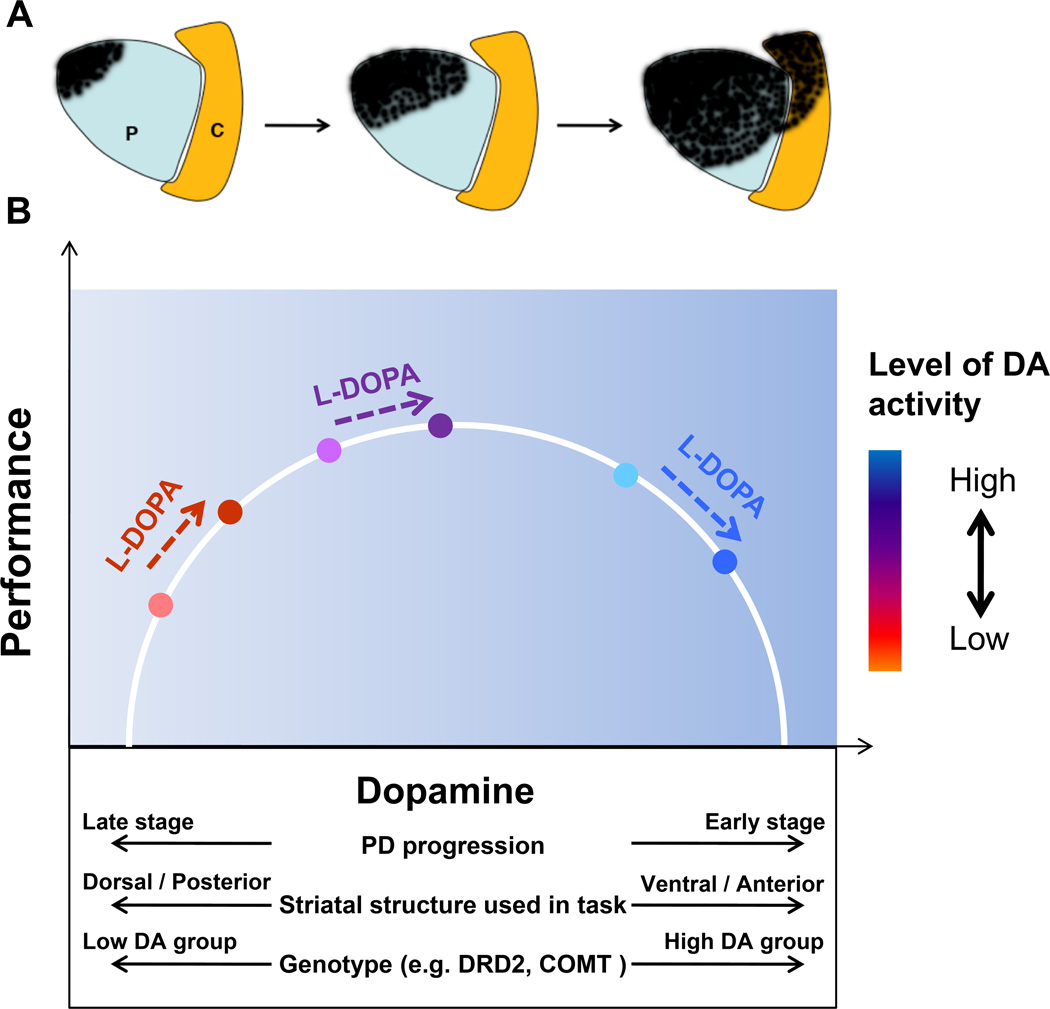
The ventral lateral tier of the substantia nigra sends dopaminergic projections primarily to the dorsal putamen, whereas the dorsal tier of the substantia nigra sends dopaminergic projections primarily to the ventral striatum. The ventral striatum receives also projections from the VTA11. Studies using 18F-dopa positron emission tomography (PET) have shown that patients with unilateral Parkinson’s disease who are Hoehn and Yahr Stage 1 have reduced dopamine storage primarily within the dorsal putamen contralateral to motor symptoms17. In a cross-sectional study, it was shown that in early Parkinson’s disease, 18F-dopa metabolism was reduced by almost 50% in the dorsal rostral putamen and dorsal caudal putamen, whereas the ventral putamen was unaffected18. With the progression of disease symptoms, there is a loss of 18F-dopa metabolism in the ventral putamen. Longitudinal multi-tracer PET studies show that the posterior-dorsal to anterior-ventral striatal gradient of nigrostriatal denervation is maintained while the asymmetry gradient becomes less prominent with progressive Parkinson's disease19. Figure 1A shows the posterior-dorsal to anterior-ventral gradient of nigrostriatal denervation in Parkinson's disease across different stages of severity of disease (from mild to severe, left to right). Early and most severe loss of nerve terminals is in the dorsal and posterior putamen. Anterior and ventral striatal regions are relatively spared, at least in the early stage of disease. This pattern of striatal degeneration from dorsal to ventral segments with the progression of Parkinson’s disease provides a fundamental basis for the dopamine overdose hypothesis. That is, regions that are less affected early in the disease could be over stimulated by exogenous dopamine administration.
Figure 1.
A, shows the posterior-dorsal to anterior-ventral gradient of nigrostriatal denervation in Parkinson's disease across different stages of severity of disease (from mild to severe, left to right). Early and most severe loss of nerve terminals is in the dorsal and posterior putamen. Anterior and ventral striatal regions are relatively spared, at least in early stage of disease. Abbreviations: P=putamen, C=Caudate. B, individual patients can experience differing performance effects in response to levodopa administration due to a combination of factors including stage of disease, striatal structure used in the task, and genotype for genetic polymorphisms that play a role in dopaminergic metabolism in striatum and prefrontal cortex (Cools, 2006). These factors collectively determine each patient’s starting location on the inverted-U shaped function describing the association between dopamine and performance, which in turn determines whether performance will worsen, improve, or show no change with dopaminergic medications.
It has been several decades since Cotzias and colleagues20 demonstrated the striking efficacy of oral levodopa for relieving motor symptoms of Parkinson’s disease. Since that time, levodopa has remained the gold standard for antiparkinsonian therapy. Nonetheless, since the dorsal putamen is mostly affected in early Parkinson’s disease whereas the ventral striatum remains relatively intact18, a given dose of levodopa that has beneficial effects for most motor tasks that rely upon dorsal putamen, can also have deleterious effects on specific cognitive and motor tasks that specifically rely upon ventral striatum5. The administration of levodopa is thought to decarboxylase in remaining dopaminergic neurons, and thus the lack of specificity of dopamine therapy can lead to a possible over stimulation of ventral striatal areas that remain intact. Figure 1B illustrates the many factors which can modulate the effects of dopaminergic medication on performance, including disease stage, striatal structure engaged by the task, and genotype for genes that regulate endogenous dopamine availability. The dopamine overdose hypothesis proposes that when patients with Parkinson’s disease are off levodopa or other dopaminergic medications, their performance on tasks such as probabilistic reversal learning is optimal, whereas their performance on cognitive tasks such as set-switching is impaired. Since reversal learning and set-switching implicate ventral and dorsal striatum respectively21, 22, these tasks have provided excellent paradigms to investigate dopaminergic effects on different striatal regions. Further, when levodopa or other dopaminergic medications are given to the patient the proposal is that performance on probabilistic reversal learning will worsen, whereas set-switching performance improves. While set-switching requires flexible swapping or switching between tasks, reversal learning requires an adaptation to a previously learned stimulus-reward contingency. The central tenet of the dopamine overdose hypothesis is that tasks that rely on the ventral striatum will be impaired by dopaminergic medications in early Parkinson’s disease because of over stimulation of the structure.
In addition to the effects of dopamine on the ventral and dorsal striatum, there is also evidence that dopamine overdose effects can occur in prefrontal cortex8. The mesocortical dopaminergic pathway projects from VTA to frontal cortex, and this pathway regulates numerous cognitive functions including set-shifting, working memory, and planning. As reviewed in subsequent sections, the studies in the literature collectively point to a cortical and subcortical role for dopamine overdose effects. In the next sections, we will review the specific evidence in support of the dopamine overdose hypothesis in several domains of learning and cognitive performance.
Reversal Learning and Dopamine in Parkinson’s Disease
Table 1 shows a summary of the studies in which reversal learning paradigms have been investigated in patients with Parkinson’s disease off and on levodopa. The consistent empirical finding has been that when on levodopa reversal learning performance is impaired compared with the performance off levodopa. Initial evidence in support of this finding was from a study by Swainson and colleagues7. The authors studied three groups of patients with Parkinson’s disease, including mild/unmedicated, mild/medicated, and severe/medicated. The reversal learning task required subjects to choose between a red pattern and a green pattern, with one pattern rewarded over the other on a probabilistic schedule. The subjects were asked to learn the task and figure out which pattern was correct, and after a series of trials the correct pattern was reversed. This was not immediately detectable given that rewards were assigned probabilistically (e.g. 80-20 ratio). When the reversal occurred, patients in the mild/medicated and severe/medicated group had difficulty adapting to the new pattern. The patients in the mild/unmedicated group had no difficulty adapting in the reversal learning task. In a subsequent study by the same laboratory3, set-switching performance and reversal learning were compared in two groups of subjects with Parkinson’s disease while either off or on dopaminergic therapy. The set-switch task required that subjects identify the correct letter or number in a well-learned sequence and did not require that subjects adapt to a new condition as in the reversal learning task. The authors identified that reaction time during the set-switch task was reduced with dopaminergic medication. During the reversal learning task, subjects with Parkinson’s disease in the off and on medication groups made more errors than control subjects at the reversal learning stage, but the patients with Parkinson’s disease off medication were able to adapt to the reversal. Patients on dopaminergic medication had more difficulty shifting their performance when the reward stimuli reversed. While the between group design utilized in these studies may make interpretations difficult, they did provide a critical advance in identifying which type(s) of task is detrimentally affected by dopaminergic medication in mild to moderate Parkinson’s disease.
Table 1.
Summary of studies in which probabilistic learning and motor sequence learning paradigms have been investigated in patients with Parkinson’s disease off and on levodopa
| Study | Outcome Measure | Results |
|---|---|---|
| Swainson et al. 20007 | Probabilistic Reversal learning | Medicated PD groups showed impairments on reversal learning tasks |
| Cools et al. 20013 | Probabilistic Reversal learning | Dopamine medication induced impairment in reversal stage of learning task |
| Shohamy et al. 200631 | Probabilistic Category learning | Impairment in initial learning, but not transfer, of association between stimuli and rewards |
| Cools et al. 200664 | Deterministic Reversal Learning | Dopaminergic medication-induced deficits on reversal shifting were restricted to reversals signaled by unexpected punishment but not by unexpected reward |
| Funkiewiez et al 200665 | Probabilistic Reversal Learning | Levodopa impaired performance on the extinction phase of reversal learning task |
| Cools et al. 200766 | Probabilistic Reversal Learning | L-Dopa disrupted activity in the nucleus accumbens but not in the dorsal striatum or prefrontal cortex during reversal learning |
| Graef et al. 201027 | Probabilistic Reversal learning | Reward based reversal learning with changing reward contingencies was impaired with dopamine medication |
| Feigin et al 200349 | Motor Sequence Learning | Levodopa infusion had no effect on motor sequence learning |
| Carbon et al 200648 | Motor Sequence Learning | Levodopa decreased measures of network activity and learning task performance in PD patients |
| Ghilardi et al 200751 | Motor Sequence Learning | L-Dopa improved scores on simple motor tasks and movement speed but had no effect on motor sequence learning |
| Argyelan et al. 200852 | Motor Sequence Learning | In de novo patients, levodopa suppressed learning-related deactivation of the ventromedial prefrontal cortex |
| Kwak et al. 20104 | Motor Sequence Learning | Dopamine medication selectively impaired early-phase motor sequence learning |
| Kwak et al. 201244 | Motor Sequence Learning | Levodopa decreased ventral striatum activation during the early learning phase; extent was correlated with levodopa-associated reductions in motor sequence learning |
| Kwak et al. 201343 | Motor Sequence Learning | Improved sequence learning only for patients carrying the minor T allele for a DRD2 polymorphism |
| Kwak et al. in press53 | Motor Sequence Learning | Spatial pattern of nigrostriatal dopaminergic denervation predicted levodopa-associated impairments in motor sequence learning |
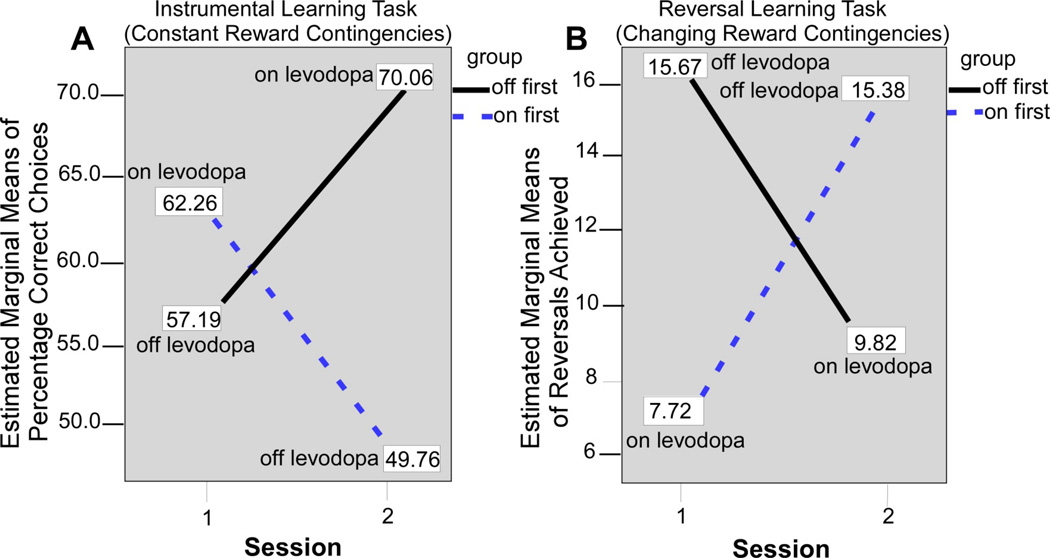
Subsequent studies by the same laboratory and others have used within-subjects designs to test subjects with Parkinson’s disease off and on levodopa. Fifteen subjects with Parkinson’s were tested off and on levodopa monotherapy using an instrumental learning task with constant stimulus-reward assocations, and a reversal learning task with changing reward contingencies. The study counterbalanced whether patients were tested in the on or off state first. A simple instrumental learning task was used since it has been linked mainly with dorsal striatum and frontal circuits23, 24, whereas the reversal learning task has been linked with ventral striatum and frontal circuits21, 25, 26. The prediction was that dopaminergic medication would have opposite effects on performance during these two tasks. Figure 2 shows experimental findings from the study during the instrumental learning task and the reversal learning task27. During the instrumental learning task (Figure 2A), which had constant reward contingencies, subjects on levodopa produced a greater percentage of correct choices compared to when off levodopa. Figure 2B shows the opposite pattern for the reversal learning task. Here, subjects on levodopa produced fewer reversal errors than when the subjects were off medication. These findings controlled for the testing order and tested the same subjects, and provide compelling evidence that reversal learning is impaired when subjects with Parkinson’s disease are on medication.
Figure 2.
Figure adapted from Graef and colleagues (2010). A, performance of On-beginner (“On first”) and Off-beginner (“Off first”) subgroups across sessions on the reversal learning task. Covariate-corrected estimated means of the number of reversals achieved are shown in boxes. B, performance of On-beginner (“On first”) and Off-beginner subgroups (“Off first”) across sessions on the instrumental learning task. Covariate-corrected estimated means of percentage of correct choices are shown in boxes.
Another probabilistic learning task, developed by Gluck and colleagues, is the Weather Prediction Task28. In this task, four “cards” have independent and probabilistic relationships to two possible outcomes, “sun” and “rain”. Patients with Parkinson’s disease exhibit impaired learning on this task when they are on dopaminergic medication, but learn at a rate similar to that of controls when off medication29, 30. In a similar paradigm, Parkinson’s patients and controls were asked to learn which of two stimuli was associated with a reward31. The stimuli varied in shape and color, with only dimension predicting reward. Parkinson’s patients were impaired at learning the initial association when on medications, but not when they were off medications. In the next phase of the study, the relevant dimension of each stimulus was held constant whereas the irrelevant feature changed. Control subjects, Parkinson’s patients on medication, and patients off medication all performed equally well during this transfer phase. Given that the weather prediction task has been shown to rely on the head caudate nucleus32, a more anterior striatal structure which exhibits dopaminergic denervation later in Parkinson’s disease than the putamen, these findings provide additional support for the dopamine overdose hypothesis playing a role in the behavior of patients with Parkinson’s disease when on levodopa.
Dopamine stimulates receptors in the basal ganglia and prefrontal cortex, and there is an important distinction for D1 and D2 receptors in these brain regions. The dopamine overdose hypothesis is also supported from studies of dopamine synthesis in animals and pharmacological studies in healthy adults. In animal models, stimulating D1 receptors with dopaminergic medication in the prefrontal cortex has revealed changes in the firing of neurons during the delay period of delayed-response tasks33–35. Computational models have provided insight into how dopamine enhances D1 receptor stimulation such that dopamine can increase the resistance to distracting stimuli36, 37. D2 receptors are more abundant in the basal ganglia than in the prefrontal cortex, and it has been suggested that the basal ganglia may serve as a gating mechanism for updating the prefrontal cortex38. D2 receptor stimulation has been tested in healthy adults using the D2 receptor agonist bromocriptine relative to their baseline dopamine synthesis capacity as determined by 18F-fluoro-L-meta-tyrosine PET tracer39. Subjects performed a reward-based reversal learning task with deterministic stimulus-outcome contingencies that required a button press to predict if the face or scene stimulus would lead to a reward or punishment. The authors found that during the placebo drug, striatal dopamine synthesis was positively related to reversal learning performance. Subjects with low striatal dopamine synthesis performed poorly on the reversal learning task, whereas high striatal dopamine synthesis was associated with high reversal learning performance. Subjects with low striatal dopamine synthesis responded positively to bromocriptine. In contrast, subjects with high baseline striatal dopamine synthesis were over-stimulated by bromocriptine, and exhibited impaired reversal learning performance when on drug.
In a study using event-related functional magnetic resonance imaging (fMRI), it was found that in healthy adults performing a probabilistic reversal learning task the ventral striatum and ventrolateral prefrontal cortex were specifically activated when subjects committed reversal errors21. A recent study evaluated the effects of bromocriptine versus sulpiride (a D2 receptor antagonist), and the combination of both drugs on reward and punishment reversal learning40. It was found that improved reward relative to punishment reversal learning performance occurred with the administration of sulpiride compared with bromocriptine. These findings provide additional insight into the specificity of the D1 and D2 receptors related to reward and punishment during reversal learning40. Next, we discuss the pertinent findings for the dopamine overdose hypothesis for motor sequence learning.
Motor Sequence Learning and Dopamine in Parkinson’s Disease
Both probabilistic reversal learning and category learning rely on more ventral subcortical structures such as the nucleus accumbens and caudate nucleus, which remain relatively intact in mild to moderate Parkinson’s disease. Thus, the dopamine overdose hypothesis provides a plausible framework from which to interpret the finding that patients perform these tasks better when off medication. There is a gradient of dopaminergic denervation within the caudate and putamen as well, with the more ventral and anterior portions of these structures affected later in the disease41, 42. The Seidler laboratory has conducted a series of experiments to investigate interactions between this gradient of denervation and anti-parkinsonian medications, working with a motor sequence learning task4, 43, 44.
Motor sequence learning refers to the capacity to combine individual elements of action into one smooth, cohesive movement. For example, patients moving into an assisted living facility may need to learn an action sequence to navigate their new surroundings. Sequential behavior is also important for skills such as driving and playing a musical instrument and motor sequence learning plays an important role in many physical rehabilitation protocols. In healthy individuals, the process of motor sequence learning is known to rely on the striatum45–47. In particular, it has been demonstrated that early in sequence learning, the more ventral and anterior portions of the striatum (i.e. associative striatum) are engaged47, whereas once a sequence becomes well learned it is represented in the more dorsal and posterior aspects of the striatum (i.e. sensorimotor striatum). Previous studies investigating the effects of medication on sequence learning in Parkinson’s patients have yielded mixed results. Table 1 shows the study by Carbon et al.48 which references the data from two prior studies from the same laboratory indicating that motor sequence learning was impaired by levodopa. The initial paper by Feigin and colleagues49 found that levodopa reduced sequence learning in Parkinson’s patients for a subjective measure of self-reported learning. When comparing objective measures, the investigators reported non-significant differences in sequence learning for patients on versus off levodopa50, 51. Although not significant for objective measures of learning, the direction of change was that levodopa may be impairing sequencing learning for some of the subjects, and that was opposite to the effects of DBS which improved sequencing learning48, 49. This line of work averaged performance across time rather than examining performance changes in the context of learning. Based on the purported inverted-U shaped relationship between dopamine levels and performance and established changes in associative and sensorimotor striatum with learning, it could be that Parkinson’s patients learn new motor sequences faster in the early stages of learning when they were off medication in comparison to on medication. In contrast, once sequences become well learned, patients could be able to perform them better when on medication as opposed to off medication.
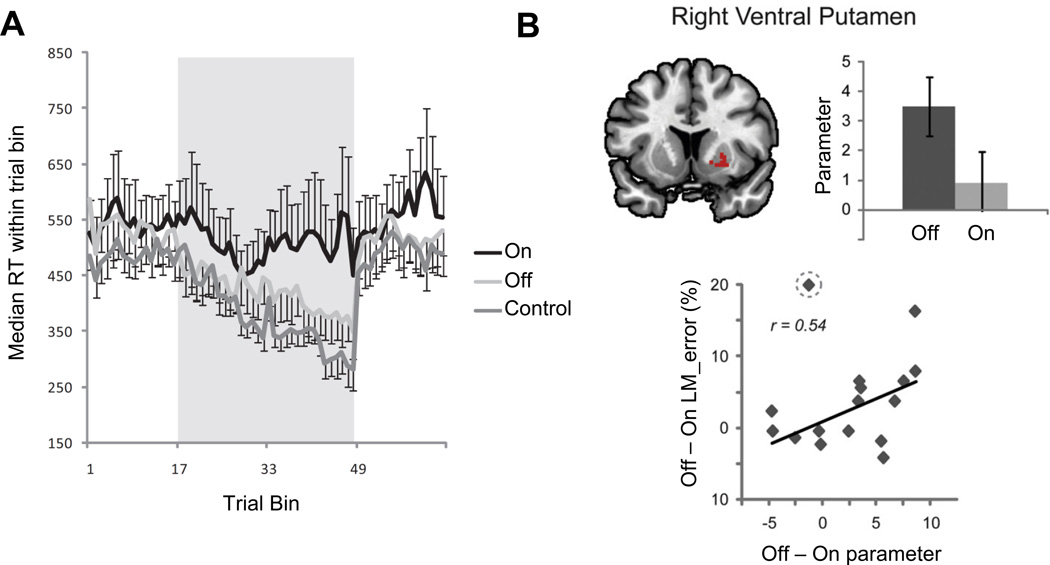
In an initial experiment4, 14 patients with mild to moderate Parkinson’s disease and 11 healthy controls were evaluated within the same age range for their ability to learn a sequence of finger movements. Patients were tested on two separate occasions, once on their anti-parkinsonian medications, which included varying combinations of medications across the participants of L-dopa and/or dopamine agonists. On the second occasion, patients were tested in the functional off state, 12–18 hours after taking their last dose of dopaminergic medications. Testing occurred in a counterbalanced fashion across medication states. As predicted by the dopamine overdose hypothesis, patients off medication exhibited early learning improvements that were equivalent to those of control subjects for the early phase of learning (see Figure 3A). As learning progressed, performance of the patients on medication began to catch up to that of their off medication performance.
Figure 3.
Panel A (from Kwak et al. 2010) illustrates that patients off their medication learn new sequences of action at the same rate as healthy controls, while patients on medication are impaired. For trials 1 – 16 and 49 – 64 participants pressed buttons in response to randomly cued stimuli; trials 17 – 48 were sequence practice trials. Panel B (from Kwak et al. 2012) illustrates a region in the right ventral putamen that is more active when patients learn a motor sequence off medication than when they are on medication. This differential activation is correlated with off-medication improvements in sequence learning.
In a follow up study44, new cohorts of 17 Parkinson’s patients and 21 healthy control subjects were studied in order to evaluate brain networks recruited during sequence learning with fMRI. Again, patients were tested across two different days. For this study a single blind placebo controlled medication design was used, with patients being given either levodopa plus carbidopa or placebo plus carbidopa after arriving to the study in the functional off state. The order of drug versus placebo testing was counterbalanced across testing days. It was found that levodopa was associated with decreased recruitment of the ventral putamen during early phase of sequence learning. Moreover, the difference in activation levels in this structure between on and off medication testing days was correlated with differences in sequence learning performance between on and off days across individual patients. That is, patients that showed the largest decrease in ventral putamen activation when on medication versus off medication exhibited the largest decrease in sequence learning abilities when on medication versus off medication, as illustrated in Figure 3B. A PET investigation by another group reported reduced brain deactivation when Parkinson’s patients were learning a sequence of actions on levodopa versus off52. Taken together, these findings suggest that both task-relevant and default network activation can be negatively impacted by administration of levodopa in mild to moderate stage Parkinson’s disease.
Another study measured dopaminergic denervation in 18 Parkinson’s patients using 11C-dihydrotetrabenazine PET scans53. Patients acquired new motor sequences on two testing days in a counterbalanced within subjects design, either on placebo or levodopa plus carbidopa. The ratio of denervation in the anterior putamen versus the posterior dorsal putamen predicted the level of levodopa-associated impairments in motor sequence learning. That is, patients with relatively less denervation in the anterior putamen showed greater on-medication sequence learning decrements. Thus, individual differences in the magnitude of motor sequence learning dopamine overdose effects can be predicted by the spatial pattern of dopaminergic denervation across the striatum.
Genotype and Dopaminergic Medication Interact to Influence Cognitive Function
Another set of studies indicates that dopaminergic medication may lead to deleterious performance on specific cognitive tasks depending on the genotype of the patient (Figure 1B). A common polymorphism in the catechol O-methyltransferase (COMT) gene has a strong influence on performance on tests of working memory, attention, and planning54–56. COMT is an important regulator of dopamine levels, and its activity is thought to primarily influence dopamine levels in the prefrontal cortex57, 58. In healthy individuals59, low-activity COMT genotypes are associated with improved performance on the Wisconsin Card Sorting Test, and fMRI studies have confirmed that the prefrontal cortex activity is influenced by the COMT genotype. However in Parkinson’s disease, performance on a Tower of London cognitive test in a large cohort of 288 patients with PD found that low COMT activity was associated with impaired performance, and the effect was more pronounced in those patients taking dopaminergic medications54. Using fMRI, it was found that patients with Parkinson’s disease who were homozygous for valine (val/val; high COMT activity) performed better and had greater activity in the frontal-parietal cortex compared with patients with Parkinson’s disease who were homozygous for methionine (met/met; low COMT activity)56. The authors did not find clear fMRI differences in the putamen and caudate nucleus.
In a task of attention control55, people with Parkinson’s disease who tested for the high COMT genotype (val/val) adopted a more optimal attention shifting strategy compared with the Parkinson’s disease group that had the low COMT genotype (met/met). The low COMT genotype group failed to adopt the attention shifting strategy. The authors also found that the fronto-parietal network typically associated with attention was less active in the low COMT genotype compared with the high COMT genotype. There were no differences observed in the putamen and caudate nucleus.
Another gene that may interact with medication effects in Parkinson’s patients is the DRD2 gene, which codes for dopamine D2 receptors in the striatum. A recent study43 evaluated whether a particular DRD2 polymorphism (rs 1076560, G > T) is associated with the on versus off medication sequence learning effects that were previously reported in patients with Parkinson’s disease. DRD2 T allele carriers of this polymorphism have reduced D2S expression (short isoform of the D2 receptor); thus in comparison G allele carriers have higher D2 receptor availability. It was predicted that Parkinson’s patients who are minor T allele carriers would exhibit a greater benefit of levodopa on early stage sequence learning. This hypothesis was evaluated in a behavioral study with 45 Parkinson’s patients; 1 patient was TT genotype (grouped together with GT patients), 10 were GT, and 34 were GG. Patients were tested on two days following a single blind placebo controlled design. Levodopa improved early sequence learning over the level of placebo pill for only the TT and GT patients, whereas GG patients did not learn better on levodopa. In contrast, levodopa improved performance on the grooved pegboard test, a simple motor execution test that should rely predominately on the sensorimotor striatum, for all patients regardless of genotype.
These findings suggest that endogenous dopamine modulating factors and exogenous dopamine interact to result in dopamine overdose effects in patients with Parkinson’s disease. That is, disease, genetics, and medication status can all influence an individual’s location on the inverted-U shaped function relating dopamine to performance. Moreover, these studies suggest that the viewpoint for the dopamine overdose hypothesis to occur only because of the ventral and dorsal striatum is too narrow and that other factors including the prefrontal cortex and parietal cortex also play a role. Also, these findings further demonstrate that the genotype of the patient is an important factor to consider when interpreting effects of dopamine on cognitive functions.
Clinical Implications and Conclusions
The clear consensus of a large body of literature is that dopaminergic therapy can improve cognitive functions in some patients but make them worse in others (see5 for an in-depth review). The dopamine overdose hypothesis provides a conceptual framework to better understand patients' individual cognitive responses to dopaminergic pharmacotherapy in Parkinson's disease. The purported inverted-U shaped relationship between dopamine levels and performance is complex and includes many contributory factors. As discussed in this review, the regional striatal topography of nigrostriatal denervation is a critical factor as supported by fMRI and dopaminergic PET studies. Second, a patient's individual genotype will determine the relative baseline position on this inverted-U curve. Third, dopaminergic pharmacotherapy and individual gene polymorphisms, such as COMT, can also affect the mesolimbic and prefrontal cortical dopaminergic functions in a comparable inverted-U dose-response relationship similar as proposed for the striatum. Therefore, at present a patient's individual cognitive response to dopaminergic pharmacotherapy cannot be predicted by a simple formula. However, a number of clinical implications may be inferred based on the available literature and experience in clinical movement disorders practice.
Striatal dopamine overdose effects have been identified for probabilistic reversal learning and motor sequence learning tasks. Implications for instrumental activities of daily living are clearly apparent. Current technological advances in society will affect persons living with Parkinson's disease. Exposure to new electronic gadgets for daily communication, financial management, bill paying, receiving healthcare, and even medication management are essentially unavoidable and will challenge patients' learning skill abilities. This will include sequential motor behaviors that include fine and precise finger movements when using these new gadgets. Navigation in new environments, whether it is driving a car in new surroundings or moving into a new place to live will be equally challenging.
A key predictor for adverse cognitive learning effects of dopaminergic therapy is the presence of early stage and/or mild severity of disease, where overdose effects of dopaminergic medications are predicted to be most prominent. Practical implications of the dopamine overdose hypothesis in the management of the individual patient with Parkinson's disease will first depend on identification of a dopaminergic dose-related cognitive or behavioral problem. If present, a simple pointer will be to implement a new motor learning challenge, such as using a new device, when in a relative medication 'off' state (assuming preservation of critical motor abilities). Apart from learning new motor behaviors in an 'off' state, a rational pharmacological therapy approach based on the principle of the lowest effective dose may also help to prevent or ameliorate dopaminergic 'on' overdose cognitive adverse effects. Most of all, clinicians need to consider a broader range of symptoms and individual patient priorities in adjusting medication dosages by suggesting medication strategies that strike a better balance between motor and cognitive symptoms60. It is also important to consider premorbid personality and psychiatric history when making clinical decisions. Future clinical treatment algorithms may include information about specific gene polymorphisms that affects the dopamine system (so-called 'personalized medicine'). It may also be prudent to consider exercise as an adjunct to dopamine therapy to reduce motor symptoms to also minimize the overdose, as recent studies have shown promise using balance training over six months61 and progressive resistance exercise over two years62.
Cognitive functions, including reward based learning, have been the major focus of research of the dopamine overdose hypothesis60. However, much is less is known into the neurobehavioral correlates of these impairments and how it affects instrumental activities of daily living. Dopamine dysregulation syndrome (DDS) is a dysfunction of the reward system in patients with Parkinson's disease who are on dopaminergic drugs, in particular dopamine agonists. Patients with DDS show evidence of impulse control disorders63. Further research is needed to determine whether an excess of exogenous dopamine release within the anteroventral striatum and related cortical projections may be a mechanistic factor underlying impulsive and/or risk taking behaviors in susceptible patients.
Acknowledgements
This review was funded in part by NIH grants (R01 NS075012, R01 NS052318, P01 NS015655 and R01 NS070856), Bachman-Strauss Foundation, Tyler’s Hope Foundation, NASA NNX11AR02G, NSBRI, Department of Veterans Affairs, and the Michael J. Fox Foundation.
Dr. Vaillancourt receives grant support from NIH, Bachmann-Straus Foundation, Tyler’s Hope Foundation, and consults for projects at UT Southwestern Medical Center, University of Illinois, and Great Lakes NeuroTechnologies. Dr. Bohnen has research support from the NIH, Department of Veteran Affairs, and the Michael J. Fox Foundation. Dr. Seidler receives grant support from NIH, NASA, NSBRI, and the Michigan Parkinson’s Foundation.
Footnotes
Financial Disclosure Statement: Daniel Schonfeld has no financial disclosures. Dr. Kwak has no financial disclosures.
Author Contributions
Dr. Vaillancourt – review design, obtaining funding, collecting articles, and writing
Mr. Schonfeld – review design, collecting articles, and writing
Dr. Kwak – review design and writing
Dr. Bohnen - review design, obtaining funding, collecting articles, and writing
Dr. Seidler - review design, obtaining funding, collecting articles, and writing
References
- 1.Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180(4596):1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- 2.Watts RL, Koller WC, editors. Movement Disorders: Neurologic Principles and Practice. 2nd ed. New York: McGraw-Hill; 2004. [Google Scholar]
- 3.Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11(12):1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- 4.Kwak Y, Muller ML, Bohnen NI, Dayalu P, Seidler RD. Effect of dopaminergic medications on the time course of explicit motor sequence learning in Parkinson's disease. J Neurophysiol. 2010;103(2):942–949. doi: 10.1152/jn.00197.2009. [DOI] [PubMed] [Google Scholar]
- 5.Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neuroscience and biobehavioral reviews. 2006;30(1):1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Gotham AM, Brown RG, Marsden CD. 'Frontal' cognitive function in patients with Parkinson's disease 'on' and 'off' levodopa. Brain. 1988;111(Pt 2):299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- 7.Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW. Probabilistic learning and reversal deficits in patients with Parkinson's disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38(5):596–612. doi: 10.1016/s0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 8.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet neurology. 2010;9(12):1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- 9.Robert G, Drapier D, Verin M, Millet B, Azulay JP, Blin O. Cognitive impulsivity in Parkinson's disease patients: assessment and pathophysiology. Mov Disord. 2009;24(16):2316–2327. doi: 10.1002/mds.22836. [DOI] [PubMed] [Google Scholar]
- 10.Wiecki TV, Frank MJ. Neurocomputational models of motor and cognitive deficits in Parkinson's disease. Prog Brain Res. 2010;183:275–297. doi: 10.1016/S0079-6123(10)83014-6. [DOI] [PubMed] [Google Scholar]
- 11.Dahlstroem A, Fuxe K. Evidence for the Existence of Monoamine-Containing Neurons in the Central Nervous System. I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons. Acta Physiol Scand Suppl. 1964;(SUPPL 232):231–255. [PubMed] [Google Scholar]
- 12.Bjorklund A, Lindvall O. Dopamin-containing systems in the CNS. In: Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. Amsterdam: Elsevier; 1984. pp. 55–122. [Google Scholar]
- 13.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 14.Vaillancourt DE, Spraker MB, Prodoehl J, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72(16):1378–1384. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du G, Lewis MM, Sen S, et al. Imaging nigral pathology and clinical progression in Parkinson's disease. Mov Disord. 2012;27(13):1636–1643. doi: 10.1002/mds.25182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaillancourt DE, Spraker MB, Prodoehl J, Zhou XJ, Little DM. Effects of aging on the ventral and dorsal substantia nigra using diffusion tensor imaging. Neurobiology of aging. 2012;33(1):35–42. doi: 10.1016/j.neurobiolaging.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrish PK, Sawle GV, Brooks DJ. Clinical and [18F] dopa PET findings in early Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry. 1995;59(6):597–600. doi: 10.1136/jnnp.59.6.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson's disease. Brain. 1996;119(Pt 2):585–591. doi: 10.1093/brain/119.2.585. [DOI] [PubMed] [Google Scholar]
- 19.Nandhagopal R, Kuramoto L, Schulzer M, et al. Longitudinal progression of sporadic Parkinson's disease: a multi-tracer positron emission tomography study. Brain. 2009;132(Pt 11):2970–2979. doi: 10.1093/brain/awp209. [DOI] [PubMed] [Google Scholar]
- 20.Cotzias GC, Papavasiliou PS, Gellene R. Modification of Parkinsonism--chronic treatment with L-dopa. The New England journal of medicine. 1969;280(7):337–345. doi: 10.1056/NEJM196902132800701. [DOI] [PubMed] [Google Scholar]
- 21.Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22(11):4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci U S A. 2000;97(24):13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. The European journal of neuroscience. 2004;19(1):181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- 24.Kimchi EY, Torregrossa MM, Taylor JR, Laubach M. Neuronal correlates of instrumental learning in the dorsal striatum. J Neurophysiol. 2009;102(1):475–489. doi: 10.1152/jn.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10(3):284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 26.Heekeren HR, Wartenburger I, Marschner A, Mell T, Villringer A, Reischies FM. Role of ventral striatum in reward-based decision making. Neuroreport. 2007;18(10):951–955. doi: 10.1097/WNR.0b013e3281532bd7. [DOI] [PubMed] [Google Scholar]
- 27.Graef S, Biele G, Krugel LK, et al. Differential influence of levodopa on reward-based learning in Parkinson's disease. Front Hum Neurosci. 2010;4:169. doi: 10.3389/fnhum.2010.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learn Mem. 1994;1(2):106–120. [PubMed] [Google Scholar]
- 29.Shohamy D, Myers CE, Kalanithi J, Gluck MA. Basal ganglia and dopamine contributions to probabilistic category learning. Neuroscience and biobehavioral reviews. 2008;32(2):219–236. doi: 10.1016/j.neubiorev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273(5280):1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- 31.Shohamy D, Myers CE, Geghman KD, Sage J, Gluck MA. L-dopa impairs learning, but spares generalization, in Parkinson's disease. Neuropsychologia. 2006;44(5):774–784. doi: 10.1016/j.neuropsychologia.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poldrack RA, Prabhakaran V, Seger CA, Gabrieli JD. Striatal activation during acquisition of a cognitive skill. Neuropsychology. 1999;13(4):564–574. doi: 10.1037//0894-4105.13.4.564. [DOI] [PubMed] [Google Scholar]
- 33.Sawaguchi T, Matsumura M, Kubota K. Catecholaminergic effects on neuronal activity related to a delayed response task in monkey prefrontal cortex. J Neurophysiol. 1990;63(6):1385–1400. doi: 10.1152/jn.1990.63.6.1385. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Goldman-Rakic PS. D2 receptor regulation of synaptic burst firing in prefrontal cortical pyramidal neurons. Proc Natl Acad Sci U S A. 2004;101(14):5093–5098. doi: 10.1073/pnas.0400954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376(6541):572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 36.Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2001;98(1):301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21(10):3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frank MJ, Loughry B, O'Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1(2):137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- 39.Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29(5):1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Schaaf ME, van Schouwenburg MR, Geurts DE, et al. Establishing the Dopamine Dependency of Human Striatal Signals During Reward and Punishment Reversal Learning. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs344. [DOI] [PubMed] [Google Scholar]
- 41.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. The New England journal of medicine. 1988;318(14):876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 42.Rakshi JS, Uema T, Ito K, et al. Frontal, midbrain and striatal dopaminergic function in early and advanced Parkinson's disease A 3D [(18)F]dopa-PET study. Brain. 1999;122(Pt 9):1637–1650. doi: 10.1093/brain/122.9.1637. [DOI] [PubMed] [Google Scholar]
- 43.Kwak Y, Bohnen NI, Muller ML, Dayalu P, Burke DT, Seidler RD. Task-dependent interactions between Dopamine D2 receptor polymorphisms and L-DOPA in patients with Parkinson's disease. Behav Brain Res. 2013;245:128–136. doi: 10.1016/j.bbr.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 44.Kwak Y, Muller ML, Bohnen NI, Dayalu P, Seidler RD. l-DOPA changes ventral striatum recruitment during motor sequence learning in Parkinson's disease. Behav Brain Res. 2012;230(1):116–124. doi: 10.1016/j.bbr.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Seidler RD, Purushotham A, Kim SG, Ugurbil K, Willingham D, Ashe J. Neural correlates of encoding and expression in implicit sequence learning. Exp Brain Res. 2005;165(1):114–124. doi: 10.1007/s00221-005-2284-z. [DOI] [PubMed] [Google Scholar]
- 46.Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Current opinion in neurobiology. 2002;12(2):217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- 47.Lehericy S, Benali H, Van de Moortele PF, et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A. 2005;102(35):12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carbon M, Eidelberg D. Functional imaging of sequence learning in Parkinson's disease. Journal of the neurological sciences. 2006;248(1–2):72–77. doi: 10.1016/j.jns.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Feigin A, Ghilardi MF, Carbon M, et al. Effects of levodopa on motor sequence learning in Parkinson's disease. Neurology. 2003;60(11):1744–1749. doi: 10.1212/01.wnl.0000072263.03608.42. [DOI] [PubMed] [Google Scholar]
- 50.Carbon M, Ghilardi MF, Feigin A, et al. Learning networks in health and Parkinson's disease: reproducibility and treatment effects. Human brain mapping. 2003;19(3):197–211. doi: 10.1002/hbm.10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghilardi MF, Feigin AS, Battaglia F, et al. L-Dopa infusion does not improve explicit sequence learning in Parkinson's disease. Parkinsonism & related disorders. 2007;13(3):146–151. doi: 10.1016/j.parkreldis.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Argyelan M, Carbon M, Ghilardi MF, et al. Dopaminergic suppression of brain deactivation responses during sequence learning. J Neurosci. 2008;28(42):10687–10695. doi: 10.1523/JNEUROSCI.2933-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwak Y, Bohnen NI, Muller MLTM, Dayalu P, Seidler RD. Striatal denervation pattern predicts levodopa effects on sequence learning in Parkinson's disease. Journal of Motor Behavior. doi: 10.1080/00222895.2013.817380. In press. [DOI] [PubMed] [Google Scholar]
- 54.Foltynie T, Goldberg TE, Lewis SG, et al. Planning ability in Parkinson's disease is influenced by the COMT val158met polymorphism. Mov Disord. 2004;19(8):885–891. doi: 10.1002/mds.20118. [DOI] [PubMed] [Google Scholar]
- 55.Williams-Gray CH, Hampshire A, Barker RA, Owen AM. Attentional control in Parkinson's disease is dependent on COMT val 158 met genotype. Brain. 2008;131(Pt 2):397–408. doi: 10.1093/brain/awm313. [DOI] [PubMed] [Google Scholar]
- 56.Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci. 2007;27(18):4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. Journal of neurochemistry. 1994;63(3):972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- 58.Mazei MS, Pluto CP, Kirkbride B, Pehek EA. Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res. 2002;936(1–2):58–67. doi: 10.1016/s0006-8993(02)02542-8. [DOI] [PubMed] [Google Scholar]
- 59.Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159(4):652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- 60.MacDonald AA, Monchi O, Seergobin KN, Ganjavi H, Tamjeedi R, MacDonald PA. Parkinson's disease duration determines effect of dopaminergic therapy on ventral striatum function. Mov Disord. 2013;28(2):153–160. doi: 10.1002/mds.25152. [DOI] [PubMed] [Google Scholar]
- 61.Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson's disease. The New England journal of medicine. 2012;366(6):511–519. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corcos DM, Robichaud JA, David FJ, et al. A two-year randomized controlled trial of progressive resistance exercise for Parkinson's disease. Mov Disord. 2013 doi: 10.1002/mds.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ray NJ, Strafella AP. Imaging impulse control disorders in Parkinson's disease and their relationship to addiction. J Neural Transm. 2013;120(4):659–664. doi: 10.1007/s00702-012-0933-5. [DOI] [PubMed] [Google Scholar]
- 64.Cools R, Altamirano L, D'Esposito M. Reversal learning in Parkinson's disease depends on medication status and outcome valence. Neuropsychologia. 2006;44(10):1663–1673. doi: 10.1016/j.neuropsychologia.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 65.Funkiewiez A, Ardouin C, Cools R, et al. Effects of levodopa and subthalamic nucleus stimulation on cognitive and affective functioning in Parkinson's disease. Mov Disord. 2006;21(10):1656–1662. doi: 10.1002/mds.21029. [DOI] [PubMed] [Google Scholar]
- 66.Cools R, Lewis SJ, Clark L, Barker RA, Robbins TW. L-DOPA disrupts activity in the nucleus accumbens during reversal learning in Parkinson's disease. Neuropsychopharmacology. 2007;32(1):180–189. doi: 10.1038/sj.npp.1301153. [DOI] [PubMed] [Google Scholar]