Abstract
Autism spectrum disorders are associated with social and emotional deficits, the aetiology of which are not well understood. A growing consensus is that the autonomic nervous system serves a key role in emotional processes, by providing physiological signals essential to subjective states. We hypothesized that altered autonomic processing is related to the socio-emotional deficits in autism spectrum disorders. Here, we investigated the relationship between non-specific skin conductance response, an objective index of sympathetic neural activity, and brain fluctuations during rest in high-functioning adults with autism spectrum disorder relative to neurotypical controls. Compared with control participants, individuals with autism spectrum disorder showed less skin conductance responses overall. They also showed weaker correlations between skin conductance responses and frontal brain regions, including the anterior cingulate and anterior insular cortices. Additionally, skin conductance responses were found to have less contribution to default mode network connectivity in individuals with autism spectrum disorders relative to controls. These results suggest that autonomic processing is altered in autism spectrum disorders, which may be related to the abnormal socio-emotional behaviours that characterize this condition.
Keywords: autism, autonomic nervous system, emotion, skin conductance, resting state
Introduction
Autism spectrum disorder (ASD) manifests early in development, and is characterized by deficits in social interaction and communication, as well as stereotyped and repetitive behaviours, and restricted interests in domains of activities (American Psychiatric Association, 2013). Individuals with ASD also exhibit difficulties in emotional processing, particularly in self-awareness of feelings (Hill et al., 2004; Silani et al., 2008) and in the interpretation of feelings of others (Hobson et al., 1988; Baron-Cohen, 1991; Bal et al., 2010). Despite the extensive investigation into the aetiology of ASD, the psychophysiological correlates of the socio-emotional deficits that characterize the disorder are not yet clear. Further exploration of the physiological and neural substrates of social and emotional processes is critical for better understanding, diagnosis, and treatment of ASD.
The autonomic nervous system regulates the physiological events associated with emotional experiences, including changes in heart rate, respiration, pupil dilation and sweating (Craig, 2002; Barrett et al., 2007). James (1884) and Lange (1885) proposed that these physiological changes are essential precursors for the subjective feeling of emotions. This idea has since been supported and extended by studies demonstrating that different emotional stimuli generate distinct autonomic activities, which are interpreted by the brain as different emotional experiences (Ekman, 1983; Rainville et al., 2006; Harrison et al., 2010). False physiological feedback affects emotional attributions (Valins, 1966; Liebhart, 1977), and emotional interpretation of ambiguous stimuli (Truax, 1983; Gray et al., 2007). As social situations are often ambiguous and require rapid interpretation of emotional cues, autonomic nervous system signals play a critical role in socio-emotional processing.
The initial processing of autonomic signals takes place in the reticular formation, brainstem and thalamic nuclei, and the hypothalamus, whereas higher-order processing of these signals takes place mainly in the somatosensory cortex, supplementary motor area, anterior insular cortex, and anterior cingulate cortex (Boucsein, 1992; Damasio et al., 2000; Craig, 2002; Porges, 2003; Critchley, 2005). Specifically, the anterior insular cortex, through bidirectional neural connections with the thalamus, amygdala, nucleus accumbens, anterior cingulate cortex and orbitofrontal cortex, is suggested to have a critical role in the integration of bottom-up interoceptive and exteroceptive signals with top-down predictions and evaluations of emotional states (Craig, 2002, 2003; Gray et al., 2007; Harrison et al., 2010; Critchley et al., 2011; Seth et al., 2011; Gu et al., 2013). The anterior insular cortex and anterior cingulate cortex are involved in socio-emotional processing (Damasio et al., 2000; Adolphs, 2002; Phillips et al., 2003; Frith and Frith, 2007; Gu et al., 2012, 2013) in addition to their role in autonomic nervous system regulation, further supporting the important relationship between socio-emotional processing and autonomic activity. Abnormal autonomic nervous system activity may, therefore, be a potential source of the socio-emotional deficits that characterize ASD.
Physiological studies have demonstrated abnormal autonomic nervous system activity in ASD. Abnormal autonomic activity related to external stimuli has been found in individuals with ASD, particularly when those stimuli were of a social or emotional nature (Hirstein et al., 2001; Kylliainen and Hietanen, 2006; Vaughan Van Hecke et al., 2009). Abnormalities in basal autonomic activity have been observed in ASD as well, including reduced baseline cardiac parasympathetic activity (Ming et al., 2005), lower amplitude of respiratory sinus arrhythmia (Bal et al., 2010), and larger baseline pupil dilation (Anderson and Colombo, 2009; Anderson et al., 2013) when compared with matched neurotypical control subjects. Gastrointestinal symptoms have also been found to be significantly more prevalent in ASD than in neurotypical samples (Horvath et al., 1999; Molloy and Manning-Courtney, 2003; White, 2003). These findings of increased sympathetic and decreased parasympathetic activity suggest an imbalance between these two systems in ASD.
Autonomic activity has also been linked to functional connectivity patterns in the resting brain (Birn et al., 2008; Iacovella and Hasson, 2011; Fan et al., 2012; Chang et al., 2013). In a recent study we showed significant contributions of skin conductance response (SCR) signal, an objective and sensitive marker of sympathetic neural activity (Vetrugno et al., 2003), to the connectivity strength of the default mode network (DMN) in a healthy cohort (Fan et al., 2012). Previous investigations of resting-state functional connectivity in ASD have demonstrated weaker connectivity of the DMN compared with neurotypical control subjects (Kennedy and Courchesne, 2008b; Monk et al., 2009; Assaf et al., 2010; Weng et al., 2010). It is possible that these findings of weaker DMN connectivity in ASD may be explained, at least in part, by differences in autonomic nervous system activity between the groups.
We hypothesized that ASD is associated with abnormal autonomic and correlated brain activities. This may stem from alterations in the central generation of autonomic response, the peripheral conductance of autonomic nervous system signals, and/or the central representations of physiological changes. To test our hypothesis, we recorded skin conductance and simultaneously measured brain activity during rest using functional MRI from high-functioning adults with ASD, as well as from demographically matched neurotypical control subjects. We predicted that the rate of non-specific (not task-evoked) SCR would differ between the groups, and that there would be differences in brain activity and connectivity associated with SCR, such that (i) SCR would be associated with subcortical and cortical brain regions that process and modulate autonomic activity to a lesser extent in ASD compared with neurotypical controls; and (ii) differences in DMN connectivity strength between ASD and neurotypical controls could be explained, at least partially, by differences in autonomic nervous system activity.
Materials and methods
Participants
Seventeen high-functioning adults with autism (n = 12) or Asperger’s syndrome (n = 5) (ASD group) and 15 matched neurotypical control participants were evaluated at the Seaver Autism Centre for Research and Treatment, Icahn School of Medicine at Mount Sinai (Table 1 for demographic data). All participants underwent a diagnostic evaluation consisting of psychiatric, medical, and developmental assessments, as well as IQ measurement using the Wechsler Adult Intelligence Scale (WAIS-III) (Wechsler, 1997). Diagnoses of autism or Asperger’s syndrome were determined by psychiatric interview according to the Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DSM-IV-TR), and confirmed by the Autism Diagnostic Observation Schedule-Generic (ADOS-G) (Lord et al., 2000), as well as the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994). Exclusion criteria included epilepsy, history of schizophrenia, schizoaffective disorder or other Axis I mental disorders, except for obsessive-compulsive disorder (given the phenotypic overlap with ASD), and use of depot neuroleptic medication or other psychoactive drugs within the 5 weeks prior to participation. Participants who had a lifetime history of substance/alcohol dependence and/or abuse within the last year were also excluded. For the healthy control group, participants were excluded based on medical illness or history in first-degree relatives of developmental disorders, learning disabilities, autism, affective disorders, and anxiety disorders. All participants provided written informed consent, approved by the Icahn School of Medicine at Mount Sinai Institutional Review Board.
Table 1.
Demographic data (means ± SD) of ASD and neurotypical control groups
| Subject characteristics | ASD | NC | t | P |
|---|---|---|---|---|
| (n = 17) | (n = 15) | |||
| Age (years) | 26.1 ± 6.5 | 27.1 ± 8.2 | 0.37 | 0.72 |
| Handedness score | 60.0 ± 53.40 | 87.3 ± 11.6 | 2.06 | 0.06 |
| Years of educationa | 14.90 ± 2.3 | 16.2 ± 1.8 | 1.7 | 0.10 |
| Parents’ socio-economic status | 88.35 ± 18.73 | 90.73 ± 23.19 | 0.32 | 0.75 |
| Full-scale IQ | 110.3 ± 18.6 | 112.6 ± 12.5 | 0.42 | 0.68 |
| ASD diagnosis (autism/Asperger’s) | 12/5 | |||
| ADI-Rb | ||||
| Social | 18.3 ± 8.0 | |||
| Verbal communication | 16.0 ± 4.8 | |||
| Repetitive behaviour | 5.9 ± 3.0 | |||
| Development | 3.0 ± 1.6 | |||
| ADOS-G | ||||
| Communication | 3.1 ± 1.5 | |||
| Social | 7.3 ± 2.6 | |||
| Imagination | 0.7 ± 0.5 | |||
| Stereotyped behaviours | 1.4 ± 1.4 |
aYears of education data was not available for five participants, therefore ASD: n = 14, neurotypical controls (NC): n = 13.
bADI-R scores were not available for one participant, therefore n = 16 for this measure.
Data acquisition
Skin conductance response acquisition
SCR was acquired according to the procedure described by Fan et al. (2012). Briefly, SCR was recorded using the GSR100C amplifier (BIOPAC Systems), together with the base module MP150 and the AcqKnowledge software (version 3.9.1.6). The GSR100C measures skin conductance by applying a constant voltage of 0.5 V between two electrodes that are attached to the skin. This allows for the measurements of both skin conductance level and SCR, which vary with sweat gland activity due to stress, arousal or emotional excitement. Skin conductance (measured in μS) was recorded using a 2000-Hz sampling rate (gain = 2 μS/V, both high-pass filters = DC, low-pass filter = 10 Hz). After cleaning the skin with alcohol swabs, two EL507 disposable EDA (isotonic gel) electrodes were placed on the palmar surface of the distal phalanges of the big and second toes of left foot. The electrode leads were shielded and the signal was low-pass filtered (using the MRI-Compatible MRI CBL/FILTER System MECMRI-TRANS) to reduce radiofrequency interference from the scanner. BIOPAC recording was synchronized to the E-Prime (Psychology Software Tools) program through the parallel port of the computers to enable precise time alignment of skin conductance recording with scan onsets.
Image acquisition
All brain images were obtained using a 3 T Siemens Allegra MRI system at the Icahn School of Medicine at Mount Sinai, and were acquired parallel to the anterior-posterior commissures axis (AC-PC). Foam padding was used to reduce head motion. Whole-brain anatomical T2-weighted images were acquired in high-resolution using a turbo spin-echo plus sequence: 40 axial slices of 4 mm thickness; skip = 0 mm; repetition time = 4050 ms; echo time = 99 ms; flip angle = 170°; field of view = 240 mm; matrix size = 448 × 512; voxel size = 0.47 × 0.47 × 4 mm. After the anatomical image acquisition, one run of T2*-weighted images was obtained during rest, corresponding to the T2-weighted images localization, using a 6 min gradient echoplanar imaging sequence for resting-state functional MRI: 40 axial slices, 4-mm thick; skip = 0 mm; repetition time = 2500 ms; echo time = 27 ms; flip angle = 82°; field of view = 240 mm; matrix size = 64 × 64; in-plane resolution = 3.75 × 3.75 mm. The resting-state functional MRI run started with two dummy volumes before the onset of the fixation to allow for equilibration of T1 saturation effects, followed by 144 image volumes. Refer to the Supplementary material for a detailed procedure of the eyes-open resting-state scan.
Data analysis
Skin conductance analysis
Skin conductance level was calculated as the average of all data points on the skin conductance waveform for each of the participants. A t-test was conducted between the skin conductance level values of the groups to examine possible differences in basal skin conductance levels. AcqKnowledge software (version 4.2) was used in order to identify and count SCRs (Supplementary material). The number of valid SCR events was determined for each participant, and a t-test was then conducted to examine possible group differences in the number of SCRs.
Regression analysis
To correlate SCR with brain fluctuations, the skin conductance waveform was down-sampled by averaging the data points in each 2.5-s bin matching the repetition time (2.5 s) of the echoplanar imaging scan of functional image acquisition. Because relaxation causes skin conductance level to decrease slowly in a linear fashion, the SCR waveform was detrended and band-pass filtered with the same frequency range (0.01–0.08 Hz) that is used in a typical resting-state functional MRI analysis. The similarity between the SCR and haemodynamic response function curves (Bach et al., 2010a, b) allows for using SCR as a regressor in the model directly without transformations, using the same filtering band for both SCR and blood oxygenation level-dependent signals (0.01–0.08 Hz) (Fan et al., 2012). SCR was then entered as a regressor in a general linear model (Friston et al., 1995) using statistical parametric mapping package (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK). Numbers of SCR were not equated between the groups to avoid specification error (Supplementary material). The echoplanar imaging scans were realigned to the first volume, timing corrected, coregistered to the T2 image, normalized to a standard template (MNI, Montreal Neurological Institute), resampled to a 2 × 2 × 2 mm voxel size, and spatially smoothed with an 8 mm full-width at half-maximum Gaussian kernel. The general linear model was then conducted with the SCR time series as a predictor of the observed blood oxygen level-dependent signals. Low-frequency drifts in signal were removed using a standard high-pass filter with a 128 s cut-off. Serial correlation was estimated using an autoregressive AR(1) model. To remove non-neural noise from the data, ventricle and white-matter signals were extracted using corresponding masks and were entered as covariates. In addition, the six parameters generated during motion correction were also entered as covariates. Head motions (for all participants) did not exceed 2.5 mm of displacement or 2.5° of rotation in any direction. Finally, mean voxel value was used for global calculation and grand mean scaling, applied with global normalization to further remove non-specific noise (Van Dijk et al., 2010). Detailed justifications for using the global mean correction can be found in the Supplementary material.
The contrast images from the participants in each group were entered into a second-level random effect group analysis. The resultant voxel-wise statistical maps were thresholded for significance using a cluster-size algorithm that protects against an inflation of the false-positive rate of multiple comparisons; an uncorrected P-value of 0.05 for the height (intensity) threshold of each activated voxel and extent threshold of k = 120 was used based on Monte Carlo simulation (Slotnick and Schacter, 2004). Assuming an individual voxel type I error of P < 0.05, a cluster extent of 120 contiguous resampled voxels (2 × 2 × 2 mm) was indicated as necessary to correct for multiple voxel comparisons at P < 0.05. Statistical results were mapped onto the standardized surface of the cerebral cortex.
Functional connectivity analysis of the default mode network
To investigate the relationship between non-specific SCR and brain connectivity during rest, a functional connectivity analysis was conducted using a seed region within the posterior cingulate cortex as in previous studies (Koshino et al., 2005). Specifically, time-series volumes of functional MRI scan images were preprocessed for each participant using the Data Processing Assistant for Resting-State fMRI (DPARSF) toolbox (Yan and Zang, 2010). This included slice timing correction, realignment, coregistration, normalization and spatial smoothing (using an 8 mm full-width at half-maximum Gaussian kernel) as in the regression analysis. In addition, de-trending (to remove the systematic drift) and temporal filtering (band-pass, 0.01–0.08 Hz, to reduce the effect of low-frequency drift and high-frequency physiological signal or noise) were applied. Time course of the posterior cingulate cortex (left and right combined) was then extracted using the automated anatomical labelling template (Tzourio-Mazoyer et al., 2002), and a voxel-wise linear correlation between the mean time course of the posterior cingulate cortex and the time course of each voxel in the whole brain was calculated using the resting-state functional MRI data analysis toolkit (Song et al., 2011). The six head motion parameters, global mean signal, white matter signal, and CSF signal were included as covariates. A two-sample t-test was then conducted to examine between-group connectivity differences.
To test the effects of SCR on brain connectivity, changes in functional connectivity of the posterior cingulate cortex before and after regressing out SCR effects were examined and compared between the groups in an ANOVA model. SCR signals were regressed out as covariates in a second voxel-wise connectivity analysis, followed by transformations of the correlation coefficients using Fisher’s r-to-z’ transformations. Posterior cingulate cortex time course was also extracted after regressing out the SCR signal. In addition, to examine SCR contributions to the posterior cingulate cortex functional connectivity within each group, a paired t-test was conducted for each group before versus after regressing out SCR signals for the posterior cingulate cortex functional connectivity maps. Posterior cingulate cortex connectivity analysis is reported here because it allows comparisons with previous studies investigating DMN connectivity in ASD that used a posterior cingulate cortex seed (Monk et al., 2009; Weng et al., 2010), and because the posterior cingulate cortex has a more focal anatomical definition than alternative seed regions. However, the ventromedial prefrontal cortex, also commonly used as a DMN seed, is potentially more relevant to ASD abnormalities, emotional processing and autonomic pathways. We, therefore, conducted an additional functional connectivity analysis for the time course of the ventromedial prefrontal cortex, using the coordinates (−1, 47, −4) reported by Fox et al. (2005), and group differences were examined. To further examine possible differences in the connectivity of areas that are related to emotional and autonomic signal processing, we also tested the functional connectivity of the anterior insular cortex (Supplementary material). It is important to note that there were no significant differences in head motion between the two groups, as was indicated by the root mean squares of both overall head motion displacement and rotation and the temporal derivatives (Supplementary material).
Functional connectivity analysis of the whole brain
To examine whether SCR contributes to the whole brain connectivity, a voxel-wise whole brain functional connectivity strength analysis was performed both before and after regressing out the SCR signal. Images were preprocessed using DPARSF, similar to the posterior cingulate cortex functional connectivity analysis, with two exceptions. First, the resolution of resultant echoplanar imaging was 3 × 3 × 3 mm after normalization to reduce computational load. Second, to reduce artificial local correlations between voxels introduced by smoothing, a 4 mm full-width at half-maximum Gaussian kernel was used (instead of 8 mm as in the posterior cingulate cortex connectivity analysis). For each voxel, functional connectivity strength was measured as the summed weights of all connections linking this voxel and every other voxel. Pearson correlation coefficients for the time series of every possible pair of voxels were calculated to obtain the whole brain correlation matrix for each participant. The calculation was constrained within a customized mask (n voxels = 48 159) including all voxels with grey matter tissue probability >20% on the averaged grey matter map of all participants. The functional connectivity strength (FCS) was computed for each voxel as follows:
where rij is the correlation coefficient of voxel i and voxel j, zij is the normalized rij value using Fisher’s r-to-z transformation, and 0.3 is the threshold set to eliminate the potential contributions of weak connections arising from noise. To evaluate the reproducibility of our results, additional functional connectivity strength maps were calculated using both 0.2 and 0.4 correlation thresholds; these computations led to no major changes in our primary results. The connectivity map was then standardized by converting to Z scores so that maps across participants could be averaged and compared. The Z score transformation is:
where  are mean and SD of the functional connectivity strength across all the voxels in the whole-brain map.
are mean and SD of the functional connectivity strength across all the voxels in the whole-brain map.
Notably, in graph theory, the functional connectivity strength is referred to as ‘degree centrality’ or ‘degree’ of weighted networks, and voxels with high functional connectivity strength usually play important roles in information transformation (Buckner et al., 2009; Cole et al., 2010; Zuo et al., 2012; Liang et al., 2013; Wang et al., 2013). To examine the SCR contribution to whole brain connectivity, functional connectivity strength maps before and after regressing out the SCR signals were compared within each group using paired t-tests. Between-group differences of SCR signal contribution to whole brain functional connectivity (i.e. functional connectivity strength maps before versus after regressing out SCR signals) were examined in an ANOVA model. All statistical maps were corrected for multiple comparisons to a significance level of P < 0.05 by combining the individual voxel P-value < 0.05 with cluster size >67 voxels, based on Monte Carlo simulation (Ledberg et al., 1998).
Relationship between autism symptom severity, skin conductance and imaging data
Correlations between the ADI-R subscale scores of each of the ASD participants and number of SCRs and overall skin conductance level were examined. In addition, correlations between the individual ADI-R subscale scores and SCR-brain association, posterior cingulate cortex connectivity, ventromedial prefrontal cortex connectivity, and whole brain connectivity maps were examined using regression analyses. The ADI-R four subscales include ratings of social interaction (subscale A), communication (subscale B), repetitive and restricted behaviour (subscale C), and early development (subscale D). Higher scores on each scale indicate higher symptom severity in each of the specific domains. Note that these exploratory correlation analyses were not corrected for multiple comparisons.
Modelling central generation and representation of skin conductance response
The central generation and representation (feedback) of SCR were modelled by shifting the SCR vector in relation to the haemodynamic response. Two models were created: a generation model in which brain activation preceded the SCR signal by one repetition time (2.5 s), and a representation model in which SCR preceded brain activation by one repetition time (Critchley et al., 2000; Patterson et al., 2002) (see Supplementary material for details of the modelling).
Results
Electrodermal activity
A t-test revealed no significant difference in skin conductance level between ASD (15.96 ± 1.83 µS) and neurotypical controls [17.27 ± 1.76 µS, t(30) = 0.51, P > 0.05, two-tailed] (Fig. 1A). However, during the scan period, the ASD group had significantly less non-specific SCRs (4.76 ± 1.34) compared with the neurotypical control group [12.2 ± 3.13, t(30) = 2.19, P < 0.05, two-tailed] (Fig. 1B).
Figure 1.
(A) Skin conductance level (SCL) and (B) number of skin conductance responses (SCR) during the entire rest session (6 min). NC = neurotypical controls.
Skin conductance response and resting state brain activity
In the neurotypical control group, there were significant positive correlations between SCR and the anterior insular cortex, dorsal anterior cingulate cortex, supplementary motor area, medial prefrontal cortex, thalamus, superior parietal lobule, calcarine cortex, cuneus, and basal ganglia, as well as negative correlations with the precentral gyrus, superior parietal lobule, posterior cingulate cortex, precuneus, and inferior parietal lobule (Table 2 and Fig. 2A). In the ASD group, SCR was positively correlated with the lingual gyrus, calcarine cortex, superior and inferior parietal lobule, precentral and postcentral gyri, middle temporal gyrus, and inferior frontal gyrus, and was negatively correlated with the anterior cingulate cortex, supplementary motor area, medial prefrontal cortex, basal ganglia, inferior parietal and temporal gyri, posterior midcingulate cortex (see Vogt, 2005 for definition), and the thalamus (Table 3 and Fig. 2B). A between-group comparison similarly revealed that medial frontal brain regions were generally more correlated with SCR in the neurotypical control group, whereas posterior and sensory regions were generally more correlated with SCR in the ASD group (Table 4 and Fig. 2C and D).
Table 2.
Positive and negative correlations between SCR and brain activation in neurotypical controls
| Region | L/R | BA | x | y | z | T | Z | P | k |
|---|---|---|---|---|---|---|---|---|---|
| Positive | |||||||||
| Mid frontal gyrus | R | 9 | 36 | 46 | 32 | 4.73 | 3.59 | 0.000 | 12334 |
| Superior parietal lobule | R | 7 | 30 | −40 | 40 | 4.66 | 3.56 | 0.000 | |
| Superior frontal gyrus | R | 6 | 22 | 6 | 62 | 3.97 | 3.20 | 0.001 | |
| Thalamus | L | −12 | −4 | 0 | 3.93 | 3.17 | 0.001 | ||
| Caudate nucleus | R | 12 | 14 | 18 | 3.87 | 3.14 | 0.001 | ||
| Thalamus | R | 14 | −8 | 12 | 3.82 | 3.11 | 0.001 | ||
| Caudate nucleus | L | −14 | 12 | 10 | 3.77 | 3.08 | 0.001 | ||
| Lateral globus pallidus | R | 24 | −18 | −2 | 3.76 | 3.08 | 0.001 | ||
| Mid frontal gyrus | R | 10 | 38 | 50 | 24 | 3.76 | 3.08 | 0.001 | |
| Caudate nucleus | L | −16 | 18 | 4 | 3.74 | 3.06 | 0.001 | ||
| Anterior cingulate cortex | R | 32 | 16 | 20 | 36 | 3.74 | 3.06 | 0.001 | |
| Anterior cingulate cortex | L | 32 | −16 | 10 | 46 | 3.65 | 3.01 | 0.001 | |
| Anterior cingulate cortex | R | 32 | 6 | 26 | 38 | 3.61 | 2.99 | 0.001 | |
| Anterior insular cortex | L | −34 | 26 | 8 | 3.59 | 2.97 | 0.001 | ||
| Anterior insular cortex | R | 32 | 18 | 4 | 3.53 | 2.93 | 0.002 | ||
| Caudate nucleus | R | 14 | 18 | 0 | 3.49 | 2.91 | 0.002 | ||
| Mid frontal gyrus | R | 6 | 42 | 4 | 32 | 3.35 | 2.83 | 0.002 | |
| Mid frontal gyrus | R | 9 | 48 | 22 | 26 | 3.35 | 2.82 | 0.002 | |
| Orbitofrontal cortex | R | 11 | 26 | 48 | −12 | 3.05 | 2.63 | 0.004 | |
| Anterior insular cortex | R | 32 | 26 | 6 | 2.97 | 2.57 | 0.005 | ||
| Mid frontal gyrus | L | 10 | −24 | 32 | 18 | 2.91 | 2.53 | 0.006 | |
| Inferior frontal gyrus | R | 44/45 | 58 | 22 | 4 | 2.88 | 2.51 | 0.006 | |
| Putamen | R | 26 | 18 | −6 | 2.87 | 2.50 | 0.006 | ||
| Superior frontal gyrus | L | 8 | −4 | 24 | 44 | 2.81 | 2.46 | 0.007 | |
| Precentral gyrus | R | 6 | 44 | 0 | 42 | 2.76 | 2.42 | 0.008 | |
| Mid temporal gyrus | R | 21/22 | 52 | −22 | −2 | 2.70 | 2.39 | 0.009 | |
| Supplementary motor area | R | 8 | 2 | 22 | 50 | 2.32 | 2.10 | 0.018 | |
| Inferior parietal gyrus | R | 40 | 56 | −40 | 52 | 4.38 | 3.42 | 0.000 | 253 |
| Supramarginal gyrus | R | 40 | 48 | −36 | 42 | 2.66 | 2.36 | 0.009 | |
| Dentate nucleus | L | −26 | −40 | −36 | 3.73 | 3.06 | 0.001 | 509 | |
| Cerebellum crus VI | L | −24 | −64 | −24 | 3.61 | 2.98 | 0.001 | ||
| Superior parietal lobule | L | 7 | −22 | −42 | 42 | 3.67 | 3.02 | 0.001 | 309 |
| Calcarine cortex | L | 17 | −16 | −76 | 8 | 3.13 | 2.68 | 0.004 | 518 |
| Cuneus | L | 18 | −4 | −94 | 10 | 3.12 | 2.67 | 0.004 | |
| Calcarine cortex | L | 17 | −12 | −82 | 12 | 2.77 | 2.43 | 0.008 | |
| Cuneus | R | 18 | 8 | −92 | 16 | 2.64 | 2.34 | 0.010 | |
| Calcarine cortex | R | 17 | 8 | −86 | 10 | 2.61 | 2.32 | 0.010 | |
| Lingual gyrus | L | 19 | −16 | −74 | 0 | 2.40 | 2.16 | 0.015 | |
| Precentral gyrus | L | 6 | −24 | −16 | 62 | 2.34 | 2.12 | 0.017 | |
| Negative | |||||||||
| Precentral gyrus | L | 6 | −2 | −20 | 72 | 3.70 | 3.04 | 0.001 | 329 |
| Precentral gyrus | R | 6 | 6 | −22 | 62 | 2.34 | 2.12 | 0.017 | |
| Superior parietal lobule | L | 7 | −22 | −58 | 58 | 2.76 | 2.42 | 0.008 | 616 |
| Posterior cingulate gyrus | L | 23 | −6 | −46 | 24 | 2.60 | 2.31 | 0.010 | |
| Posterior cingulate gyrus | R | 23 | 4 | −54 | 22 | 2.60 | 2.31 | 0.011 | |
| Precuneus | L | 7 | −8 | −58 | 42 | 2.25 | 2.05 | 0.020 | |
| Posterior central gyrus | R | 31 | 12 | −54 | 38 | 2.19 | 2.00 | 0.023 | |
| Posterior cingulate gyrus | L | 31 | −2 | −54 | 36 | 2.15 | 1.97 | 0.024 | |
| Posterior cingulate gyrus | R | 31 | 4 | −58 | 30 | 2.02 | 1.86 | 0.031 | |
| Inferior parietal lobule | L | 40/22 | −58 | −42 | 24 | 2.74 | 2.41 | 0.008 | 133 |
| Inferior parietal lobule | R | 40 | 64 | −22 | 32 | 2.66 | 2.35 | 0.009 | 161 |
Height threshold: T = 1.76, P < 0.05.
Extent threshold: k = 120.
L = left; R = right; BA = Brodmann area.
Figure 2.
Positive and negative correlations between non-specific SCR and brain fluctuations during rest in (A) neurotypical control subjects, and (B) adults with ASD. Red indicates voxels with positive correlations, whereas blue indicates voxels with negative correlations. (C) Stronger correlations in neurotypical control subjects compared with ASD (neurotypical controls > ASD), and (D) stronger correlations in ASD compared with neurotypical control subjects (ASD > neurotypical controls). These hemispheric surfaces were visualized using BrainNet Viewer (http://www.nitrc.org/projects/bnv/, Xia et al., 2013).
Table 3.
Positive and negative correlations between SCR and brain activation in ASD
| Region | L/R | BA | x | y | z | T | Z | P | k |
|---|---|---|---|---|---|---|---|---|---|
| Positive | |||||||||
| Lingual gyrus | R | 19 | 14 | −58 | −6 | 3.67 | 3.08 | 0.001 | 4196 |
| Superior occipital gyrus | R | 19 | 16 | −82 | 20 | 3.48 | 2.96 | 0.002 | |
| Lingual gyrus | R | 18/19 | 16 | −80 | −4 | 3.44 | 2.93 | 0.002 | |
| Calcarine cortex | L | 17 | −14 | −82 | 2 | 3.28 | 2.82 | 0.002 | |
| Inferior parietal lobule | L | 40 | −48 | −50 | 48 | 3.27 | 2.82 | 0.002 | |
| Cerebellum crus VI | R | 24 | −58 | −26 | 3.21 | 2.78 | 0.003 | ||
| Superior parietal lobule | L | 7 | −30 | −64 | 58 | 3.17 | 2.75 | 0.003 | |
| Fusiform gyrus | L | 19 | −22 | −62 | −10 | 2.95 | 2.60 | 0.005 | |
| Inferior parietal lobule | L | 40 | −32 | −52 | 42 | 2.89 | 2.55 | 0.005 | |
| Fusiform gyrus | R | 19 | 28 | −68 | −10 | 2.61 | 2.35 | 0.009 | |
| Parahippocampal gyrus | L | 28 | −14 | −44 | 2 | 2.52 | 2.28 | 0.011 | |
| Lingual gyrus | R | 37 | 28 | −48 | −6 | 2.42 | 2.20 | 0.014 | |
| Cerebellum crus IV | R | 2 | −50 | −16 | 2.4 | 2.19 | 0.014 | ||
| Calcarine cortex | L | 17/18 | −6 | −66 | 18 | 2.36 | 2.15 | 0.016 | |
| Parahippocampal gyrus | R | 27 | 12 | −38 | −4 | 2.35 | 2.15 | 0.016 | |
| Cerebellum crus VI | L | −2 | −74 | −10 | 2.3 | 2.11 | 0.018 | ||
| Mid temporal gyrus | R | 21 | 58 | −32 | −10 | 3.58 | 3.03 | 0.001 | 222 |
| Precentral gyrus | L | 6 | −36 | −2 | 56 | 3.29 | 2.84 | 0.002 | 127 |
| Postcentral gyrus | L | 2 | −64 | −18 | 32 | 3.19 | 2.76 | 0.003 | 352 |
| Superior temporal gyrus | L | 42 | −52 | −14 | 14 | 2.84 | 2.52 | 0.006 | |
| Mid temporal gyrus | L | 21 | −52 | −46 | −2 | 2.92 | 2.58 | 0.005 | 483 |
| Mid temporal gyrus | L | 21 | −62 | −26 | 2 | 2.39 | 2.18 | 0.015 | |
| Inferior frontal gyrus | L | 47 | −40 | 40 | −8 | 2.54 | 2.29 | 0.011 | 173 |
| Inferior parietal lobule | R | 40 | 38 | −58 | 48 | 2.3 | 2.11 | 0.018 | 121 |
| Negative | |||||||||
| Posterior mid cingulate cortex | L | 31 | −4 | −18 | 42 | 3.33 | 2.86 | 0.002 | 850 |
| Anterior cingulate cortex | L | 24 | −4 | 6 | 38 | 2.6 | 2.34 | 0.010 | |
| Supplementary motor area | L | 6 | −4 | −22 | 58 | 2.21 | 2.04 | 0.021 | |
| Anterior cingulate cortex | R | 32 | 2 | 28 | 30 | 1.95 | 1.83 | 0.034 | |
| Superior frontal gyrus (medial) | R | 10 | 8 | 60 | 6 | 3.19 | 2.76 | 0.003 | 2221 |
| Superior frontal gyrus (medial) | R | 6 | 2 | 12 | 60 | 3.17 | 2.75 | 0.003 | |
| Superior frontal gyrus (medial) | R | 9 | 6 | 62 | 30 | 3.03 | 2.65 | 0.004 | |
| Superior frontal gyrus | R | 8 | 12 | 28 | 60 | 2.88 | 2.54 | 0.005 | |
| Anterior cingulate cortex (pregenual) | L | 24 | −14 | 46 | −4 | 2.57 | 2.32 | 0.010 | |
| Superior frontal gyrus (medial) | R | 8 | 2 | 48 | 44 | 2.51 | 2.27 | 0.012 | |
| Superior temporal gyrus | L | 42 | −48 | −28 | 18 | 3.15 | 2.74 | 0.003 | 279 |
| Inferior parietal lobule | L | 40 | −58 | −40 | 34 | 2.82 | 2.50 | 0.006 | |
| Putamen | R | 16 | 16 | −6 | 3 | 2.63 | 0.004 | 294 | |
| Putamen | R | 24 | −2 | 4 | 2.08 | 1.93 | 0.027 | ||
| Red nucleus | 0 | −22 | 2 | 2.99 | 2.62 | 0.004 | 208 | ||
| Inferior parietal gyrus | R | 40 | 56 | −32 | 32 | 2.78 | 2.48 | 0.007 | 203 |
| Thalamus | R | 8 | −14 | 14 | 2.72 | 2.43 | 0.008 | 311 | |
| Caudate nucleus | L | −10 | −4 | 16 | 2.68 | 2.40 | 0.008 | ||
| Inferior temporal gyrus | R | 37 | 34 | −74 | 6 | 2.55 | 2.30 | 0.011 | 136 |
| Parahippocampal gyrus | L | 36 | −30 | 2 | −30 | 2.47 | 2.24 | 0.013 | 124 |
| Putamen | L | −20 | 6 | −8 | 2.08 | 1.94 | 0.026 | ||
| Cerebellum crus VII | L | −22 | −70 | −40 | 2.39 | 2.18 | 0.015 | 178 | |
| Anterior cingulate cortex (subgenual) | R | 32 | 10 | 30 | −8 | 2.37 | 2.16 | 0.015 | 213 |
Height threshold: T = 1.74, P < 0.05.
Extent threshold: k = 120.
L = left; R = right; BA = Brodmann area.
Table 4.
Two-sample t-test between neurotypical control subjects and ASD for SCR correlations with brain activation
| Region | L/R | BA | x | y | z | T | Z | P | k |
|---|---|---|---|---|---|---|---|---|---|
| Controls > ASD | |||||||||
| Superior temporal gyrus | L | 42 | −48 | −28 | 18 | 3.89 | 3.48 | 0.000 | 202 |
| Supramarginal gyrus | L | 40 | −58 | −42 | 26 | 2.21 | 2.12 | 0.017 | |
| Superior frontal gyrus (medial) | R | 10 | 4 | 60 | 10 | 3.86 | 3.45 | 0.000 | 12511 |
| Supplementary motor area | R | 6 | 4 | 14 | 58 | 3.79 | 3.40 | 0.000 | |
| Thalamus | R | 10 | −10 | 12 | 3.63 | 3.28 | 0.001 | ||
| Red nucleus | L | −2 | −22 | 0 | 3.60 | 3.25 | 0.001 | ||
| Anterior cingulate cortex | L | 24 | −4 | −18 | 42 | 3.46 | 3.15 | 0.001 | |
| Anterior cingulate gyrus (pregenual) | L | 32 | −14 | 46 | −4 | 3.42 | 3.11 | 0.001 | |
| Caudate nucleus | L | −10 | −4 | 16 | 3.08 | 2.85 | 0.002 | ||
| Anterior cingulate gyrus (subgenual) | R | 32 | 10 | 30 | −8 | 3.03 | 2.81 | 0.002 | |
| Superior frontal gyrus (medial) | R | 9 | 4 | 46 | 42 | 3.02 | 2.80 | 0.003 | |
| Putamen | R | 22 | 8 | 2 | 2.91 | 2.71 | 0.003 | ||
| Anterior cingulate cortex | 24 | 0 | 6 | 32 | 2.85 | 2.66 | 0.004 | ||
| Anterior cingulate gyrus (pregenual) | R | 24 | 6 | 38 | 10 | 2.82 | 2.63 | 0.004 | |
| Caudate nucleus | L | −4 | 8 | 6 | 2.80 | 2.62 | 0.004 | ||
| Mid frontal gyrus | R | 10 | 28 | 60 | 20 | 2.79 | 2.61 | 0.005 | |
| Putamen | R | 28 | −8 | 6 | 2.71 | 2.55 | 0.005 | ||
| Anterior cingulate cortex | R | 24 | 4 | 26 | 30 | 2.64 | 2.48 | 0.007 | |
| Anterior insular cortex | R | 28 | 18 | −10 | 2.56 | 2.42 | 0.008 | ||
| Posterior cingulate cortex | L | 31 | −8 | −38 | 46 | 2.55 | 2.41 | 0.008 | |
| Inferior frontal gyrus | R | 45 | 52 | 24 | 8 | 2.53 | 2.39 | 0.008 | |
| Putamen | L | −20 | 6 | −8 | 2.47 | 2.34 | 0.010 | ||
| Supplementary motor area | R | 6 | 14 | 0 | 68 | 2.46 | 2.33 | 0.010 | |
| Posterior cingulate cortex | R | 31 | 16 | −32 | 42 | 2.32 | 2.21 | 0.013 | |
| Superior frontal gyrus | R | 8 | 26 | 32 | 50 | 2.30 | 2.19 | 0.014 | |
| Supplementary motor area | L | 6 | −10 | 8 | 72 | 2.29 | 2.18 | 0.015 | |
| Mid frontal gyrus | R | 46 | 42 | 28 | 42 | 2.27 | 2.16 | 0.015 | |
| Mid frontal gyrus | R | 6 | 36 | 4 | 38 | 3.00 | 2.79 | 0.003 | 679 |
| Precentral gyrus | R | 6 | 44 | −6 | 50 | 2.57 | 2.42 | 0.008 | |
| Cerebellum crus VIII | L | −20 | −68 | −38 | 2.98 | 2.77 | 0.003 | 410 | |
| Mid frontal gyrus | L | 46 | −24 | 46 | 26 | 2.90 | 2.70 | 0.003 | 351 |
| Inferior occipital gyrus | R | 19 | 42 | −78 | 0 | 2.78 | 2.61 | 0.005 | 193 |
| Supramarginal gyrus | R | 40 | 56 | −32 | 30 | 2.71 | 2.54 | 0.006 | 570 |
| Anterior insular cortex | L | −38 | 24 | 4 | 2.45 | 2.33 | 0.010 | 172 | |
| Cerebellum crus IX | L | −4 | −46 | −46 | 2.34 | 2.23 | 0.013 | 202 | |
| Pons | L | −10 | −20 | −44 | 2.28 | 2.17 | 0.015 | ||
| ASD > Controls | |||||||||
| Cuneus | R | 19 | 16 | −82 | 20 | 3.96 | 3.52 | 0.000 | 5113 |
| Lingual gyrus | R | 18 | 14 | −70 | −2 | 3.56 | 3.23 | 0.001 | |
| Superior parietal lobule | L | 7 | −30 | −64 | 58 | 3.41 | 3.11 | 0.001 | |
| Inferior parietal lobule | L | 40 | −46 | −54 | 48 | 3.23 | 2.97 | 0.001 | |
| Cerebellum crus VI | R | 24 | −56 | −24 | 3.19 | 2.93 | 0.002 | ||
| Mid occipital gyrus | L | 19 | −44 | −76 | 14 | 2.91 | 2.71 | 0.003 | |
| Cerebellum crus IV | R | 10 | −50 | −10 | 2.82 | 2.63 | 0.004 | ||
| Lingual gyrus | L | 18 | −8 | −84 | −6 | 2.80 | 2.62 | 0.004 | |
| Calcarine sulcus | L | 17 | −4 | −68 | 20 | 2.54 | 2.40 | 0.008 | |
| Fusiform gyrus | L | 19 | −24 | −62 | −14 | 2.22 | 2.12 | 0.017 | |
| Lingual gyrus | R | 37 | 26 | −44 | −8 | 2.19 | 2.09 | 0.018 | |
| Calcarine cortex | R | 17 | 18 | −64 | 14 | 2.12 | 2.03 | 0.021 | |
| Cerebellum crus VI | L | −12 | −72 | −20 | 2.07 | 1.99 | 0.023 | ||
| Cerebellum crus IV | L | −8 | −42 | −4 | 2.03 | 1.96 | 0.025 | ||
| Postcentral gyrus | L | 2 | −64 | −18 | 32 | 3.94 | 3.51 | 0.000 | 456 |
| Precentral gyrus | L | 6 | −34 | 2 | 56 | 2.89 | 2.69 | 0.004 | 233 |
| Mid temporal gyrus | L | 21 | −60 | −34 | 4 | 2.88 | 2.69 | 0.004 | 487 |
| Inferior frontal gyrus | L | 45 | −42 | 42 | 0 | 2.87 | 2.67 | 0.004 | 234 |
| Mid temporal gyrus | R | 21 | 64 | −40 | −4 | 2.80 | 2.62 | 0.004 | 233 |
| Inferior parietal lobule | R | 39 | 42 | −64 | 44 | 2.35 | 2.24 | 0.013 | 139 |
Height threshold: T = 1.7, P < 0.05.
Extent threshold: k = 120.
L = left; R = right; BA = Brodmann area.
Skin conductance response and resting state functional connectivity
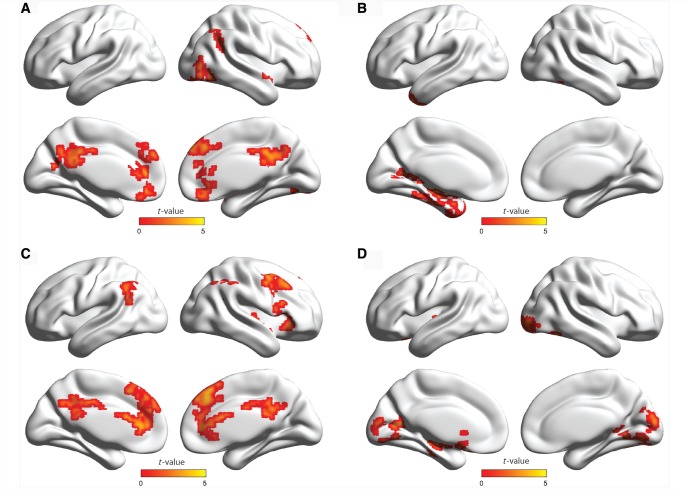
Posterior cingulate cortex seed-based analysis results
Using the posterior cingulate cortex as a seed region, the neurotypical control group had stronger connectivity between the seed and areas of the DMN, compared to the ASD group, mainly with the ventromedial prefrontal cortex and the left inferior parietal lobule (Table 5 and Fig. 3A). Stronger connectivity was found in ASD between the posterior cingulate cortex and the superior and inferior occipital gyri, temporal pole, lingual gyrus, fusiform gyrus, amygdala, hippocampus, posterior insular cortex, supplementary motor area, and precentral and postcentral gyri (Table 5 and Fig. 3B). The general linear model revealed a significant interaction between group (ASD versus neurotypical control subjects) and SCR (before versus after regressing out SCR), wherein SCR had greater positive effects on the connectivity between posterior cingulate cortex and medial prefrontal and orbitofrontal cortices, precentral and postcentral gyri, supramarginal gyrus, and superior and inferior parietal gyri in neurotypical control subjects than in ASD (Table 6 and Fig. 3C). In contrast, SCR had a greater positive impact on the connectivity between the posterior cingulate cortex and the posterior insular cortex, lingual gyrus, amygdala, hippocampus, parahippocampal and fusiform gyri, superior and middle occipital gyri, and superior and inferior temporal gyri in ASD than in neurotypical control subjects (Table 6 and Fig. 3D). For paired t-test results within each group (before > after regressing out SCR signal), see Supplementary Table 1 and Supplementary Fig. 1. For ventromedial prefrontal cortex and anterior insular cortex functional connectivity results, see Supplementary Tables 2 and 3, and Supplementary Figs 2 and 3.
Table 5.
Two sample t-test between neurotypical control subjects and ASD for the functional connectivity of the posterior cingulate cortex
| Region | L/R | BA | x | y | z | T | Z | P | k |
|---|---|---|---|---|---|---|---|---|---|
| Controls > ASD | |||||||||
| Mid frontal gyrus | L | 10 | −24 | 44 | 22 | 5.35 | 4.45 | 0.000 | 7314 |
| Superior frontal gyrus | R | 10 | 16 | 52 | 20 | 4.34 | 3.79 | 0.000 | |
| Anterior cingulate cortex | R | 24 | 20 | 10 | 30 | 3.99 | 3.54 | 0.000 | |
| Superior frontal gyrus | L | 6 | −14 | 16 | 60 | 3.29 | 3.01 | 0.001 | |
| Anterior cingulate cortex (subgenual) | R | 32 | 8 | 28 | −4 | 3.27 | 3.00 | 0.001 | |
| Superior frontal gyrus | L | 6 | −6 | 28 | 58 | 3.21 | 2.96 | 0.002 | |
| Anterior cingulate cortex | R | 32 | 14 | 28 | 30 | 3.18 | 2.92 | 0.002 | |
| Mid frontal gyrus | L | 6 | −38 | 2 | 58 | 3.11 | 2.87 | 0.002 | |
| Superior frontal gyrus | R | 8 | 10 | 32 | 58 | 3.06 | 2.83 | 0.002 | |
| Superior frontal gyrus (medial) | 32 | 0 | 44 | 28 | 2.84 | 2.65 | 0.004 | ||
| Caudate nucleus | L | −6 | 2 | 12 | 2.58 | 2.43 | 0.007 | ||
| Caudate nucleus | R | 6 | 2 | 14 | 2.49 | 2.36 | 0.009 | ||
| Orbitofrontal cortex | L | 11 | −6 | 38 | −18 | 2.26 | 2.15 | 0.016 | |
| Anterior cingulate cortex (subgenual) | L | 32 | −6 | 30 | −4 | 2.17 | 2.07 | 0.019 | |
| Caudate nucleus | L | −12 | 8 | 16 | 2.12 | 2.03 | 0.021 | ||
| Cerebral peduncle | R | 14 | −20 | −32 | 3.56 | 3.23 | 0.001 | ||
| Cerebral peduncle | L | −18 | −20 | −30 | 3.34 | 3.06 | 0.001 | ||
| Posterior cingulate cortex | L | 31 | −10 | −46 | 36 | 3.97 | 3.53 | 0.000 | 1852 |
| Posterior cingulate cortex | R | 31 | 8 | −44 | 28 | 3.37 | 3.08 | 0.001 | |
| Precuneus | R | 7 | 2 | −72 | 30 | 2.89 | 2.69 | 0.004 | |
| Precuneus | L | 7 | −8 | −62 | 36 | 2.49 | 2.36 | 0.009 | |
| Temporal parietal junction | L | 22 | −52 | −56 | 24 | 3.48 | 3.16 | 0.001 | 1193 |
| Cerebellum crus II | R | 32 | −78 | −46 | 3.42 | 3.12 | 0.001 | 682 | |
| Mid temporal gyrus | L | 21 | −64 | −34 | −10 | 3.37 | 3.08 | 0.001 | 783 |
| Inferior temporal gyrus | L | 21 | −58 | −22 | −20 | 3.01 | 2.79 | 0.003 | |
| Superior frontal gyrus | R | 6 | 22 | 2 | 74 | 3.36 | 3.07 | 0.001 | 128 |
| Mid temporal gyrus | L | 21 | −52 | 4 | −34 | 3.02 | 2.80 | 0.003 | 147 |
| Superior parietal lobule | R | 7 | 14 | −58 | 74 | 2.63 | 2.47 | 0.007 | 131 |
| Inferior frontal gyrus | L | 47 | −50 | 34 | −2 | 2.42 | 2.29 | 0.011 | 196 |
| ASD > Controls | |||||||||
| Cuneus | L | 18 | −18 | −92 | 14 | 4.55 | 3.94 | 0.000 | 16754 |
| Superior occipital gyrus | L | 19 | −24 | −80 | 14 | 4.47 | 3.88 | 0.000 | |
| Inferior occipital gyrus | R | 18 | 28 | −86 | −8 | 4.31 | 3.77 | 0.000 | |
| Superior parietal lobule | R | 7 | 26 | −70 | 38 | 4.07 | 3.60 | 0.000 | |
| Superior occipital gyrus | R | 19 | 24 | −70 | 24 | 3.94 | 3.51 | 0.000 | |
| Fusiform gyrus | R | 19 | 42 | −70 | −14 | 3.90 | 3.48 | 0.000 | |
| Cerebellum crus IV | R | 14 | −40 | −18 | 3.79 | 3.40 | 0.000 | ||
| Inferior longitudinal fasciculus | R | 41 | 40 | −30 | 0 | 3.71 | 3.34 | 0.000 | |
| Medial occipital gyrus | R | 18 | 28 | −80 | 10 | 3.70 | 3.33 | 0.000 | |
| Fusiform gyrus | R | 19 | 32 | −76 | 0 | 3.69 | 3.32 | 0.000 | |
| Inferior temporal gyrus | R | 37 | 52 | −58 | −10 | 3.64 | 3.29 | 0.001 | |
| Cerebellum crus IV | R | 2 | −50 | −2 | 3.22 | 2.96 | 0.002 | ||
| Superior occipital gyrus | R | 19 | 16 | −82 | 30 | 3.21 | 2.95 | 0.002 | |
| Inferior occipital gyrus | R | 19 | 40 | −78 | 0 | 3.17 | 2.92 | 0.002 | |
| Parahippocampal gyrus | R | 36 | 28 | 0 | −28 | 3.08 | 2.85 | 0.002 | |
| Lingual gyrus | R | 19 | 16 | −76 | −10 | 3.05 | 2.82 | 0.002 | |
| Pons | R | 10 | −24 | −26 | 2.91 | 2.71 | 0.003 | ||
| Hippocampus | L | 34 | −30 | −20 | −8 | 2.88 | 2.68 | 0.004 | |
| Inferior occipital gyrus | L | 19 | −40 | −80 | 2 | 2.87 | 2.67 | 0.004 | |
| Pons | L | −2 | −30 | −14 | 2.86 | 2.67 | 0.004 | ||
| Fusiform gyrus | L | 19 | −28 | −66 | −14 | 2.78 | 2.60 | 0.005 | |
| Superior parietal lobule | L | 19 | −22 | −64 | 30 | 2.78 | 2.60 | 0.005 | |
| Lingual gyrus | L | 18 | −8 | −64 | −4 | 2.77 | 2.59 | 0.005 | |
| Amygdala | R | 22 | 0 | −18 | 2.66 | 2.50 | 0.006 | ||
| Temporal pole | R | 38 | 32 | 8 | −40 | 2.44 | 2.31 | 0.010 | |
| Cerebellum crus VII | L | 4/43 | −8 | −72 | −36 | 3.53 | 3.21 | 0.001 | 450 |
| Rolandic operculum | L | −40 | −8 | 18 | 3.37 | 3.08 | 0.001 | 757 | |
| Superior temporal gyrus | L | 22 | −64 | −6 | 6 | 2.78 | 2.60 | 0.005 | |
| Posterior insular cortex | L | −44 | 0 | 4 | 2.56 | 2.42 | 0.008 | ||
| Inferior frontal gyrus | R | 45 | 48 | 20 | 8 | 3.15 | 2.90 | 0.002 | 945 |
| Rolandic operculum | R | 4/43 | 42 | 0 | 14 | 2.71 | 2.54 | 0.006 | |
| Posterior insular cortex | R | 44 | 8 | 2 | 2.35 | 2.24 | 0.013 | ||
| Temporal pole | L | 38 | −28 | 18 | −34 | 3.14 | 2.90 | 0.002 | 227 |
| Precentral gyrus | R | 4 | 44 | −10 | 34 | 2.91 | 2.71 | 0.003 | 412 |
| Precentral gyrus | R | 6 | 50 | −4 | 46 | 2.72 | 2.55 | 0.005 | |
| Postcentral gyrus | L | 3 | −42 | −22 | 48 | 2.90 | 2.70 | 0.003 | 263 |
| Supplementary motor area | L | 6 | −6 | −4 | 60 | 2.88 | 2.68 | 0.004 | 424 |
| Supplementary motor area | R | 6 | 8 | 6 | 58 | 2.67 | 2.51 | 0.006 | |
| Inferior parietal lobule | R | 40 | 42 | −38 | 60 | 2.69 | 2.53 | 0.006 | 309 |
Height threshold: T = 1.7, P < 0.05.
Extent threshold: k = 120.
L = left; R = right; BA = Brodmann area.
Figure 3.
Functional connectivity of the posterior cingulate cortex, and an interaction between group (neurotypical controls versus ASD) and SCR (before versus after regressing out SCR signal) on posterior cingulate cortex connectivity. (A) Stronger connectivity in neurotypical controls compared with ASD (neurotypical controls > ASD). (B) Stronger connectivity in ASD, compared to neurotypical controls (ASD > neurotypical controls). (C) Stronger effects of SCR on posterior cingulate cortex connectivity in neurotypical control subjects compared to ASD [neurotypical controls (with-without SCR) > ASD (with-without SCR)]. (D) Stronger effects of SCR on posterior cingulate cortex connectivity in ASD compared with neurotypical control subjects [ASD (with-without SCR) > neurotypical controls (with-without SCR)].
Table 6.
Interaction of group (neurotypical controls versus ASD) and SCR (with SCR versus without SCR) for the functional connectivity of the posterior cingulate cortex
| Region | L/R | BA | x | y | z | T | Z | P | k |
|---|---|---|---|---|---|---|---|---|---|
| Controls (with-without) > ASD (with-without) | |||||||||
| Posterior cingulate cortex | R | 31 | 6 | −48 | 36 | 4.85 | 4.13 | 0.000 | 3566 |
| Posterior cingulate cortex | L | 31 | −12 | −40 | 40 | 4.63 | 3.99 | 0.000 | |
| Superior parietal lobule | L | 7 | −34 | −36 | 66 | 4.17 | 3.67 | 0.000 | |
| Supramarginal gyrus | L | 40 | −62 | −42 | 30 | 4.04 | 3.58 | 0.000 | |
| Inferior parietal lobule | L | 40 | −42 | −36 | 56 | 3.86 | 3.45 | 0.000 | |
| Posterior cingulate cortex | R | 23 | 4 | −28 | 40 | 3.64 | 3.29 | 0.001 | |
| Precuneus | R | 7 | 2 | −46 | 46 | 3.58 | 3.24 | 0.001 | |
| Postcentral gyrus | L | 3 | −20 | −38 | 54 | 3.22 | 2.96 | 0.002 | |
| Postcentral gyrus | L | 2 | −42 | −20 | 56 | 3.16 | 2.91 | 0.002 | |
| Precentral gyrus | L | 6 | −36 | −22 | 66 | 3.05 | 2.82 | 0.002 | |
| Postcentral gyrus | R | 2 | 62 | −8 | 38 | 2.69 | 2.52 | 0.006 | |
| Precentral gyrus | L | 6 | −54 | 0 | 44 | 4.07 | 3.60 | 0.000 | 266 |
| Mid frontal gyrus | L | 10 | −8 | 54 | 20 | 3.98 | 3.54 | 0.000 | 443 |
| Mid frontal gyrus | 10 | 0 | 58 | 22 | 3.66 | 3.30 | 0.000 | ||
| Mid frontal gyrus | R | 9 | 2 | 46 | 24 | 2.70 | 2.53 | 0.006 | |
| Superior frontal gyrus | L | 6 | −14 | −6 | 72 | 3.92 | 3.49 | 0.000 | 256 |
| Gyrus rectus | L | 11 | −10 | 20 | −16 | 3.11 | 2.87 | 0.002 | |
| Cerebellum crus VIIIB | R | 16 | −58 | −58 | 3.67 | 3.31 | 0.000 | 128 | |
| Posterior cingulate cortex | L | 26 | −6 | −42 | 6 | 3.60 | 3.25 | 0.001 | 124 |
| Cerebellum crus VI | R | 14 | −70 | −24 | 3.57 | 3.23 | 0.001 | 264 | |
| Cerebellum crus I | R | 36 | −68 | −30 | 2.91 | 2.71 | 0.003 | ||
| Angular gyrus | L | 39 | −46 | −70 | 32 | 3.57 | 3.23 | 0.001 | 215 |
| Dentate nucleus | R | 12 | −48 | −26 | 3.48 | 3.16 | 0.001 | 178 | |
| Cerebellum crus I | L | −28 | −88 | −30 | 3.32 | 3.04 | 0.001 | 146 | |
| Superior parietal lobule | R | 7 | 34 | −48 | 58 | 3.28 | 3.01 | 0.001 | 895 |
| Postcentral gyrus | R | 2 | 16 | −40 | 58 | 3.06 | 2.83 | 0.002 | |
| Superior parietal lobule | R | 7 | 20 | −62 | 60 | 2.69 | 2.52 | 0.006 | |
| Mid temporal gyrus | R | 21 | 60 | −52 | 4 | 3.05 | 2.82 | 0.002 | |
| Inferior temporal gyrus | R | 37 | 52 | −62 | 2 | 2.58 | 2.43 | 0.008 | |
| Precentral gyrus | R | 6 | 26 | −14 | 66 | 2.92 | 2.72 | 0.003 | 203 |
| Mid frontal gyrus | R | 6 | 30 | −8 | 60 | 2.89 | 2.69 | 0.004 | |
| Inferior frontal gyrus | L | 47 | −50 | 32 | −14 | 2.88 | 2.69 | 0.004 | 126 |
| Inferior frontal gyrus | L | 47 | −34 | 24 | −16 | 2.80 | 2.62 | 0.004 | |
| Supplementary motor area | R | 6 | 8 | −8 | 58 | 2.86 | 2.67 | 0.004 | 194 |
| ASD (with-without) > Controls (with-without) | |||||||||
| Posterior insular cortex | R | 42 | −10 | −12 | 4.98 | 4.21 | 0.000 | 1464 | |
| Putamen | R | 26 | −10 | 8 | 4.73 | 4.06 | 0.000 | ||
| Superior temporal gyrus | R | 42 | 46 | −28 | 6 | 3.30 | 3.02 | 0.001 | |
| Posterior insular cortex | R | 46 | 2 | 0 | 3.24 | 2.97 | 0.001 | ||
| Hippocampus | R | 34 | 32 | −10 | −20 | 2.94 | 2.73 | 0.003 | |
| Parahippocampal gyrus | R | 28 | 30 | −10 | −32 | 2.62 | 2.47 | 0.007 | |
| Amygdala | R | 32 | −2 | −28 | 2.45 | 2.32 | 0.010 | ||
| Posterior insular cortex | L | −44 | 0 | 2 | 4.72 | 4.05 | 0.000 | 5420 | |
| Transverse temporal gyrus | L | 41 | −40 | −26 | 6 | 4.66 | 4.00 | 0.000 | |
| Fusiform gyrus | R | 37 | 22 | −52 | −10 | 4.39 | 3.82 | 0.000 | |
| Parahippocampal gyrus | L | 28 | −28 | −22 | −30 | 4.12 | 3.64 | 0.000 | |
| Mid temporal gyrus | L | 21 | −50 | −14 | −4 | 3.84 | 3.44 | 0.000 | |
| Lingual gyrus | R | 19 | 14 | −56 | −4 | 3.81 | 3.41 | 0.000 | |
| Lingual gyrus | L | 19 | −12 | −70 | 0 | 3.80 | 3.40 | 0.000 | |
| Hippocampus | L | 34 | −16 | −32 | −2 | 3.56 | 3.23 | 0.001 | |
| Fusiform gyrus | L | 20 | −36 | −32 | −10 | 3.36 | 3.07 | 0.001 | |
| Superior temporal gyrus | L | 42 | −56 | −28 | 6 | 3.23 | 2.97 | 0.001 | |
| Precuneus | R | 31 | 6 | −66 | 26 | 2.99 | 2.78 | 0.003 | |
| Superior parietal lobule | L | 19 | −22 | −80 | 46 | 2.94 | 2.74 | 0.003 | |
| Calcarine cortex | L | 17/18 | −24 | −64 | 4 | 2.91 | 2.71 | 0.003 | |
| Precuneus | L | 7 | −2 | −78 | 42 | 2.55 | 2.41 | 0.008 | |
| Anterior cingulate cortex | L | 24 | −12 | 8 | 38 | 4.36 | 3.80 | 0.000 | 512 |
| Anterior cingulate cortex | R | 24 | 2 | 6 | 42 | 3.10 | 2.86 | 0.002 | |
| Superior occipital gyrus | R | 19 | 22 | −92 | 24 | 4.22 | 3.71 | 0.000 | 846 |
| Superior occipital gyrus | L | 18 | −16 | −96 | 20 | 3.96 | 3.52 | 0.000 | |
| Mid occipital gyrus | R | 18 | 30 | −86 | 18 | 3.68 | 3.32 | 0.000 | |
| Mid frontal gyrus | L | 11 | −34 | 62 | −12 | 4.08 | 3.61 | 0.000 | 295 |
| Fusiform gyrus | R | 37 | 34 | −38 | −20 | 3.90 | 3.48 | 0.000 | 826 |
| Inferior temporal gyrus | R | 37 | 54 | −62 | −10 | 3.57 | 3.23 | 0.001 | |
| Cerebellum crus I | R | 50 | −68 | −28 | 3.33 | 3.05 | 0.001 | ||
| Superior temporal gyrus | R | 22 | 66 | −22 | 4 | 3.72 | 3.34 | 0.000 | 344 |
| Mid frontal gyrus | R | 10 | 36 | 52 | −6 | 3.19 | 2.94 | 0.002 | 180 |
| Paracentral lobule | L | 4 | −8 | −28 | 68 | 2.59 | 2.44 | 0.007 | 170 |
| Caudate nucleus | R | 14 | 20 | 0 | 2.50 | 2.36 | 0.009 | 197 | |
Height threshold: T = 1.7, P < 0.05.
Extent threshold: k = 120.
L = left; R = right; BA = Brodmann area.
Voxel-wise whole brain connectivity
A between-group comparison revealed that the neurotypical control group had higher functional connectivity strength in the posterior cingulate cortex, precuneus, medial prefrontal cortex, anterior cingulate cortex, gyrus rectus, posterior insular cortex, superior temporal gyrus, and inferior temporal, parietal, and occipital gyri (Table 7 and Fig. 4A). The ASD group had higher functional connectivity strength in the hippocampus, inferior temporal gyrus, temporal pole, lingual gyrus, calcarine cortex and amygdala, all on the left, as well as the cerebellum bilaterally (Table 7 and Fig. 4B). A significant interaction (Group × SCR) was also found; SCR signal contributed significantly to the connectivity of the posterior cingulate cortex, medial prefrontal cortex, anterior cingulate cortex, inferior parietal lobule, and the right anterior and posterior insular cortex in neurotypical control subjects, compared with ASD (Table 8 and Fig. 4C). In ASD there was significant contribution of SCR signal to the connectivity of the hippocampus, amygdala, nucleus accumbens, calcarine cortex, and the fusiform, lingual, and inferior occipital gyri, compared with neurotypical control subjects (Table 8 and Fig. 4D). For within-group paired t-test results (before > after SCR regression), see Supplementary Table 4 and Supplementary Fig. 4.
Table 7.
Two sample t-test between neurotypical controls and ASD for whole-brain functional connectivity
| Region | L/R | BA | x | y | z | T | Z | P | k |
|---|---|---|---|---|---|---|---|---|---|
| Controls > ASD | |||||||||
| Superior temporal gyrus | R | 38 | 42 | 6 | −15 | 4.88 | 4.15 | 0.000 | 104 |
| Posterior insular cortex | R | 45 | −12 | 0 | 3.03 | 2.81 | 0.002 | ||
| Inferior temporal gyrus | R | 37 | 42 | −63 | −9 | 3.77 | 3.38 | 0.000 | 119 |
| Inferior occipital gyrus | R | 19 | 48 | −75 | 0 | 3.31 | 3.03 | 0.001 | |
| Gyrus rectus | L | 11 | −3 | 36 | −18 | 3.75 | 3.37 | 0.000 | 361 |
| Superior frontal gyrus (medial) | R | 8 | 9 | 33 | 39 | 3.60 | 3.26 | 0.001 | |
| Anterior cingulate cortex | L | 32 | −9 | 42 | 9 | 3.52 | 3.19 | 0.001 | |
| Anterior cingulate cortex (subgenual) | R | 32 | 6 | 30 | −6 | 3.42 | 3.12 | 0.001 | |
| Superior frontal gyrus (medial) | R | 9 | 6 | 51 | 36 | 3.17 | 2.92 | 0.002 | |
| Anterior cingulate cortex | R | 24 | 12 | 36 | 9 | 3.08 | 2.85 | 0.002 | |
| Superior frontal gyrus (medial) | L | 9 | −3 | 45 | 24 | 3.07 | 2.84 | 0.002 | |
| Inferior parietal lobule | R | 39 | 45 | −54 | 36 | 3.45 | 3.14 | 0.001 | 101 |
| Posterior cingulate cortex | L | 23 | −3 | −36 | 39 | 3.34 | 3.05 | 0.001 | 376 |
| Precuneus | L | 31 | −9 | −57 | 36 | 2.74 | 2.57 | 0.005 | |
| Precuneus | R | 19 | 9 | −54 | 30 | 2.56 | 2.42 | 0.008 | |
| ASD > Controls | |||||||||
| Hippocampus | L | −30 | −21 | −12 | 4.61 | 3.98 | 0.000 | 120 | |
| Inferior temporal gyrus | L | 20 | −45 | −27 | −21 | 2.65 | 2.49 | 0.006 | |
| Amygdala | L | −27 | 3 | −18 | 2.29 | 2.18 | 0.015 | ||
| Calcarine cortex | L | 17 | −3 | −63 | 9 | 4.02 | 3.57 | 0.000 | 79 |
| Lingual gyrus | L | 19 | −12 | −48 | 3 | 3.63 | 3.28 | 0.001 | |
| Cerebellum crus VIII | L | −36 | −63 | −54 | 4.02 | 3.57 | 0.000 | 119 | |
| Cerebellum crus II | R | 30 | −78 | −48 | 3.99 | 3.55 | 0.000 | 112 | |
| Temporal pole | L | 38 | −39 | 15 | −30 | 2.99 | 2.77 | 0.003 | |
| Cerebellum crus VII | L | −30 | −39 | −39 | 3.60 | 3.25 | 0.001 | 130 | |
| Cerebellum crus I | R | 39 | −57 | −33 | 3.41 | 3.11 | 0.001 | 81 | |
Height threshold: T = 1.7, P < 0.05.
Extent threshold: k = 67.
L = left, R = right; BA = Brodmann area.
Figure 4.
Voxel-wise whole brain functional connectivity, and an interaction between group (neurotypical controls versus ASD) and SCR (before versus after regressing out the SCR signal) in voxel-wise whole-brain connectivity. (A) Stronger connectivity in neurotypical controls compared with ASD (neurotypical controls > ASD). (B) Stronger connectivity in ASD, compared to neurotypical control subjects (ASD > neurotypical controls). (C) Stronger effects of SCR on whole-brain connectivity in neurotypical control subjects compared to ASD [neurotypical controls (with-without SCR) > ASD (with-without SCR)]. (D) Stronger effects of SCR on whole-brain connectivity in ASD compared to neurotypical control subjects [ASD (with-without SCR) > neurotypical controls (with-without SCR)].
Table 8.
Interaction of group (neurotypical controls versus ASD) and SCR (with SCR versus without SCR) for the functional connectivity of the whole brain
| Region | L/R | BA | x | y | z | T | Z | P | k |
|---|---|---|---|---|---|---|---|---|---|
| Controls (with-without) > ASD (with-without) | |||||||||
| Anterior cingulate cortex | R | 32 | 6 | 42 | 18 | 5.08 | 4.28 | 0.000 | 606 |
| Superior frontal gyrus (medial) | L | 8 | −3 | 30 | 60 | 4.13 | 3.65 | 0.000 | |
| Superior frontal gyrus (medial) | R | 8 | 6 | 33 | 42 | 4.03 | 3.57 | 0.000 | |
| Anterior cingulate cortex (pregenual) | L | 24 | −6 | 33 | 6 | 3.95 | 3.51 | 0.000 | |
| Anterior cingulate cortex (subgenual) | R | 25 | 6 | 24 | −6 | 2.96 | 2.75 | 0.003 | |
| Inferior frontal gyrus | R | 45 | 48 | 21 | 3 | 4.17 | 3.67 | 0.000 | 157 |
| Anterior insular cortex | R | 33 | 21 | −9 | 2.76 | 2.59 | 0.005 | ||
| Posterior insular cortex | R | 42 | −15 | −6 | 4.13 | 3.65 | 0.000 | 78 | |
| Cerebellum crus II | L | −9 | −84 | −24 | 3.26 | 2.99 | 0.001 | 87 | |
| Mid frontal gyrus | R | 8 | 33 | 15 | 54 | 3.20 | 2.95 | 0.002 | 197 |
| Mid frontal gyrus | R | 8 | 42 | 24 | 42 | 3.08 | 2.85 | 0.002 | |
| Posterior cingulate cortex | 23 | 0 | −12 | 30 | 3.20 | 2.94 | 0.002 | 230 | |
| Posterior cingulate cortex | R | 26 | 3 | −42 | 27 | 2.97 | 2.76 | 0.003 | |
| Posterior cingulate cortex | L | 31 | −3 | −27 | 36 | 2.89 | 2.69 | 0.004 | |
| Precuneus | L | 31 | −12 | −57 | 36 | 2.81 | 2.62 | 0.004 | |
| Inferior parietal lobule | L | 40 | −51 | −51 | 39 | 3.12 | 2.88 | 0.002 | 99 |
| Angular gyrus | L | 39 | −57 | −60 | 27 | 2.58 | 2.43 | 0.007 | |
| Inferior parietal lobule | R | 40 | 36 | −51 | 42 | 2.96 | 2.75 | 0.003 | 92 |
| Vermis IV | R | 6 | −54 | −21 | 2.64 | 2.48 | 0.007 | 78 | |
| ASD (with-without) > Controls (with-without) | |||||||||
| Hippocampus | L | −30 | −21 | −12 | 4.43 | 3.86 | 0.000 | 74 | |
| Amygdala | L | −21 | −3 | −15 | 2.16 | 2.07 | 0.019 | ||
| Cerebellum crus VII | R | 39 | −69 | −54 | 2.76 | 2.58 | 0.005 | 80 | |
| Nucleus accumbens | L | −21 | 9 | −15 | 3.67 | 3.31 | 0.000 | 92 | |
| Caudate nucleus | L | −6 | 12 | −3 | 3.08 | 2.85 | 0.002 | ||
| Calcarine cortex | R | 18 | 3 | −90 | 12 | 3.53 | 3.20 | 0.001 | 203 |
| Calcarine cortex | L | 17 | −9 | −66 | 9 | 3.34 | 3.05 | 0.001 | |
| Cuneus | L | 19 | 3 | −81 | 36 | 2.51 | 2.37 | 0.009 | |
| Cerebellum crus VI | L | −12 | −72 | −18 | 3.19 | 2.93 | 0.002 | 92 | |
| Inferior occipital gyrus | L | 18 | −18 | −90 | −6 | 2.33 | 2.22 | 0.013 | |
| Fusiform gyrus | R | 37 | 42 | −60 | −15 | 3.11 | 2.87 | 0.002 | 145 |
| Lingual gyrus | R | 18 | 15 | −54 | 0 | 2.93 | 2.73 | 0.003 | |
| Inferior occipital gyrus | R | 18 | 33 | −93 | −12 | 3.06 | 2.83 | 0.002 | 109 |
Height threshold: T = 1.7, P < 0.05.
Extent threshold: k = 67.
L = left; R = right; BA = Brodmann area.
Correlations with clinical symptoms
No significant correlations were found between the number of SCRs, or overall skin conductance level, and any of the four ADI-R subscales, all P’s > 0.05. However, ADI-R subscales for social interaction and communication were negatively correlated with posterior cingulate cortex connectivity with the medial and lateral prefrontal cortices, and with the posterior cingulate cortex and precuneus, respectively. Positive correlations with posterior cingulate cortex connectivity were found in sensory and temporal regions, as well as within the thalamus (Supplementary Table 5 and Supplementary Fig. 5) with all four subscales. Symptom severity on the social interaction and communication subscales was also correlated with decreased ventromedial prefrontal cortex connectivity with the posterior cingulate cortex (i.e. decreased DMN connectivity), as well as increased connectivity with the anterior insular cortex and anterior cingulate cortex (i.e. reduced anticorrelations with the task-positive network) (Supplementary Table 6 and Supplementary Fig. 6A and B). ADI-R correlations with whole brain connectivity revealed negative correlations with the posterior cingulate cortex/precuneus and supplementary motor area (social interaction and communication subscales), and positive correlations with the anterior cingulate cortex and medial prefrontal cortex (restricted behaviour and early development subscales) (Supplementary Table 7 and Supplementary Fig. 7). For ADI-R subscale correlation with brain activity that is associated with SCR, see Supplementary Table 8 and Supplementary Fig. 8.
Central generation and representation of skin conductance responses
One-sample t-tests of both generation and representation models revealed comparable brain activation associated with SCR to the original data in both groups (i.e. anterior brain regions in neurotypical controls, and posterior brain activation in ASD). When the two models were compared within each group, no significant differences were found between representation and generation (representation > generation) in neurotypical control subjects, or between the two models in ASD. There was, however, significant activation in the right thalamus and the left cerebellum in the generation model, as compared with the representation model (generation > representation), in neurotypical control subjects (Supplementary Table 9 and Supplementary Fig. 9).
Discussion
Skin conductance response and its correlations with brain activity
The results demonstrate a reduced number of spontaneous SCRs, as well as abnormal correlations of SCR with brain activation during rest in ASD. This could result from either abnormal peripheral conductance of autonomic nervous system signals, or from alterations in the central generation and/or representation of visceral autonomic arousal states. Previous studies have found abnormal basal autonomic nervous system activity in ASD (Horvath et al., 1999; Molloy and Manning-Courtney, 2003; White, 2003; Ming et al., 2005; Anderson and Colombo, 2009; Bal et al., 2010; Anderson et al., 2013). However, all of these findings indicate increased sympathetic/parasympathetic balance. Our finding of decreased number of SCRs in ASD, therefore, is unlikely to be a result of hypoactivation of the sympathetic autonomic nervous system. Moreover, skin conductance level did not differ between groups, consistent with previous studies (Zahn et al., 1987; Schoen et al., 2008; Mathersul et al., 2013), further suggesting that the electrodermal abnormality in ASD is not of peripheral origin. Thus, the results are likely to reflect alterations in the central generation and/or representation of SCR signals.
SCR is a sensitive index of sympathetic neural activity and can detect even subtle changes in autonomic arousal due to mental activity and thought processes (Nikula, 1991). Our results may, therefore, reflect differences in the central generation of SCR, stemming from different mental activity. More specifically, in neurotypical controls, SCR was positively correlated with the anterior insular cortex, a brain region involved in interoceptive awareness (Craig, 2002, 2009; Critchley et al., 2002; Pollatos et al., 2007; Gu et al., 2013), and with the medial prefrontal cortex, which is implicated in self-referential processing (Craik et al., 1999; Kelley et al., 2002; Macrae et al., 2004; Northoff et al., 2006; Jenkins and Mitchell, 2011). In ASD, however, SCR was highly correlated with visual and auditory cortices. Thus, it is possible that when neurotypical control participants lay in the scanner at rest, they were aware of their inner bodily sensations and were engaged in self-referential thoughts, which drove their SCRs. On the other hand, when participants with ASD were resting in the scanner, they may have concentrated on the noises and the visual information inside the scanner and that may have been the driving force of their autonomic responses.
The results may also reflect alterations in the central representation of autonomic signals. The thalamus, supplementary motor area, anterior insular cortex, and anterior cingulate cortex were positively correlated with SCR in neurotypical controls, but were either not correlated, or negatively correlated with SCR in individuals with ASD. These brain regions were previously implicated in autonomic nervous system signal processing (Boucsein, 1992; Damasio et al., 2000; Craig, 2002; Porges, 2003; Critchley, 2005; Gu et al., 2013). The anterior insular cortex, specifically, was indicated to have a key role in the representation of autonomic signals. Anterior insular cortex was not associated with SCR in the ASD group, further supporting abnormal central autonomic nervous system representation in ASD.
Results from our additional analyses did not reveal significant differences in cortical regions that were uniquely associated with the generation or representation of SCR in both groups, suggesting the involvement of similar brain regions in both the generation and the representation of SCR. These regions corresponded with brain areas that were positively correlated with SCR in each of the groups in our original regression analysis, suggesting that the differences that were originally found between the groups are present both in the generation phase and the representation phase of SCR signals. The higher activation in the right thalamus and left cerebellum that was found during the generation of SCRs in neurotypical controls may suggest that there is an increased autonomic arousal during the generation phase, compared with the representation phase. These results differ from previous findings of brain regions that are uniquely activated in the generation or representation of SCR in a healthy cohort (Critchley et al., 2000). However, differences in methods may account for the discrepancy between the results of these studies. For a more detailed discussion of the afferent and efferent SCR pathways see the online Supplementary material.
Skin conductance response contribution to default mode network and voxel-wise connectivity
Our functional connectivity findings replicated recent work showing reduced connectivity of the DMN in individuals with ASD compared with neurotypical controls (Kennedy and Courchesne, 2008a, b; Monk et al., 2009; Assaf et al., 2010; Weng et al., 2010), and added a critical component: the difference in DMN connectivity between the groups was significantly associated with SCR. Thus, by examining the interaction between SCR signal and group difference in posterior cingulate cortex connectivity, our data indicated significantly higher contributions of the SCR signal to the DMN connectivity in the neurotypical control group, compared to the ASD group. Decreased DMN connectivity in ASD was also found using the ventromedial prefrontal cortex as a seed; however, SCR did not have significant contributions to this network in this analysis. Because the ventromedial prefrontal cortex is a relatively large brain region, these negative results may be associated with the specific seed region of the ventromedial prefrontal cortex that was used in this analysis [based on the coordinates reported by Fox et al. (2005)]. Nevertheless, differences in autonomic-related brain activity may account, at least in part, for the previously observed under-connectivity of the DMN in ASD.
Voxel-wise whole-brain connectivity analysis results were strikingly similar to those obtained for the posterior cingulate cortex connectivity analysis, providing converging evidence for a decreased connectivity in DMN-related brain regions, and increased connectivity along the medial temporal lobe in ASD. Moreover, symptom severity on the social interaction and communication subscales of the ADI-R were negatively correlated with DMN connectivity strength in the posterior cingulate cortex, ventromedial prefrontal cortex and whole brain connectivity analyses. The analysis of the interaction between whole-brain connectivity and SCR revealed significant contributions of SCR signals to the connectivity patterns in both groups, suggesting that differences in autonomic-related brain activity between the groups may account, at least in part, for the differences in brain connectivity.
Possible differences in thought content, as previously discussed, may also account for the differences in DMN connectivity. Previous studies have linked the DMN to the generation of spontaneous thoughts (Mason et al., 2007; Andrews-Hanna et al., 2010) and self-referential mental activity (Whitfield-Gabrieli et al., 2011). Thus, the weaker connectivity of the DMN and higher connectivity of visual and auditory cortices in ASD may indicate an inability to shift attention from exteroceptive to interoceptive signals during rest in those individuals. This interpretation supports a recent model in which ASD is associated with abnormalities in the salience network (Uddin and Menon, 2009; Menon and Uddin, 2010). The salience network is comprised of the anterior insular cortex and anterior cingulate cortex, and is postulated to play a key role in switching between the externally oriented executive control network and the internally oriented DMN (Uddin and Menon, 2009; Menon and Uddin, 2010). Indeed, several studies have found neuropathological and functional abnormalities of the anterior insular cortex in ASD (Di Martino et al., 2009; Ebisch et al., 2011; Santos et al., 2011), and altered functional connectivity patterns of the anterior insular cortex in the ASD group were found in our data set too. It is possible, therefore, that the focus of the ASD participants on visual and auditory information during the scan may have not only driven their SCR, but in fact influenced whole brain connectivity patterns, including connectivity of the DMN. This model, together with the significant contributions of SCR to the connectivity patterns, supports the importance of autonomic activity in modulating resting-state functional connectivity.
This work adds an important element to an emerging line of research showing significant contributions of autonomic nervous system signals to resting state network connectivity in the normal population (Birn et al., 2008; Iacovella and Hasson, 2011; Fan et al., 2012). These studies suggest that incorporating measurements of autonomic signals in resting-state functional MRI connectivity analysis, rather than regressing them out as noise, may provide better insight into the driving forces of brain fluctuations, and the representation of brain activity and connectivity during ‘rest’. The current findings further suggest that examining autonomic nervous system contributions to brain connectivity during rest may significantly improve our understanding of alterations in resting state functional connectivity patterns that were previously observed in a variety of patient populations, such as in individuals with depression (Greicius et al., 2007; Bluhm et al., 2009), schizophrenia (Garrity et al., 2007; Whitfield-Gabrieli et al., 2009; Öngür et al., 2010), bipolar disorder (Ongur et al., 2010) and attention-deficit hyperactivity disorder (Uddin et al., 2008; Cao et al., 2009), in addition to ASD (Kennedy and Courchesne, 2008b; Monk et al., 2009; Assaf et al., 2010; Weng et al., 2010).
Clinical implications
Our data may have important clinical implications for the diagnosis and treatment of ASD. Autonomic measures are relatively easy and inexpensive to obtain, even with individuals of a very young age. Detecting autonomic abnormalities may aid in an early diagnosis of ASD, a process that could be critical to the effectiveness of existing behavioural treatments (Eaves and Ho, 2004; Granpeesheh et al., 2009). In addition, treatments targeting autonomic abnormalities may also reduce autistic symptoms (Murphy et al., 2000; Binstock, 2001; Porges, 2003; Field and Diego, 2008). For example, Murphy et al. (2000) observed that autistic symptoms were significantly reduced after vagal nerve stimulation in four patients with severe autistic behaviours. More research is needed, however, to determine the benefits of such treatments for ASD.
Conclusion
Our data provide evidence for abnormal associations between spontaneous SCR and brain activity and connectivity during rest in ASD. The observed autonomic abnormalities may contribute to the socio-emotional deficits in ASD because autonomic signals are essential to emotional processing. Taking autonomic measurements during investigation of resting state brain activity and connectivity may be critical for between-group comparisons, especially if one of the groups exhibits emotional abnormality, as is the case in many forms of psychopathology.
Funding
This research was supported by the National Institute of Health (NIH) Grant R21 MH083164 and two Research Enhancement Awards from Queens College, City University of New York, to J.F., along with the National Center for Research Resources (NCRR) Grant UL1 RR029887, and a James S. McDonnell Foundation grant (22002078, to P.R.H.). Two additional grants, from the National Natural Science Foundation (Grant No. 81030028) and the National Science Fund for Distinguished Young Scholars (Grant No. 81225012) of China to Y.H., helped in supporting this study. The contents are the sole responsibility of the authors and do not necessarily represent the official views of the aforementioned funding agencies. We would like to acknowledge the Beatrice and Samuel A. Seaver Foundation for their support. The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- ADI-R
Autism Diagnostic Interview-Revised
- ASD
autism spectrum disorders
- DMN
default mode network
- SCR
skin conductance response
References
- Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12:169–77. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders. DSM-5. 5th edn. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Anderson CJ, Colombo J. Larger tonic pupil size in young children with autism spectrum disorder. Dev Psychobiol. 2009;51:207–11. doi: 10.1002/dev.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CJ, Colombo J, Unruh KE. Pupil and salivary indicators of autonomic dysfunction in autism spectrum disorder. Dev Psychobiol. 2013;55:465–82. doi: 10.1002/dev.21051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. J Neurophysiol. 2010;104:322–35. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M, Jagannathan K, Calhoun VD, Miller L, Stevens MC, Sahl R, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. NeuroImage. 2010;53:247–56. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Daunizeau J, Friston KJ, Dolan RJ. Dynamic causal modelling of anticipatory skin conductance responses. Biol Psychol. 2010a;85:163–70. doi: 10.1016/j.biopsycho.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Flandin G, Friston KJ, Dolan RJ. Modelling event-related skin conductance responses. Int J Psychophysiol. 2010b;75:349–56. doi: 10.1016/j.ijpsycho.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal E, Harden E, Lamb D, Van Hecke AV, Denver JW, Porges SW. Emotion recognition in children with autism spectrum disorders: relations to eye gaze and autonomic state. J Autism Dev Disord. 2010;40:358–70. doi: 10.1007/s10803-009-0884-3. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Do people with autism understand what causes emotion? Child Dev. 1991;62:385–95. [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annu Rev Psychol. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binstock T. Anterior insular cortex: linking intestinal pathology and brain function in autism-spectrum subgroups. Med Hypotheses. 2001;57:714–17. doi: 10.1054/mehy.2001.1440. [DOI] [PubMed] [Google Scholar]
- Birn RM, Murphy K, Bandettini PA. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Hum Brain Mapp. 2008;29:740–50. doi: 10.1002/hbm.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R, Williamson P, Lanius R, Theberge J, Densmore M, Bartha R, et al. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci. 2009;63:754–61. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- Boucsein W. Electrodermal activity. New York: Plenum; 1992. [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Cao Q, Long X, Sun L, Sui M, Zhu C, et al. Abnormal resting-state functional connectivity patterns of the putamen in medication-naive children with attention deficit hyperactivity disorder. Brain Res. 2009;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M. Association between heart rate variability and fluctuations in resting-state functional connectivity. NeuroImage. 2013;68:93–104. doi: 10.1016/j.neuroimage.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Pathak S, Schneider W. Identifying the brain's most globally connected regions. NeuroImage. 2010;49:3132–48. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, et al. In search of the self: a positron emission tomography study. Psychol Sci. 1999;10:26–34. [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci. 2000;20:3033–40. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Volitional control of autonomic arousal: a functional magnetic resonance study. NeuroImage. 2002;16:909–19. doi: 10.1006/nimg.2002.1147. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Nagai Y, Gray MA, Mathias CJ. Dissecting axes of autonomic control in humans: insights from neuroimaging. Auton Neurosci. 2011;161:34–42. doi: 10.1016/j.autneu.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LC, Ho HH. The very early identification of autism: outcome to age 4 1/2-5. J Autism Dev Disord. 2004;34:367–78. doi: 10.1023/b:jadd.0000037414.33270.a8. [DOI] [PubMed] [Google Scholar]
- Ebisch SJ, Gallese V, Willems RM, Mantini D, Groen WB, Romani GL, et al. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp. 2011;32:1013–28. doi: 10.1002/hbm.21085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Levenson RW, Friesen WV. Autonomic nervous-system activity distinguishes among emotions. Science. 1983;221:1208–10. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- Fan J, Xu P, Van Dam NT, Eilam-Stock T, Gu X, Luo YJ, et al. Spontaneous brain activity relates to autonomic arousal. J Neurosci. 2012;32:11176–86. doi: 10.1523/JNEUROSCI.1172-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M. Vagal activity, early growth and emotional development. Infant Behav Dev. 2008;31:361–73. doi: 10.1016/j.infbeh.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Frith CD, Frith U. Social cognition in humans. Curr Biol. 2007;17:R724–32. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–7. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Gray MA, Harrison NA, Wiens S, Critchley HD. Modulation of emotional appraisal by false physiological feedback during fMRI. PLoS one. 2007;2:e546. doi: 10.1371/journal.pone.0000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granpeesheh D, Dixon DR, Tarbox J, Kaplan AM, Wilke AE. The effects of age and treatment intensity on behavioral intervention outcomes for children with autism spectrum disorders. Res Autism Spectr Disord. 2009;3:1014–22. [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Gao Z, Wang X, Liu X, Knight RT, Hof PR, et al. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135(Pt 9):2726–35. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, Fan J. Anterior insular cortex and emotional awareness. J Comp Neurol. 2013;521:3371–88. doi: 10.1002/cne.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Gray MA, Gianaros PJ, Critchley HD. The embodiment of emotional feelings in the brain. J Neurosci. 2010;30:12878–84. doi: 10.1523/JNEUROSCI.1725-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E, Berthoz S, Frith U. Brief report: cognitive processing of own emotions in individuals with autistic spectrum disorder and in their relatives. J Autism Dev Disord. 2004;34:229–35. doi: 10.1023/b:jadd.0000022613.41399.14. [DOI] [PubMed] [Google Scholar]
- Hirstein W, Iversen P, Ramachandran VS. Autonomic responses of autistic children to people and objects. Proc Biol Sci. 2001;268:1883–8. doi: 10.1098/rspb.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson RP, Ouston J, Lee A. Emotion recognition in autism: coordinating faces and voices. Psychol Med. 1988;18:911–23. doi: 10.1017/s0033291700009843. [DOI] [PubMed] [Google Scholar]
- Horvath K, Papadimitriou JC, Rabsztyn A, Drachenberg C, Tildon JT. Gastrointestinal abnormalities in children with autistic disorder. J Pediatr. 1999;135:559–63. doi: 10.1016/s0022-3476(99)70052-1. [DOI] [PubMed] [Google Scholar]
- Iacovella V, Hasson U. The relationship between BOLD signal and autonomic nervous system functions: implications for processing of “physiological noise". J Magn Reson Imag. 2011;29:1338–45. doi: 10.1016/j.mri.2011.03.006. [DOI] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- Jenkins AC, Mitchell JP. Medial prefrontal cortex subserves diverse forms of self-reflection. Soc Neurosci. 2011;6:211–18. doi: 10.1080/17470919.2010.507948. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Soc Cogn Affect Neurosci. 2008a;3:177–90. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. NeuroImage. 2008b;39:1877–85. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–21. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Kylliainen A, Hietanen JK. Skin conductance responses to another person's gaze in children with autism. J Autism Dev Disord. 2006;36:517–25. doi: 10.1007/s10803-006-0091-4. [DOI] [PubMed] [Google Scholar]
- Lange CG. The mechanism of the emotions. In: Rand B, editor. The classical psychologist. Boston, MA: Houghton Mifflin; 1885. pp. 672–85. [Google Scholar]
- Ledberg A, Akerman S, Roland PE. Estimation of the probabilities of 3D clusters in functional brain images. NeuroImage. 1998;8:113–28. doi: 10.1006/nimg.1998.0336. [DOI] [PubMed] [Google Scholar]
- Liang X, Zou Q, He Y, Yang Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci USA. 2013;110:1929–34. doi: 10.1073/pnas.1214900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebhart EH. Effects of false heart rate feedback and task instructions on information search, attributions, and stimulus ratings. Psychol Res. 1977;39:185–202. doi: 10.1007/BF00309286. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14:647–54. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathersul D, McDonald S, Rushby JA. Autonomic arousal explains social cognitive abilities in high-functioning adults with autism spectrum disorder. Int J Psychophysiol. 2013;89:475–482. doi: 10.1016/j.ijpsycho.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Julu PO, Brimacombe M, Connor S, Daniels ML. Reduced cardiac parasympathetic activity in children with autism. Brain Dev. 2005;27:509–16. doi: 10.1016/j.braindev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Manning-Courtney P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism. 2003;7:165–71. doi: 10.1177/1362361303007002004. [DOI] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage. 2009;47:764–72. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JV, Wheless JW, Schmoll CM. Left vagal nerve stimulation in six patients with hypothalamic hamartomas. Pediatr Neurol. 2000;23:167–8. doi: 10.1016/s0887-8994(00)00170-3. [DOI] [PubMed] [Google Scholar]
- Nikula R. Psychological correlates of nonspecific skin conductance responses. Psychophysiology. 1991;28:86–90. doi: 10.1111/j.1469-8986.1991.tb03392.x. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Öngür D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, et al. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183:59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JC, II, Ungerleider LG, Bandettini PA. Task-independent functional brain activity correlation with skin conductance changes: an fMRI study. NeuroImage. 2002;17:1797–806. doi: 10.1006/nimg.2002.1306. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Hum Brain Mapp. 2007;28:9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic contributions to social behavior. Physiol Behav. 2003;79:503–13. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Rainville P, Bechara A, Naqvi N, Damasio AR. Basic emotions are associated with distinct patterns of cardiorespiratory activity. Int J Psychophysiol. 2006;61:5–18. doi: 10.1016/j.ijpsycho.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Santos M, Uppal N, Butti C, Wicinski B, Schmeidler J, Giannakopoulos P, et al. Von Economo neurons in autism: a stereologic study of the frontoinsular cortex in children. Brain Res. 2011;1380:206–17. doi: 10.1016/j.brainres.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Schoen SA, Miller LJ, Brett-Green B, Hepburn SL. Psychophysiology of children with autism spectrum disorder. Res Autism Spectr Disord. 2008;2:417–29. [Google Scholar]
- Seth AK, Suzuki K, Critchley HD. An interoceptive predictive coding model of conscious presence. Front Psychol. 2011;2:395. doi: 10.3389/fpsyg.2011.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: an fMRI study. Soc Neurosci. 2008;3:97–112. doi: 10.1080/17470910701577020. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Schacter DL. A sensory signature that distinguishes true from false memories. Nat Neurosci. 2004;7:664–72. doi: 10.1038/nn1252. [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truax SR. Active search, mediation, and the manipulation os cue dimensions: emotion attribution in the false feedback paradigm. Motiv Emot. 1983;7:41–59. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 2008;169:249–54. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Menon V. The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev. 2009;33:1198–203. doi: 10.1016/j.neubiorev.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valins S. Cognitive effects of false heart-rate feedback. J Pers Soc Psychol. 1966;4:400–8. doi: 10.1037/h0023791. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan Van Hecke A, Lebow J, Bal E, Lamb D, Harden E, Kramer A, et al. Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Dev. 2009;80:1118–33. doi: 10.1111/j.1467-8624.2009.01320.x. [DOI] [PubMed] [Google Scholar]
- Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003;13:256–70. doi: 10.1007/s10286-003-0107-5. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dai Z, Peng H, Tan L, Ding Y, He Z, et al. Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum Brain Mapp. 2013 doi: 10.1002/hbm.22241. ; doi: 10.1002/hbm.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3rd edn. New York: Psychological Corporation; 1997. [Google Scholar]
- Weng SJ, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, et al. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–14. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JF. Intestinal pathophysiology in autism. Exp Biol Med (Maywood) 2003;228:639–49. doi: 10.1177/153537020322800601. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Moran JM, Nieto-Castanon A, Triantafyllou C, Saxe R, Gabrieli JD. Associations and dissociations between default and self-reference networks in the human brain. NeuroImage. 2011;55:225–32. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y. BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLoS ONE. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C-G, Zang Y-F. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn TP, Rumsey JM, Van Kammen DP. Autonomic nervous system activity in autistic, schizophrenic, and normal men: effects of stimulus significance. J Abnorm Psychol. 1987;96:135–44. doi: 10.1037//0021-843x.96.2.135. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Ehmke R, Mennes M, Imperati D, Castellanos FX, Sporns O, et al. Network centrality in the human functional connectome. Cereb Cortex. 2012;22:1862–75. doi: 10.1093/cercor/bhr269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.