Abstract
Context and objective
Most case reports suggest an association between autistic spectrum disorders (ASD) and celiac disease (CD) or positive CD serology, but larger studies are contradictory. We examined the association between ASD and CD according to small intestinal histopathology.
Setting
Sweden.
Design
Nationwide case-control study.
Participants and outcome measure
Through 28 Swedish biopsy registers, we collected data on 26,995 individuals with CD (equal to villous atrophy, Marsh histopathology stage 3), 12,304 individuals with inflammation (Marsh 1–2), and 3,719 individuals with normal mucosa (Marsh 0) but positive CD serology (IgA/IgG gliadin, endomysium, tissue transglutaminase) and compared them with 213,208 age-and sex-matched controls. Conditional logistic regression estimated odds ratios (ORs) for having a prior diagnosis of ASD according to the Swedish Patient Register. In a second analysis we used Cox regression to estimate hazard ratios (HRs) for future ASD in individuals undergoing small intestinal biopsy.
Results
Prior ASD was not associated with CD (OR=0.93; 95% CI=0.51–1.68) or inflammation (OR=1.03; 95% CI=0.40–2.64) but was associated with a markedly increased risk of having a normal mucosa but positive CD serology (OR=4.57; 95% CI=1.58–13.22).
Restricting our data to individuals without a diagnosis of ASD at the time of biopsy, CD (HR=1.39; 95% CI=1.13–1.71) and inflammation (HR=2.01; 95% CI=1.29–3.13) were both associated with moderate excess risks of later ASD, whereas the HR for later ASD in individuals with normal mucosa but positive CD serology was 3.09 (95% CI=1.99–4.80).
Conclusion
Although this study found no association between CD or inflammation and earlier ASD, there was a markedly increased risk of ASD in individuals with a normal mucosa but positive CD serology.
Keywords: autism, autistic spectrum disorder, autoimmunity, celiac, coeliac, inflammation
Context
Autism or autistic spectrum disorders (ASD) is a complex developmental disorder characterized by the triad of symptoms comprising impaired social interaction, impaired communication, and repetitive behaviors. ASD was previously estimated to occur in less than 1 per 1000 individuals,1 but increasing disease awareness, new diagnostic criteria, and a potentially true increase in ASD incidence have led some to suggest that more than 1% of the US child population have some kind of ASD.2 ASD, typically apparent before age 3 years, includes infantile autism, Asperger syndrome, and pervasive development disorders (atypical autism).3 Many children with ASD show feeding symptoms from infancy.3
While there is a strong genetic component in ASD4, 5 (siblings to individuals with ASD are at a highly increased risk of ASD themselves),6 no single mutation has been identified that can explain more than a small percentage of all disorders, and emerging research has linked various determinants including an array of toxicant exposures with subsequent ASD.7, 8 The fact that not only pregnancy-related and neonatal adverse events9 but also intrauterine exposure to certain teratogenic drugs may increase the risk of ASD10 strongly suggest that ASD begins early in life. Of note, we have recently demonstrated a link between parental autoimmunity and ASD.11
Celiac disease (CD) is an immune-mediated disorder that occurs in 1–2% of the Western population.12 It is triggered by gluten exposure in genetically susceptible individuals.12 Individuals with CD usually have small intestinal villous atrophy (VA) and inflammation, but in the past years it has become evident that some individuals with CD or CD-related disorders have only minor, if any, mucosal changes.12 The vast majority of CD individuals are serologically positive, especially for endomysium and tissue transglutaminase antibodies but also for the less CD-specific antigliadin antibodies.13 CD is associated with substantial comorbidity, including neurological and psychiatric disorders.14
Several case reports suggest a positive association between CD and ASD,15, 16 but more systematic research findings have been contradictory and widely challenged,17–20 with most studies indicating no association between the diseases.18–20 Still, the negative studies were underpowered to demonstrate a positive relation between CD and ASD.18–20 Furthermore, although two studies18, 20 detected individuals with ASD and positive CD serology, none of the studies was designed to examine the association between ASD and CD without VA.
The purpose of this study was to examine the association between ASD and CD in individuals grouped by their small intestinal histopathology at biopsy: I) VA (equal to CD), II) inflammation without VA, or III) normal mucosa but positive CD serology.
Methods
Autistic spectrum disorders (ASD)
ASD was defined as having a relevant ICD-9 or ICD-10 code (international classification of disease code in the Swedish Patient Register (Appendix)). The Swedish Patient Register started in 1964, becoming nationwide in 1987. Since 2001, it also includes hospital-based outpatient care. In Sweden, physicians examine all 4-year-old children as part of the national child health surveillance system. When an ASD is suspected at this mandatory screening, or earlier by parents or in infant care, the child is referred to a child psychiatrist specialist unit. The validity of the registry-based diagnosis is high. Two of the present authors (AR and CMH) recently conducted a medical record review by ascertainment of a random sample of cases (n=88) with ASD that appear in the Patient Registry and implemented a validation protocol developed by the Centers for Disease Control and Prevention (CDC).21 In 94.3% (83/88) could we substantiate the presence of DSM-IV autism according to medical records reviews, identical to findings from Denmark.22
Celiac disease, inflammation, and normal mucosa
We searched computerized biopsy registers at Sweden’s 28 pathology departments and obtained data on personal identity number,23 date of biopsy, and morphology (see Appendix for a list of histopathology codes according to the Swedish SnoMed system) in all individuals undergoing a duodenal or jejunal biopsy. CD was defined as having VA (equivalent to Marsh histopathology stage 3, see Supplementary Table 1).24 We also collected data on individuals with a lesser degree of mucosal damage, such as inflammation without VA (equivalent to Marsh 1–2)24 and normal mucosa (Marsh 0).24 In total, we identified 287,586 unique individuals who had undergone a biopsy (29,148 with VA, 13,446 with inflammation, and 244,992 with normal mucosa)(Figure 1).25 Data on normal biopsies performed at 10 university hospitals (n=121,952) were then sent for matching with CD serology at the 8 biochemistry departments responsible for the same catchment area as the university hospitals. We were thus able to identify 3,736 individuals with normal mucosa but positive IgA/IgG gliadin, endomysium, or tissue transglutaminase antibodies at time of biopsy (≤180 days before biopsy until ≤30 days after biopsy).26 A detailed description of the data collection procedure has been published elsewhere.25, 26 We then excluded individuals with data irregularities (Figure 1), and those undergoing a biopsy (or entering the study) before 1 January 1987, since only then could a patient be diagnosed with ASD according to our definitions. In all, this study included 26,995 individuals with CD, 12,304 individuals with inflammation but no VA, and 3,719 individuals with normal mucosa but positive CD serology. (Of the latter 3,719 individuals, 359 had positive IgA endomysium or IgA tissue transglutaminase while 3,360 had positive IgA gliadin or positive IgG antibodies against endomysium, transglutaminase, or gliadin. See appendix for detailed information)
Figure 1. Flowchart of study participants.
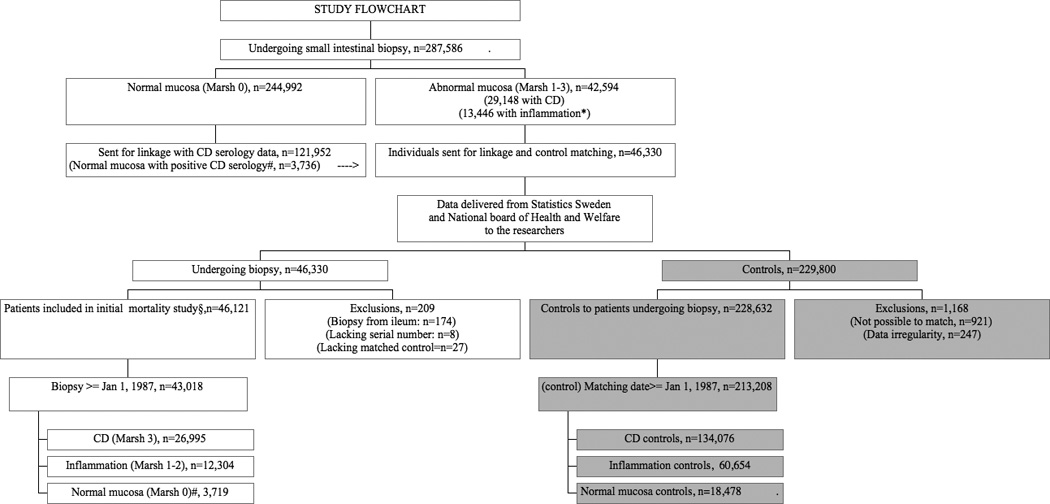
CD, Coeliac disease
* Marsh pathology grade 1–2.
# Marsh pathology grade 0 in individuals with positive CD serology (antigliadin, antiendomysial or anti-transglutaminase antibodies).
We did not require a positive CD serology for the diagnosis of CD, and data on positive CD serology were only collected in a sub-sample of individuals with CD (n=3,388 with positive serology) and inflammation (n=141). However, a patient chart validation found that 88% of individuals with available data on CD serology were positive at the time of biopsy.25 This same evaluation found that the positive predictive value of VA for CD was 95% (n=108/114).25
Controls
For each patient undergoing biopsy, the government agency Statistics Sweden (SCB, responsible for producing official statistics), identified up to five controls from the total population register, matched for sex, county, age, and calendar year. Controls were only selected from among individuals without a previous record of small intestinal biopsy. Finally, for arguments given above, we only kept controls entering the study in 1987 or later. In all, this study was based on 213,208 controls (CD controls: n=134,076, inflammation: n=60,654, and normal mucosa but positive CD serology: n=18,478). We did not have data on CD serology status in controls.
Analyses
In this paper we used two statistical approaches to examine the relationship between CD and ASD.
Case control study
In our main analyses we calculated odds ratios (ORs) using logistic regression conditional on sex, age, calendar year, and county (analyses performed stratum-wise). In the analyses we examined the proportion of individuals with a prior diagnosis of ASD among those undergoing biopsy. The calculated ORs equal the risk of future CD, inflammation or normal mucosa with positive CD serology in individuals with a diagnosis of ASD.
In separate analyses we restricted ASD to individuals I) diagnosed before the age of 10 years, II) with an inpatient record of ASD, III) with an outpatient record of ASD, IV) with at least two records of ASD, out of which at least one occurred before small intestinal biopsy (and corresponding date in matched controls), V) with ASD according to a narrower definition corresponding to “infantile autism” (ICD 9: 299A and ICD-10: F840), and VI) when only looking at study participants born in 1987 or later since only these individuals were at risk of having a recorded diagnosis of ASD throughout their entire life (ICD-9 began in 1987).
In individuals with CD and their controls we also estimated the association with ASD according to age, sex, and calendar period of CD diagnosis. We calculated the risk of having a biopsy with CD according to time since first diagnosis with ASD (first year, beyond first years). To determine whether the association between ASD and CD was influenced by country of birth (Nordic vs. not Nordic) or education (four predefined categories) we added these two variables as covariates in one analysis. If the individual had no education, we used the highest available parental education. We also adjusted for intrauterine growth retardation in individuals with available birth data from the Swedish Medical Birth Register (individuals with CD: n=11,647; controls: n=56,440) because this exposure is associated with an increased risk of both ASD9 and CD27.
Cohort study
To further examine the temporal relationship between CD and ASD we used Cox regression to estimate hazard ratios (HRs) for ASD in the future in individuals undergoing small intestinal biopsy. Also in the Cox regression did we compare each celiac individual only with his/her matched controls (that is the analysis was performed within each stratum thereby eliminating the influence of sex, age, calendar year, and county) and our HR was the result of all these stratum-specific HRs. We restricted our analyses to individuals without a previous record of ASD and those who were biopsied in 1987 or later (when ICD-9 was introduced and ASD could be diagnosed). Furthermore, we excluded individuals who belonged to strata where earlier exclusion meant that there were either no index cases (those with biopsy) or controls. Hence, the cohort analyses were based on 26,981 individuals with CD, 12,299 with inflammation but not VA, and 3,713 with normal mucosa but positive CD serology. These three groups were compared with 213,055 matched controls.
We used SPSS version 20 to analyze all the data (SPSS Inc., Chicago, Il, USA). P-values <0.05 (two-tailed) were considered statistically significant.
Ethics
The current study was approved by the Ethics review board in Stockholm, Sweden (2006/633-31/4; approved June 14th, 2006). The board did not require individual informed consent because data were strictly register-based.
Results
Background data
About half of the study participants underwent biopsy in 2000 or later (47.9% of CD individuals had been diagnosed since 2000). The majority were females (Supplementary Table 2). Some 40% of individuals with CD were diagnosed in childhood. Controls were matched on sex, age, and calendar period; thus, their distribution was identical to that of the individuals undergoing biopsy (Supplementary Tables 2 and 3).
Conditional logistic regression: prior ASD
Having a prior diagnosis of ASD was not associated with CD (OR=0.93; 95% CI=0.51–1.68) or inflammation (OR=1.03; 95% CI=0.40–2.64) but with a highly increased risk of having a normal mucosa but positive CD serology (OR=4.57; 95% CI=1.58–13.22) (Table 1). The number of individuals with ASD is given in Supplementary Table 2. Six individuals with normal mucosa but positive CD serology had an earlier diagnosis of ASD. These individuals were positive for different antibodies (IgA Gliadin: n=3; IgG Gliadin: n=2; and IgA Endomysium: n=1) Adjusting for intrauterine growth retardation did not influence the risk estimate for CD (adjusted OR=1.04; 95% CI=0.56–1.95); nor did adjustment for education or country of birth (data not shown).
Table 1.
Prior autistic spectrum disorders (ASD) and small intestinal histopathology (subanalyses).
| Subgroup | Any ASD | ASD diagnosed <10 years of age |
Inpatient diagnosis with ASD b |
Outpatient diagnosis with ASD c |
At least two records of ASDd |
Narrow definition of ASD e |
ASD – study participants born ≥1 Jan 1987 |
|---|---|---|---|---|---|---|---|
| Celiac disease | 0.93; 0.51–1.68 | 1.06; 0.48–2.31 | 0.90; 0.36–2.27 | 0.91; 0.46–1.80 | 1.15; 0.60–2.21 | 0.79; 0.32–1.98 | 1.07; 0.53–2.13 |
| Inflammation | 1.03; 0.40–2.64 | 1.50; 0.46–13.64 | 0.93; 0.28–3.11 | 1.14; 0.34–3.87 | 1.52; 0.58–4.02 | 1.44; 0.42–4.98 | 1.24; 0.27–5.68 |
| Normal mucosa but positive CD serology a | 4.57; 1.58–13.22 | 5.59; 1.27–24.56 | 5.00; 1.25–19.99 | 2.82; 0.72–11.03 | 3.14; 0.94–10.51 | 4.72; 1.25–17.86 | 7.05; 1.80–27.53f |
ASD, Autistic spectrum disorder, see text for definition
Includes positive IgA/IgG antigliadin, endomysium, and transglutaminase antibodies ≤180 days before biopsy and ≤30 days after biopsy.
The patient had at least one diagnosis of ASD as an inpatient. This need not be his/her first diagnosis of autism.
The patient had at least one diagnosis of ASD as an outpatient. This need not be his/her first diagnosis of autism.
At least one diagnosis of autism had to be recorded before biopsy.
See text for definition.
3/785 with normal mucosa but positive CD serology vs. 2/3,925 controls had an earlier diagnosis of ASD.
Sensitivity analyses showed similar ORs to the above (Table 1), with the exception of a slightly lower OR for normal mucosa and positive CD serology in individuals with at least two records of ASD (OR=3.14; 95% CI=0.94–10.51) and a slightly higher OR in the same patient group when we restricted study participants to those born in 1987 or later (OR=7.05; 95% CI=1.80–27.53) (Table 1).
No individual with CD who was diagnosed from 40 years of age or later had a record of earlier ASD. With this exception, and our finding of a non-significantly lower OR for CD in females with ASD, there were no differences in ORs based on age, sex, or calendar period (Supplementary Table 4).
In a posthoc analysis we estimated the OR for earlier ASD in patients with villous atrophy (here classified as CD) who also had positive CD serology. We found no association between serologically positive villous atrophy and earlier ASD (OR=1.47; 95%CI=0.52–4.15). None of the 141 individuals with inflammation and positive CD serology had an earlier diagnosis of ASD and hence no risk estimate could be calculated for that association.
In a second posthoc analysis we calculated the OR for earlier ASD in individuals who either had villous atrophy (here classified as CD) or positive IgA tissue transglutaminase/endomysium at time of biopsy. This combined group was not associated with earlier ASD (OR=1.21; 95%CI=0.74–1.97).
Cohort study: future ASD
Individuals with CD and inflammation were between 1.5 and 2 -fold increased risk of having a later diagnosis of ASD (Table 2), whereas the highest HRs for ASD were seen in individuals with normal mucosa but positive CD serology (Table 2).
Table 2.
Subanalyses: Small intestinal biopsy and risk of later autistic spectrum disorders (ASD) (overall and according to time since biopsy)
| Subgroup | ASD: Observed vs. expected* |
Overall*, HR with 95% CI |
First year | Beyond first year |
|---|---|---|---|---|
| Celiac disease | 113 vs. 81 | 1.39; 1.13–1.71 | 1.44; 0.48–4.28 | 1.39; 1.12–1.71 |
| Inflammation | 25 vs. 12 | 2.01; 1.29–3.13 | 3.20; 0.84–12.18 | 1.91; 1.19–3.06 |
| Normal mucosa but positive celiac serology | 26 vs. 8 | 3.09; 1.99–4.80 | 7.05; 1.31–37.87 | 2.95; 1.87–4.65 |
Main analysis. Hazard ratios estimated through Cox Regression.
ASD, Autistic spectrum disorder, see text for definition
Comment
This study found no association between CD and ASD before diagnosis of CD and only a weak relation thereafter. In contrast, we found a strong association between having normal mucosa but positive CD serology and ASD both before and after biopsy.
Comparison with earlier literature
To our knowledge, the first large case series with autistic individuals undergoing investigation for CD was reported in 1973.28 In that study 18 children with ASD were examined, of which 7 had a history of gastrointestinal symptoms.28 Three children underwent small intestinal biopsy, but none of these children had VA.28 In a second study eight autistic children with steatorrhea and alleged behavioral improvements on gluten restriction underwent biopsy but neither in this group did the researchers identify any patient with CD.29
Two larger studies have investigated the association between CD and ASD. In the first Pavone et al evaluated 120 CD individuals from Catania, Italy.18 Parents were asked to answer 16 DSM-IIIR questions relating to their child's behavior.18 The researchers concluded that the prevalence of ASD was not elevated in CD. The same researchers tested 11/22 children with infantile autism in the same hospital for antigliadin and endomysium antibodies. Although two children were serologically positive, both had normal small intestinal mucosa. In a second study Batista et al examined 211 individuals with biopsy-proven CD for ASD.20 Two of these 211 children had an ASD, resulting in a prevalence of ASD of 0.95% (95% CI=0.11–3.82%). Of 147 individuals with diagnosed ASD, 6 had positive antigliadin or transglutaminase antibodies, but all were negative for the more CD-specific endomysium antibodies. In contrast, Barcia et al reported that 5/150 individuals with ASD had both serological and histopathological findings of CD (p=0.014).17
Our data are consistent with earlier research in that we found no convincing evidence that CD is associated with ASD,18–20 except for a small excess risk noted after CD diagnosis. A possible explanation for the excess risk of ASD after CD diagnosis is surveillance bias. We found a strong association (OR>l4) between positive serology (with normal mucosa) and later ASD. These individuals may suffer from non-celiac gluten sensitivity,12 in which a gluten-free diet can be beneficial.30 Markers of gluten sensitivity has been linked also to other neurological31 or psychiatric32 disorders such as schizophrenia.33, 34 Interestingly many antibody-positive patients are negative for HLA DQ2/835 suggesting that the response to gliadin in psychiatric and neurological disease33 may be typical of non-celiac gluten sensitivity rather than CD. Sensitivity-related illnesses are increasing,36 and effects from gluten may be mediated by a number of mechanisms.
Part of the positive association between positive serology and ASD could also be due to an increased likelihood of serological testing for CD in children with ASD but not altogether since with increased serological testing more individuals with true CD should also have been diagnosed. The association between serologically positive CD and earlier ASD was neutral although this analysis was limited in power.
The role of gluten and a gluten-free diet in individuals with ASD is under debate. One study reported that a gluten-free diet in 15 children with ASD had no effect; however, after the trial, parents of 9 of the children wanted to continue with the diet because they thought their autistic children had improved.37, 38 A Cochrane review found only small effect of gluten- and casein-free diets on ASD,39 whereas a later randomized single-blind study reported that dietary intervention with a gluten- and casein-free diet had a beneficial effect in some children with ASD.40
The current paper has some limitations, including the fact that we did not have data on symptoms in individuals with ASD. If these individuals have more gastrointestinal symptoms because of other disorders than CD, that could result in surveillance bias. Awareness of the potential effect of dietary interventions in ASD may also have led to more testing for CD. However, as stated previously, this should have resulted in a higher OR for all three pathology groups and not just for having a positive serology with normal mucosa.
At the same time, impaired communication in autistic individuals may lead to difficulties in communicating gastrointestinal symptoms, but could also make physicians hesitant to perform a biopsy before CD diagnosis in individuals with ASD. A major strength of our paper is therefore our use of several patient groups in which the high risk for positive serology in those with normal mucosa shows that the lack of association with CD is unlikely to be due to physicians refusing to perform small intestinal biopsies.
We used nationwide registers to ascertain ASD. Although the prevalence of ASD in our study is lower than in smaller data sets in which individuals are screened for ASD, our prevalence of ASD (either before or after study entry) in our reference population (controls) was nevertheless 2.8/1000 (606 divided by 213,208), which is consistent with American register-based data on ASD prevalence (0.6–4.6/1000).41
Another shortcoming is that we lacked data on gluten-free diet. A gluten-free diet usually results in negative CD serology and a lower probability of undergoing small intestinal biopsy. Hence, if a large number of autistic individuals were on a gluten-free diet already when being investigated for CD that could underestimate the association with CD. However, the strong positive association between ASD and positive serology makes such an explanation unlikely. Antibody levels will usually decrease before the mucosa heals in persons on a gluten-free diet.42
At the same time, we cannot rule out that the moderate excess risk for ASD seen in individuals with diagnosed CD (HR=1.49) may be restricted to CD individuals with poor compliance, although we believe surveillance bias is a more likely explanation. In a validation of a random subset of individuals with CD 17% had evidence of poor dietary adherence.25
Another limitation is that we did not have access to data on family history and could not adjust for parental autoimmunity. We have previously shown that parental autoimmunity is a risk factor for ASD. However, different patterns of parental autoimmunity are unlikely to explain the highly increased risk for positive serology and normal mucosa, as well as a largely neutral risk for CD. Finally we did not have data on serology in all individuals with CD and inflammation.
Apart from the different comparison groups, the main strength of our paper is its statistical power. This study included more cases of individuals with both CD and ASD than all previous papers taken together (12 before CD diagnosis and 127 after, n=139). The large power allowed us to calculate narrow confidence intervals and to test the association with positive CD serology in various sensitivity analyses. We also had data on a number of potential confounders, including country of birth, education, and, in a subset of individuals, intrauterine growth retardation. Adjustment for these variables had little effect on the risk estimates.
Other advantages include the high sensitivity and specificity of VA as proof of diagnosed CD. More than 95% of Swedish gastroenterologist and pediatricians perform a biopsy before celiac diagnosis,25 and Swedish pathologists classify 90% of all slides with VA correctly.25
Another strength is our long follow-up. This study was based on data on ASD recorded in the Swedish National Patient Register over a period of more than 20 years.
Potential mechanisms
Given that ASD is linked to early brain development, the reason for the positive association with positive CD serology is not self-evident. Because most individuals with positive CD serology are likely to have the same HLA as CD individuals with VA (where little association with ASD was seen), shared genetics is unlikely to explain our findings. Instead, we speculate that the positive association between ASD and positive celiac serology observed in this study may be due to increased mucosal permeability noted in some individuals with early CD43, 44 or individuals with elevated levels of more non-specific antigliadin antibodies.45
A recent Italian study found a significantly higher intestinal permeability in patients with ASD and their first-degree relatives compared to healthy controls.46 However, this study also showed that patients with ASD who avoided gluten and casein had lower intestinal permeability compared to those consuming gluten and casein.46 A high intestinal permeability may allow an increased absorption of short peptides that trigger the immune system leading to ASD. We cannot, however, explain the different pattern in individuals with CD vs. those with normal mucosa but positive CD serology. Unfortunately we had no information on antibodies to other food antigens that could perhaps shed light on whether this is a broad sensitization.
In conclusion, we found weak evidence of a link between ASD and CD but a strong association between ASD and positive CD serology in individuals with normal mucosa.
Supplementary Material
Acknowledgments
Acknowledgement and Grant Support (Funding)
JFL: Örebro University Hospital, Karolinska Institutet, the Swedish Society of Medicine, the Swedish Research Council – Medicine (522-2A09-195), the Swedish Celiac Society, the Fulbright commission.
JAM: The National Institutes of Health – DK071003 and DK057892.
CH: The Swedish Research Council (2011-4659)
JAM: Grant support: Alba Therapeutics (>$50,000); Advisory board: Alvine Pharmaceuticals, Inc. (<$10,000), Nexpep (<$10,000), Consultant (none above 10,000 USD): Ironwood, Inc., Flamentera, Actogenix, Ferring Research Institute inc., Bayer Healthcare Pharmaceuticals, Vysera Biomedical, 2G Pharma, Inc, ImmunosanT, Inc and Shire US Inc.
AR: Speaker Honoraria: Astra Zeneca
Abbreviations used in this article
- CD
Celiac disease
- CI
Confidence interval
- HR
Hazard ratio
- OR
Odds ratio
- VA
Villous atrophy
Footnotes
Previous presentation: None
Details of ethics approval: This project (2006/633-31/4) was approved by the Research Ethics Committee of the Karolinska Institutet, Sweden on June 14th, 2006.
Conflict of Interest
(The other authors have no conflict of interest to declare).
Statements
JFL had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. JFL performed the statistical analyses.
None of the funding organization(s) or sponsor(s) had any role in the "design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Author contributions:
ICMJE criteria for authorship read and met: JFL, JAM, CH.
Agree with the manuscript’s results and conclusions: JFL, JAM, CH
Designed the experiments/the study: JFL, JAM, CH.
Collected data: JFL
Analyzed the data: JFL
Wrote the first draft of the paper: JFL.
Contributed to study design, interpretation of data and writing: JAM, CH.
Interpretation of data; approved the final version of the manuscript: JFL, JAM, CH
Responsible for data integrity: JFL.
Obtained funding: JFL.
References
- 1.Wing L, Yeates SR, Brierley LM, Gould J. The prevalence of early childhood autism: comparison of administrative and epidemiological studies. Psychol Med. 1976 Feb;6(1):89–100. doi: 10.1017/s0033291700007522. [DOI] [PubMed] [Google Scholar]
- 2.Prevalence of autism spectrum disorders - autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012 Mar 30;61(3):1–19. [PubMed] [Google Scholar]
- 3.Emond A, Emmett P, Steer C, Golding J. Feeding symptoms, dietary patterns, and growth in young children with autism spectrum disorders. Pediatrics. 2010 Aug;126(2):e337–e342. doi: 10.1542/peds.2009-2391. [DOI] [PubMed] [Google Scholar]
- 4.Geschwind DH. Advances in autism. Annu Rev Med. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtenstein P, Carlstrom E, Rastam M, Gillberg C, Anckarsater H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. 2010 Nov;167(11):1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- 6.Jorde LB, Hasstedt SJ, Ritvo ER, Mason-Brothers A, Freeman BJ, Pingree C, McMahon WM, Petersen B, Jenson WR, Mo A. Complex segregation analysis of autism. Am J Hum Genet. 1991 Nov;49(5):932–938. [PMC free article] [PubMed] [Google Scholar]
- 7.McCanlies EC, Fekedulegn D, Mnatsakanova A, Burchfiel CM, Sanderson WT, Charles LE, Hertz-Picciotto I. Parental occupational exposures and autism spectrum disorder. J Autism Dev Disord. 2012 Nov;42(11):2323–2334. doi: 10.1007/s10803-012-1468-1. [DOI] [PubMed] [Google Scholar]
- 8.Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the san francisco bay area. Environ Health Perspect. 2006 Sep;114(9):1438–1444. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchmayer S, Johansson S, Johansson A, Hultman CM, Sparen P, Cnattingius S. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics. 2009 Nov;124(5):e817–e825. doi: 10.1542/peds.2008-3582. [DOI] [PubMed] [Google Scholar]
- 10.Dufour-Rainfray D, Vourc'h P, Tourlet S, Guilloteau D, Chalon S, Andres CR. Fetal exposure to teratogens: evidence of genes involved in autism. Neurosci Biobehav Rev. 2011 Apr;35(5):1254–1265. doi: 10.1016/j.neubiorev.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Keil A, Daniels JL, Forssen U, Hultman C, Cnattingius S, Soderberg KC, Feychting M, Sparen P. Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiology. 2010 Nov;21(6):805–808. doi: 10.1097/EDE.0b013e3181f26e3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, Hadjivassiliou M, Kaukinen K, Kelly CP, Leonard JN, Lundin KE, Murray JA, Sanders DS, Walker MM, Zingone F, Ciacci C. The Oslo definitions for coeliac disease and related terms. Gut. 2013 Jan;62(1):43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill ID. What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations? Gastroenterology. 2005 Apr;128(4) Suppl 1:S25–S32. doi: 10.1053/j.gastro.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Lionetti E, Francavilla R, Pavone P, Pavone L, Francavilla T, Pulvirenti A, Giugno R, Ruggieri M. The neurology of coeliac disease in childhood: what is the evidence? A systematic review and meta-analysis. Dev Med Child Neurol. 2010 Aug;52(8):700–707. doi: 10.1111/j.1469-8749.2010.03647.x. [DOI] [PubMed] [Google Scholar]
- 15.Dohan FC. Coeliac disease and schizophrenia. Lancet. 1970 Apr 25;1(7652):897–898. doi: 10.1016/s0140-6736(70)91729-0. [DOI] [PubMed] [Google Scholar]
- 16.Genuis SJ, Bouchard TP. Celiac disease presenting as autism. J Child Neurol. 2010 Jan;25(1):114–119. doi: 10.1177/0883073809336127. [DOI] [PubMed] [Google Scholar]
- 17.Barcia G, Posar A, Santucci M, Parmeggiani A. Autism and coeliac disease. J Autism Dev Disord. 2008 Feb;38(2):407–408. doi: 10.1007/s10803-007-0480-3. [DOI] [PubMed] [Google Scholar]
- 18.Pavone L, Fiumara A, Bottaro G, Mazzone D, Coleman M. Autism and celiac disease: failure to validate the hypothesis that a link might exist. Biol Psychiatry. 1997 Jul 1;42(1):72–75. doi: 10.1016/S0006-3223(97)00267-9. [DOI] [PubMed] [Google Scholar]
- 19.Black C, Kaye JA, Jick H. Relation of childhood gastrointestinal disorders to autism: nested case-control study using data from the UK General Practice Research Database. BMJ. 2002 Aug 24;325(7361):419–421. doi: 10.1136/bmj.325.7361.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batista IC, Gandolfi L, Nobrega YK, Almeida RC, Almeida LM, Campos Junior D, Pratesi R. Autism spectrum disorder and celiac disease: no evidence for a link. Arq Neuropsiquiatr. 2012 Jan;70(1):28–33. doi: 10.1590/s0004-282x2012000100007. [DOI] [PubMed] [Google Scholar]
- 21.Bertrand J, Mars A, Boyle C, Bove F, Yeargin-Allsopp M, Decoufle P. Prevalence of autism in a United States population: the Brick Township, New Jersey, investigation. Pediatrics. 2001 Nov;108(5):1155–1161. doi: 10.1542/peds.108.5.1155. [DOI] [PubMed] [Google Scholar]
- 22.Lauritsen MB, Jorgensen M, Madsen KM, Lemcke S, Toft S, Grove J, Schendel DE, Thorsen P. Validity of childhood autism in the Danish Psychiatric Central Register: findings from a cohort sample born 1990–1999. J Autism Dev Disord. 2010 Feb;40(2):139–148. doi: 10.1007/s10803-009-0818-0. [DOI] [PubMed] [Google Scholar]
- 23.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue') Gastroenterology. 1992;102(1):330–354. [PubMed] [Google Scholar]
- 25.Ludvigsson JF, Brandt L, Montgomery SM, Granath F, Ekbom A. Validation study of villous atrophy and small intestinal inflammation in Swedish biopsy registers. BMC Gastroenterol. 2009 Mar 11;9(1):19. doi: 10.1186/1471-230X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludvigsson JF, Brandt L, Montgomery SM. Symptoms and signs in individuals with serology positive for celiac disease but normal mucosa. BMC Gastroenterol. 2009;9:57. doi: 10.1186/1471-230X-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marild K, Stephansson O, Montgomery S, Murray JA, Ludvigsson JF. Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology. 2012 Jan;142(1):39–45 e33. doi: 10.1053/j.gastro.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker-Smith J. Gastrointestinal disease and autism-the result of a survey. Paper presented at: Symposium on Autism. 1973 Sydney. [Google Scholar]
- 29.McCarthy DM, Coleman M. Response of intestinal mucosa to gluten challenge in autistic subjects. Lancet. 1979 Oct 27;2(8148):877–878. doi: 10.1016/s0140-6736(79)92688-6. [DOI] [PubMed] [Google Scholar]
- 30.Biesiekierski JR, Newnham ED, Irving PM, Barrett JS, Haines M, Doecke JD, Shepherd SJ, Muir JG, Gibson PR. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011 Mar;106(3):508–514. doi: 10.1038/ajg.2010.487. quiz 515. [DOI] [PubMed] [Google Scholar]
- 31.Bushara KO. Neurologic presentation of celiac disease. Gastroenterology. 2005 Apr;128(4) Suppl 1:S92–S97. doi: 10.1053/j.gastro.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Ruggieri M, Incorpora G, Polizzi A, Parano E, Spina M, Pavone P. Low prevalence of neurologic and psychiatric manifestations in children with gluten sensitivity. J Pediatr. 2008 Feb;152(2):244–249. doi: 10.1016/j.jpeds.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 33.Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, Yang S, Krivogorsky B, Alaedini A, Yolken R. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry. 2010 Jul 1;68(1):100–104. doi: 10.1016/j.biopsych.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Cascella NG, Kryszak D, Bhatti B, Gregory P, Kelly DL, Mc Evoy JP, Fasano A, Eaton WW. Prevalence of celiac disease and gluten sensitivity in the United States clinical antipsychotic trials of intervention effectiveness study population. Schizophr Bull. 2011 Jan;37(1):94–100. doi: 10.1093/schbul/sbp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundin KE, Alaedini A. Non-celiac gluten sensitivity. Gastrointest Endosc Clin N Am. 2012 Oct;22(4):723–734. doi: 10.1016/j.giec.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Genuis SJ. Sensitivity-related illness: the escalating pandemic of allergy, food intolerance and chemical sensitivity. Sci Total Environ. 2010 Nov 15;408(24):6047–6061. doi: 10.1016/j.scitotenv.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 37.Elder JH, Shankar M, Shuster J, Theriaque D, Burns S, Sherrill L. The gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trial. J Autism Dev Disord. 2006 Apr;36(3):413–420. doi: 10.1007/s10803-006-0079-0. [DOI] [PubMed] [Google Scholar]
- 38.Buie T, Campbell DB, Fuchs GJ, 3rd, Furuta GT, Levy J, Vandewater J, Whitaker AH, Atkins D, Bauman ML, Beaudet AL, Carr EG, Gershon MD, Hyman SL, Jirapinyo P, Jyonouchi H, Kooros K, Kushak R, Levitt P, Levy SE, Lewis JD, Murray KF, Natowicz MR, Sabra A, Wershil BK, Weston SC, Zeltzer L, Winter H. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010 Jan;125(Suppl 1):S1–S18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- 39.Millward C, Ferriter M, Calver S, Connell-Jones G. Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database Syst Rev. 2008;(2) doi: 10.1002/14651858.CD003498.pub3. CD003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiteley P, Haracopos D, Knivsberg AM, Reichelt KL, Parlar S, Jacobsen J, Seim A, Pedersen L, Schondel M, Shattock P. The ScanBrit randomised, controlled, single-blind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutr Neurosci. 2010 Apr;13(2):87–100. doi: 10.1179/147683010X12611460763922. [DOI] [PubMed] [Google Scholar]
- 41.Mandell DS, Palmer R. Differences among states in the identification of autistic spectrum disorders. Arch Pediatr Adolesc Med. 2005 Mar;159(3):266–269. doi: 10.1001/archpedi.159.3.266. [DOI] [PubMed] [Google Scholar]
- 42.Hopper AD, Hadjivassiliou M, Hurlstone DP, Lobo AJ, McAlindon ME, Egner W, Wild G, Sanders DS. What is the role of serologic testing in celiac disease? A prospective, biopsy-confirmed study with economic analysis. Clin Gastroenterol Hepatol. 2008 Mar;6(3):314–320. doi: 10.1016/j.cgh.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Monsuur AJ, Bakker PI, Alizadeh BZ, Zhernakova A, Bevova MR, Strengman E, Franke L, Slot RV, Belzen MJ, Lavrijsen IC, Diosdado B, Daly MJ, Mulder CJ, Mearin ML, Meijer JW, Meijer GA, Oort E, Wapenaar MC, Koeleman BP, Wijmenga C. Myosin IXB variant increases the risk of celiac disease and points toward a primary intestinal barrier defect. Nat Genet. 2005 Dec;37(12):1341–1344. doi: 10.1038/ng1680. [DOI] [PubMed] [Google Scholar]
- 44.Wapenaar MC, Monsuur AJ, van Bodegraven AA, Weersma RK, Bevova MR, Linskens RK, Howdle P, Holmes G, Mulder CJ, Dijkstra G, van Heel DA, Wijmenga C. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut. 2008 Apr;57(4):463–467. doi: 10.1136/gut.2007.133132. [DOI] [PubMed] [Google Scholar]
- 45.Troncone R, Starita A, Coletta S, Mayer M, Greco L. Antigliadin antibody, D-xylose, and cellobiose/mannitol permeability tests as indicators of mucosal damage in children with coeliac disease. Scand J Gastroenterol. 1992 Aug;27(8):703–706. doi: 10.3109/00365529209000144. [DOI] [PubMed] [Google Scholar]
- 46.de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, Carteni M, De Rosa M, Francavilla R, Riegler G, Militerni R, Bravaccio C. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010 Oct;51(4):418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


