Abstract
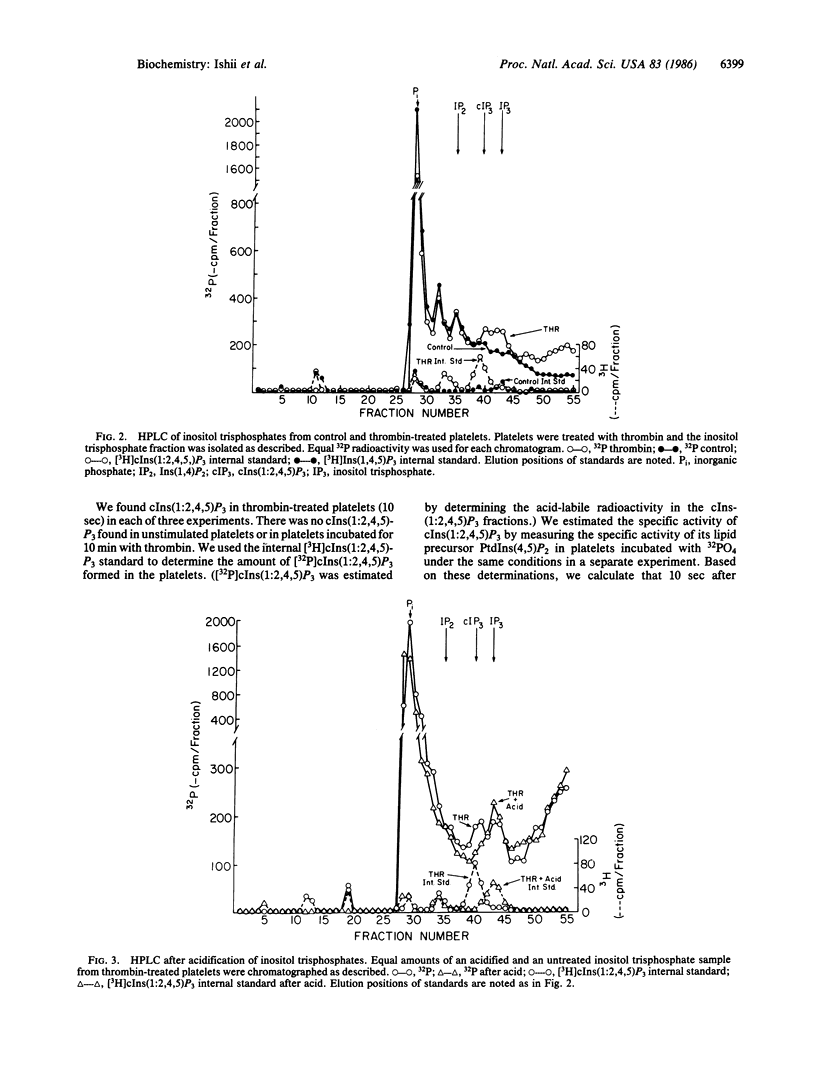
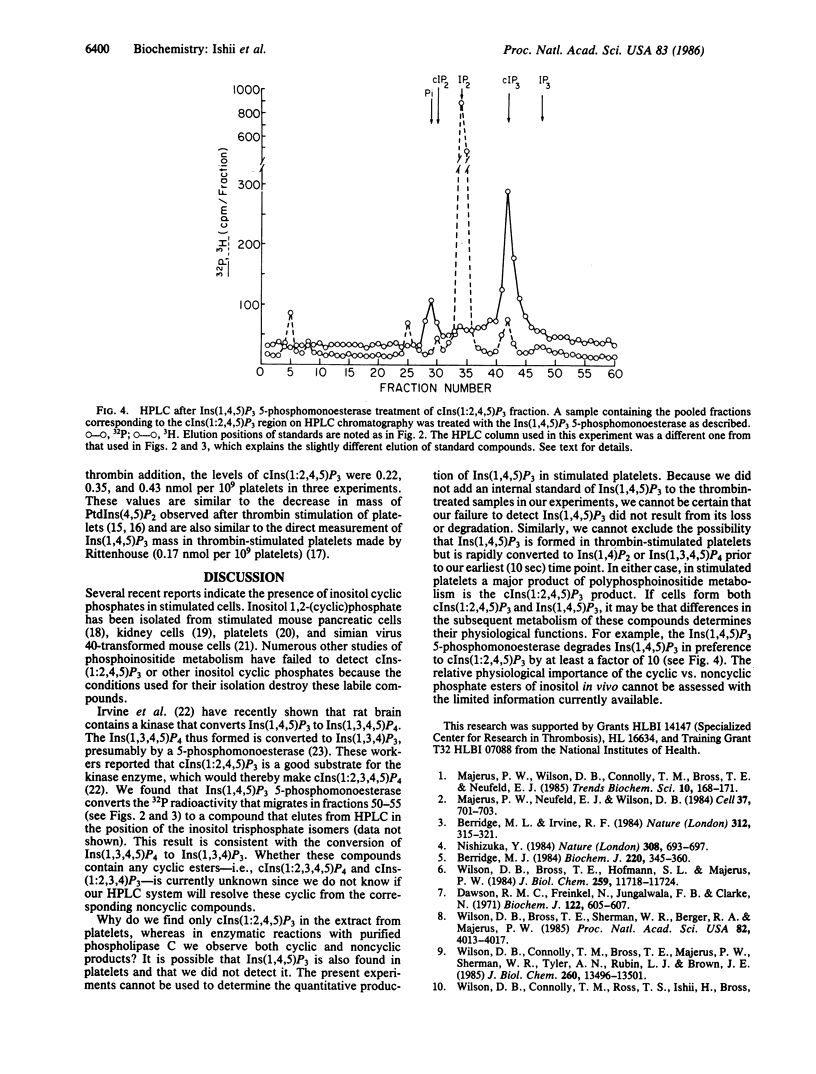
Cleavage of polyphosphoinositides in vitro by phospholipase C results in formation of both cyclic and noncyclic inositol phosphates. We have now isolated the cyclic product of phosphatidylinositol 4,5-bisphosphate cleavage, inositol 1,2(cyclic)-4,5-triphosphate [cIns(1:2,4,5)P3], from thrombin-treated platelets. We found 0.2-0.4 nmol of cIns-(1:2,4,5)P3 per 10(9) platelets at 10 sec after thrombin; none was found in unstimulated platelets or in platelets 10 min after thrombin addition. We conclude that cIns(1:2,4,5)P3 is a major product of polyphosphoinositide metabolism in thrombin-stimulated platelets.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger N. L., Majerus P. W. Isolation of human platelets and platelet surface membranes. Methods Enzymol. 1974;31:149–155. doi: 10.1016/0076-6879(74)31015-4. [DOI] [PubMed] [Google Scholar]
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Binder H., Weber P. C., Siess W. Separation of inositol phosphates and glycerophosphoinositol phosphates by high-performance liquid chromatography. Anal Biochem. 1985 Jul;148(1):220–227. doi: 10.1016/0003-2697(85)90649-9. [DOI] [PubMed] [Google Scholar]
- Broekman M. J. Phosphatidylinositol 4,5-bisphosphate may represent the site of release of plasma membrane-bound calcium upon stimulation of human platelets. Biochem Biophys Res Commun. 1984 Apr 16;120(1):226–231. doi: 10.1016/0006-291x(84)91437-2. [DOI] [PubMed] [Google Scholar]
- Connolly T. M., Bross T. E., Majerus P. W. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyzes the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem. 1985 Jul 5;260(13):7868–7874. [PubMed] [Google Scholar]
- Connolly T. M., Wilson D. B., Bross T. E., Majerus P. W. Isolation and characterization of the inositol cyclic phosphate products of phosphoinositide cleavage by phospholipase C. Metabolism in cell-free extracts. J Biol Chem. 1986 Jan 5;261(1):122–126. [PubMed] [Google Scholar]
- Dawson R. M., Freinkel N., Jungalwala F. B., Clarke N. The enzymic formation of myoinositol 1:2-cyclic phosphate from phosphatidylinositol. Biochem J. 1971 May;122(4):605–607. doi: 10.1042/bj1220605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J. F., Hokin L. E. The formation of inositol 1,2-cyclic phosphate on agonist stimulation of phosphoinositide breakdown in mouse pancreatic minilobules. Evidence for direct phosphodiesteratic cleavage of phosphatidylinositol. J Biol Chem. 1985 Dec 25;260(30):16068–16071. [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Koch M. A., Diringer H. Isolation of cyclic inositol-1,2-phosphate from mammalian cells and a probable function of phosphatidylinositol turnover. Biochem Biophys Res Commun. 1974 May 20;58(2):361–367. doi: 10.1016/0006-291x(74)90373-8. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Neufeld E. J., Wilson D. B. Production of phosphoinositide-derived messengers. Cell. 1984 Jul;37(3):701–703. doi: 10.1016/0092-8674(84)90405-7. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S. E., Sasson J. P. Mass changes in myoinositol trisphosphate in human platelets stimulated by thrombin. Inhibitory effects of phorbol ester. J Biol Chem. 1985 Jul 25;260(15):8657–8660. [PubMed] [Google Scholar]
- Walseth T. F., Gander J. E., Eide S. J., Krick T. P., Goldberg N. D. 18O labeling of adenine nucleotide alpha-phosphoryls in platelets. Contribution by phosphodiesterase-catalyzed hydrolysis of cAMP. J Biol Chem. 1983 Feb 10;258(3):1544–1558. [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Hofmann S. L., Majerus P. W. Hydrolysis of polyphosphoinositides by purified sheep seminal vesicle phospholipase C enzymes. J Biol Chem. 1984 Oct 10;259(19):11718–11724. [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Sherman W. R., Berger R. A., Majerus P. W. Inositol cyclic phosphates are produced by cleavage of phosphatidylphosphoinositols (polyphosphoinositides) with purified sheep seminal vesicle phospholipase C enzymes. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4013–4017. doi: 10.1073/pnas.82.12.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B., Connolly T. M., Bross T. E., Majerus P. W., Sherman W. R., Tyler A. N., Rubin L. J., Brown J. E. Isolation and characterization of the inositol cyclic phosphate products of polyphosphoinositide cleavage by phospholipase C. Physiological effects in permeabilized platelets and Limulus photoreceptor cells. J Biol Chem. 1985 Nov 5;260(25):13496–13501. [PubMed] [Google Scholar]
- Wilson D. B., Neufeld E. J., Majerus P. W. Phosphoinositide interconversion in thrombin-stimulated human platelets. J Biol Chem. 1985 Jan 25;260(2):1046–1051. [PubMed] [Google Scholar]


