Abstract
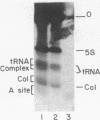
Initiation complexes formed by E. coli ribosomes in the presence of 32P-labeled A protein initiator region from R17 bacteriophage Rna have been treated with colicin E3 and disassembled by exposure to 1% sodium dodecyl sulfate. Electrophoresis on 9% polyacrylamide gels reveals a dissociable complex containing the 30-nucleotide-long messenger fragment and the 50-nucleotide-long colicin fragment, which arises from the 3' terminus of the 16S RNA. The complex is a pure RNA-RNA hybird; it is apparently maintained by a seven-base complementarity between the two RNA fragments. Detection of this mRNA-rRNA complex strongly supports the hypothesis that during the initiation step of protein biosynthesis the 3' end of 16S RNA base pairs with the polypurine stretch common to initiator regions in E. coli and bacteriophage mRNAs. The implications of our findings with respect to the molecular mechanism of initiation site selection and mRNA binding to ribosomes, the role of rRNA in ribosome function, and species specificity in translation are explored.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrand J. R., Hindley J. Nucleotide sequence of a ribosome binding site on RNA synthesized in vitro from coliphage T7. Nat New Biol. 1973 Jul 4;244(131):10–13. doi: 10.1038/newbio244010a0. [DOI] [PubMed] [Google Scholar]
- Barrell B. G., Weith H. L., Donelson J. E., Robertson H. D. Sequence analysis of the ribosome-protected bacteriophase phiX174 DNA fragment containing the gene G initiation site. J Mol Biol. 1975 Mar 5;92(3):377–393. doi: 10.1016/0022-2836(75)90287-9. [DOI] [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Bollen A., Heimark R. L., Cozzone A., Traut R. R., Hershey J. W. Cross-linking of initiation factor IF-2 to Escherichia coli 30 S ribosomal proteins with dimethylsuberimidate. J Biol Chem. 1975 Jun 10;250(11):4310–4314. [PubMed] [Google Scholar]
- Bowman C. M., Dahlberg J. E., Ikemura T., Konisky J., Nomura M. Specific inactivation of 16S ribosomal RNA induced by colicin E3 in vivo. Proc Natl Acad Sci U S A. 1971 May;68(5):964–968. doi: 10.1073/pnas.68.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. The origin of the genetic code. J Mol Biol. 1968 Dec;38(3):367–379. doi: 10.1016/0022-2836(68)90392-6. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Dahlberg J. E. Binding of ribosomal protein S1 of Escherichia coli to the 3' end of 16S rRNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2940–2944. doi: 10.1073/pnas.72.8.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg A. E., Dingman C. W., Peacock A. C. Electrophoretic characterization of bacterial polyribosomes in agarose-acrylamide composite gels. J Mol Biol. 1969 Apr 14;41(1):139–147. doi: 10.1016/0022-2836(69)90131-4. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Lund E., Kjeldgaard N. O., Bowman C. M., Nomura M. Colicin E3 induced cleavage of 16S ribosomal ribonucleic acid; blocking effects of certain antibiotics. Biochemistry. 1973 Feb 27;12(5):948–950. doi: 10.1021/bi00729a025. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E. Two forms of the 30 S ribosomal subunit of Escherichia coli. J Biol Chem. 1974 Dec 10;249(23):7673–7678. [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Ebel J. P. Sequence analysis of the 3'-T1 oligonucleotide of 16S ribosomal RNA from Escherichia coli. FEBS Lett. 1974 Dec 1;49(1):47–48. doi: 10.1016/0014-5793(74)80628-9. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Mackie G. A., Zimmermann R. A., Ebel J. P., Fellner P. Primary sequence of the 16S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1975 Feb;2(2):265–278. doi: 10.1093/nar/2.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann V. A., Sprinzl M., Pongs O. The involvement of 5S RNA in the binding of tRNA to ribosomes. Biochem Biophys Res Commun. 1973 Oct 1;54(3):942–948. doi: 10.1016/0006-291x(73)90785-7. [DOI] [PubMed] [Google Scholar]
- Grollman A. P., Stewart M. L. Inhibition of the attachment of messenger ribonucleic acid to ribosomes. Proc Natl Acad Sci U S A. 1968 Oct;61(2):719–725. doi: 10.1073/pnas.61.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. L., Chen J., Schaefer L., Lengyel P., Weissman S. M. Nucleotide sequence of a ribosome attachment site of bacteriophage f2 RNA. Biochem Biophys Res Commun. 1970 Jun 5;39(5):883–888. doi: 10.1016/0006-291x(70)90406-7. [DOI] [PubMed] [Google Scholar]
- Haselkorn R., Rothman-Denes L. B. Protein synthesis. Annu Rev Biochem. 1973;42:397–438. doi: 10.1146/annurev.bi.42.070173.002145. [DOI] [PubMed] [Google Scholar]
- Hawley D. A., Slobin L. I., Wahba A. J. The mechanism of action of initiation factor 3 in protein synthesis. II. Association of the 30S ribosomal protein S12 with IF-3. Biochem Biophys Res Commun. 1974 Nov 27;61(2):544–550. doi: 10.1016/0006-291x(74)90991-7. [DOI] [PubMed] [Google Scholar]
- Held W. A., Gette W. R., Nomura M. Role of 16S ribosomal ribonucleic acid and the 30S ribosomal protein S12 in the initiation of natural messenger ribonucleic acid translation. Biochemistry. 1974 May 7;13(10):2115–2122. doi: 10.1021/bi00707a019. [DOI] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Change in methylation of 16S ribosomal RNA associated with mutation to kasugamycin resistance in Escherichia coli. Nat New Biol. 1971 Sep 1;233(35):12–14. doi: 10.1038/newbio233012a0. [DOI] [PubMed] [Google Scholar]
- Hindley J., Staples D. H. Sequence of a ribosome binding site in bacteriophage Q-beta-RNA. Nature. 1969 Dec 6;224(5223):964–967. doi: 10.1038/224964a0. [DOI] [PubMed] [Google Scholar]
- Jakes K., Zinder N. D., Boon T. Purification and properties of colicin E3 immunity protein. J Biol Chem. 1974 Jan 25;249(2):438–444. [PubMed] [Google Scholar]
- Kenner R. A. A protein-nucleic acid crosslink in 30S ribosomes. Biochem Biophys Res Commun. 1973 Apr 16;51(4):932–938. doi: 10.1016/0006-291x(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Rosenberg M., Steitz J. A. Nucleotide sequences of the 5' and 3' termini of bacteriophage T7 early messenger RNAs synthesized in vivo: evidence for sequence specificity in RNA processing. J Mol Biol. 1974 Nov 15;89(4):767–776. doi: 10.1016/0022-2836(74)90051-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Robertson H. D. Regulation of in vitro translation of bacteriophage f2 RNA. Cold Spring Harb Symp Quant Biol. 1969;34:655–673. doi: 10.1101/sqb.1969.034.01.076. [DOI] [PubMed] [Google Scholar]
- Maizels N. E. coli lactose operon ribosome binding site. Nature. 1974 Jun 14;249(458):647–649. doi: 10.1038/249647b0. [DOI] [PubMed] [Google Scholar]
- Musso R. E., de Crombrugghe B., Pastan I., Sklar J., Yot P., Weissman S. The 5'-terminal nucleotide sequence of galactose messenger ribonucleic acid of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4940–4944. doi: 10.1073/pnas.71.12.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F., Herr W. Nucleotide sequence of the 3' terminus of E. coli 16S ribosomal RNA. Mol Biol Rep. 1974 Dec;1(8):437–439. doi: 10.1007/BF00360668. [DOI] [PubMed] [Google Scholar]
- Pieczenik G., Model P., Robertson H. D. Sequence and symmetry in ribosome binding sites of bacteriophage f1 RNA. J Mol Biol. 1974 Dec 5;90(2):191–124. doi: 10.1016/0022-2836(74)90368-4. [DOI] [PubMed] [Google Scholar]
- Platt T., Yanofsky C. An intercistronic region and ribosome-binding site in bacterial messenger RNA. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2399–2403. doi: 10.1073/pnas.72.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel M., Greenshpan H. Specificity in the binding of Escherichia coli ribosomes to natural messenger RNA. Eur J Biochem. 1970 Sep;16(1):117–122. doi: 10.1111/j.1432-1033.1970.tb01061.x. [DOI] [PubMed] [Google Scholar]
- Richter D., Erdmann V. A., Sprinzl M. Specific recognition of GTpsiC loop (loop IV) of tRNA by 50S ribosomal subunits from E. coli. Nat New Biol. 1973 Dec 5;246(153):132–135. doi: 10.1038/newbio246132a0. [DOI] [PubMed] [Google Scholar]
- Santer U. V., Santer M. The sequence of the 3'-OH end of the 16 S RNA of Escherichia coli. FEBS Lett. 1972 Apr 1;21(3):311–314. doi: 10.1016/0014-5793(72)80191-1. [DOI] [PubMed] [Google Scholar]
- Senior B. W., Holland I. B. Effect of colicin E3 upon the 30S ribosomal subunit of Escherichia coli. Proc Natl Acad Sci U S A. 1971 May;68(5):959–963. doi: 10.1073/pnas.68.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Identical 3'-terminal octanucleotide sequence in 18S ribosomal ribonucleic acid from different eukaryotes. A proposed role for this sequence in the recognition of terminator codons. Biochem J. 1974 Sep;141(3):609–615. doi: 10.1042/bj1410609a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague K. U., Steitz J. A. The 3' terminal oligonucleotide of E. coli 16S ribosomal RNA: the sequence in both wild-type and RNase iii- cells is complementary to the polypurine tracts common to mRNA initiator regions. Nucleic Acids Res. 1975 Jun;2(6):787–798. doi: 10.1093/nar/2.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley W. M., Jr, Salas M., Wahba A. J., Ochoa S. Translation of the genetic message: factors involved in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jul;56(1):290–295. doi: 10.1073/pnas.56.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples D. H., Hindley J., Billeter M. A., Weissmann C. Localization of Q-beta maturation cistron ribosome binding site. Nat New Biol. 1971 Sep 15;234(50):202–204. doi: 10.1038/newbio234202a0. [DOI] [PubMed] [Google Scholar]
- Staples D. H., Hindley J. Ribosome binding site of Q-beta RNA polymerase cistron. Nat New Biol. 1971 Sep 15;234(50):211–212. doi: 10.1038/newbio234211a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Discriminatory ribosome rebinding of isolated regions of protein synthesis initiation from the ribonucleic acid of bacteriophage R17. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2605–2609. doi: 10.1073/pnas.70.9.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A. Oligonucleotide sequence of replicase initiation site in Q RNA. Nat New Biol. 1972 Mar 22;236(64):71–75. doi: 10.1038/newbio236071a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature. 1969 Dec 6;224(5223):957–964. doi: 10.1038/224957a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Specific recognition of non-initiator regions in RNA bacteriophage messengers by ribosomes of Bacillus stearothermophilus. J Mol Biol. 1973 Jan;73(1):1–16. doi: 10.1016/0022-2836(73)90155-1. [DOI] [PubMed] [Google Scholar]
- Szer W., Leffler S. Interaction of Escherichia coli 30S ribosomal subunits with MS2 phage RNA in the absence of initiation factors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3611–3615. doi: 10.1073/pnas.71.9.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- van Duin J., van Knippenberg P. H. Functional heterogeneity of the 30 S ribosomal subunit of Escherichia coli. 3. Requirement of protein S1 for translation. J Mol Biol. 1974 Mar 25;84(1):185–195. doi: 10.1016/0022-2836(74)90221-6. [DOI] [PubMed] [Google Scholar]