Abstract
One third of the world is infected with Mycobacterium tuberculosis (Mtb) with eight million new cases of active tuberculosis (TB) each year. Development of a new vaccine to augment or replace the only approved TB vaccine, BCG, is needed to control this disease. Mtb infection is primarily controlled by TH1 cells through the production of IFN-γ and TNF which activate infected macrophages to kill the bacterium. Here we examine an array of adjuvant formulations containing the TLR4 agonist GLA to identify candidate adjuvants to pair with ID93, a lead TB vaccine antigen, to elicit protective TH1 responses. We evaluate a variety of adjuvant formulations including alum, liposomes, and oil-in-water emulsions to determine how changes in formulation composition alter adjuvant activity. We find that alum and an aqueous nanosuspension of GLA synergize to enhance generation of ID93-specific TH1 responses, whereas neither on their own are effective adjuvants for generation of ID93-specific TH1 responses. For GLA containing oil-in-water emulsions, the selection of the oil component is critical for adjuvant activity, whereas a variety of lipid components may be used in liposomal formulations of GLA. The composition of the liposome formulation of ID93/GLA does alter the magnitude of the TH1 response. These results demonstrate that there are multiple solutions for an effective formulation of a novel TB vaccine candidate that enhances both TH1 generation and protective efficacy.
Keywords: Vaccine formulation, Liposomes, Oil-in-water emulsions, Alum, Mycobacterium tuberculosis
1. Introduction
One third of the world is latently infected with Mycobacterium tuberculosis (Mtb). There are approximately 8 million new cases of active tuberculosis (TB), leading to 1.5 million deaths annually [1]. The only approved vaccine for Mtb, Bacillus Calmette–Guérin (BCG) is routinely given shortly after birth. BCG is effective in limiting the development of miliary TB in children, but does not prevent development of active pulmonary TB in adults [2]. Mtb is primarily spread by the patients with active pulmonary TB, thus a new vaccine that will limit this manifestation is desperately needed. Mathematical modeling of the impact of implementing a hypothetical new vaccine against TB with 60% efficacy predicts an 80% drop in incidence by 2050 [3]. Thus there is an urgent need for a new TB vaccine to either boost immunity primed by BCG or replace BCG.
Most vaccines against human pathogens work by eliciting protective antibody responses. For Mtb, antibodies are thought to play a limited role in controlling the infection because Mtb is an intracellular pathogen, primarily infecting macrophages. Immune control of Mtb is largely mediated by IFN-γ and TNF producing CD4 T cells [4,5]. Both of these cytokines induce production of reactive nitrogen and oxygen species in Mtb-infected macrophages, which kill the bacterium. Some studies have found that the frequency of TB-specific multifunctional CD4 T helper 1 (TH1) cells, i.e. cells that make a combination of IFN-γ, TNF, and IL-2 upon stimulation, correlates with vaccine efficacy, although this is not always the case [6-8]. Under some conditions CD8 T cells also contribute to Mtb control, although not to the same degree as CD4 T cells [9].
Adjuvant selection is a critical step for the development of effective subunit vaccines. The two most commonly used adjuvants in approved vaccines are aluminum salts collectively referred to as alum and oil-in-water emulsions such as MF59 (Novartis) and AS03 (GSK), both of which contain squalene as an oil component [10]. These adjuvants efficiently enhance humoral responses, but provide little enhancement of TH1 immunity. Next generation adjuvants are being developed to enhance T cell immunity. Inclusion of TLR agonists is one effective method of enhancing T cell responses elicited by vaccines. The recently approved vaccine adjuvant for the Cervarix vaccine for human papilloma virus, AS04 (GSK), contains the TLR4 agonist MPL and alum [11]. However, as with alum and oil-in-water adjuvants, the development of AS04 was based on enhanced antibody production [12].
Two additional MPL-containing adjuvants are currently being developed for T cell-based vaccines against Mtb and malaria. AS01 and AS02 both contain MPL and the saponin QS21 [13]. AS01 consists of a liposome formulation of MPL and QS21 whereas AS02 is a squalene-containing emulsion formulation of the same immunostimulants. Clinical trials comparing the immunogenicity and efficacy of the RTS,S and M72 antigens for malaria and TB respectively found that AS01 facilitated greater TH1 responses against both antigens and enhanced RTS,S efficacy [12-14]. These studies highlight the need to examine how vaccine formulation affects vaccine efficacy.
To develop a new TB vaccine we have identified a number of Mtb proteins that are antigenic in healthy Mtb-infected or BCG-immunized humans and protective in mouse challenge models when paired with an appropriate adjuvant [15]. We have generated a fusion protein of four of these proteins as our lead vaccine antigen. This fusion protein, ID93, consists of three proteins associated with virulence, Rv2608, Rv3619 and Rv3620 and one latency-associated antigen Rv1813 [16]. When combined with the synthetic TLR4 agonist GLA and formulated in a squalene-in-water stable emulsion (SE) ID93 protects against aerosol challenge with Mtb in both mouse and guinea pig models [17]. ID93/GLA-SE also shows efficacy as a therapeutic vaccine given as an adjunct to drug treatment of Mtb-infected mice and non-human primates [18].
Proper formulation of immunostimulatory molecules such as GLA and MPL can enhance both the stability and efficacy of adjuvants. For example many TLR4 agonists are insoluble and prone to aggregation without proper formulation. Besides squalene-based nanoemulsion formulations of GLA, we have previously reported on the development of triglyceride-based nanoemulsions [19-22], aqueous nanosuspensions [19,23], liposomal formulations [24], and an alum-adsorbed microparticulate formulation of GLA [25]. Each of these formulations includes lipid-based excipients but differs significantly in particle morphology, surface characteristics, and size. Formulation selection criteria include compatibility with the antigen and immunostimulant, the nature of the desired immune response (i.e. TH1 for a TB vaccine), ease of manufacture, and stability after manufacture. In the present study we examine how vaccine formulation affects the immunogenicity and efficacy of candidate ID93/GLA vaccines. We examine how oil and lipid composition affect oil-in-water and liposome formulations as well as examine the efficacy of GLA formulated with alum or as an aqueous nanosuspension. These adjuvant formulations were evaluated for antibody and cellular immunogenicity as well as protective efficacy in mice when combined with a recombinant tuberculosis antigen, ID93, and injected intramuscularly. Moreover, the extent of association of the antigen with the adjuvant was examined, and whether these interactions could have an effect on vaccine bioactivity. Besides evaluating the adjuvant activity of the various formulations, we have also characterized their physicochemical nature and stability.
2. Materials and methods
2.1. Adjuvant manufacturing
Glucopyranosyl lipid adjuvant (GLA), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-Dipalmitoyl-sn-glycero-3-phosphoglycerol (DPPG), 1,2-dipalmitoyl-3-trimethylammonium-propane (DPTAP), 1,2 dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(succinyl) (succinyl-functionalized DPPE), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), and egg yolk phosphatidylcholine (Egg PC) were purchased from Avanti Polar Lipids Inc. (Alabaster, AL). DPPG was also purchased from Corden Pharma (Liestal, Switzerland). Squalene was purchased from Sigma-Aldrich (St. Louis, MO). Grapeseed oil by Napa Valley Naturals (San Francisco, CA) was obtained from a local grocer. Miglyol 810 was obtained courtesy of Sasol (Johannesburg, South Africa). Poloxamer 188 and glycerol were purchased from Spectrum Chemical (Gardena, CA). Cholesterol, ammonium phosphate mono and dibasic were purchased from J.T. Baker (San Francisco, CA). Phosphate buffered saline (PBS) of 1 × concentration at pH 7.2 was purchased from Invitrogen (Grand Island, NY). Alhydrogel® 85 (aluminum oxyhydroxide gel) was purchased from E.M. Sergeant Pulp & Chemical Co. (Kalamazoo, MI). Cross linking reagents Sulfo-NHS N-Hydroxyssulfosuccinimide and EDC 1-Ethyl-3-(3 dimethylaminopropyl) carbodimide HCL were both purchased from Thermo Scientific.
Aqueous GLA was manufactured by first combining DPPC and GLA (2:1 molar ratio of DPPC:GLA) in minimal amount of chloroform needed to dissolve all solutes. The GLA/DPPC/Chloroform mixture was then evaporated using a Buchi Rotovapor R-114 (Flawil, Switzerland) or Genevac EZ-2 centrifugal evaporator (Stone Ridge, NY) to remove the chloroform. The dried DPPC and GLA were then rehydrated in ultrapure water at a concentration of 0.25 mg/ml GLA and 0.21 mg/ml DPPC, and sonicated at ~60 °C with either a VWR 75D (West Chester, PA) or Crest Powersonic CP230D (Trenton, NJ) waterbath sonicator for approximately 1–2 h or until it appeared to be a single-phase, translucent formulation. Aqueous GLA was diluted 5× with antigen and buffer prior to immunization studies. Aluminum oxyhydroxide formulation with GLA was prepared by mixing aqueous GLA with Alhydrogel® 85 for a GLA concentration of 0.1 mg/ml and an aluminum concentration of 2 mg/ml. The alum formulations were diluted 2× with antigen and buffer prior to immunization.
GLA-SE emulsions were manufactured by mixing a buffered aqueous phase and an oil phase with a Silverson Heavy Duty Laboratory Mixer Emulsifier (3/4 in. tubular square hole high shear screen attachment; East Longmeadow, MA) at ~7000–10,000 rpm for ~10 min, then microfluidizing the mixture using the Microfluidics M110P (Newton, MA) for 12 passes at 30,000 psi. The buffered aqueous phase was prepared by combining poloxamer 188 and glycerol in ammonium phosphate buffer (pH 5.1). The oil phase was prepared by dispersing DMPC or Egg PC and GLA into squalene, grapeseed oil, or Miglyol 810. The oil phase was then sonicated at ~70 °C for 1–3 h or until evenly dispersed. Component concentrations in the emulsions consisted of 10% v/v oil, 1.9% w/v phosphatidylcholine, 0.1% w/v poloxamer 188, 2.3% w/v glycerol, and 25 mM ammonium phosphate buffer. Prior to mixing with antigen for immunization, the formulations were diluted 5× with phosphate buffered saline and antigen.
GLA-liposome formulations were manufactured by first combining cholesterol and DPPC with either DPPG (anionic), DPTAP (cationic), or succinyl-functionalized DPPE (covalent) and with or without GLA in minimal amount of chloroform or chloroform:methanol:water, which was then evaporated off. The dried components were rehydrated in PBS, then sonicated at ~60 °C until all the lipids were suspended in the buffer at a concentration of 18 mg/ml DPPC, 5 mg/ml cholesterol, and 2 mg/ml DPPG, DPTAP, or succinyl-functionalized DPPE. For small volume batches (<20 ml), the liposomes were sonicated until they appeared to be single phase, translucent formulation. Larger batch sizes (100 ml) were prepared by transferring the crude suspension to the Microfluidics M110P (Newton, MA) for high-pressure homogenization for ~12 passes at 10,000 psi. Post sonication, the covalent liposomes were crosslinked to ID93 with the aid of two reagents, Sulfo-NHS N-Hydroxyssulfosuccinimide prepared in phosphate saline at a final concentration of 1 mM in liposome solution and EDC 1-Ethyl-3-(3 dimethylaminopropyl) carbodiimde HCL prepared in 50 mM MES at a final concentration of 0.5 mM in solution. Mixture of both intermediary liposome solutions and ID93 were allowed to incubate for 2 h at room temperature. Finally, any unbound ID93 was separated from the resulting ID93-bound covalent liposomes by passing it through an SEC column manually packed with 20 ml Sepharose™ CL-4B, an agarose-based gel filtration matrix obtained from GE Healthcare Bio-Sciences AB (Uppsala, Sweden). The liposome formulations were diluted 5× prior to immunization studies. All formulations were stored at 5 °C.
2.2. Adjuvant characterization
With the exception of Alum, GLA-Alum, and covalent liposomes with and without GLA, all other formulations were monitored by HPLC with charged aerosol detection (CAD) to determine GLA concentration and dynamic light scattering (DLS) to determine particle size. Particle size was determined using the Malvern Instruments (Worcestershire, UK) Zetasizer Nano-S or Nano-ZS. For oil-in water emulsions and liposome formulations, samples was prepared at 1:100 dilution by combining 5 μl of each formulation with 500 μl of ultrapure water in a 1.5 ml polystyrene disposable cuvette. For aqueous GLA, a 1:10 dilution was accomplished by combining 50 μl of formulation with 450 μl ultrapure water in a 1.5 ml polystyrene disposable cuvette. In general, three separate cuvettes were prepared. All DLS measurements were then made three times with each of the three separate cuvettes. Zeta potential measurements were also obtained using the Malvern Instruments (Worcestershire, UK) Zetasizer Nano-ZS. Samples were prepared for a 1:20 dilution by combining 50 μl of each formulation with 950 μl of ultrapure water in a 1.5 ml eppitube before being transferred to a disposable capillary cell for analysis.
For HPLC-CAD, samples were prepared for analysis in a 1.5 ml capacity HPLC glass vials obtained from Agilent Technologies (Santa Clara, CA), by combining 50 μl of formulation with 950 μl mobile phase B (1:1 [v:v] methanol:chloroform, 20 mM ammonium acetate, 1% acetic acid) for GLA-emulsion and GLA-liposome samples or mobile phase A (75:15:10 [v:v:v] methanol:chloroform:water, 20 mM ammonium acetate, 1% acetic acid) for aqueous GLA samples. Three separate vials were prepared for each formulation. Samples were then placed on que for injection into a Waters Co. (Milford, MA) Atlantis T3 column attached to an Agilent Model 1100 HPLC (Santa Clara, CA). A gradient consisting of mobile phases A and B was used spanning a 25-minute time period. Detection was performed by a Charged Aerosol Detector obtained from ESA Biosciences (Chelmosford, MA). An 11-point nonlinear quadratic standard curve fit was obtained of GLA standards ranging from 1 to 100 μg/ml GLA.
2.3. Adjuvant–antigen association
With the exception of covalent liposome formulations which already incorporated this step in the manufacturing process, ID93 association with all other formulation platforms was evaluated by incubating 0.1 mg/mL of ID93 with each formulation and PBS for 30 min in 4 ml capacity BD Falcon tubes obtained from BD Biosciences (San Jose, CA) and placed in a beaker filled with ice to mimic post-mixing/pre-immunization conditions. Each ID93-formulation mixture was then introduced into a size exclusion gel chromatography column packed with Sepharose CL-4B and eluted with PBS. Eluted fractions were collected from ID93 control (no adjuvant) and ID93-adjuvant mixtures. Fractions eluting between 11 and 16 ml were pooled as representative of unassociated ID93. A 20 μl sample from each of these pooled fractions was then combined in an eppitube with 20 μl LDS Sample Buffer (4×) and 40 μl of 20% SDS solution obtained from Invitrogen (Grand Island, NY). The sample mixture was then very briefly vortexed, heated at 85 °C for 5 min, and assayed by SDS-PAGE, followed by transfer to a PVDF membrane and finally stained with Colloidal Gold Total Protein Stain from BioRad (Hercules, CA). Stained membranes were scanned on a Konica Minolta bizhub C552 printer/scanner. Pixel intensity histograms of the ID93 bands were collected using ImageJ (NIH), and the area of the ID93 band peak was integrated using a baseline approximation of the background pixel intensity gradient and normalized to the observed intensity of the ID93 control in each membrane in GRAMS/AI (Galactic Software). The association percentage was calculated as a ratio of band intensity of eluted ID93 from adjuvant formulation mixtures compared to ID93 control.
2.4. Animals and immunizations
6–8-week-old female C57Bl/6 mice were purchased from Charles River and maintained in Specific Pathogen Free conditions. After infection the animals were maintained in BL3 containment according to the regulations and guidelines of the IDRI Institutional Animal Care and Use Committee. Mice were immunized three times three weeks apart by intramuscular injection. Each immunization contained 0.5 μg of ID93 recombinant protein (either by admixing ID93 to the preformed adjuvant or for the covalent liposome including sufficient ID93-liposome to obtain ~0.5 μg of ID93) with or without 5 μg of GLA. For BCG immunization 5 × 104 CFU (Pasteur strain, Sanofi Pasteur) were injected intradermally once at the time of the first subunit immunization.
2.5. Antibody titers
ID93-specific endpoint titers for IgG1 and IgG2c were determined one week after the final immunization. Nunc Polysorp plates were coated with ID93 (2 μg/ml) in 0.1 M bicarbonate and blocked overnight at 4 °C with 0.05% PBS-Tween 20 + 1% BSA. Serial dilutions of mouse serum were incubated for 2 h at room temperature, washed and incubated for 1 h with anti-mouse IgG1 or IgG2c conjugated to streptavidin (VWR). Plates were washed and developed using SureBlue tetramethylbenzidine substrate (Kirkegaard & Perry Laboratories). The enzymatic reaction was stopped with 1N H2SO4. Plates were read within 30 min at 450 nm with a reference filter set at 650 nm using a microplate ELISA reader (Molecular Devices) and Soft Max Pro5 software with a cutoff of 0.1 absorbance unit.
2.6. ELISpots
Four weeks after the final immunization splenocytes were isolated from three animals per group. Red blood cells were lysed using Red Blood Cell Lysis Buffer (eBioscience) and resuspended in RPMI 1640 and 10% FBS. A MultiScreen 96-well filtration plate (Millipore) was coated with 10 μg/ml rat anti-mouse IL-5 capture antibody (eBioscience) and incubated overnight at 4 °C. Plates were washed with PBS, blocked with RPMI 1640 and 10% FBS for at least 1 h at room temperature, and washed again. Splenocytes were plated in triplicate at 2 × 105 cells/well and stimulated with media or ID93 (10 μg/ml) for 48 h at 37 °C. The plates were then washed with 0.1% PBS-Tween 20 and incubated overnight with a biotin-conjugated rat anti-mouse IL-5 secondary Ab (eBioscience) diluted 1:250 in 0.1% PBS-Tween 20/0.5% BSA. The filters were developed using the VectaStain ABC avidin peroxidase conjugate and Vectastain AEC substrate kits (Vector Laboratories, Burlingame, CA) according to the manufacturer’s protocol. The reaction was stopped by washing the plates with deionized water. Plates were dried in the dark, and spots were counted on an automated ELISPOT reader (C.T.L. Series 3A Analyzer; Cellular Technology) and analyzed with ImmunSpot software (CTL Analyzer).
2.7. Intracellular cytokine staining
One to four weeks after the final immunization splenocytes were isolated from three animals per group. Red blood cells were lysed using Red Blood Cell Lysis Buffer (eBioscience) and resuspended in RPMI 1640 and 10% FBS. To isolate lymphocytes from the lungs immunized mice were perfused with PBS. Lungs were digested for one hour with Liberase TM (Roche) and dissociated using a GentleMACS system (Miltenyi). Cells were plated at 2 × 106 cells/well in 96-well plates and were stimulated for 1 h with media or ID93 (10 μg/mL) at 37 °C. GolgiPlug (BD Biosciences) was added and the cells were incubated for an additional 7 h at 37 °C. Cells were washed with PBS and stained with LIVE/DEAD Fixable Stain (Invitrogen) for 30 min at 4 °C. Cells were washed and surface stained with fluorochrome labeled antibodies to CD4 (clone GK1.5) and CD8 (clone 53-6. 7) (BioLegend and eBioscience) in the presence of 20% normal mouse serum for 20 min at 4 °C. Cells were washed and permeabilized with Cytofix/Cytoperm (BD Biosciences) for 20 min at room temperature. Cells were washed twice with Perm/Wash (BD Biosciences) and stained intracellularly with fluorochrome labeled antibodies to IFN-γ (clone XMG-1.2), IL-2 (JES6-5H4), TNF (MP6-XT22), and IL-17A (clone TC11-18H10.1) BioLegend and eBioscience) for 20 min at room temperature. Cells were washed and resuspended in PBS. Up to 106 events were collected on a four laser LSRFortessa flow cytometer (BD Biosciences). Data were analyzed with FlowJo. Cells were gated as singlets > live > lymphocytes > CD4 + CD8− > cytokine positive. Analysis and presentation of distributions were performed using SPICE version 5.2, downloaded from <http://exon.niaid.nih.gov/spice.
2.8. MHC class II tetramer production and staining
ID93-specific I-Ab tetramers with the immunodominant epitope from Rv3619 (VIYEQANAHGQ) were produced using methods previously described [26,27]. One week after the final immunization splenocytes were stained for one hour at room temperature with 10 μM tetramer. Cells were washed and stained for surface CD4, CD8, CD44 (clone IM7), Ly6C (clone HK1.4), PD-1 (clone 29F.1A12), KLRG1 (clone 2F1), CD27 (clone LG.3A10), and CD62L (clone MEL14) (BioLegend and eBioscience). Cells were then washed, fixed, permeabilized and stained for T-bet (clone 4B10) using the FoxP3 Fixation/Permeabilization buffer and protocol (eBioscience).
2.9. Mtb aerosol challenge and enumeration
Four weeks after the last immunization, mice (n = 7/group) were aerogenically infected with M. tuberculosis H37Rv (ATCC No. 35718; American Type Culture Collection) using a GlasCol aerosol generator calibrated to deliver 50–100 bacteria into the lungs. To confirm the amount of bacteria delivered an additional three unimmunized animals per infection were euthanized one day later and bacterial burden in the lungs were enumerated. Protection was determined three to four weeks after challenge by harvesting the lungs and spleens from the infected mice, homogenizing the tissue in 0.1% PBS-Tween 80, and plating 5-fold serial dilutions on7H10 agar plates (Molecular Toxicology) for bacterial growth. Bacterial colonies were counted after incubation at 37 °C with 5% CO2 for 14–21 days.
2.10. Statistical methods
Bacterial burdens were normalized by log10 transformation. Statistical significance of differences in ELISpot values and bacterial burden were determined using one-way analysis of variance. Differences relative to ID93 immunized animals were calculated using Dunnett’s post-test using Prism 5 (GraphPad Software). Differences between immunized animals were calculated with the Bonferroni Multiple Comparison Test using Prism 5 (GraphPad Software).
3. Results
3.1. Physicochemical characteristics of adjuvant formulations
To explore the formulation space for effective ID93/GLA vaccines we developed multiple adjuvant formulations based on distinct platforms in order to produce a library of formulations with different size, charge, and compositional properties (Table 1 and Fig. S1). We produced oil-in-water stable emulsions, liposomes, and aluminum oxyhydroxide (Alum)-based formulations. Oil-in-water emulsions are comprised of homogeneous ~75 to 85 nm average diameter droplets consisting of an oily core emulsified by phospholipid and nonionic block copolymer. The oily core was comprised of squalene, long chain triglyceride (grapeseed oil), or medium chain triglyceride (Miglyol 810), although the emulsifiers remained constant for each type of oil. We also produced anionic and cationic liposome formulations depending on whether DPPG or DPTAP was included. Alternatively, we produced liposomes covalently linked to ID93 through a succinyl-functionalized DPPE. The liposomes demonstrated average diameter in a similar range as the emulsions, that is from ~76 to 93 nm. Another lipid-based formulation consisting of an aqueous nanosuspension of GLA with DPPC was manufactured at 90 nm and termed aqueous GLA. This same suspension was completely adsorbed to aluminum oxyhydroxide particles to create GLA-Alum, a formulation with a heterogeneous size distribution of large crystalline particles with a highly positive surface charge and ranging from ~1 to 10 μm in size according to the literature [28]. In general, emulsion, liposome, and aqueous formulation platforms demonstrated little change in particle size or GLA concentration over several months at 5 °C, although we note that some particle precipitation was visually apparent over time in one cationic liposome batch and one aqueous batch (data not shown).
Table 1.
Formulation composition.
| Formulation name | Formulation type | Composition | Particle size
|
Zetapotential
|
pH of adjuvant at time of zeta measurement | pH (after mixing w/antigen) | Estimated % antigen associated w/adjuvant | |
|---|---|---|---|---|---|---|---|---|
| Z-Ave (d.nm) | PdI | (mV) | ||||||
| Alum | Aluminum salt | Aluminum oxyhydroxide | ~1000–10000** | NM | 15.7 (±0.6) | 5.6 | 7.1 | 97 |
| GLA-AF | Aqueous suspension | GLA, DPPC | 90 (±3)* | 0.257 (±0.011)* | −4.9 (±1.1)* | 5.5 | 7.2 | 11 |
| GLA-Alum | Aluminum salt | GLA, DPPC, aluminum oxyhydroxide | ~1000–10000** | NM | 27.0 (±0.7) | 5.4 | 6.9 | 97 |
| GLA-SE (Squalene) | Emulsion | GLA, squalene, Egg PC or DMPC, poloxamer 188, glycerol, ammonium phosphate buffer | 84 (±8)* | 0.047 (±0.019)* | −11.6 (±4.2)* | 5.5 | 6.4 | 96 |
| GLA-SE (Miglyol) | Emulsion | GLA, Miglyol 810, DMPC, poloxamer 188, glycerol, ammonium phosphate buffer | 75 (±1) | 0.053 (±0.014) | −5.9 (±1.7) | 5.6 | 6.5 | 25 |
| GLA-SE (Grapeseed) | Emulsion | GLA, grapeseed oil, DMPC, poloxamer 188, glycerol, ammonium phosphate buffer | 85 (±2) | 0.070 (±0.017) | −13.7 (±0.3) | 5.3 | 6.4 | 76 |
| LS (DPPG) | Liposome | DPPC, DPPG, cholesterol, PBS | 76 (±1) | 0.111 (±0.011) | −25.3 (±1.2) | 6.9 | 7.1 | 5 |
| GLA-LS (DPPG) | Liposome | GLA, DPPC, DPPG, cholesterol, PBS | 85 (±7)* | 0.192 (±0.062)* | −37.7 (±12.4)* | 6.9 | 7.1 | 0 |
| LS (DPTAP) | Liposome | DPPC, DPTAP, cholesterol, PBS | 77 (±2)* | 0.175 (±0.056)* | 33.2 (±3.4)* | 6.2 | 6.9 | 99 |
| GLA-LS (DPTAP) | Liposome | GLA, DPPC, DPTAP, cholesterol, PBS | 76 (±7)* | 0.167 (±0.011)* | 33.8 (±7.7)* | 3.6 | 6.2 | 95 |
| LS (covalent) | Liposome | DPPC, succinyl-functionalized DPPE, cholesterol, PBS | 83 (±21)† | 0.202 (±0.032) | NM | NM | 7.9 | NM*** |
| GLA-LS (covalent) | Liposome | GLA, DPPC, succinyl-functionalized DPPE, cholesterol, PBS | 93 (±15)† | 0.233 (±0.009) | NM | NM | 8.1 | NM*** |
Particle size was measured within approximately one week after formulation manufacture. Zeta potential and pH were measured up to 3 years after manufacture.
Values listed are an average of data obtained for two or more lots of the same formulation type.
Estimated from literature.
Abbreviations: NM [not measured], PdI [polydispersity index], GLA [glucopyransoyl lipid adjuvant], Egg PC [egg phosphatidylcholine], DMPC [1,2-dimyristoyl-sn-glycero-3-phosphocholine], DPPC [1,2-dipalmitoyl-sn-glycero-3-phosphocholine], DPPG [1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt)], DPTAP [1,2-dipalmitoyl-3-trimethylammonium-propane (chloride salt)], succincyl-functionalized DPPE [1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(succinyl) (sodium salt)], PBS [phosphate buffered saline].
ID93 concentration in final product too low to detect, but association level is assumed to be high since unassociated ID93 was separated during manufacture.
Particle size measured before binding to ID93.
Due to the wide variation in charge, size, and composition of the formulations developed, we examined the interaction of each formulation with ID93 to determine the extent of association (Table 1). Each formulation was mixed with ID93 followed by size exclusion chromatography to separate ID93-associated adjuvant particles from unassociated ID93. The eluted fraction volume representing unassociated ID93 was collected and assayed by SDS-PAGE with gold stain and compared to ID93 control (not containing any adjuvant) to estimate the amount of ID93 unassociated with each adjuvant formulation. As expected, the anionic ID93 (pI ~4.7) adsorbed completely to Alum and GLA-Alum after simple mixing. The cationic liposomes (with or without GLA) also associated efficiently with ID93 (95–99% of the ID93 was bound to the liposome after mixing). Association data is not shown for the liposomes covalently linked to ID93 since these were separated from unassociated protein by size exclusion chromatography in the course of manufacture. In contrast, the anionic liposomes (with or without GLA) and the aqueous GLA showed little association with the protein (although it must be noted here that the latter includes a population of very small particles [data not shown] which could potentially coelute with unassociated ID93). The above patterns are interpretable based on electrostatic interactions between the formulations and the negatively charged ID93. However, the various oil-in-water emulsions showed unexpected differences in association with ID93 depending on the oil component. Thus, GLA-SE showed high association with ID93 (96%), with the GLA-grapeseed emulsion having somewhat lower association (76%), and the Miglyol-based emulsion associating with only a fraction of the total antigen concentration (25%). It should be noted that it was necessary to increase the ID93 concentration in the association assay to 0.1 mg/ml in order to detect the protein, compared to the concentration employed in the in vivo studies (0.005 mg/ml). The disparate physicochemical characteristics of the various adjuvant formulations as well as the extent of their association with ID93 provide a potential context for interpreting the effects of formulation parameters on immune responses in immunized mice.
3.2. Alum: GLA and alum synergize to enhance a protective ID93-specific TH1 response
We have previously found that ID93 in the SE formulation elicits a strong CD4+ T helper 2 (TH2) cell response, characterized by IL-5 and IgG1 antibody, which was not beneficial for control of Mtb. Inclusion of the TLR4 agonist GLA into this vaccine formulation switches the ID93 specific T cell response to a predominantly IFN-γ producing T cell response and balanced IgG1 and IgG2 antibody response [17]. This correlated with an increase in protective efficacy against low dose aerosolized Mtb challenge. The SE adjuvant used in those studies is similar to MF59 adjuvant included in human influenza vaccines approved in the EU since 1997 [29]. Despite a good overall safety profile after millions of doses administered, MF59 and other emulsions induce some local reactogenicity and there are still no approved emulsion-based adjuvants in the US. The only FDA-approved human vaccines containing adjuvants are alum-based formulations or in the case of Cervarix MPL and alum (AS04). In fact, over a billion doses of vaccines containing alum have been administered to people of all ages [30]. Alum-based formulations may also offer stability advantages, since antigens may adsorb to the alum particles, which may enhance the immunogenicity of the antigen.
To determine whether alum-based adjuvant formulations with or without a TLR4 agonist would be beneficial for an ID93-based vaccine we immunized mice with either unadjuvanted ID93, ID93 adjuvanted with alum, ID93 adjuvanted with an aqueous nanosuspension of GLA or ID93 adjuvanted with a combination of GLA and alum (GLA-Alum) where the GLA and ID93 are both adsorbed to the alum (Table 1) [28]. One month after the final immunization all immunized groups had substantial anti-ID93 IgG1 titers (Fig. S2A). Inclusion of alum with or without GLA enhanced this response, whereas aqueous GLA did not boost IgG1 responses. Conversely IgG2c titers were only elicited when ID93 was adjuvanted with aqueous GLA or GLA-Alum (Fig. S2B). Formulation of ID93 in alum alone did not elicit IgG2c responses and alum did not enhance responses to ID93/GLA (Fig. S2B).
Immunization with ID93 or ID93-alum elicited ID93-specific IL-5 producing splenocytes (Fig. 1A). Both the aqueous GLA and GLA-Alum adjuvants inhibited IL-5 responses compared to ID93 alone or ID93-Alum, indicating that GLA can override the TH2 programming elicited by protein alone or protein plus alum immunization. Although neither the alum nor the aqueous GLA adjuvant enhanced IFN-γ or TNF production, the combination of GLA and alum elicited a significant IFN-γ and TNF response by CD4 T cells to ID93 stimulation, indicating that these two adjuvants can synergize to elicit TH1 responses following immunization (Fig. 1B). Alum and aqueous GLA, as well as the combination GLA-alum slightly enhanced the frequency of IL-2 producing cells compared to unadjuvanted ID93 (Fig. 1B). Alum also elicited a low frequency of IL-17-producing CD4 T cells and this was not impacted by inclusion of GLA with the alum. Of the ID93-specific TH1 cells, only ID93/GLA-alum produced multifunctional TH1 cells characterized by co-production of IFN-γ and TNF. More than half of these cells also produced IL-2 (Fig. 1C).
Fig. 1.
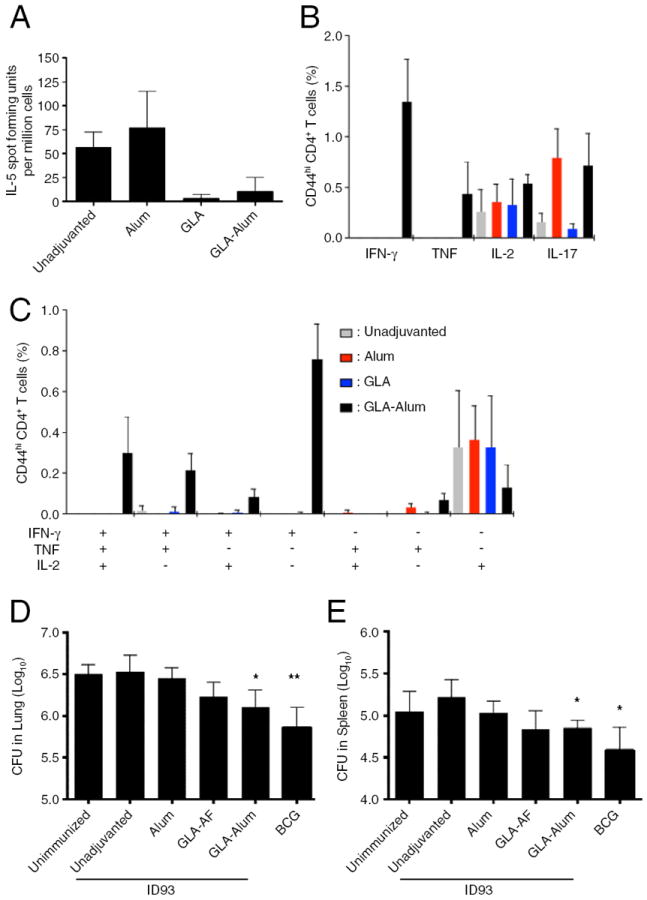
Alum and GLA synergize to promote protective TH1 responses to ID93. One month after the third immunization splenocytes from immunized mice were stimulated with ID93 and analyzed for (A) IL-5 production or (B and C) cytokine production by CD4 T cells. (D and E) Mtb burdens in the lung and spleen were determined four weeks after challenge with a low dose of aerosolized Mtb. Data are representative at least two experiments with three to four mice per group for immunogenicity and seven mice per group for Mtb challenge. * indicates P < 0.05 compared to the unadjuvanted group.
Compared to immunization with ID93 alone, immunization with ID93/GLA-alum significantly reduced the bacterial burden in the lungs and spleens of animals after a low dose aerosol challenge with virulent Mtb (Fig. 1D and E). There was no demonstrable benefit from immunization with ID93-alum, whereas immunization with ID93/GLA provided a non-significant trend towards protection in the lung and spleen. Taken together these data demonstrate that GLA and alum can synergize to promote the generation of multi-functional TH1 cells and provide significant protection against aerosolized Mtb, whereas neither GLA nor alum alone elicited a TH1 response or provided a protective benefit.
3.3. Emulsions: Squalene is essential for the generation of protective TH1 responses by ID93 adjuvanted with GLA in an oil-in-water emulsion
When adjuvanted with a squalene-based SE, ID93 elicited a TH2 response that was either non-protective in mice or detrimental to guinea pigs following Mtb challenge [17]. These studies led us to speculate that, while squalene-based emulsions produce unique adjuvant activity, when used in combination with a TLR ligand such as GLA it may be desirable to substitute squalene with an immunologically inert oil. We hypothesized that a non-squalene emulsion could serve as a delivery vehicle for a TH1 inducing TLR agonist adjuvant while not inducing separate unwanted TH2 adjuvant activity of its own.
To determine whether other oils could be substituted for squalene in an oil-in-water formulation of ID93 and GLA we created stable emulsions using either long-chain triglyceride (grapeseed oil) or a medium-chain triglyceride (Miglyol). These alternate emulsions were very similar to the squalene-based emulsions in terms of physicochemical characteristics (Table 1). Mice were immunized three times with either unadjuvanted ID93, ID93 adjuvanted with aqueous GLA, GLA-SE, GLA-Miglyol, GLA-grapeseed, GLA-Alum or unimmunized. As expected, immunization with ID93 elicited a TH2 response (Fig. 2A). Addition of any of the GLA containing formulations ablated this response, indicating that the presence of the TLR4 agonist was sufficient to inhibit TH2 induction, regardless of the delivery system. However, only the squalene containing GLA-SE adjuvant enhanced robust multi-functional TH1 responses (Fig. 2B). The most common response produced by ID93/GLA-SE was CD4 T cells that co-produced IFN-γ and TNF with or without IL-2 upon restimulation (Fig. 2C). The identity of the oil was critical to TH1 induction as changing the oil from squalene to Miglyol or grapeseed oil greatly limited the induction of TH1 responses after immunization. Additionally, the magnitude of the TH1 response elicited by ID93/GLA-SE was substantially greater than that elicited by the ID93/GLA-alum formulation, although the latter did elicit measurable multi-functional TH1 cells (Fig. 2).
Fig. 2.

Squalene is required for an oil-in-water emulsion formulation of GLA to augment protective TH1 responses to ID93. One month after the third immunization splenocytes from immunized mice were stimulated with ID93 and analyzed for (A) IL-5 production or (B and C) cytokine production by CD4 T cells. (D and E) Mtb burdens in the lung and spleen were determined three weeks after challenge with a low dose of aerosolized Mtb. Data are representative at least two experiments with three to four mice per group for immunogenicity and seven mice per group for Mtb challenge. * and ***indicate P < 0.05 and P < 0.001 compared to the unadjuvanted group, respectively.
Inclusion of any of the GLA containing adjuvants dramatically enhanced the production of ID93 specific IgG2c antibodies, whereas IgG1 titers were not augmented by the inclusion of the adjuvant (Fig. S3). GLA-SE provided the greatest enhancement of IgG2c. Formulation of ID93/GLA in either the grapeseed or Miglyol emulsion did not alter the responses compared to the oil-free aqueous GLA adjuvant suggesting that these oils are immunologically inert as far as effecting adaptive immune responses and that IgG2c enhancement is dependent on the presence of GLA, but can be augmented when formulated in SE.
One month after the final immunization we challenged cohorts of animals with a low dose of aerosolized Mtb to determine how the oil content impacts vaccine efficacy. There was a trend towards lower bacterial burden in the lungs of mice immunized with unadjuvanted ID93 compared to unimmunized mice, although this was not statistically significant. Compared to the animals that were immunized with unadjuvanted ID93, only the ID93/GLA-SE or ID93/GLA-Alum immunizations significantly lowered the lung bacterial burden (Fig. 2D). There was a slight trend towards lower bacterial burden with the other adjuvanted vaccines, but these differences did not reach statistical significance. The pattern of protection was similar in the spleens of these animals as only ID93/GLA-SE and ID93/GLA-Alum immunizations significantly reduced the Mtb burden (Fig. 2E). These data show that inclusion of squalene in an oil-in-water stable emulsion of ID93/GLA is critical for production of a strong multi-functional TH1 response that correlates with protection against Mtb challenge. ID93/GLA-Alum also elicited multi-functional TH1 cells albeit at a much reduced level. However the immune response elicited by this regimen was substantial enough to limit Mtb infection.
3.4. Liposomes: Lipid content alters the immunogenicity of ID93/GLA liposome vaccines
Liposomes are an attractive class of formulations for vaccine delivery due to their versatility, ease of manufacture and low cost of materials [31]. Liposome-based adjuvants are currently employed in many countries in approved influenza and hepatitis A vaccines. Liposomes do not contain an oil component but rather consist of a phospholipid bilayer surrounding an aqueous core. To assess whether liposomes would be a beneficial delivery platform for an ID93-based vaccine we prepared three different DPPC-based liposome formulations of ID93 with and without GLA. The liposome formulations differed in their minor lipid component, which produced either an anionic or cationic liposome as well as whether they were covalently bound to ID93, electrostatically associated (cationic) or not associated (anionic) (Table 1). We also hypothesized that associating the ID93 antigen to the liposome via covalent binding would enhance the liposome efficacy by increasing antigen uptake.
In the absence of GLA the liposome formulations enhanced the number of ID93-specific IL-5 producing splenocytes, although anionic liposomes provided only a marginal enhancement (Fig. 3A). Addition of GLA to any of the liposome formulations ablated this response. Both IFN-γ and TNF production by CD4 T cells were enhanced by addition of GLA to liposome formulations. Surprisingly the anionic liposomes produced the greatest frequency of TH1 cells when combined with ID93/GLA (Fig. 3B). Only the anionic liposome formulation of GLA substantially augmented the frequency of IL-2 producing CD4 T cells, whereas there was little IL-17 induction detectable over background levels. All three liposome formulations of ID93/GLA elicited multifunctional TH1 cells characterized by IFN-γ and TNF production with or without simultaneous IL-2 production (Fig. 3C). These cells were most frequent following immunization with ID93/GLA-anionic. In the absence of GLA none of the liposome formulations significantly enhanced ID93-specific TH1 responses.
Fig. 3.

Liposomal formulations of GLA enhance protective TH1 responses to ID93. One month after the third immunization splenocytes from immunized mice were stimulated with ID93 and analyzed for (A) IL-5 production or (B and C) cytokine production by CD4 T cells. (D and E) Mtb burdens in the lung and spleen were determined three weeks after challenge with a low dose of aerosolized Mtb. Data are representative at least two experiments with three to four mice per group for immunogenicity and seven mice per group for Mtb challenge. * indicates P < 0.05 compared to the unadjuvanted group.
The choice of liposome or the inclusion of GLA had little impact on ID93-specific IgG1 titers following immunization (Fig. S4A). Surprisingly, both the cationic and covalently-associated liposomes enhanced ID93-specific IgG2 titers compared to unadjuvanted ID93, even in the absence of GLA, particularly when the liposome was covalently linked to the antigen (Fig. S4B). Addition of GLA further increased these responses in both the anionic and cationic formulations (P < 0.01 for both comparisons).
To determine whether the differences in the magnitude of the TH1 response impacted the protective efficacy of the different ID93/GLA-liposome formulations we challenged immunized mice with a low dose of Mtb. Compared to unadjuvanted ID93, all three GLA-liposome formulations promoted control of Mtb in the lung and trended towards protection in the spleen (Fig. 3D and E). Among the ID93/GLA-liposome groups there was no clear correlation between the magnitude of the TH1 response and the magnitude of protection. However inclusion of GLA was essential to the protective efficacy as ID93 formulated in liposomes alone provided no protective benefit (data not shown). Taken together, these data show that liposome formulations of ID93/GLA enhance TH1 responses following vaccination, particularly the anionic formulation, which correlated with significant protection against Mtb challenge.
3.5. Squalene SE is a more effective formulation than anionic liposomes for enhancing the immunogenicity of ID93/GLA
In humans the liposomal formulation of MPL and QS21, AS01, has proven to enhance the immune responses to two different antigens more robustly than the oil-in-water formulation of the same immunostimulants, AS02. To determine whether an SE or liposome formulation of ID93/GLA would prove more immunogenic and protective we directly compared CD4 T cell responses in animals immunized with either ID93/GLA-SE or ID93/GLA-liposomes. We focused on the anionic liposome formulation because it showed the strongest enhancement of TH1 responses among the various GLA-liposome formulations and there was no clear difference in protective efficacy against pulmonary Mtb between these formulations (Fig. 3). Additionally the anionic liposome formulation was substantially easier to produce and is more amenable to scale up than the covalent liposomes. Due to the lower TH1 response elicited by ID93/GLA-Alum compared to ID93/GLA-SE the former formulation was not evaluated further (Fig. 2).
One week after the final immunization both ID93/GLA regimens induced multi-functional TH1 responses in the spleen and lung (Fig. 4A and B). There was a greater frequency of response following ID93/GLA-SE immunization in both organs, particularly of IFN-γ and TNF co-expressing cells. To further characterize these cells we stained splenocytes with an MHC class II tetramer bound to the dominant epitope from Rv3619, one of the component proteins of ID93 (Fig. 4C). As expected there was a higher frequency of Rv3619 specific cells in animals immunized with ID93/GLA-SE (Fig. 4D). It has been proposed that the long-term fate of antigen experienced T cells can be predicted based on expression of different constellations of surface markers and transcription factors. Kaech and colleagues have suggested that short-lived effector (SLEC) CD4 T cells express high levels of the TH1 committing transcription factor T-bet and the surface marker Ly6C and do not persist, whereas memory precursor effector cells (MPEC) express lower levels of these two proteins and persist to transition into memory T cells [32]. Using expression of two inhibitory surface molecules Woodland and colleagues proposed that SLEC are distinguished as being PD-1- KLRG1+ whereas MPEC are PD-1+ and KLRG1- [33]. The costimulatory molecule CD27 and CD62L which facilitates lymph node homing have also been suggested to be expressed on memory precursor cells [34,35]. One week following immunization ID93/GLA-SE induced more terminally differentiated SLEC CD4 T cells than ID93/GLA-liposome as defined by any of three phenotypes: CD27− CD62L−, KLRG1+ PD-1−, or Ly6Chi T-bethi, whereas ID93/GLA-liposome immunization induced a greater frequency of MPECs defined as either: CD27+ CD62L+, KLRG1− PD-1+, or Ly6Clo T-betlo (Fig. 4E). Importantly there was a large overlap of these phenotypes indicating that the populations identified by different groups using different markers may largely be the same.
Fig. 4.

The SE formulation induces more terminally differentiated ID93 specific TH1 responses than the liposome formulation of ID93/GLA. (A) Splenocytes and (B) lung from immunized mice were analyzed for ID93 specific CD4 T cell responses one week after the final immunization. (C) Splenocytes were also analyzed for the frequency of ID93 specific CD4 T cells by tetramer staining. Representative samples for ID93/GLA-SE and ID93/GLA-liposome immunizations are shown on the top and bottom rows respectively. (D) The frequency of tetramer positive cells was determined for each group. (E) The frequencies of MPEC and SLEC were determined based on expression of either: CD27 and CD62L, PD1 and KLRG1, or Ly6C and T-bet. Data are representative of at least two experiments with three to four mice per group.
One month after immunization the frequency of multi-functional ID93-specific TH1 cells in the spleen and lung were more similar between the two immunization regimens than at one week post-immunization, although responses were still higher in animals that received ID93/GLA-SE (Fig. 5A and B). This likely reflects the increased induction of long-lived MPEC by ID93/GLA-liposome. Both ID93/GLA-SE and ID93/GLA-liposome provided significant protection in the lung following aerosol challenge with Mtb (Fig. 5C and D). Protective efficacy in the lung was indistinguishable between animals immunized with ID93/GLA-SE and ID93/GLA- liposome. The Mtb burdens in the lungs of immunized mice were not significantly different than in mice receiving BCG, which is the gold standard for protection in the mouse model of Mtb challenge. Compared to ID93/GLA-liposome, immunization with ID93/GLA-SE did provide a more robust protective benefit against Mtb dissemination to the spleen, where it was not significantly different than BCG. Taken together we find that immunization with ID93/GLA-SE elicits a greater frequency of ID93 specific TH1 cells and provides more effective protection against disseminated Mtb at the time points tested. However ID93/GLA-liposome elicits a higher frequency of MPEC CD4 T cells and also is protective against experimental challenge.
Fig. 5.
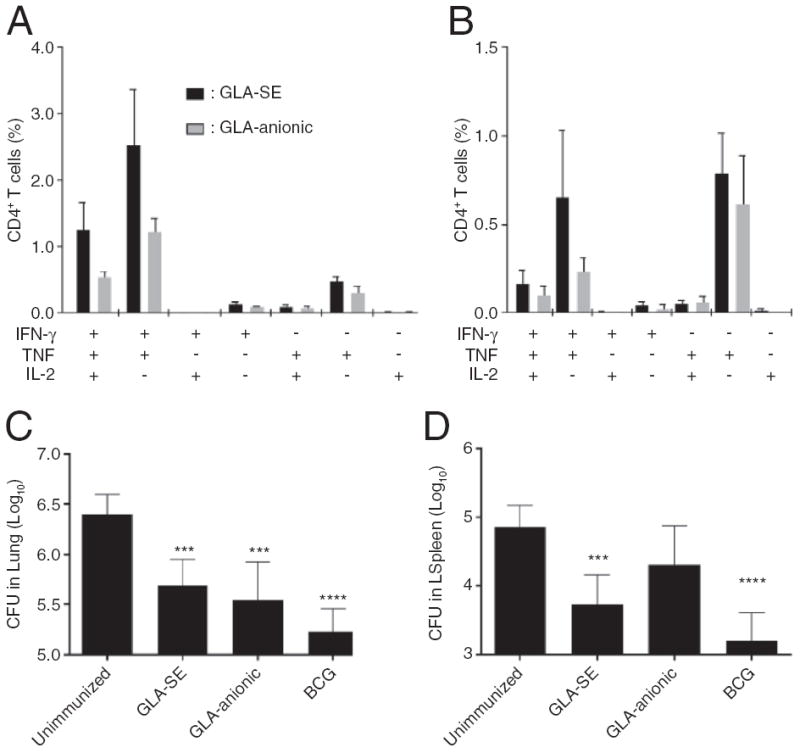
ID93/GLA-SE is more immunogenic and protective than ID93/GLA-anionic. One month after the third immunization (A) splenocytes and (B) lung lymphocytes from immunized mice were stimulated with ID93 and analyzed for cytokine production by CD4 T cells. (C and D) Mtb burdens in the lung and spleen were determined four weeks after challenge with a low dose of aerosolized Mtb. Data are representative of at least two experiments with three to four mice per group for immunogenicity and seven mice per group for Mtb challenge. *, **, ***, and **** indicate P < 0.05, 0.01, 0.001 and 0.0001 compared to the saline immunized group, respectively.
4. Discussion
With one third of the world infected with Mtb and eight million new cases of active TB annually there is a pressing need to develop new vaccines against Mtb. We have taken a systematic approach to develop a defined subunit vaccine consisting of the ID93 fusion protein antigen and the synthetic TLR4 agonist GLA. In this study we explore a variety of formulations for ID93/GLA to identify those that are the most effective in enhancing ID93-specific TH1 responses and protection against Mtb challenge.
Vaccine formulation had a profound impact on the ability of ID93/GLA to induce the multi-functional TH1 cells hypothesized to protect against Mtb. There were no clear physico-chemical parameters that were consistently predictive of those formulations that elicited multi-functional TH1 cells and protected against aerosolized Mtb and those that did not. All of the formulations that were protective induced at least a modest level of multifunctional TH1 cells, primarily cells producing both IFN-γ and TNF with or without IL-2, although the magnitude of the TH1 responses was not predictive of the magnitude of protection. This was most evident when comparing ID93/GLA-SE to either ID93/GLA-Alum or ID93/GLA-liposomes. All three formulations produced similar levels of control of Mtb challenge despite ID93/GLA-SE inducing by far the greatest frequency of multi-functional TH1 cells. Others have also reported a lack of clear correlation in the magnitude of the splenic TH1 response and protective efficacy [6]. As we are using an aerosol challenge model we hypothesized that the frequency of lung resident TH1 cells may be a better correlate of protective efficacy. However ID93/GLA-SE and ID93/GLA-liposome were equally protective in the lung, despite the former having a higher frequency of TH1 cells in both the lung and spleen at the time of challenge. This lack of direct correlation may be a limitation of the mouse model of aerosol Mtb challenge or may be indicative that other immune parameters may be more indicative of protective efficacy. Alternatively this may reflect the different qualities of the memory CD4 T cells elicited by the two vaccine candidates. ID93/GLA-SE elicited a greater frequency of short-lived effector cells as identified by expression of KLRG1, Tbet and Ly6C, such that the frequencies of MPEC elicited by the two candidates were not substantially different.
Despite similar average particle size and size polydispersity only the squalene-based oil-in-water emulsion among the three oils tested elicited detectable TH1 responses and provided protection against Mtb when paired with ID93. It is notable that the squalene-based emulsion also showed the highest level of antigen association, although it is not clear what the mechanism of association is, since the same emulsifiers were employed in the three emulsions and zeta potentials appeared similar, with each formulation showing a slightly negative charge. The contribution of squalene itself to the adjuvant effect of oil-in-water emulsions is also unclear. A similar emulsion, MF59, has been shown to enhance cellular infiltration to the immunization and antigen uptake [36]. Further MF59 appears to activate the inflammasome via an ASC- and MyD88-dependent, NLRP3-independent process, although it is unclear if this activation is caused by direct recognition of the adjuvant or secondary recognition of an intermediate molecule induced by MF59 [37,38].
Emulsions in licensed vaccines or in advanced development including MF59, AS02 and AS03 are based on the metabolizable oil squalene [39]. However, some reports continue to point to potential safety concerns regarding squalene, although the merit of these concerns is unclear. Several other metabolizable oils are employed in approved pharmaceutical oil-based injectable products and may offer an alternative to squalene. We previously compared stable oil-in-water emulsion formulations consisting of different oils for adjuvant activity when combined with a recombinant malaria antigen or an inactivated influenza vaccine [21]. A squalene-based emulsion clearly elicited enhanced overall antibodies as well as functional antibody activity compared to emulsions based on various other metabolizable oils, including long chain triglyceride oils (such as grapeseed), a medium chain triglyceride oil (Miglyol), or a perfluorocarbon oil. In a related study, squalene emulsion dose titration resulted in some reduction in humoral and cellular immune responses to a recombinant malaria antigen except when formulated with a TLR4 ligand, in which case the squalene emulsion dose could be reduced (to 1% or 0.5% v/v oil) without compromising immunogenicity compared to a 2% v/v emulsion dose formulated with the TLR4 ligand [23]. Thus we were surprised that of the oil-in-water emulsions tested here only the squalene-containing emulsion, which on its own enhanced TH2 responses, provided an effective formulation for TH1 induction when paired with GLA. Thus the same parameters that improve the antibody inducing capacity of squalene based SE may also be necessary for induction of a strong TH1 response by GLA formulated in an oil-in-water emulsion. Based on our current data neither Miglyol nor grapeseed emulsions provide any advantage over the aqueous nanosuspension of GLA and appear to be truly inert. On its own the aqueous GLA formulation may be beneficial to other vaccine applications where IgG2c induction is more important than the magnitude of the TH1 responses.
Others have found that direct association of a vaccine antigen with the lipid component of liposomes significantly enhances vaccine efficacy [40-42]. For GLA-liposomes we expected either the covalently associated liposomes or the cationic liposomes that showed strong association with the antigen would produce the highest TH1 responses. Based on this we were surprised that the anionic liposome formulation of GLA provided the greatest enhancement of ID93-specific TH1 responses, even though there was little detectable association with ID93. However another study found that anionic liposomes, such as the DPPG-containing liposomes used here, are effective adjuvant formulations despite lack of antigen association [43]. The mechanisms of this activity are unclear but may affect the site of vaccine injection or cells that traffic to the injection site and take up the antigen. An additional benefit of the anionic liposomes over the covalent liposome formulation is that the former exhibits longer term stability and requires a simpler manufacturing process (data not shown), both parameters that are important in the selection of a clinically usable adjuvant.
The series of adjuvant formulations that we have developed here is somewhat analogous to the AS01, AS02, AS03, and AS04 series of adjuvants developed by GSK (reviewed in [12,44]). Key differences between the two systems include the nature of the TLR4 agonist; MPL is a heterogeneous mixture of molecules from a biological source including both agonists and antagonists for TLR4 whereas GLA is a synthetic mono-species of a defined structure; and the inclusion of QS21 in AS01 and AS02, but not in any of the adjuvants studied here. The AS adjuvants have been compared head-to-head in a number of clinical trials evaluating both antibody and CD4 T cell responses to select the lead adjuvant for each vaccine candidate. AS04 (MPL and alum) is included in the HPV vaccine Cervarix and the HBV vaccine Fendrix based on superior antibody production compared to alum [45,46]. However when AS04 (MPL and alum) was compared to AS02 (MPL QS21 and squalene emulsion) as an adjuvant for HBV the latter adjuvant produced higher antibody titers and a greater frequency of patients producing detectable antibodies against the antigen [47]. Unfortunately T cell responses were not reported in this study. It would be interesting to know if AS02 elicited a more robust TH1 response than AS04, similar to our findings presented here. Similarly early studies with the RTS,S malaria antigen found that AS02, but not AS04 or AS03 (a squalene-in-water emulsion without MPL or QS21) provided a protective benefit against malaria challenge in human volunteers [48]. Importantly AS02 contains the saponin QS21 whereas AS04 does not thus the benefit of AS02 could not be definitely attributed to the difference in formulation (Alum vs. oil-in-water emulsion). Multiple clinical trials have evaluated the relative performance of AS01 and AS02 for the induction of CD4 T cell responses. Across a variety of antigens including those for malaria (RTS,S, and LSA-1), tuberculosis (M72), and HIV (gp120/Nef/Tat) there is a consistent finding that the liposome formulation AS01 induces a greater frequency of antigen specific TH1 cells than the squalene containing AS02 [13,14,49,50]. Most of these cells are multi-functional expressing combinations of IFN-γ, TNF, IL-2 and/or CD40L. Importantly this clinical finding was predicted in pre-clinical comparisons of AS01 and AS02 with the RTS,S antigen. In both mouse and primate models TH1 responses were enhanced to a greater degree with AS01 than AS02 [51,52], which correlated with the magnitude of protection against sporozoite challenge. The inclusion of the saponin QS21 in both AS01 and AS02, but not included in the adjuvants studied here may shape the selection of the optimal adjuvant formulation. Thus the selection of the optimal AS adjuvant cannot be directly compared to the present studies, yet both sets of data clearly demonstrate that adjuvant formulation plays a critical role in optimal vaccine development.
5. Conclusion
Based on the reproducible protective efficacy and strong TH1 response elicited by ID93/GLA-SE we have advanced the SE formulation of ID93/GLA into clinical trials to evaluate safety and immunogenicity in humans. However there were no clear reasons to eliminate either ID93/GLA-liposome or ID93/GLA-Alum based on the current data. The final selection of the optimal formulation of ID93/GLA among SE, liposome and Alum may require side-by-side testing in humans similar to what was required to select AS01 over AS02 for 72F and RTS,S for TB and malaria respectively. Other considerations for final adjuvant selection will depend on safety considerations and the likelihood of regulatory approval. To date the FDA has not approved oil-in-water based emulsions as vaccine adjuvants (although an FDA advisory committee recently recommended approval of GSK’s pandemic influenza vaccine containing AS03), whereas both alum and the alum-containing AS04 adjuvant are included in approved vaccines. Thus GLA-Alum may have a straightforward regulatory pathway compared to other adjuvant formulation platforms. In conclusion, adjuvant formulation is a critical consideration in the development of vaccines intended to elicit multi-functional TH1 cells and control intracellular pathogens such as Mtb.
Supplementary Material
Acknowledgments
We thank Valerie Reese, David Argilla, Charles Davis, Tom Hudson, Traci Mikasa, Sarah Parker, Lucien Barnes V, and Millie Fung for their excellent technical assistance. This work was supported by the National Institutes of Health grants A1044373 and A1078054 and contract HHSN272200800045C.
Abbreviations
- Mtb
Mycobacterium tuberculosis
- TB
tuberculosis
- BCG
Bacillus Calmette–Guérin
- GLA
glucopyranosyl lipid adjuvant
- SE
stable emulsion
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jconrel.2013.07.030.
References
- 1.WHO global tuberculosis control report, Summary. Cent Eur J Public Health. 2010;18:237. [PubMed] [Google Scholar]
- 2.Andersen P, Doherty TM. The success and failure of BCG — implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Raddad LJ, Sabatelli L, Achterberg JT, Sugimoto JD, Longini IM, Jr, Dye C, Halloran ME. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci U S A. 2009;106:13980–13985. doi: 10.1073/pnas.0901720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 5.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol. 2008;181:4955–4964. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittrucker HW, Steinhoff U, Kohler A, Krause M, Lazar D, Mex P, Miekley D, Kaufmann SH. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci U S A. 2007;104:12434–12439. doi: 10.1073/pnas.0703510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kagina BM, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, Gamieldien H, Sidibana M, Hatherill M, Gelderbloem S, Mahomed H, Hawkridge A, Hussey G, Kaplan G, Hanekom WA. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette–Guerin vaccination of newborns. Am J Respir Crit Care Med. 2010;182:1073–1079. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodworth JS, Wu Y, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J Immunol. 2008;181:8595–8603. doi: 10.4049/jimmunol.181.12.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alving CR, Peachman KK, Rao M, Reed SG. Adjuvants for human vaccines. Curr Opin Immunol. 2012;24:310–315. doi: 10.1016/j.coi.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J, Losonksy G, Martin MT, Dubin G, Wettendorff MA. Enhanced humoral and memory B cellular immunity using HPV16/18L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Garcon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines. 2011;10:471–486. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- 13.Leroux-Roels I, Forgus S, De Boever F, Clement F, Demoitie MA, Mettens P, Moris P, Ledent E, Leroux-Roels G, Ofori-Anyinam O. Improved CD4(+) T cell responses to Mycobacterium tuberculosis in PPD-negative adults by M72/AS01 as compared to the M72/AS02 and Mtb72F/AS02 tuberculosis candidate vaccine formulations: a randomized trial. Vaccine. 2013;13:2196–2206. doi: 10.1016/j.vaccine.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 14.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, Schwenk R, Nielsen RA, Debebe Z, Pinelis E, Juompan L, Williams J, Dowler M, Stewart VA, Wirtz RA, Dubois MC, Lievens M, Cohen J, Ballou WR, Heppner DG., Jr Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS, S/AS01B and RTS, S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200:337–346. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 15.Bertholet S, Ireton GC, Kahn M, Guderian J, Mohamath R, Stride N, Laughlin EM, Baldwin SL, Vedvick TS, Coler RN, Reed SG. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J Immunol. 2008;181:7948–7957. doi: 10.4049/jimmunol.181.11.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, Phan T, Orme IM, Vedvick TS, Baldwin SL, Coler RN, Reed SG. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. 2010;2:53ra74. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baldwin SL, Bertholet S, Reese VA, Ching LK, Reed SG, Coler RN. The importance of adjuvant formulation in the development of a tuberculosis vaccine. J Immunol. 2012;188:2189–2197. doi: 10.4049/jimmunol.1102696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coler RN, Bertholet S, Pine SO, Orr MT, Reese V, Windish HP, Davis C, Kahn M, Baldwin SL, Reed SG. Therapeutic immunization against Mycobacterium tuberculosis is an effective adjunct to antibiotic treatment. J Infect Dis. 2013;207:1242–1252. doi: 10.1093/infdis/jis425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson RC, Fox CB, Dutill TS, Shaverdian N, Evers TL, Poshusta GR, Chesko J, Coler RN, Friede M, Reed SG, Vedvick TS. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids Surf B: Biointerfaces. 2010;75:123–132. doi: 10.1016/j.colsurfb.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Fox CB, Anderson RC, Dutill TS, Goto Y, Reed SG, Vedvick T. Monitoring the effects of component structure and source and formulation stability and adjuvant activity of oil-in-water emulsions. Colloids Surf B: Biointerfaces. 2008;65:98–105. doi: 10.1016/j.colsurfb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Fox CB, Baldwin SL, Duthie MS, Reed SG, Vedvick TS. Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions. Vaccine. 2011;29:9563–9572. doi: 10.1016/j.vaccine.2011.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox CB, Barnes L, Evers VT, Chesko JD, Vedvick TS, Coler RN, Reed SG, Baldwin SL. Adjuvanted pandemic influenza vaccine: variation of emulsion components affects stability, antigen structure, and vaccine efficacy. Influenza Other Respir Viruses. 2013;7:815–826. doi: 10.1111/irv.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox CB, Baldwin SL, Vedvick TS, Angov E, Reed SG. Effects on immunogenicity by formulations of emulsion-based adjuvants for malaria vaccines. Clin Vaccine Immunol. 2012;19:1633–1640. doi: 10.1128/CVI.00235-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox CB, Friede M, Reed SG, Ireton GC. Synthetic and natural TLR4 agonists as safe and effective vaccine adjuvants. In: Wang X, Quinn PJ, editors. Endotoxins: Structure, Function and Recognition. Springer; New York: 2010. pp. 303–321. [DOI] [PubMed] [Google Scholar]
- 25.Fox CB. Characterization of TLR4 agonist effects on alhydrogel sedimentation: a novel application of laser scattering optical profiling. J Pharm Sci. 2012;101:4357–4364. doi: 10.1002/jps.23307. [DOI] [PubMed] [Google Scholar]
- 26.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox CB. Characterization of TLR4 agonist effects on alhydrogel(R) sedimentation: a novel application of laser scattering optical profiling. J Pharm Sci. 2012;101:4357–4364. doi: 10.1002/jps.23307. [DOI] [PubMed] [Google Scholar]
- 29.O’Hagan DT, Ott GS, Nest GV, Rappuoli R, Giudice GD. The history of MF59((R)) adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccines. 2013;12:13–30. doi: 10.1586/erv.12.140. [DOI] [PubMed] [Google Scholar]
- 30.Schubert C. Illuminating alum. Nat Med. 2009;15:985. doi: 10.1038/nm0909-985. [DOI] [PubMed] [Google Scholar]
- 31.Alving CR, Rao M, Steers NJ, Matyas GR, Mayorov AV. Liposomes containing lipid A: an effective, safe, generic adjuvant system for synthetic vaccines. Expert Rev Vaccines. 2012;11:733–744. doi: 10.1586/erv.12.35. [DOI] [PubMed] [Google Scholar]
- 32.Marshall HD, Chandele A, Jung YW, Meng H, Poholek AC, Parish IA, Rutishauser R, Cui W, Kleinstein SH, Craft J, Kaech SM. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity. 2011;35:633–646. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard G, Moon JJ, Jenkins MK, Urdahl KB, Winslow GM, Woodland DL. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 2010;107:19408–19413. doi: 10.1073/pnas.1006298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 35.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O’Hagan DT, De Gregorio E, Seubert A, Wack A. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29:1812–1823. doi: 10.1016/j.vaccine.2010.12.090. [DOI] [PubMed] [Google Scholar]
- 37.Ellebedy AH, Lupfer C, Ghoneim HE, DeBeauchamp J, Kanneganti TD, Webby RJ. Inflammasome-independent role of the apoptosis-associated speck-like protein containing CARD (ASC) in the adjuvant effect of MF59. Proc Natl Acad Sci U S A. 2011;108:2927–2932. doi: 10.1073/pnas.1012455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seubert A, Calabro S, Santini L, Galli B, Genovese A, Valentini S, Aprea S, Colaprico A, D’Oro U, Giuliani MM, Pallaoro M, Pizza M, O’Hagan DT, Wack A, Rappuoli R, De Gregorio E. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci U S A. 2011;108:11169–11174. doi: 10.1073/pnas.1107941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox CB. Squalene emulsions for parenteral vaccine and drug delivery. Molecules. 2009;14:3286–3312. doi: 10.3390/molecules14093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henriksen-Lacey M, Christensen D, Bramwell VW, Lindenstrom T, Agger EM, Andersen P, Perrie Y. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J Control Release. 2010;145:102–108. doi: 10.1016/j.jconrel.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 41.Watson DS, Platt VM, Cao L, Venditto VJ, Szoka FC., Jr Antibody response to polyhistidine-tagged peptide and protein antigens attached to liposomes via lipid-linked nitrilotriacetic acid in mice. Clin Vaccine Immunol. 2011;18:289–297. doi: 10.1128/CVI.00425-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahum E, Therien HM. Immunopotentiation of the humoral response by liposomes: encapsulation versus covalent linkage. Immunology. 1988;65:315–317. [PMC free article] [PubMed] [Google Scholar]
- 43.Yanasarn N, Sloat BR, Cui Z. Negatively charged liposomes show potent adjuvant activity when simply admixed with protein antigens. Mol Pharm. 2011;8:1174–1185. doi: 10.1021/mp200016d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines. 2007;6:723–739. doi: 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- 45.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van Mechelen M, Garcon N. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 46.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, Dubin G. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccin. 2009;5:705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 47.Surquin M, Tielemans CL, Kulcsar I, Ryba M, Voros P, Mat O, Treille S, Dhaene M, Stolear JC, Kuriyakose SO, Leyssen MX, Houard SA. Rapid, enhanced, and persistent protection of patients with renal insufficiency by AS02(V)-adjuvanted hepatitis B vaccine. Kidney Int. 2010;77:247–255. doi: 10.1038/ki.2009.454. [DOI] [PubMed] [Google Scholar]
- 48.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, Wellde BT, Garcon N, Krzych U, Marchand M. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS, S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 49.Cummings JF, Spring MD, Schwenk RJ, Ockenhouse CF, Kester KE, Polhemus ME, Walsh DS, Yoon IK, Prosperi C, Juompan LY, Lanar DE, Krzych U, Hall BT, Ware LA, Stewart VA, Williams J, Dowler M, Nielsen RK, Hillier CJ, Giersing BK, Dubovsky F, Malkin E, Tucker K, Dubois MC, Cohen JD, Ballou WR, Heppner DG., Jr Recombinant Liver Stage Antigen-1 (LSA-1) formulated with AS01 or AS02 is safe, elicits high titer antibody and induces IFN-gamma/IL-2 CD4+ T cells but does not protect against experimental Plasmodium falciparum infection. Vaccine. 2010;28:5135–5144. doi: 10.1016/j.vaccine.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 50.Leroux-Roels I, Koutsoukos M, Clement F, Steyaert S, Janssens M, Bourguignon P, Cohen K, Altfeld M, Vandepapeliere P, Pedneault L, McNally L, Leroux-Roels G, Voss G. Strong and persistent CD4+ T-cell response in healthy adults immunized with a candidate HIV-1 vaccine containing gp120, Nef and Tat antigens formulated in three Adjuvant Systems. Vaccine. 2010;28:7016–7024. doi: 10.1016/j.vaccine.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 51.Mettens P, Dubois PM, Demoitie MA, Bayat B, Donner MN, Bourguignon P, Stewart VA, Heppner DG, Jr, Garcon N, Cohen J. Improved T cell responses to Plasmodium falciparum circumsporozoite protein in mice and monkeys induced by a novel formulation of RTS,S vaccine antigen. Vaccine. 2008;26:1072–1082. doi: 10.1016/j.vaccine.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 52.Stewart VA, McGrath SM, Walsh DS, Davis S, Hess AS, Ware LA, Kester KE, Cummings JF, Burge JR, Voss G, Delchambre M, Garcon N, Tang DB, Cohen JD, Heppner DG., Jr Pre-clinical evaluation of new adjuvant formulations to improve the immunogenicity of the malaria vaccine RTS, S/AS02A. Vaccine. 2006;24:6483–6492. doi: 10.1016/j.vaccine.2006.06.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


