SUMMARY
The Staphylococcus aureus leukotoxin ED (LukED) is a pore-forming toxin required for the lethality associated with bacteremia in murine models. LukED targets the chemokine receptor CCR5 to kill T lymphocytes, macrophages and dendritic cells. LukED also kills CCR5-deficient cells like neutrophils, suggesting the existence of additional cellular receptors. Here we identify the chemokine receptors CXCR1 and CXCR2 as the targets of LukED on neutrophils. The LukE subunit binds neutrophils in a specific and saturable manner and this interaction is inhibited by CXCL8, the high affinity endogenous ligand of CXCR1 and CXCR2. LukED recognition of CXCR1 and CXCR2 promotes the killing of monocytes and neutrophils in vitro. LukED-mediated targeting of CXCR1/CXCR2+ cells contributes to S. aureus pathogenesis and facilitates lethality in systemically infected mice. Thus, LukED is a versatile toxin that endows S. aureus with the ability to simultaneously disarm both innate and adaptive compartments of the host immune response.
INTRODUCTION
Staphylococcus aureus (S. aureus) is a Gram positive bacterium that is responsible for significant morbidity and mortality worldwide (DeLeo and Chambers, 2009). The pathogenesis of this organism depends on the production of an arsenal of virulence factors that are thought to contribute to immune evasion and subsequent manifestation of disease (Nizet, 2007; Otto, 2010; Spaan et al., 2013b). Strains associated with human infection can produce up to five different bi-component leukotoxins (LukSF-PV/PVL, HlgAB, HlgCB, LukED, and LukAB, also known as LukHG) (Alonzo and Torres, 2013). These toxins potently target and kill human neutrophils (polymorphonuclear cells; PMNs), innate immune cells critical for defense against bacterial infections (Rigby and DeLeo, 2012; Spaan et al., 2013b). For many years cellular targeting by the leukotoxins was thought to be redundant, however the recent identification of cellular factors that facilitate their unique cellular tropism has proven otherwise (Alonzo et al., 2013; Dumont et al., 2013a; Spaan et al., 2013a).
CCR5 was recently identified as a LukED receptor on T cells, macrophages and dendritic cells (Alonzo et al., 2013), yet the identification of this cellular target failed to explain how LukED targets leukocytes that lack CCR5, which include PMNs. Here we report that LukED targets and kills CCR5-deficient leukocytes via recognition of CXCR1/CXCR2. We demonstrate the importance of CXCR1/CXCR2 targeting in vitro, ex vivo and in vivo, further highlighting the importance of LukED to S. aureus pathogenesis.
RESULTS
LukED Targets Monocytes and PMNs in a CCR5-Independent Manner
While investigating the effects of LukED on primary human peripheral blood mononuclear cells (PBMCs) we observed that monocytes within PBMCs isolated from a Δ32Ccr5 individual, which naturally lacks CCR5 on the cell surface (Liu et al., 1996; Samson et al., 1996) are targeted in a LukED-mediated, CCR5-independent manner (Figure 1A). Similarly, monocytes from PBMCs isolated from Ccr5+/+ individuals were susceptible to LukED even in the presence of maraviroc, a CCR5 antagonist known to block LukED-mediated killing of CCR5+ T cells (Figure S1A) (Alonzo et al., 2013). Moreover, we found that primary human PMNs from Ccr5+/+ and Δ32Ccr5 donors were equally susceptible to LukED (Figures 1B and S1B), indicating that LukED targets human monocytes and PMNs in a CCR5-independent manner.
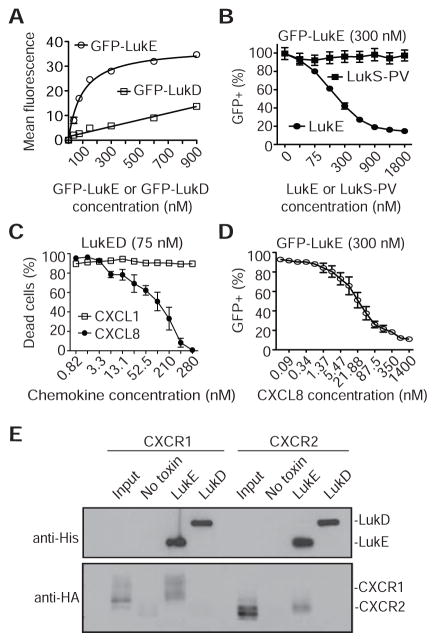
Figure 1. LukED Targets CXCR1 and CXCR2 to Kill Monocytes and PMNs.
(A) PBMCs isolated from a Δ32Ccr5 donor incubated with PBS or LukED (75 nM) and gated for CD14 and CD3 positivity. (B) Viability of primary human neutrophils (PMNs) isolated from a Ccr5+/+ or a Δ32Ccr5 donor in the presence of LukE, LukD or LukED (75 nM). (C) Bacterial burden (CFUs) from livers of Ccr5+/+ mice systemically infected with isogenic S. aureus WT (n = 14), ΔlukED (n = 15) or ΔlukED::lukED (n = 15) strains and Ccr5−/− mice systemically infected with S. aureus WT (n = 10), ΔlukED (n = 11) or ΔlukED::lukED (n = 3). **P < 0.01, ***P < 0.001 or ****P < 0.0001 by unpaired Student’s t-test. (D) Viability of transfected HEK293T cells incubated with LukED or LukSF-PV (600 nM). Means ± SEM are shown (n = 3). (E–F) CXCR1 and CXCR2 levels on the surface of PMNs (E) or monocytes (F) as determined by flow cytometry. (G) Viability of THP-1 cells transduced with non-target or Cxcr2 shRNA treated with LukED. Means ± SEM are shown (n = 3). See also Figure S1.
To evaluate the relevance of the CCR5-independent contribution of LukED to S. aureus virulence in vivo, we systemically infected Ccr5+/+ and Ccr5−/− mice with wild type S. aureus, an isogenic ΔlukED mutant or an isogenic ΔlukED mutant containing the lukED gene expressed from its native promoter integrated in single copy within the S. aureus chromosome (ΔlukED::lukED) (Alonzo et al., 2012; Alonzo et al., 2013) and evaluated the bacterial burden in infected livers 96 hours post-infection. ΔlukED-infected Ccr5+/+ mice displayed a 2-log reduction in CFU compared to those infected with WT or the complementation strain (ΔlukED::lukED) (Figure 1C). Consistent with prior studies, the bacterial burden in Ccr5−/− mice infected with WT S. aureus was reduced 1-log compared to Ccr5+/+ mice infected with WT S. aureus (Alonzo et al., 2013). Interestingly, we observed that Ccr5−/− mice infected with the ΔlukED strain showed ~3-log reduction in bacterial burden compared to Ccr5−/− mice infected with WT S. aureus, a phenotype fully complemented upon infection with ΔlukED::lukED (Figure 1C).
LukED Targets CXCR1 and CXCR2 to Kill Leukocytes
The ex vivo experiments with Δ32Ccr5 human leukocytes and the in vivo experiments with Ccr5−/− mice (Figure 1A–C), suggest the existence of alternate LukED receptors on the surface of PMNs and monocytes, whose targeting contributes to establishment of systemic S. aureus infection. To identify these targets, a collection of chemokine receptors present on the surface of leukocytes were ectopically expressed on Human Embryonic Kidney 293T cells (HEK293T) followed by incubation with LukED. We discovered that as with CCR5, the chemokine receptors CXCR1 or CXCR2, but not CXCR4, were sufficient to render HEK293T cells susceptible to LukED, but not to the homologous leukotoxin LukSF-PV (Figures 1D and S1C), which does not target CXCR2 (Spaan et al., 2013a). Consistent with their susceptibility to LukED, the surface of the majority of primary human PMNs and peripheral blood monocytes are decorated with both CXCR1 and CXCR2 (Figures 1E and 1F). To determine if these receptors are necessary to render host cells susceptible to LukED, a loss of function approach was employed using lentiviral-based knockdown and the human monocytic cell line THP-1, which displays only CXCR2 (Figures S1D and S1E). We observed that Cxcr2 shRNA rendered THP-1 cells markedly resistant to LukED compared to non-target shRNA controls (Figure 1G). These data demonstrate that CXCR1 and/or CXCR2 are necessary and sufficient for LukED-mediated killing of mammalian cells.
LukE Specifically Binds to CXCR1/CXCR2 on Host Cells
Because of their primary role in defense against S. aureus (Rigby and DeLeo, 2012), we focused the remainder of our studies on LukED-mediated targeting of CXCR1/CXCR2 on primary PMNs. A binding assay was employed where PMNs were incubated with green fluorescent protein-fused LukE or LukD (GFP-LukE or GFP-LukD) (Alonzo et al., 2013). Only GFP-LukE bound to PMNs in a dose-dependent and saturable manner, while GFP-LukD displayed nonsaturable surface association (Figure 2A). GFP-LukE binding was competed off with LukE but not the equivalent subunit of LukSF-PV, LukS-PV (Figure 2B), suggesting specific interaction with CXCR1/CXCR2.
Figure 2. LukED Targets PMNs Via LukE Binding to CXCR1 and CXCR2.
(A) Binding of GFP-LukE or GFP-LukD to the surface of PMNs evaluated by flow cytometry. Means ± SEM are shown (n = 3). (B) Binding of GFP-LukE (300 nM) to PMNs in the presence of unlabeled LukE or LukS-PV as determined by flow cytometry. Means ± SEM are shown (n = 3). (C) Viability of PMNs challenged with a lethal dose of LukED (75 nM) in the presence of CXCL8 or CXCL1. Means ± SEM are shown (n = 3). (D) Binding of GFP-LukE (300 nM) to the surface of PMNs in the presence of CXCL8. Means ± SEM are shown (n = 3). (E) Interaction of His-LukE or His-LukD with cell lysates containing HA-tagged CXCR1 or CXCR2. Immunoblots are representative of at least three independent experiments. See also Figure S2.
The CXCR1/CXCR2 receptors respond primarily to the chemokine ligand CXCL8, which is produced by the host in response to injury and infection (Stillie et al., 2009). In addition to CXCL8, CXCR2 also responds to the chemokine CXCL1 (Stillie et al., 2009). To test whether these chemokines are able to inhibit LukED-mediated cytotoxicity, PMNs were treated with LukED in the presence of either CXCL8 or CXCL1. CXCL8 prevented LukED-mediated death of PMNs but not CXCL1 (Figure 2C), suggesting that blockade of both receptors is required to protect PMNs from LukED-mediated killing. CXCL8 protected PMNs from LukED by preventing LukE binding to the cell surface (Figure 2D), a prerequisite for cytotoxicity. While LukE and CXCL8 both target CXCR1/CXCR2 they do not appear to engage the receptors to the same capacity, as LukE is unable to elicit calcium mobilization upon incubation with PMNs (Figure S2). Consistent with the LukED-PMN binding studies, pull-down experiments revealed that LukE but not LukD interacts with both CXCR1 and CXCR2 (Figure 2E).
LukE Divergence Region 4 (DR4) is Required for LukED-mediated Killing of CXCR1/CXCR2+ Cells
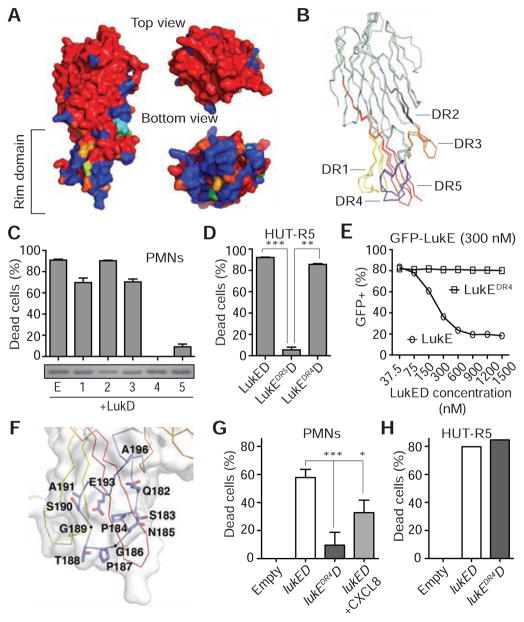
LukE and LukS-PV share ~71% amino acid identity (Figure S3A), yet LukS-PV uses the C5a receptors (Spaan et al., 2013a) instead of CXCR1/CXCR2 to target human PMNs (Figure 1D). Amino acid sequence alignment of LukE and LukS-PV revealed five regions containing significant sequence divergence (divergence regions 1–5; DR1–5) (Figure S3A). These DRs are located in the rim domain of the LukE and LukS-PV structures (Figures 3A and 3B), which has been hypothesized to play a role in receptor recognition (Guillet et al., 2004; Olson et al., 1999; Pedelacq et al., 1999). We generated hybrid LukE/S-PV toxins to test whether any of these five DRs are involved in conferring specificity of LukE toward human PMNs. The LukE/S-PV hybrid proteins were purified, mixed at equimolar ratio with LukD, and incubated with PMNs to evaluate their activity. We found that only the LukEDR4D and LukEDR5D toxins were impaired in cytotoxic activity towards PMNs compared to WT LukED (Figures 3C and S3B).
Figure 3. LukE Amino Acids 182–196 in Divergence Region 4 Are Required for LukED Targeting of CXCR1/CXCR2+ cells.
(A) The structures of LukE and LukS-PV differ primarily in the rim domain surface as indicated by a color ramp in which highly divergent residues are colored in dark blue, identical residues are colored in red, and conservative substitutions have an intermediate color. (B) LukE (3ROH, light blue) and LukS (1T5R, light green) structural alignment with DRs 1–5 amino acids highlighted as follows: DR1 (yellow, 57–75), DR2 (grey, 139–150), DR3 (orange, 164–178), DR4 (blue, 182–196) and DR5 (red, 237–271). (C) Viability of PMNs treated with WT and LukEDR hybrids (DRs 1–5, 300 nM). Insert is a coomassie blue-stained gel of purified LukEDR hybrids. Means ± SEM are shown (n = 9). (D) Viability of CCR5+ cells treated with the indicated toxins (300 nM). Means ± SEM are shown (n = 3). Data shown are representative of one out of three experiments done in triplicate. **P ≤ 0.001 and ***P ≤ 0.0001 by one-way analysis of variance with Tukey’s multiple comparison test. (E) Binding of GFP-LukE (300 nM) in the presence of unlabeled LukE or LukEDR4 as determined by flow cytometry. Means ± SEM are shown (n = 3). (F) LukEDR4 (blue) structure with residues that differ between LukE and LukS-PV depicted as sticks. (G–H) Ex vivo infection of PMNs (G) or HUT-R5 (H) with S. aureus Newman ΔΔΔΔ (ΔlukED, ΔhlgACB::tet, ΔlukAB::spec, Δhla::ermC) strains containing the pOS1-PlukAB-lukAs.s.-6xHis vector either empty (empty), or with lukED or lukEDR4D at a multiplicity of infection of 10. Data shown are representative of one out of three experiments done in triplicate. *P < 0.05; **P ≤ 0.0001 by one-way analysis of variance (G). See also Figure S3 and Table S3.
To evaluate whether the lack of cytotoxicity exhibited by the LukEDR4D and LukEDR5D hybrids was specific towards CXCR1/CXCR2+ cells, we also tested their activity towards a CCR5+ T cell line (Alonzo et al., 2013). We observed that LukEDR5D was also impaired in killing CCR5+ cells, suggesting that LukE’s DR5 is required for toxin activity rather than receptor targeting (Figures 3C, 3D, S3B and S3C). Remarkably, LukEDR4D was able to target CCR5+ cell lines and primary human T cells at similar potency to that of WT LukED (Figures 3D, and S3C and S3D). Further analysis of PBMCs revealed that LukED also targets a subset of CD8+ T cells and primary human CXCR1+ NK cells in a DR4-dependent manner (Figure S3D). LukE DR4 was also required for LukED-mediated killing of primary human monocytes despite also displaying CCR5 on their surface (Figure S3D). These data demonstrate that CXCR1/CXCR2 are likely the preferred receptors for LukED-mediated targeting of monocytes, a finding consistent with the susceptibility of Δ32Ccr5 monocytes to LukED (Figure 1A).
We found that in contrast to LukE, LukEDR4 was unable to compete with GFP-LukE for binding to the plasma membrane of PMNs, establishing the role of the DR4 domain in recognition of CXCR1/CXCR2+ cells (Figure 3E). The 15 amino acid sequence of LukE’s DR4, residues 182–196 of the mature protein, forms a loop containing two glycine residues (G186 and G189) and two proline residues (P184 and P187) that present a polar surface sufficiently distinct from that of LukS-PV’s DR4, which likely determines the tropism of LukE towards CXCR1/CXCR2.
We next investigated the contribution of CXCR1/CXCR2 targeting by LukED to S. aureus-mediated killing of PMNs during ex vivo infection. Since S. aureus produces an array of toxins capable of killing PMNs (Alonzo and Torres, 2013) and LukED is produced at sublytic quantities by S. aureus in broth culture (Alonzo et al., 2012; DuMont et al., 2011), we opted to use an engineered S. aureus strain lacking all the pore-forming toxins (hla, lukAB, hlgACB, and lukED). The lukED or lukEDR4D loci were expressed in trans from a plasmid using the lukAB promoter (Figure S4A), as proof of concept that LukED is targeting CXCR1 and CXCR2 through the DR4 domain. As expected, the toxinless S. aureus strain was impaired in killing PMNs, whereas the strain complemented in trans with lukED was able to kill these cells (Figure 3G). The cytotoxic activity of the LukED-producing S. aureus strain was inhibited by CXCL8 and required LukE’s DR4 domain as the LukEDR4D-producing strain exhibited reduced PMN killing compared to the WT LukED-producing strain (Figure 3G). Importantly, the defect in cell killing exhibited by the LukEDR4D-producing strain was specific towards CXCR1/CXCR2+ cells, as LukEDR4D-producing strains retained the ability to kill CCR5+ cells (Figure 3H).
To evaluate if LukED also kills murine leukocytes in a CXCR1/CXCR2-dependent manner, murine peritoneal exudate cells (PEC) were treated with LukED or LukEDR4D. While LukED killed ~79% of the PMNs, LukEDR4D was significantly impaired and only killed ~8% of these cells. In contrast to the effects on PMNs, CCR5+ macrophages from within the PEC population were equally susceptible to both LukED and LukEDR4D (Figures 4A and S4B), consistent with the finding that LukED kills these cells in a strictly CCR5-dependent manner (Alonzo et al., 2013). We also assessed the viability of PMNs from tissues infected with an S. aureus Newman ΔlukED deletion mutant or a ΔlukED mutant complemented with a single chromosomal copy of either the WT lukED (Alonzo et al., 2012) or the lukEDR4D operon with expression driven by the endogenous lukED promoter (ΔlukED, ΔlukED::lukED or ΔlukED::lukEDR4D, respectively). Importantly, the ΔlukED::lukED and ΔlukED::lukEDR4D isogenic strains produced similar levels of toxin in vitro (Figure S4C). We observed that PMNs isolated from S. aureus ΔlukED- or ΔlukED::lukEDR4D-infected mice were largely protected from toxin-mediated death compared to that of ΔlukED::lukED-infected mice (Figures 4B and S4D), demonstrating that the DR4 domain is required for LukED-mediated PMN targeting in vivo.
Figure 4. LukED-mediated Killing of CXCR1/CXCR2+ Cells Contributes to S. aureus Pathogenesis in Mouse Models of Systemic Infection.
(A) Viability of peritoneal elicited murine PMNs (CXCR2+) or macrophages (CCR5+) in the presence of PBS (No Toxin), LukED or LukEDR4D (300 nM). FACS plots are representative of one of 3 mice per treatment. (B) Murine PMNs isolated from the liver (top panel) and kidneys (bottom panel) of mice systemically infected with isogenic S. aureus Newman ΔlukED, ΔlukED::lukED or ΔlukED::lukEDR4D strains. FACS plots are representative of one of 10 infected mice per strain. (C) ‘Survival’ of mice infected with isogenic S. aureus ΔlukED (n = 10), ΔlukED::lukED (+lukED, n = 16) or ΔlukED::lukEDR4D (+lukEDR4D, n = 16) strains. Statistical analysis for ΔlukED versus +lukED, ****P = 0.0001; ΔlukED versus +lukEDR4D, P = 0.0577; +lukED versus +lukEDR4D, ***P = 0.0003 by Log-Rank (Mantel-Cox test). See also Figure S4.
LukED Targeting of CXCR1/CXCR2 Contributes to S. aureus Pathogenesis
LukED contributes to the mortality observed in mice infected systemically with S. aureus, including MRSA (Alonzo et al., 2012; Alonzo et al., 2013). To evaluate the role of LukED-mediated targeting of CXCR1/CXCR2 in conferring this phenotype, we monitored the survival of mice infected systemically with isogenic S. aureus ΔlukED, ΔlukED::lukED, or ΔlukED::lukEDR4D strains described above. As expected (Alonzo et al., 2012; Alonzo et al., 2013), mice infected with the ΔlukED strain survived the infection, while mice infected with the ΔlukED::lukED complemented strain succumbed to infection (Figure 4C). In contrast, we observed that the ΔlukED::lukEDR4D-infected mice were markedly protected compared to the ΔlukED::lukED complemented strain (Figure 4C). Altogether, these findings demonstrate that LukED-mediated targeting of CXCR1 or CXCR2 promotes S. aureus pathogenesis in vivo.
DISCUSSION
The identification of the chemokine receptors CXCR1 and CXCR2 as LukED targets provides an explanation for the ability of this toxin to kill immune cells that lack CCR5, substantiates previous studies demonstrating PMN targeting in vitro by LukED, and directly links PMN cytotoxicity by a leukotoxin to S. aureus pathogenesis in vivo. It is well established that purified S. aureus bi-component leukotoxins (LukSF-PV/PVL, HlgAB, HlgCB, LukED, and LukAB) kill PMNs in vitro. As such, they have been presumed to play strictly redundant roles during S. aureus infection. However, demonstrating toxin redundancy or lack thereof using in vivo models has proven difficult. For example, LukSF-PV/PVL and LukAB target cells in a species-specific manner (Dumont et al., 2013a; Spaan et al., 2013a), limiting the implementation of murine models to study their mechanism of action. In contrast, the broad host range and specific cellular targets (CCR5, CXCR1 and CXCR2) of LukED allows detailed in vivo mechanistic studies of toxin-mediated pathobiology via leukocyte targeting. Thus, through the study of LukED, we can now begin to model leukotoxin activity in vivo using small animal infection models.
Support for a non-redundant in vivo mechanism of action of the leukotoxins is provided by recent studies that suggest that not all leukotoxins are produced by S. aureus simultaneously or at equal levels (Dumont et al., 2013b). For example, LukSF-PV/PVL and LukAB are produced in a manner that depends on specific growth conditions (Dumont et al., 2013b), while LukED is produced only at low levels using similar conditions (Alonzo et al., 2012; Dumont et al., 2013b). We suspect that in an animal model where the host is fully sensitive to each leukotoxin, a model currently unavailable at this time, a profound effect on S. aureus pathogenesis would be seen due to combined toxin targeting of PMNs, as well as toxin-specific targeting of other leukocytes dictated by environmental conditions, as well as the receptor profiles of specific immune cell targets. Importantly, the recent identification of unique leukotoxin receptors for LukED, LukSF-PV/PVL and LukAB implies that although each toxin can target PMNs, they do so in a non-redundant manner (Alonzo et al., 2013; Dumont et al., 2013a; Spaan et al., 2013a).
Since PMNs are the first responders to infection and defects in PMN function result in extraordinary susceptibility to S. aureus infection (Rigby and DeLeo, 2012), it is logical that a pathogen like S. aureus would produce virulence factors such as leukotoxins to kill these cells (Alonzo and Torres, 2013; Spaan et al., 2013b). Nevertheless, the sustained function of PMNs is also dependent on their continuous recruitment and enhanced potency or lifespan through inflammatory mediators secreted at the site of infection (Cho et al., 2010; Kolaczkowska and Kubes, 2013; Lin et al., 2009). Indeed, quantitative and qualitative disruption of either PMNs or T cells, especially effector subsets that secrete IL-17 or IFNγ, greatly increase the susceptibility to S. aureus infection (Alonzo et al., 2012; Alonzo et al., 2013; Cho et al., 2010; Lin et al., 2009). As such, through the use of CXCR1/CXCR2, LukED targets largely innate defenses mediated by PMNs, monocytes, and NK cells. Whereas by targeting CCR5+ cells, LukED eliminates T cell subsets including memory cells and Th1/Th17 cells, as well as professional antigen presenting cells (Alonzo et al., 2013), all of which are critical in anti-Staph immunity.
Due to the temporal nature of the host immune response and the diverse cell types involved in infection resolution, we observed that blockade of LukED targeting of either CXCR1/CXCR2 or CCR5 (Alonzo et al., 2013) leads to enhanced survivability in vivo in a murine model of systemic infection. These findings support a role for toxin-mediated PMN killing as an efficient immune evasion mechanism that ultimately facilitates pathogenesis. Importantly, we cannot exclude other roles for LukED as they relate to animal death at this time. It is however well established that antibody-mediated depletion of PMNs in systemic models of infection renders mice hyper-susceptible to S. aureus-mediated death (Alonzo et al., 2012; Corbin et al., 2008; Gresham et al., 2000). Thus, we suggest that S. aureus produces LukED to function in a similar manner, as a suppressor of the host’s ability to contain infection by eliminating leukocytes involved in infection control and resolution.
EXPERIMENTAL PROCEDURES
Ethics Statement
All experiments involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of New York University. All experiments were performed according to NIH guidelines, the Animal Welfare Act, and US Federal law.
Blood was obtained from de-identified, consenting healthy adult donors as buffy coats from the New York Blood Center.
Binding and Competition Assays
For binding assays, a dose response of GFP-LukE or GFP-LukD were added to 5 × 104 human PMNs and incubated for 30 minutes on ice, then cells were washed once in FACS buffer (PBS + 2% FBS + 0.05% sodium azide) and fixed for 15 minutes at room temperature followed by flow cytometric analysis. Mean fluorescence intensity (MFI) of GFP+ cells was measured to establish the toxin concentration required to achieve saturable binding. For remaining competition assays, a dose response of either LukE or LukS-PV was co-incubated with a constant saturable concentration of LukE-GFP (300 nM) for 30 minutes. Cells were washed once in FACS buffer, fixed for 15 minutes in FACS fixing buffer (FACS buffer + 2% paraformaldehyde + 0.05% sodium azide), washed again in FACS buffer and binding assessed by flow cytometry. The mean GFP fluorescent intensity is represented as % GFP+, based on the maximal fluorescence observed upon incubation with 300 nM GFP-LukE. For competition assays using CXCL8 or CXCL1, a dose response of either chemokine was added to human PMNs for 30 minutes on ice, followed by addition of 300 nM GFP-LukE. Cells were washed once in FACS buffer, fixed for 15 minutes in FACS fixing buffer, washed again in FACS buffer and binding assessed by flow cytometry as described above. Results for these assays were depicted graphically using GraphPad PRISM software (version 5.0f, GraphPad PRISM Software, Inc.).
Ex Vivo Infection Experiments
S. aureus Newman ΔΔΔΔ strains lacking all four pore-forming toxins (ΔlukED Δhla::ermC ΔhlgACB::tet ΔlukAB::spec) containing the pOS1-PlukAB-lukAs.s.-6xHis vector construct with either empty vector (empty), lukED or lukEDR4D (Tables S1 and S2) were subcultured for 4.5 hours, followed by normalization to 1 × 109 CFU per ml in RPMI + 10% FBS. Cells were then diluted 1:10 and 20 μl were added to 80 μl of media containing 2 × 105 PMNs seeded into 96-well plates. Infections were carried out for 3.5 hours at 37°C with shaking at 180 RPM. 2 μg ml−1 of lysostaphin was added for 20 minutes at 37°C with shaking at 180 RPM to kill all bacteria. Cells were centrifuged for 5 minutes at 1,500 RPM and 4°C, followed by fixing in FACS fixing buffer. To analyze toxin-mediated killing by flow cytometry, cellular depletion from gated live cells was evaluated. Percent cell death was calculated by comparing cells remaining in the live gate to that of Newman ΔΔΔΔ strain containing the pOS1-PlukAB modified empty vector (no toxin), which was set to 0% dead.
Generation of S. aureus Chromosomal Integration Strains
For complementation with WT LukED, the entire lukED locus was amplified from S. aureus Newman genomic DNA using the primers VJT605 and VJT299 (Table S3) and chromosomal integration performed as described (Alonzo et al., 2012).
To generate the lukEDR4D integration construct, the lukED promoter region was amplified from S. aureus Newman genomic DNA using primers VJT605 and VJT1019. The lukEDR4 coding region was amplified using purified plasmid from strain VJT34.58 containing lukEDR4 with primers VJT1020 and VJT1021. S. aureus Newman genomic DNA was used to amplify lukD and the intergenic region between lukE and lukD using primers VJT1022 and VJT299. A final overlap PCR reaction was preformed with the resultant DNA fragments and primers VJT605 and VJT299. The lukED and lukEDR4D constructs were transformed into E. coli DH5α and clones selected by ampicillin resistance. The purified plasmids were cloned into pJC1112 using BamHI and PstI restriction sites and transformed into DH5α. The resulting recombinant plasmids were introduced by electroporation into strain RN9011, containing plasmid pRN7023 which encodes the SaPI1 integrase to facilitate single copy chromosomal integration into the S. aureus SaPI1 site (Ruzin et al., 2001) and selected for chloramphenicol and erythromycin resistance, as previously described (Alonzo et al., 2012). The SaPI1 integrated constructs were then transduced into strain VJT8.16, Newman ΔlukED, using previously described methods (Alonzo et al., 2012).
To generate an empty vector-containing ΔlukED strain, the pJC1112 vector was electroporated into RN9011 as above. Bacteriophage-mediated transduction was then used to introduce the integrated complementation vector into S. aureus strain Newman ΔlukED using previously described methods (Alonzo et al., 2012).
Murine In Vitro and In Vivo Experiments
To evaluate LukED-mediated killing of murine cells in vitro, C57BL/6 WT mice (Taconic) were injected intraperitoneally with 1 × 107 CFU of heat-killed S. aureus Newman ΔlukED. Twenty-four hours post injection, another dose of 1 × 107 CFU of heat-killed S. aureus Newman ΔlukED was injected as before. After an additional twenty-four hours, mice were sacrificed and S. aureus elicited immune cells were collected by peritoneal cavity lavage using 8 ml of PBS. Red blood cells were lysed using 2 ml ACK lysis buffer (Gibco) followed by resuspension of remaining peritoneal exudate cells in RPMI + 10% FBS. Cells were incubated with PBS, LukED or LukEDR4D (300 nM) and incubated for 30 minutes on ice. After incubation, the cells were washed three times with PBS then stained with the fixable viability dye eFluor-450, followed by cell surface staining with CD11b, B220, F480, CD3, Ly6G, CCR5 and CXCR2 antibodies. Cell viability of specific immune cell populations was subsequently analyzed on an LSRII flow cytometer. FACS plots are representative of results obtained from cells isolated from at least 3 independent infected mice. Cell death was quantified and displayed graphically as the percentage of eFluor-450+ cells.
For in vivo experiments, 8-week old female C57BL/6 mice (Taconic) were anesthetized with 250 μl of Avertin (2,2,2-tribromoethanol dissolved in tert-amyl-alcohol and diluted to a final concentration of 2.5% v/v in sterile saline), followed by retro-orbital injection of 1 × 107 CFU of isogenic S. aureus Newman strains containing a lukED deletion complemented in single copy with either the empty integrase-encoding vector pJC1112 (ΔlukED) or WT lukED or lukEDR4D loci (ΔlukED::lukED and ΔlukED::lukEDR4D, respectively) in the SaPI1 site of S. aureus (Alonzo et al., 2012), resulting in single copy chromosomal complementation of lukED or lukEDR4D with endogenous gene expression driven by the native lukED promoter. 96-hours post infection, mice were sacrificed and organs were harvested and homogenized to evaluate the bacterial burden (colony forming units, CFUs). To determine the effects of infection with these strains on immune cells, organ immune cell suspensions were purified using a 40/80 Percoll (GE Healthcare) density gradient centrifugation and were subsequently processed and stained as described before (Alonzo et al., 2013). Cell viability of specific immune cell populations was determined by flow cytometric analysis on an LSRII flow cytometer. FACS plots are representative of results obtained from at least 9 infected animals per group. Cell death was quantified and displayed graphically as the percentage of eFluor450+ cells.
For survival experiments, 3-hour subcultures of isogenic S. aureus strains Newman ΔlukED, ΔlukED::lukED and ΔlukED::lukEDR4D described above were normalized to 5 × 108 CFU per milliliter using PBS. 5–6 week old, female ND4 mice (Harlan) were anesthetized intraperitoneally with 250 μl of Avertin, followed by retro-orbital injection of 100 μl of normalized bacteria, for a final CFU count of 5 × 107. Survival of mice was monitored over time until signs of morbidity, such as hunched posture, ruffled fur, weight loss, inability to acquire food or water, ataxia and hind limb paralysis were reached, at which point the mice were immediately sacrificed and survival curves plotted over time (hours).
Structural Modeling of LukE/LukS-PV Structural Diversity
The LukE and LukS-PV amino acid sequences were aligned with ClustalW and scored with a Risler matrix according to the extent of sequence variation using ESPript. Scores were displayed on the LukE structure surface with a color ramp (red, orange, yellow, green, light blue, dark blue) in which strictly conserved residues are colored red, and the most divergent residues are colored dark blue. Conservative substitutions are represented by intermediate colors. All structural figures were prepared using PyMOL.
Supplementary Material
HIGHLIGHTS.
S. aureus LukED targets CXCR1/CXCR2 to kill monocytes and PMNs.
LukE-CXCR1/CXCR2 binding on PMNs is blocked by CXCL8.
LukE Divergence Region 4 is required for LukED targeting of CXCR1/CXCR2+ cells.
LukED targeting of CXCR1/CXCR2 contributes to S. aureus pathogenesis.
Acknowledgments
We thank members of the Torres Laboratory for helpful discussion. We are grateful to Nara Chhua and Nadine Bode for generating the Cxcr2 shRNA THP-1 cells and the S. aureus lukED overexpressing strain, respectively. We are also grateful to Dr. Richard P. Novick and Dr. John Chen providing the pJC1112 plasmid and the RN9011 strain. This work was supported in part by New York University School of Medicine Development Funds, an American Heart Association Scientist Development Grant (09SDG2060036) to V.J.T., and National Institutes of Health grants R01-AI099394-A1 to V.J.T., T32-AI007180 to T.R.-R. and F.A., F32-AI098395-01A1 to F.A., and R21-AI087973 and R01-AI065303 grants to D.U.
Footnotes
COMPETING INTEREST STATEMENT
T.R.-R., F.A., D.U., and V.J.T. are co-inventors on patent applications filed by New York University School of Medicine, which are currently under commercial license.
Supplemental information includes four Figures, three Tables, additional Experimental Procedures and supplemental references.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonzo F, 3rd, Benson MA, Chen J, Novick RP, Shopsin B, Torres VJ. Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Mol Microbiol. 2012;83:423–435. doi: 10.1111/j.1365-2958.2011.07942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo F, 3rd, Kozhaya L, Rawlings SA, Reyes-Robles T, DuMont AL, Myszka DG, Landau NR, Unutmaz D, Torres VJ. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature. 2013;493:51–55. doi: 10.1038/nature11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo F, 3rd, Torres VJ. Bacterial survival amidst an immune onslaught: the contribution of the Staphylococcus aureus leukotoxins. PLoS Pathog. 2013;9:e1003143. doi: 10.1371/journal.ppat.1003143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, et al. Metal Chelation and Inhibition of Bacterial Growth in Tissue Abscesses. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- DeLeo FR, Chambers HF. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clin Invest. 2009;119:2464–2474. doi: 10.1172/JCI38226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuMont AL, Nygaard TK, Watkins RL, Smith A, Kozhaya L, Kreiswirth BN, Shopsin B, Unutmaz D, Voyich JM, Torres VJ. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol. 2011;79:814–825. doi: 10.1111/j.1365-2958.2010.07490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont AL, Yoong P, Day CJ, Alonzo F, 3rd, McDonald WH, Jennings MP, Torres VJ. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci U S A. 2013a;110:10794–10799. doi: 10.1073/pnas.1305121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont AL, Yoong P, Surewaard BG, Benson MA, Nijland R, van Strijp JA, Torres VJ. Staphylococcus aureus elaborates the leukotoxin LukAB to mediate escape from within human neutrophils. Infect Immun. 2013b doi: 10.1128/IAI.00095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol. 2000;164:3713–3722. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- Guillet V, Roblin P, Werner S, Coraiola M, Menestrina G, Monteil H, Prevost G, Mourey L. Crystal structure of leucotoxin S component: new insight into the Staphylococcal beta-barrel pore-forming toxins. J Biol Chem. 2004;279:41028–41037. doi: 10.1074/jbc.M406904200. [DOI] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE, Jr, Spellberg B. Th1–Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Nizet V. Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J Allergy Clin Immunol. 2007;120:13–22. doi: 10.1016/j.jaci.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Olson R, Nariya H, Yokota K, Kamio Y, Gouaux E. Crystal structure of staphylococcal LukF delineates conformational changes accompanying formation of a transmembrane channel. Nat Struct Biol. 1999;6:134–140. doi: 10.1038/5821. [DOI] [PubMed] [Google Scholar]
- Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol. 2010;64:143–162. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- Pedelacq JD, Maveyraud L, Prevost G, Baba-Moussa L, Gonzalez A, Courcelle E, Shepard W, Monteil H, Samama JP, Mourey L. The structure of a Staphylococcus aureus leucocidin component (LukF-PV) reveals the fold of the water-soluble species of a family of transmembrane pore-forming toxins. Structure. 1999;7:277–287. doi: 10.1016/s0969-2126(99)80038-0. [DOI] [PubMed] [Google Scholar]
- Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol. 2012;34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzin A, Lindsay J, Novick RP. Molecular genetics of SaPI1--a mobile pathogenicity island in Staphylococcus aureus. Mol Microbiol. 2001;41:365–377. doi: 10.1046/j.1365-2958.2001.02488.x. [DOI] [PubMed] [Google Scholar]
- Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- Spaan AN, Henry T, van Rooijen WJ, Perret M, Badiou C, Aerts PC, Kemmink J, de Haas CJ, van Kessel KP, Vandenesch F, et al. The staphylococcal toxin panton-valentine leukocidin targets human c5a receptors. Cell Host Microbe. 2013a;13:584–594. doi: 10.1016/j.chom.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Spaan AN, Surewaard BG, Nijland R, van Strijp JA. Neutrophils Versus Staphylococcus aureus: A Biological Tug of War. Annu Rev Microbiol. 2013b doi: 10.1146/annurev-micro-092412-155746. [DOI] [PubMed] [Google Scholar]
- Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol. 2009;86:529–543. doi: 10.1189/jlb.0208125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.