Abstract
Subcortical hyperintensities (SH) on brain MRI are associated with cognitive and gait impairment in elderly but their impact on dual-tasking (performing cognitive tasks while walking) in patients with Alzheimer’s disease (AD) is unknown. This study explored the costs of dual-tasking in relation to SH severity in AD and normal controls (NC). Cadence while walking on a treadmill, and speed-accuracy-tradeoff (SAT), on three working memory tasks, were measured during single- and dual-task conditions. Dual-task costs (DTC) on SAT, cadence and overall DTC were measured for each of these tasks. On visual rating of SH severity, AD and NC groups were subdivided into high- and low-SH subgroups. Compared to the NC, the AD group performed poorly on all working memory tasks across both conditions, decreased cadence on dual-tasking and showed a decrement in overall DTC (all p< 0.01). When grouped according to SH severity, the low-SH-NC group performed superiorly on working-memory tasks (p<0.001) and the high-SH-AD group (p=0.001) showed a decrease in dual task costs of cadence. While the AD group showed a decrement in overall DTC (p<0.01) compared to NC, when assessed in terms of SH severity, the high-SH-AD group showed the largest decrement in DTC (p<0.01). Greater SH severity is associated with a decrement in overall dual-tasking ability in AD.
Keywords: Alzheimer’s Disease, subcortical, hyperintensities, gait, working memory, white matter disease, white matter hyperintensities, dual-tasking, cadence, cognition, walking, treadmill
INTRODUCTION
Subcortical hyperintensities (SH), as the term suggests, are hyperintense areas in the white matter and deep nuclei seen on MRI and are indicative of vascular disease in the brain1. SH are common in the elderly with reports suggesting a prevalence as high as 96%2. In AD, similar prevalence has been reported, not significantly different from elderly controls 3-5. Clinical manifestation of SH include gait impairment and deficits in executive functioning6-10. The presence of SH in strategic white matter pathways in the brain is hypothesized to interfere with brain connectivity and therefore impede the performance of these functions or interact with AD to amplify the severity of dementia 11, 12. Therefore, SH may also lead to impede the performance of two functions when performed simultaneously in patients with AD.
Dual-tasking while walking has been shown to affect the performance of gait in mixed elderly samples13-15 and in patients with AD16-18. Some studies looked at the effect of concurrent speech on gait noted that some patients stopped walking while talking, while those that continued walking, slowed down and/or had a greater variability in velocity, stride-length and/or double-support13-18. Experts have suggested that the costs of walking under dual-task conditions are due to competition for attention resources 19. Furthermore, few studies have reported that patients with AD have impairment in the performance of two cognitive tasks simultaneously and that this dual-task impairment in AD is likely to arise from specific cognitive processes that involve encoding and retrieval rather than a general cognitive deficit. 20, 21
Evidence suggests that gait relies on executive function abilities and that performance of executive function tasks may interfere with gait performance under dual-task conditions16, 22-24. For example, decrease in gait speed and increase gait variability have been reported in patients with mild AD while performing fluency tasks, digit recall tasks and forward digit span tasks while walking16-18. Working memory is an executive function that involves transient maintenance and the concurrent mental manipulation of information in service of a particular task 25, 26. Experimental data exists to show that working memory tasks have a greater influence on gait in young adults than fluency tasks 27. The costs of performing a working memory task while walking in patients with AD is not well understood. Furthermore, the impact of underlying SH on dual-task interaction has not been reported.
Studying dual-task interaction is challenging for several reasons. Firstly, researchers suggest that in order to study dual-task interaction the individual tasks must share common neuronal resources 28. Therefore, we studied the interaction of specifically working-memory that potentially shares common neuronal resources as that of gait, which can provide a biological basis for the possible competition of these common substrates 26, 29-33. Additionally, changes in gait during dual-tasking are known to occur when the secondary task necessitates speech articulation, possibly due to rhythmic changes in respiratory cycle which can influence gait 34, 35. Several studies on dual-tasking in AD largely rely on the eliciting of speech to perform the cognitive task 16-18. To overcome this potential confound we used a working memory paradigm that elicits reaction-time and accuracy measures on a button press. Finally, reducing speed or cessation of walking during regular over-ground gait, which intuitively should increase stability 36, has been associated with higher likelihood of falls13. By enforcing gait on a treadmill and therefore constraining velocity, we proposed to observe for changes in cadence in patients with AD during dual-tasking. We postulated that under threatened gait stability, one would increase the number of steps (i.e., cadence) and therefore take smaller strides to maintain stability when the velocity is fixed. Therefore, the specific hypotheses of this study were: 1) performing working memory tasks while walking will increase cadence (steps/minute) measured on the treadmill in both patients with AD and in normal controls (NC); 2) walking on the treadmill will affect working memory performance in both groups; and 3) those with higher SH severity would have poorer working memory and maladaptive responses in gait reflected on dual-task costs on cognitive and gait performance measures.
MATERIALS AND METHODS
Participants
Participants between ages of 60 and 80 years who were enrolled in the Sunnybrook Dementia Study, a longitudinal study on cognitive impairment including AD and other dementias, were screened for inclusion in this sub-study. Participants had to be able to walk independently for 15 minutes without stopping. Patients with AD had to meet NINDS-ADRDA criteria for probable AD37. Normal controls (NC) were community volunteers who performed within normal limits on all cognitive tests, were functionally independent in all activities of daily living, had no history of neurological or psychological disorder and were in a stable healthy condition. All participants gave informed consent to the protocol which was approved by the Research Ethics Board. Additionally, participants were only screened if they had a brain magnetic resonance imaging (MRI) within a six-month window of the gait assessments, as little change in SH volume was expected over this time period in this particular population.
Patients with a Mini-mental Status Exam (MMSE) score of ≤ 20, history of other neurological disorders or coexistent neurodegenerative conditions such as Lewy Body disease or Parkinson’s Disease, major depression, hip-fractures, significant arthritis, clinically significant joint deformity, recent hip/knee replacement, sedative medication use, dependence on alcohol and/or neuroleptics drugs, use of assistive devices such as cane/walker and presence of significant sensory and/or motor neuropathy on neurological examination were considered exclusionary. MMSE38 and the Mattis Dementia Rating Scale (MDRS) 39were also used to characterize the stage of AD. All participants underwent training on the working memory tasks and those with an accuracy of <80% after 5 trials of the 2-back working memory task were excluded.
Assessments
At the time of gait assessments, data pertaining to previous falls, concomitant medical conditions, cardiovascular risk factors, exercise history, current medications were obtained followed by a physical and neurological examination that included measurement of body-mass index, leg-length, mid-calf girth, blood pressure and resting heart rate. Timed-up-and-go (TUG) test 40 is a validated bedside measure of mobility that measures the time required to arise from a chair, walk 3 meters, turn, and return to the seated position. Unified Parkinson’s Disease Rating Scale (UPDRS)41 is a standardized scale that includes rating severity of parkinsonism. In this study, the TUG, motor sub-scale of the UPDRS and the Tinetti gait scale 42 were also scored. Additionally, neuropsychological and neuroimaging data were available to ascertain cognitive, functional and other disease characteristics.
SH severity rating
SH was rated on the Age-related White Matter Change (ARWMC) scale, a four-point scale that assesses SH severity by aggregating scores in five bilateral regions as delineated by Wahlund et al.43. The rating scale was applied to T2-weighted MRI scans obtained within six-months of the gait assessments. All brain images were acquired using a 1.5 T Signa MR imager (GE Medical systems, Milwaukee, WI). Three image sets were acquired in the same imaging session: T1-weighted (axial 3D SPGR with 5ms TE, 35ms TR, 35° flip angle, 1 NEX, 22 × 16.5 cm FOV, 0.859 × 0.859mm in-plane resolution, and 1.2 to 1.4mm slice thickness), proton-density (PD) and T2-weighted images (interleaved axial spin echo, with TEs of 30 and 80 ms, 3s TR, 0.5 NEX, 20 × 20cm FOV, 0.781 × 0.781mm in-plane resolution, and 3mm slice thickness).
Gait assessment
Cadence was measured using footswitches (B&L Engineering) placed in the insoles of participant’s shoes as they walked on a motorized treadmill (Biodex™ RTM400, Biodex Medical Systems, Inc., NY). Foot-switch data was digitized at the rate of 500 samples per second through an analog-to-digital converter. Digitized signals were processed (Labview®, National Instruments, Austin, TX) to measure the cadence.
All participants were instructed to wear comfortable walking shoes for the experiment. Footswitches made of pliable material were placed in both shoes such that the shoes once worn would fit comfortably. A non-weight bearing safety-harness (The Biodex Unweighing System®, Biodex Medical Systems, Inc., NY) was strapped across the trunk for safety reasons after which participants were asked to alight on the treadmill. The target self-selected gait speed on the treadmill was determined as follows. While holding on to the hand rails participants were instructed to start walking slowly and gradually increase their walking speed to reach a pace that they would normally walk at while on a leisure stroll. As the participant started walking, the treadmill speed was gradually increased to attain the most comfortable speed as dictated by each participant. When this was attained, participants were instructed to let go of the hand rails, one at a time, and continue walking at this speed with no incline on the treadmill until they felt familiarized to it. This speed was used as the target gait speed for subsequent walking conditions of the experiment. To account for the need to endure this target self-paced gait speed for the duration of the experiment, we ensured that the self-selected gait speed on the treadmill was at least 25% less than their over-ground gait speed measured separately on an automated walkway. After acclimatization on the treadmill, the speed was gradually reduced until the participant came to a halt in order to provide adequate rest prior to data recording. For every subsequent experimental condition, the protocol for gradually increasing the treadmill speed to attain the target self-selected gait speed was adhered to. Data recording began with walking only condition followed by walking while performing the working memory tasks described below. Prior to start of the dual-task condition, participants were instructed and encouraged to perform the cognitive task to the best of their ability both in terms of accuracy and speed of responses. For each condition, three trials each lasting 65 seconds were captured at target gait speed. Between trials, the participants were provided adequate rest periods, the duration of which was dictated by the participants.
Working memory task
The working memory task was based on a n-back letter paradigm consisting of three tasks, X-task (control), 1-back and 2-back, that were administered to participants while standing and while walking to capture their single-task and dual-task performance respectively44. The working memory tasks included a display of a continuous stream of letters on the screen placed in front of the treadmill. Participants registered their responses by pressing a button held in their preferred hand. Three working memory tasks, ‘X’ [simple working memory], ‘1-back’ and ‘2-back’ [active working memory load tasks], were presented in random order. The display duration was 1500ms and the inter-stimulus interval (ISI) was 2000ms. All the three tasks required participants to maintain information in memory for immediate retrieval, continuously update this information, and register responses on the button-press while keeping track of each letter displayed on the monitor.
During the X-condition, participants were instructed to press the button whenever they saw a letter ‘X’ in the continuous stream of letters. For the 1-back condition, participants were instructed to press the button whenever a letter was the same as the one that came just before it in the sequence [for example, M-T-T or W-B-B]. For the 2-back task, participants were instructed to press the button whenever the letter was the same as the one that appeared two stimuli prior in the sequence of letters [for example U-T-U or B-Q-B]. The letter ‘X’ did not appear in any of the two active working memory tasks. Three trials for each working memory task, each trial lasting approximately 1 minute, with at least one potential response every 5-6 seconds were administered. Reaction times and task accuracy data were recorded from signals obtained by button-presses.
Statistical analysis
Demographic variables between the two groups were compared using Student’s t test and chi-square where applicable. To relate the effect of working memory performance on gait parameters to the SH load in the two groups (AD and NC), the median distribution of total SH score was determined for each group and was used as the cut-off. Those that were above the cut-off were denoted as AD+ and NC+ and those that equaled or fell below the cut-off were referred to as AD− and NC− respectively. ANOVA was used to compare the demographic variables in the four groups.
Single-task cadence, i.e., walking with gaze fixed on the screen (single-task walking), was compared with cadence while dual tasking across three dual-task conditions: the X-task, 1-back task and the 2-back task. To determine the costs on cadence while dual-tasking, a percentage change in the dual-task costs (DTC) of cadence on dual-tasking was calculated relative to participant’s single-task cadence as done in other such studies45, 46:
Performance on working memory tasks was determined by combining accuracy and reaction time (RT) into one performance score, the speed-accuracy trade-off (SAT), calculated by the formula: (Accuracy/RT) × 100. This method has been used in other studies to obtain a composite score incorporating both speed and accuracy of performance on cognitive tasks 24. SAT was calculated for each task during both the standing and walking conditions. Dual-task costs on SAT were calculated as a percentage of change relative to participant’s single-task SAT as above using the formula:
To permit a measure of overall demands on dual-tasking and compare groups we combined the dual-task costs on cadence and working memory task performance, i.e., SAT, similar to Logie et al21. Therefore, to obtain an overall DTC measure we obtained the mean of DTC on cadence and SAT for each of the three working memory tasks.
RESULTS
Baseline characteristics
Patients with AD (n=24) were not significantly different from the NC (n=20) on most baseline characteristics (age, gender, BMI, waist circumference, leg-length, mid-calf girth, blood-pressure and heart rate) but differed as expected on the MMSE (p<0.01) and DRS (p<0.01) scores. The AD group showed a trend towards a higher UPDRS score (p=0.06) and significantly longer time to complete the TUG test (p<0.01) (Table 1).
Table 1.
Baseline characteristics of patients with mild AD and normal controls (NC) .
| AD (n=24) | NC (n=20) | p value | |
|---|---|---|---|
| Age (years) | 75±9 | 72±8 | 0.16 |
| Gender (female%) | 60 | 47 | 0.25 |
| Blood pressure (mmHG) | 130±17/70±9 | 127±16/74±10 | 0.5/0.2 |
| MMSE score | 25±3 | 29±1 | <0.001 |
| Dementia Rating Scale (DRS) | 122±10 | 141±2 | <0.001 |
| Body mass index (BMI) | 25±5 | 25±5 | 0.7 |
| Waist circumference (cm) | 94±10 | 88±18 | 0.17 |
| Leg Length (cm) | 92±5 | 91±7 | 0.43 |
| UPDRS-motor subscore | 6±7 | 3±4 | 0.056 |
| Tinetti gait score | 12±0.6 | 12±0.4 | 0.15 |
| Timed-up-go (sec) | 12±3 | 9±1 | <0.01 |
| SH score | 8±7 | 6±4 | <0.01 |
| Over-ground gait speed (m/sec) | 1.02±1.9 | 1.24±1.6 | <0.001 |
MMSE: Mini-mental Status Examination
UPDRS: Unified Parkinson’s Disease Rating Scale
SH score: Total score on the ARWMC (Age-related White Matter Change) scale
The total ARWMC score in the AD group (range: 1-24, median: 7) was significantly greater than that of the NC group (range: 1-15, median: 5) (p<0.01). Therefore, based on the median of the distribution on the total ARWMC score for each group, those above the median were compared with those equal to or below the median. Thus from the two groups, four groups were derived: AD+ and AD− (with cut-off of 7 on the total ARWMC score for the AD group) and NC+ and NC− (with the cut-off of 5 on the total ARWMC score for the NC group).
The demographic differences between the four sub-groups were evaluated using ANOVA and are highlighted in Table 2. There were no statistically significant differences in baseline body characteristics such as leg-length, BMI and waist circumference. Statistically significant differences between groups were observed in age (p<0.01), MMSE (p<0.01), DRS (p<0.01), UPDRS score (p<0.01) and TUG (p<0.01). Post-hoc Tukey’s test revealed that the NC− group was significantly younger than NC+ (p<0.05) and AD+ and AD−(p<0.01) groups. The two AD groups had significantly lower MMSE and DRS scores than NC but there were no statistically significant differences between AD+ and AD− and between NC+ and NC− on these baseline cognitive measures. The AD+ group had significantly higher UPDRS score than the other three groups (p<0.01). The AD+ group also took significantly longer time to complete the TUG as compared to the NC− and NC+ groups. However, there were no statistically significant differences between AD+ and AD− and between NC+ and AD− groups on the TUG test.
Table 2.
Differences in AD and NC subdivided on high (AD+ and NC+) and low (AD− and NC−) SH severity
| NC− (n=13) |
AD− (n =14) |
NC+ (n =7) |
AD+ (n =10) |
F value |
P | |
|---|---|---|---|---|---|---|
| Age (years) | 68±6 | 72±9 | 76±5 | 79±6 | 5.8 | 0.002* |
| Female | 19% | 36% | 19% | 26% | n/a | 0.7 |
| Blood pressure (mmHg) |
129±18/74±11 | 134±13/72±8 | 124±11/75±8 | 124±20/68±9 | 1.1/0.9 | 0.4/0.4 |
| MMSE | 29±1 | 24±3 | 28±1 | 26±2 | 10.4 | <0.001† |
| DRS | 141±2 | 119±11 | 141±2 | 126±9 | 24.2 | <0.001‡ |
| BMI | 25±5 | 25±4 | 25±3 | 24±8 | 0.1 | 0.9 |
| Waist | 86±21 | 93±9 | 92±10 | 96±11 | 1 | 0.4 |
| Leg Length | 90±8 | 93±4 | 92±6 | 92±5 | .6 | 0.6 |
| UPDRS | 2±3 | 3±3 | 4±4 | 11±7 | 9.3 | <0.001∥ |
| Tinetti gait score |
12±0 | 12±1 | 10±1 | 13±4 | 0.7 | 0.6 |
| Timed-up-go | 9±2 | 11±2 | 10±1 | 13±4 | 6.2 | 0.001§ |
| SH score | 3±2 | 3±2 | 11±2 | 14±3 | 60.2 | <0.001# |
| Over-ground gait speed (m/sec) |
1.27±1.6 | 1.03±1.9 | 1.22±1.5 | 0.99±2.0 | 6.5 | 0.001^ |
AD+ vs NC− (p=0.001)
NC− vs AD− (p<0.001), NC− vs AD + (p<0.05), NC+ vs AD− (p=0.002)
AD− vs NC + and NC− (both p<0.001) and AD+ vs NC+ and NC− (both p<0.01)
AD+ vs AD− (p=0.001), NC− (p<0.001), NC+ (p=0.02)
AD+ vs NC− (p=0.001) and AD+ vs NC+ (p=0.03)
AD+ vs AD−(p<0.001), NC− (p<0.001), NC+ (0.03) and NC+ vs NC− and AD− (p<0.001)
NC− vs AD− (0.009), NC− vs AD+ (p=0.004), NC+ vs AD+ (p=0.05)
MMSE: Mini-mental Status Examination
UPDRS: Unified Parkinson’s Disease Rating Scale
SH score: Total score on the ARWMC (Age-related White Matter Change) scale
DRS: Dementia rating scale
BMI: Body mass index
There were no differences in the two groups on cadence, cycle time and double-support during the ‘walk only’ condition though the AD group had a significantly slower self-selected treadmill speed compared to the NC group (0.61 m/sec vs 0.77 m/sec, p=0.02).
Effect of working memory task performance on cadence
A positive dual task cost on cadence implies that the cadence increased while dual-tasking compared to their cadence during the walking without cognitive tasking condition. Similarly, a negative dual-task cost on cadence implies that the cadence actually decreased compared to the walking only condition. There was a significant effect of dual task costs of cadence between AD and NC groups (F(2,42)=11.6, p=0.001, η2p=0.21). The dual task costs on cadence were significantly higher in the NC group compared to the AD group [(X task: 4.4±5.0 vs 0.75±5.8 (p=0.026); 1-back: 4.9±5.7 vs −0.82±5.2 (p=0.001), 2-back: 5±8.7 vs −0.28±4.5 (p=0.002)]. In fact, the AD group had negative dual task costs on cadence in the 1-back and 2-back conditions indicating that their cadence decreased while performing the 1-back and 2-back tasks.
Next, the dual-task costs on cadence were compared between four groups using repeated measures ANOVA. There was a significant effect of dual task costs on cadence in the four groups (F(3, 41)=5.3, p=0.003, η2p = 0.28). Post hoc tests revealed that the AD+ group showed a significant decline in terms of their mean dual-task costs on cadence compared NC− (p=0.001), NC+ (p=0.004) and AD− (p=0.05) groups.
Effect of Treadmill Walking on Working Memory Task
A higher number on the SAT denotes better performance and lower numbers denote a poorer performance. The SAT scores were compared between AD and NC groups and then between the four groups subdivided on their SH load. Table 3a highlights the differences in SAT during standing (single-task) and walking (dual-task) conditions in the AD and NC groups. The performance of NC group on all working memory tasks, indicated by the respective SAT scores, on both single- and dual-task conditions was significantly better than the AD group (all p<0.01). When the four sub-groups were compared (table 3b), the NC− group alone performed significantly better on all three working memory tasks during both single- and dual-task conditions compared to the other three groups (NC+, AD+ and AD−).
Table 3a.
Speed-accuracy tradeoff (SAT) [(Accuracy/RT)*100] on performance of three working memory tasks across standing and walking conditions in Alzheimer’s Disease (AD) group and normal controls (NC).
| Condition | Working Memory task | AD | NC | t | p value |
|---|---|---|---|---|---|
| Control (X) | 17.4 ± 0.8 | 23.1 ± 1.2 | −3.978 | <0.01 | |
| STANDING | 1-back | 15.3 ± 4.1 | 20 ± 4.8 | −3.801 | <0.01 |
| 2-back | 11.8 ± 3.8 | 17.1 ± 5.8 | −3.820 | <0.01 | |
|
| |||||
| Control (X) | 17.6 ± 4 | 22.7 ± 4.6 | −4.416 | <0.01 | |
| WALKING | 1-back | 14 ± 4.1 | 19.7 ± 4.3 | −4.971 | <0.01 |
| 2-back | 11.1 ± 5 | 17.8 ± 5.2 | −4.656 | <0.01 | |
Table 3b.
Speed-accuracy tradeoff (SAT) in the four subgroups of AD patients and normal controls (NC) based on their subcortical hyperintensities (SH) severity (AD+ and NC+ denote subgroups with higher SH burden, AD− and NC− denote subgroups with lower SH burden).
| Condition | Working Memory task |
NC− | AD− | NC+ | AD+ | F | p value |
|---|---|---|---|---|---|---|---|
| Control (X) | 24.9±5.1 | 17.2±4.9 | 19.6±6.6 | 17.7±3.3 | 6.1 | 0.002† | |
| STANDING | 1-back | 20.9±5 | 15.1±4 | 18.6±4.6 | 15.1±4.6 | 4.2 | 0.01‡ |
| 2-back | 17.9±6.5 | 11.4±3.5 | 13.8±4.7 | 12.1±4.2 | 4.4 | 0.009§ | |
|
| |||||||
| Control (X) | 24.7±4.7 | 17.9±4.6 | 19.6±3.6 | 17.5±3.6 | 7.3 | <0.001¶ | |
| WALKING | 1-back | 21.4±4.3 | 14.4±3.6 | 16.6±3.9 | 13.1±4.8 | 9.4 | <0.001¶ |
| 2-back | 20.0±5.5 | 11.3±5 | 15.1±2 | 10.7±5 | 9.0 | <0.001* | |
NC− vs AD− (p<0.001), NC+ (p=0.04), AD+ (p=0.002)
NC− vs AD− (p=0.003) and AD+ (p=0.007)
NC− vs AD− (p=0.001) and AD+ (p=0.008)
NC− vs AD− (p<0.001), NC+ (p=0.02), AD+ (p<0.001)
NC− vs AD− (p<0.001), NC+ (p=0.04), AD+ (p<0.001)
When performance on the cognitive task was compared within-groups, no significant differences emerged between single-task and dual task conditions within the NC and AD groups. There were no significant within-group differences between single and dual-task conditions in the sub-groups (NC−, NC+, AD− and AD+).
We then compared the DTC of SAT for the three tasks (x-task, 1-bac, 2-back) during dual-tasking compared to standing condition. There was an overall effect of DTC on SAT between groups (F= 4, p=0.05, η2p =0.1) but not within-groups. When the four SH subgroups were compared there were no statistically significant differences in the DTC on SAT (F=2.5, p=0.07, η2p = 0.1)
Overall dual-task costs on the three working memory tasks
With the overall dual-task costs for the control task, 1-back and 2-back as dependent variables on the repeated measures ANOVA and diagnostic group (AD vs NC) as between-subjects factor, there was a statistically significant effect between-groups (F (1,43)=7, p= 0.012, η2p =0.14) but not within-groups (p=0.1). Further analysis revealed that the differences in overall DTC between AD and NC were statistically significant only for active working memory tasks - the 1-back (t=−2.3, p=0.03) and 2-back tasks (t=−2.7, p=0.01) [Figure 1].
Figure 1.
Differences in overall dual-task costs of dual-tasking on three working memory tasks (control, 1-back, 2-back) in patients with AD and normal controls (NC)
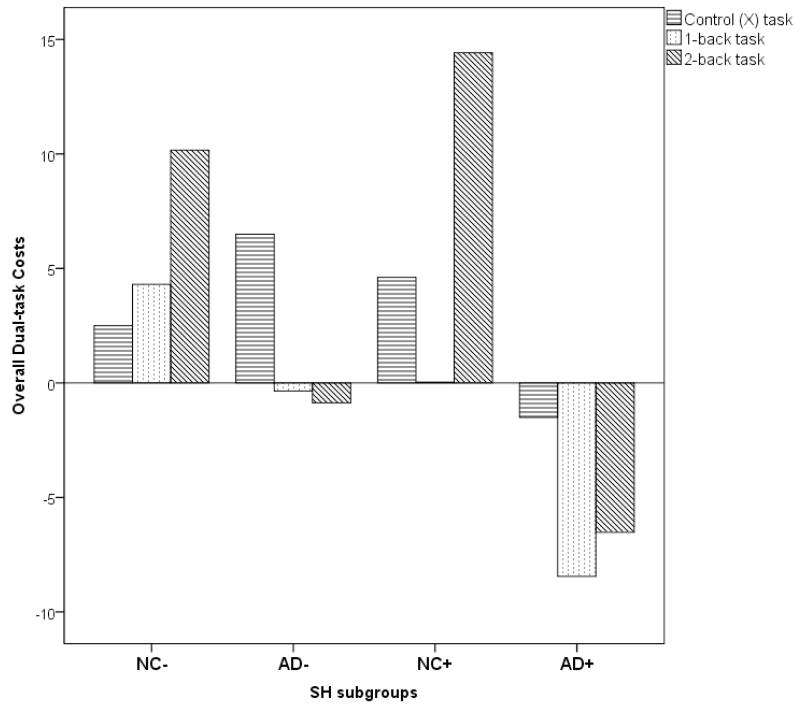
Repeated measures ANOVA for the four subgroups with the three overall DTC variables as dependent variables with SH subgroup as between-group factor revealed a significant effect of overall DTC between groups (F (3, 40) =4.2, p=0.01, η2p =0.24) but not within groups (p=0.7). Post hoc tests revealed that the AD+ group differed significantly from their AD− counterparts (p=0.04), NC− (p=0.002) and NC+ (p=0.01) groups [Figure 2]. There were no statistically significant differences between the NC−, NC+ and AD− groups.
Figure 2.
Differences in overall dual-task costs of dual-tasking on three working memory tasks (control, 1-back, 2-back) in patients with AD and normal controls (NC) subdivided on their severity of subcortical hyperintensities (SH) scores ( AD+ and NC+ ; high SH severity, AD− and NC−: low SH severity)
DISCUSSION
This study investigated the effect of performing working memory tasks while walking in patients with mild AD and healthy older adults. The impact of SH severity on costs of dual tasking was then assessed in these two groups by further subdividing them based on the severity rating of SH on their MRI. To the best of our knowledge, this is the first study that looked at costs of dual-tasking in relation to the burden of SH in AD.
Previous literature consistently shows that gait velocity decreases in order to maintain stability while dual-tasking in older adults and in patients with AD 13-18. However, on a treadmill when compensations in gait velocity to maintain dynamic stability is not an option the system is constrained to maintain the velocity by decreasing the stride-length 47. Increase in double-support and decrease in stride length are considered to be compensatory mechanisms to improve stability in aging 36. A decrease in stride-length enables more time spent with both feet on the ground (double-support time) and therefore improves stability, which could be a compensatory mechanism to improve stability of gait. Decrease in stride-length is one means of increasing dynamic gait stability while walking akin to maneuvering an ice patch on the pavement.
In this study, gait velocity was unchanged while on the treadmill throughout the experiment therefore an increase in cadence at a constant velocity would imply a decrease in stride-length 47. In studying the changes in cadence on dual-tasking, we found that the NC group significantly increased their cadence compared to the AD group during the dual-task conditions, further implying that their relative stride-length decreased. When AD and NC groups were divided based on their SH load and the dual tasks costs were compared in these four groups, only the AD+ group, i.e., patients with higher proportion of SH, decreased their cadence implying that there was an increase in relative stride-length. The increase in cadence in NC+, NC− and AD− groups may be considered as a safe compensatory mechanism to maintain dynamic stability during dual-tasking and a decrease in cadence suggests that the AD+ group were unable to make this compensatory strategy. The purpose of the experiment was not to study gait instability on dual-tasking. Nevertheless, participants were in a safety-harness, which could have prevented any likelihood of falling. These results suggests that the presence of SH may interfere with the adaptive responses to maintain dynamic stability especially in AD.
The results of cognitive performance on dual-tasking in the two groups suggest that the AD group performed poorly on all working memory tasks compared to the NC in keeping with previous studies 25. Interestingly, when study groups were assessed based on their SH load, there was a considerable overlap in the performance of AD patients with high and lower SH burden suggesting that the presence of increased SH load did not adversely affect the performance on the three working memory tasks in both conditions in AD. In the NC group, there were no statistically significant differences in performance on the standing condition but on the dual task condition, NC− group showed a significantly better performance compared to the NC+ group indicating that SH may adversely affect cognitive performance under more challenging conditions such as dual-tasking. However, when costs of dual-tasking on cognitive performance on the working memory tasks were examined, we found no statistically significant differences between the AD and NC groups or between the four SH subgroups.
The results of this study also indicate that AD participants with higher SH load decreased cadence when dual-tasking, suggesting that a high SH load in AD patients appears to adversely affect their walking ability while dual tasking. Gait in AD, especially under dual-task conditions, relies upon executive functions and the influence of executive functions on gait increases with increasing complexity of the dual-task 17, 48, 49. The concomitant performance of the functions supported by these neurons overtax the systems responsible for the performance of the required tasks and in the presence of structural brain damage such as cerebrovascular disease, the dual-task performance may be more attenuated.
The overall dual-task cost that incorporates both the DTC on cadence and DTC on cognitive task performance further suggests that when compared to NC, AD patients show significant decline in both components of the dual-task. It is noteworthy that AD patients with higher SH burden show greater magnitude of decline in overall dual-tasking ability compared to healthy elderly as well as the AD counterparts with lesser SH burden. This indicates that the overall ability to efficiently dual-task is affected in AD and that the burden of SH in these patients may further negatively impact this ability.
These results can be further explained as follows. By the time AD is clinically apparent, the distribution of disease pathology, neurofibrillary tangles and plaques, spreads beyond the medial temporal and enterorhinal cortex to involve the dorsolateral prefrontal and subcortical areas 50, and the presence of SH may not additionally interfere with the afferent connectivity of the frontal cortex as it may be already disrupted by the disease course. Therefore, performance of working-memory, that activates these regions, may not be further impede by higher SH burden.
Performance of a verbal working memory task and gait rely on common neuronal substrates. Specifically, the dorsolateral prefrontal and parietal cortices plays an important role in working memory function 26 as well as in the adaptation of gait to varying environmental conditions31. Evidence suggests that gait speed is slower in patients with AD and that higher SH burden is linked to slower gait speed in AD51, 52. It is also suggested that the presence of a slow gait requires larger cognitive processing 53. The simultaneous performance of these functions could potentially interfere with patient’s ability to execute safe adaptive responses as areas such as the dorsolateral prefrontal cortex may have a double hit, from the disease process of AD as well as the interference in connectivity by SH in these regions. In healthy elderly, the connectivity between cortical areas involved in working memory and gait performance under dual task conditions may be disrupted by the presence of SH alone and this may explain the lack of significant differences between NC+ and the two AD groups on the working memory performance. Therefore, results of this study suggest that SH burden may play a more important role in processing-speed in healthy cognitively intact adults consistent with other reports54-58. When overall dual-task costs are considered, AD patients with higher SH burden may fair poorly due to the same reason that a wider distribution of SH may affect multiple areas involved in dual-tasking. It is also possible that the decline in costs of cadence under dual-task conditions outweighs the dual-task changes in working memory performance and therefore demonstrating an overall decline in dual-task ability in this group in particular.
This study has certain limitations that need to be considered. The subdivision of groups in to those with higher and lower SH burden lead to smaller groups and comparisons between groups. However, we were able to detect a signal even with this small sample suggesting that larger studies with similar protocols are warranted. Secondly, the SH burden was rated on a visual rating scale and not quantified using automated methods; this is generally less sensitive in detecting small differences between groups59. Nevertheless, we used a well-validated rating scale 43and is found to be satisfactory in differentiating groups based on high and low SH severity 59, 60. While we strived to limit motor interference of the secondary task by limiting speech, the use of button-press to obtain accuracy and reaction times limited our ability to negate the effect of motor interference altogether. Amongst other conditions that interfere with gait, in those with hip/knee replacements, only those with recent interventions were excluded from this study. However, this study assessed dual-task changes in gait relative to their single-task gait performance, hence the effects of knee replacement on individual’s gait was factored in the assessment. Similarly, AD patients with higher SH burden (the AD+ group) had higher UPDRS scores and even though scores were indicative of mild degree of parkinsonism, the bearing of UPDRS on gait changes during dual-tasking was accounted for as changes in gait were assessed in relation to their gait performed without concurrent cognitive tasking. Finally, while dual-tasking on the treadmill may not be considered as a “real-world” setting, it does help to underscore the relationship between cognition and involuntary gait changes in a velocity constrained environment.
The results of this study extend the existing knowledge of the interaction between gait and executive function by suggesting that SH severity influences changes in gait during dual tasking in patients with AD. Prospective studies looking at fall occurrence and its relationship with the adaptive changes in gait while dual-tasking may help understand whether these changes affect stability in a clinically significant manner61, 62. This study also helps to understand the behavioral characteristics of SH suggesting an association between SH load and working memory and stepping frequency in healthy older adults and patients with AD. Volumetric assessments of SH and their specific locations in the brain using automated methods on larger samples may further help better understand these interrelationships between SH burden and dual-tasking.
ACKNOWLEDGEMENTS
We acknowledge the assistance of E. Mawji, S. Rafi-Tari, G. Szilagyi, J. Bray, I. Lam, R. Harry, A. Ganda, M. Morum, J. Lawrence, C. Scott, S. vanGaal, F. Leibovitch and F.Q. Gao with data collection and technical support.
Research funding support:
Heart and Stroke Foundation - Centre for Stroke Recovery, Canadian Institute of Health Research, Alzheimer’s Society of Canada, Alzheimer’s Association – USA. NKN is supported by the University of Pittsburgh-Pittsburgh Claude D. Pepper Older American’s Independence Center grant (P30 AG024827).
(This work was conducted at the Heart and Stroke Foundation-Centre of Stroke Recovery and the L. C. Campbell Cognitive Neurology Research Unit at Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Ontario, Canada)
REFERENCES
- [1].Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40:S48–52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- [2].Longstreth WT, Jr., Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- [3].Bondareff W, Raval J, Woo B, Hauser DL, Colletti PM. Magnetic resonance imaging and the severity of dementia in older adults. Arch Gen Psychiatry. 1990;47:47–51. doi: 10.1001/archpsyc.1990.01810130049007. [DOI] [PubMed] [Google Scholar]
- [4].Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- [5].Erkinjuntti T, Ketonen L, Sulkava R, Vuorialho M, Palo J. CT in the differential diagnosis between Alzheimer’s disease and vascular dementia. Acta Neurol Scand. 1987;75:262–270. doi: 10.1111/j.1600-0404.1987.tb07931.x. [DOI] [PubMed] [Google Scholar]
- [6].Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- [8].Baloh RW, Yue Q, Socotch TM, Jacobson KM. White matter lesions and disequilibrium in older people. I. Case-control comparison. Arch Neurol. 1995;52:970–974. doi: 10.1001/archneur.1995.00540340062013. [DOI] [PubMed] [Google Scholar]
- [9].Masdeu JC, Wolfson L, Lantos G, et al. Brain white-matter changes in the elderly prone to falling. Arch Neurol. 1989;46:1292–1296. doi: 10.1001/archneur.1989.00520480034016. [DOI] [PubMed] [Google Scholar]
- [10].de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology. 2001;56:1539–1545. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- [11].DeCarli C. The role of cerebrovascular disease in dementia. Neurologist. 2003;9:123–136. doi: 10.1097/00127893-200305000-00001. [DOI] [PubMed] [Google Scholar]
- [12].Benson RR, Guttmann CR, Wei X, et al. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology. 2002;58:48–55. doi: 10.1212/wnl.58.1.48. [DOI] [PubMed] [Google Scholar]
- [13].Lundin-Olsson L, Nyberg L, Gustafson Y. “Stops walking when talking” as a predictor of falls in elderly people. Lancet. 1997;349:617. doi: 10.1016/S0140-6736(97)24009-2. [DOI] [PubMed] [Google Scholar]
- [14].Bootsma-van der Wiel A, Gussekloo J, de Craen AJ, van Exel E, Bloem BR, Westendorp RG. Walking and talking as predictors of falls in the general population: the Leiden 85-Plus Study. J Am Geriatr Soc. 2003;51:1466–1471. doi: 10.1046/j.1532-5415.2003.51468.x. [DOI] [PubMed] [Google Scholar]
- [15].de Hoon EW, Allum JH, Carpenter MG, et al. Quantitative assessment of the stops walking while talking test in the elderly. Arch Phys Med Rehabil. 2003;84:838–842. doi: 10.1016/s0003-9993(02)04951-1. [DOI] [PubMed] [Google Scholar]
- [16].Cocchini G, Della Sala S, Logie RH, Pagani R, Sacco L, Spinnler H. Dual task effects of walking when talking in Alzheimer’s disease. Rev Neurol (Paris) 2004;160:74–80. [PubMed] [Google Scholar]
- [17].Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc. 2003;51:1633–1637. doi: 10.1046/j.1532-5415.2003.51516.x. [DOI] [PubMed] [Google Scholar]
- [18].Camicioli R, Howieson D, Lehman S, Kaye J. Talking while walking: the effect of a dual task in aging and Alzheimer’s disease. Neurology. 1997;48:955–958. doi: 10.1212/wnl.48.4.955. [DOI] [PubMed] [Google Scholar]
- [19].Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- [20].Della Sala S, Cocchini G, Logie RH, Allerhand M, MacPherson SE. Dual task during encoding, maintenance, and retrieval in Alzheimer’s disease. J Alzheimers Dis. 2010;19:503–515. doi: 10.3233/JAD-2010-1244. [DOI] [PubMed] [Google Scholar]
- [21].Logie RH, Cocchini G, Delia Sala S, Baddeley AD. Is there a specific executive capacity for dual task coordination? Evidence from Alzheimer’s disease. Neuropsychology. 2004;18:504–513. doi: 10.1037/0894-4105.18.3.504. [DOI] [PubMed] [Google Scholar]
- [22].Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342. doi: 10.1002/mds.21720. quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Giladi N. Gait and mental function: the interplay between walking, behavior and cognition. J Neural Transm. 2007;114:1241–1242. doi: 10.1007/s00702-007-0802-9. [DOI] [PubMed] [Google Scholar]
- [24].Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord. 2006;21:950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- [25].Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- [26].Stuss DT, Levine B. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- [27].Beauchet O, Dubost V, Aminian K, Gonthier R, Kressig RW. Dual-task-related gait changes in the elderly: does the type of cognitive task matter? J Mot Behav. 2005;37:259–264. [PubMed] [Google Scholar]
- [28].Huang H-J, Mercer VS. Dual-Task Methodology: Applications in Studies of Cognitive and Motor Performance in Adults and Children. Pediatric Physical Therapy. 2001;13:133–140. [PubMed] [Google Scholar]
- [29].Fukuyama H, Ouchi Y, Matsuzaki S, et al. Brain functional activity during gait in normal subjects: a SPECT study. Neurosci Lett. 1997;228:183–186. doi: 10.1016/s0304-3940(97)00381-9. [DOI] [PubMed] [Google Scholar]
- [30].Miyai I, Tanabe HC, Sase I, et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage. 2001;14:1186–1192. doi: 10.1006/nimg.2001.0905. [DOI] [PubMed] [Google Scholar]
- [31].Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp. 2003;19:47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Suzuki M, Miyai I, Ono T, et al. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: an optical imaging study. Neuroimage. 2004;23:1020–1026. doi: 10.1016/j.neuroimage.2004.07.002. [DOI] [PubMed] [Google Scholar]
- [33].Rushworth MF, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. Neuroimage. 2003;20(Suppl 1):S89–100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- [34].Dault MC, Yardley L, Frank JS. Does articulation contribute to modifications of postural control during dual-task paradigms? Brain Res Cogn Brain Res. 2003;16:434–440. doi: 10.1016/s0926-6410(03)00058-2. [DOI] [PubMed] [Google Scholar]
- [35].Yardley L, Gardner M, Leadbetter A, Lavie N. Effect of articulatory and mental tasks on postural control. Neuroreport. 1999;10:215–219. doi: 10.1097/00001756-199902050-00003. [DOI] [PubMed] [Google Scholar]
- [36].Alexander NB. Gait disorders in older adults. J Am Geriatr Soc. 1996;44:434–451. doi: 10.1111/j.1532-5415.1996.tb06417.x. [DOI] [PubMed] [Google Scholar]
- [37].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- [38].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [39].Mattis S. Mental status examination for organic mental syndrome in the elderly patient. In: Bellack R, Karasu B, editors. Geriatric Psychiatry. Grune & Stratton; New York: 1976. pp. 77–121. [Google Scholar]
- [40].Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- [41].Martinez-Martin P, Gil-Nagel A, Gracia LM, Gomez JB, Martinez-Sarries J, Bermejo F, The Cooperative Multicentric Group Unified Parkinson’s Disease Rating Scale characteristics and structure. Mov Disord. 1994;9:76–83. doi: 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- [42].Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80:429–434. doi: 10.1016/0002-9343(86)90717-5. [DOI] [PubMed] [Google Scholar]
- [43].Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- [44].Nadkarni NK, Zabjek K, Lee B, McIlroy WE, Black SE. Effect of working memory and spatial attention tasks on gait in healthy young and older adults. Motor Control. 2010;14:195–210. doi: 10.1123/mcj.14.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li KZ, Lindenberger U, Freund AM, Baltes PB. Walking while memorizing: age-related differences in compensatory behavior. Psychol Sci. 2001;12:230–237. doi: 10.1111/1467-9280.00341. [DOI] [PubMed] [Google Scholar]
- [46].Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol Aging. 2000;15:417–436. doi: 10.1037//0882-7974.15.3.417. [DOI] [PubMed] [Google Scholar]
- [47].Prince F, Corriveau H, Hebert R, Winter DA. Gait in Elderly. Gait Posture. 1997;5:128–135. [Google Scholar]
- [48].Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson’s disease. J Geriatr Psychiatry Neurol. 2003;16:53–58. doi: 10.1177/0891988702250580. [DOI] [PubMed] [Google Scholar]
- [49].Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:125–137. doi: 10.1159/000105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Delacourte A, David JP, Sergeant N, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology. 1999;52:1158–1165. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- [51].Nadkarni NK, Mawji E, McIlroy WE, Black SE. Spatial and temporal gait parameters in Alzheimer’s disease and aging. Gait Posture. 2009;30:452–454. doi: 10.1016/j.gaitpost.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nadkarni NK, McIlroy WE, Mawji E, Black SE. Gait and subcortical hyperintensities in mild Alzheimer’s disease and aging. Dement Geriatr Cogn Disord. 2009;28:295–301. doi: 10.1159/000245158. [DOI] [PubMed] [Google Scholar]
- [53].Faulkner KA, Redfern MS, Rosano C, et al. Reciprocal influence of concurrent walking and cognitive testing on performance in older adults. Gait Posture. 2006;24:182–189. doi: 10.1016/j.gaitpost.2005.08.004. [DOI] [PubMed] [Google Scholar]
- [54].Schmidt R, Fazekas F, Offenbacher H, et al. Neuropsychologic correlates of MRI white matter hyperintensities: a study of 150 normal volunteers. Neurology. 1993;43:2490–2494. doi: 10.1212/wnl.43.12.2490. [DOI] [PubMed] [Google Scholar]
- [55].van den Heuvel DM, ten Dam VH, de Craen AJ, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry. 2006;77:149–153. doi: 10.1136/jnnp.2005.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- [57].Junque C, Pujol J, Vendrell P, et al. Leuko-araiosis on magnetic resonance imaging and speed of mental processing. Arch Neurol. 1990;47:151–156. doi: 10.1001/archneur.1990.00530020047013. [DOI] [PubMed] [Google Scholar]
- [58].Ylikoski R, Ylikoski A, Erkinjuntti T, Sulkava R, Raininko R, Tilvis R. White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Arch Neurol. 1993;50:818–824. doi: 10.1001/archneur.1993.00540080029009. [DOI] [PubMed] [Google Scholar]
- [59].van Straaten EC, Fazekas F, Rostrup E, et al. Impact of white matter hyperintensities scoring method on correlations with clinical data: the LADIS study. Stroke. 2006;37:836–840. doi: 10.1161/01.STR.0000202585.26325.74. [DOI] [PubMed] [Google Scholar]
- [60].Gouw AA, Van der Flier WM, van Straaten EC, et al. Simple versus complex assessment of white matter hyperintensities in relation to physical performance and cognition: the LADIS study. J Neurol. 2006;253:1189–1196. doi: 10.1007/s00415-006-0193-5. [DOI] [PubMed] [Google Scholar]
- [61].Schwenk M, Zieschang T, Oster P, Hauer K. Dual-task performances can be improved in patients with dementia. A randomized controlled trial. Neurology. 2010 doi: 10.1212/WNL.0b013e3181e39696. [DOI] [PubMed] [Google Scholar]
- [62].Nordin E, Moe-Nilssen R, Ramnemark A, Lundin-Olsson L. Changes in step-width during dual-task walking predicts falls. Gait Posture. 2010;32:92–97. doi: 10.1016/j.gaitpost.2010.03.012. [DOI] [PubMed] [Google Scholar]