A current strategy to harness invariant natural killer T (iNKT) cells for cancer treatment is endogenous iNKT cell activation using patient-derived dendritic cells (DCs). However, the limited number and functional defects of patient DCs are still the major challenges for this therapeutic approach. In this study, we investigated whether human embryonic stem cells (hESCs) with ectopically expressed CD1d gene could be exploited to address this issue.
Keywords: Stem cell, Dendritic cell, Invariant natural killer T cell, CD1d, Genetic modification, Immunotherapy
Abstract
Invariant natural killer T (iNKT) cells are a unique lymphocyte subpopulation that mediates antitumor activities upon activation. A current strategy to harness iNKT cells for cancer treatment is endogenous iNKT cell activation using patient-derived dendritic cells (DCs). However, the limited number and functional defects of patient DCs are still the major challenges for this therapeutic approach. In this study, we investigated whether human embryonic stem cells (hESCs) with an ectopically expressed CD1d gene could be exploited to address this issue. Using a lentivector carrying an optimized expression cassette, we generated stably modified hESC lines that consistently overexpressed CD1d. These modified hESC lines were able to differentiate into DCs as efficiently as the parental line. Most importantly, more than 50% of such derived DCs were CD1d+. These CD1d-overexpressing DCs were more efficient in inducing iNKT cell response than those without modification, and their ability was comparable to that of DCs generated from monocytes of healthy donors. The iNKT cells expanded by the CD1d-overexpressing DCs were functional, as demonstrated by their ability to lyse iNKT cell-sensitive glioma cells. Therefore, hESCs stably modified with the CD1d gene may serve as a convenient, unlimited, and competent DC source for iNKT cell-based cancer immunotherapy.
Introduction
Active immunotherapy aimed at mobilizing the human immune system to obtain therapeutic benefits has been exploited to treat cancers for decades [1]. One example of the clinical use of such a therapeutic strategy is the autologous dendritic cell (DC)-based vaccine for treatment of metastatic and asymptomatic hormone refractory prostate cancer that was recently approved by the U.S. Food and Drug Administration [2]. A possible mechanism of this prostate cancer vaccine is the induction of a tumor antigen-specific cytotoxic T lymphocyte (CTL) response that directly kills the tumor cells [3]. Despite this recent progress, the clinical application of active immunotherapy is still in its infancy. To further expand the application of immunotherapy to a broad variety of cancers, it is necessary to develop a therapeutic strategy that targets effector cells in the immune system other than CTLs.
Invariant natural killer T (iNKT) cells are a unique subpopulation of lymphocytes. In humans, these cells express invariant T-cell receptor (TCR) α chain Vα24-Jα18 paired with the semi-invariant TCR β chain Vβ11 and recognize glycolipid antigen such as α-galactosylceramide (αGC) in a CD1d-restricted manner [4]. On activation, human iNKT cells mediate antitumor activity directly by recognition of target cells or indirectly by activation of other immune cells such as natural killer (NK) cells [5–8]. Therefore, modulation of iNKT cell response may present a promising strategy to induce antitumor immunity.
Various approaches have been investigated to manipulate iNKT cell response in cancer patients. In an early study, αGC was administrated intravenously to boost iNKT cell response. This approach, however, resulted in rapid disappearance of iNKT cells from the peripheral blood, which could not be recovered by repetitive injection of αGC [9]. To improve iNKT cell response, αGC-pulsed immature or mature autologous monocyte-derived DCs (moDCs) were tested, which led to transient activation [10, 11] or sustainable expansion [12] of iNKT cells, respectively. These findings suggest that the use of αGC presented by DCs is more likely to generate preferable iNKT cell response than directly administrated αGC, and the functional status of the αGC-presenting DCs is important for this approach.
Because of the challenge of producing a clinically relevant number of competent autologous DCs for cancer patients who may need multiple injections during treatment, another cell source capable of presenting αGC, such as peripheral blood mononuclear cells (PBMCs) that had been cultured with interleukin-2 (IL-2) and granulocyte macrophage-colony stimulating factor (GM-CSF), was investigated [13, 14]. Pulsed with αGC, these PBMCs increased the number of interferon-γ (IFN-γ)-producing cells in patients, which was significantly associated with prolonged medium survival time [14]. However, a large quantity (1 × 109/m2) of such cells was required for each intravenous administration, and at least four injections were necessary to achieve immunological and clinical response [14]. Furthermore, to generate a sufficient number of these cells, two leukapheresis procedures were required [13, 14], and these procedures are invasive for patients with advanced-stage cancer. The requirement for a large number of these cells probably reflects the fact that the cells prepared by this method are a mixed cell population that contains DCs, B cells, T cells, and macrophages. Previous studies have demonstrated that distinct antigen-presenting cells (APCs) induce differential iNKT cell responses such as iNKT cell anergy [15, 16]. Using a defined population of professional APCs in the form of DCs to activate iNKT cells is more appropriate for cancer immunotherapy, as this may avoid the induction of regulatory iNKT cell response that is undesirable in such clinical circumstances. Therefore, the provision of an unlimited number of human DCs that can efficiently present iNKT cell ligands to stimulate therapeutic iNKT cell response is the key for the success of iNKT cell-based immunotherapy.
Human embryonic stem cells (hESCs) have long been used as a reliable source of human pluripotent stem cells (hPSCs) to generate functional human DCs [17–22] despite the recent use of human induced pluripotent stem cells (hiPSCs) as another source [23–25]. Previously, we have demonstrated the use of baculoviral vector for the transient overexpression of the CD1d gene in hESC-derived DCs (hESC-DCs) to enhance iNKT cell response [22]. In the current study, we investigated whether stably modified hESCs using the CD1d gene could be a cell source to produce an unlimited number of functional CD1d-overexpressing human DCs to stimulate iNKT cell response for therapeutic benefit.
Materials and Methods
Cell Culture and Generation of DCs
A hESC line, H1 (WiCell Research Institute, Madison, WI, http://www.wicell.org), was maintained on Matrigel–coated six-well plates (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) using mTeSR1 medium (StemCell Technologies, Vancouver, BC, Canada, http://www.stemcell.com) according to the manufacturer’s technical manual. A mouse bone marrow stromal cell line OP9 (American Type Culture Collection [ATTC], Manassas, VA, http://www.atcc.org) was maintained with α-MEM (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) supplemented with 20% fetal bovine serum (FBS) (HyClone, Logan, UT, http://www.hyclone.com). Human glioma cell lines U87 (ATCC) and T98G (ATCC) were cultured in MEM (Invitrogen) with 10% FBS and A172 (ATCC) in Dulbecco’s modified Eagle’s medium (Invitrogen) with 10% FBS.
To generate human DCs from hESCs, a three-step protocol described previously was used [18, 22]. First, OP9 cells were seeded on a T75 flask coated with 0.1% gelatin (Sigma-Aldrich, St Louis, MO, http://www.sigmaaldrich.com). When the culture was confluent, half of the medium was changed and the OP9 cells were overgrown for an additional 4–6 days. Hematopoietic differentiation of hESCs was then induced by coculturing 1–1.5 × 106 H1 with the overgrown OP9 cells in hESC differentiation medium composed of α-MEM supplemented with 10% FBS and 100 mM monothioglycerol (Sigma-Aldrich). The coculture was fed by replacing half of the medium on days 4 and 6. After incubation for 9 days, the differentiated hESCs were harvested by sequential digestion with 1 mg/ml collagenase IV (Invitrogen) and 0.05% trypsin-0.5 mM EDTA (Invitrogen) and further expanded in hESC differentiation medium containing 100 ng/ml GM-CSF (Peprotech, Rocky Hill, NJ, http://www.peprotech.com) in a T75 flask coated with poly 2-hydroxyethyl methacrylate (Sigma-Aldrich). After 10 days, the cells were purified by density gradient centrifugation using 25% Percoll solution (Sigma-Aldrich). To obtain DCs, the Percoll-purified cells were further cultured in DC differentiation medium composed of StemSpan serum-free expansion medium (StemCell Technologies) supplemented with lipid mixture 1 (Sigma-Aldrich), 100 ng/ml GM-CSF and 100 ng/ml IL-4 (Peprotech) for 8–12 days. To generate human moDCs, frozen human PBMCs (StemCell Technologies) were thawed and cultured on a T75 flask for 2 hours. The plastic-adherent cells were used to produce moDCs by culturing in DC differentiation medium for 6 days.
To study iNKT cell response, frozen human PBMCs from healthy donors were thawed and cultured in complete RPMI 1640 medium, which contains RPMI 1640 (Invitrogen) supplemented with 10% heat-inactivated human serum AB (Gemini Bio-Products, West Sacramento, CA, http://www.gembio.com), 2 mM l-glutamine (Invitrogen), 0.1 mM nonessential amino acids (Invitrogen), and 0.1 mM 2-mercaptoethanol (Invitrogen). The human peripheral blood lymphocytes (PBLs) were generated from PBMCs by plastic adherence for 2 hours.
Lentivector Preparation, Transduction, and Modification of hESCs
To produce lentivectors, two transfer plasmids were generated. To construct a transfer plasmid containing the CD1d gene driven by human cytomegalovirus immediate early promoter (CMV promoter), the coding sequence of human CD1d was cloned from pORF9-hCD1D (Invivogen, San Diego, CA, http://www.invivogen.com) by PCR and inserted into pLenti6/V5-D-TOPO (Invitrogen). To construct another transfer plasmid containing the CD1d gene driven by human elongation factor-1α promoter (EF1α promoter), the CD1d sequence was cloned by PCR to include the Kozak sequence upstream of its start codon and NheI and BamHI restriction sites at its termini. These two sites were used to insert the CD1d gene into pCDH-EF1-MCS-IRES-Puro (System Biosciences, Mountain View, CA, http://www.systembio.com). Lentivectors, named LV.pCMV.CD1d and LV.pEF1α.CD1d, were produced by cotransfecting 293FT cells (Invitrogen) using the above-described constructs together with packaging plasmids according to the user manual for ViraPower Lentiviral Expression Systems (Invitrogen). Virus titers were determined using 293FT by transduction with virus after serial dilution and subsequent antibiotic selection.
To test the transduction efficiency, the lentivectors were used to infect U87 cells or H1 cells with the indicated multiplicity of infection (MOI) during the 6-hour incubation. Two to 5 days after transduction, the transduced cells were harvested and stained with PE mouse anti-human CD1d antibody (BD Biosciences). The CD1d expression was analyzed by a FACSCalibur flow cytometer (BD Biosciences). In some experiments, the transduced H1 cells using LV.pCMV.CD1d were selected under 2 μg/ml Blasticidin (Invitrogen) for 2 weeks before analysis of CD1d expression. To generate homogeneous H1 lines with CD1d overexpression, small H1 clumps were seeded on Matrigel-coated six-well plate at a low density. Two days later, the H1 cells were transduced by incubating with LV.pEF1α.CD1d at an MOI of 0.1 for 6 hours. Antibiotic selection was started with 1 μg/ml puromycin (Merck KGaA, Darmstadt, Germany, http://www.emdgroup.com) 3 days after transduction. One week after drug selection, to further increase the homogeneity, small and isolated H1 colonies were further dissected into smaller pieces using glass capillaries and transferred to organ culture dishes (BD Biosciences) (one piece per dish) under a stereomicroscope (Leica, Wetzlar, Germany, http://www.leica.com) for amplification. These genetically modified H1 lines were maintained with 1 μg/ml puromycin or in some experiments further selected with 3 μg/ml puromycin. The CD1d expressions in these modified hESC lines during long-term maintenance were monitored by flow cytometry.
Expansion, Detection, and Cytotoxicity of iNKT Cells
Human DCs were generated from various modified hESC lines and tested for their capability to expand iNKT cells in human PBLs. First, these hESC-DCs were pulsed with 100 ng/ml αGC (Axxora, Lausen, Switzerland, http://www.axxora.com). One day later, 1 × 105 αGC-pulsed DCs were washed and cocultured with 1 × 106 PBLs in complete RPMI 1640 medium. To detect iNKT cells, the samples were collected 7 days after coculture, stained with APC mouse anti-human CD3 (BD Biosciences), PE anti-Vα24 TCR (Beckman Coulter, Fullerton, CA, http://www.beckmancoulter.com), and FITC anti-Vβ11 TCR (Beckman Coulter), and analyzed by a FACSAria flow cytometer (BD Biosciences). For each sample, 500,000 events were collected. To investigate the effect of CD1d overexpression on DCs, these hESC-DCs were stained with both APC mouse anti-human CD209 antibody (BD Biosciences) and PE mouse anti-human CD1d antibody and sorted into CD209+CD1dlow subset with low-level to undetectable CD1d expression and CD209+CD1dhigh subset with high-level CD1d expression using the FACSAria flow cytometer. In some experiments, the hESC-DCs were pretreated with anti-CD1d blocking antibody (eBioscience, San Diego, CA, http://www.ebioscience.com) or its isotype control (eBiosciecne). These sorted hESC-DC subsets or the pretreated hESC-DCs were then used to stimulate iNKT cell expansion.
To study the cytotoxicity of the expanded iNKT cells against tumor cells, bulk cocultures were set up using 2 × 106 αGC-pulsed hESC-DCs and 20 × 106 PBLs. After incubation for 10 days, the cocultures were washed and restimulated with 2 × 106 αGC-pulsed hESC-DCs. Four days after restimulation, the bulk cultures were harvested and used as effector cells to incubate with tumor cells T98G or A172 at desired effector to target ratios. Cell-mediated cytotoxicity was measured using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI, http://www.promega.com) after a 6-hour incubation.
Detection of Allostimulatory Function, Cytokine Production, and CTL Expansion
To measure the allostimulatory function of hESC-DCs, frozen human peripheral blood pan-T cells (StemCell Technologies) were thawed and used as responders. The pan-T cells were resuspended in phosphate-buffered saline (PBS) containing 5% heat-inactivated FBS (107 cells per milliliter). Carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen) was added at a final concentration of 0.5 μM to label the cells in the dark at room temperature for 5 minutes. After washing three times with PBS containing 5% heat-inactivated FBS, the cells were resuspended in complete RPMI 1640 medium at a density of 106/ml. Cocultures were set up with hESC-DCs and 2 × 105 CFSE-labeled pan-T cells at the desired DC:T cell ratios. After incubation for 5 days, the cells were stained with APC mouse anti-human CD3 antibody and the T cell proliferation was measured by CFSE dilution using the FACSAria flow cytometer.
To study the cytokine profile during the coculture of hESC-DCs and PBLs, 105 hESC-DCs were incubated with 106 PBLs in 300 µl of complete RPMI 1640 medium. Three days later, the supernatants were collected. The cytokine concentration was then measured with a cytometric bead array (CBA) assay using the CBA Human Soluble Protein Master Buffer Kit and the CBA Flex Sets that detect INF-γ, IL-4, and tumor necrosis factor (TNF) (BD Biosciences).
To stimulate tumor antigen-specific CTL response, the HLA-A2+ H1.DCs or C1.3.DCs were used to present the HLA-A2-restricted epitope MART-126-35A27L (ELAGIGILTV; MART-1 peptide; ProImmune, Oxford, U.K., http://proimmune.com/ecommerce/index.php). The hESC-DCs were first pulsed with or without 100 ng/ml α-GC for 24 hours. The hESC-DCs were then washed and pulsed with 10 μg/ml MART-1 peptide for 4 hours. After washing, 105 hESC-DCs were cocultured with 106 HLA-A2+ PBLs in complete RPMI 1640 medium. To detect MART-1 peptide-specific CTLs, the samples were collected 9 days after coculture, stained with APC mouse anti-human CD3, FITC-labeled anti-CD8 (ProImmune) and R-PE-labeled A*0201/ELAGIGILTV Pentamer (ProImmune), and analyzed using the FACSAria flow cytometer.
Statistical Analysis
The statistical significance of difference was determined by the two-sided Student’s t test. A p value of <.05 was considered statistically significant.
Results
Transgene Expression Construct Is Crucial in hESC Engineering
To stably express the human CD1d gene in hESCs and thereafter in their DC derivatives, a transfer plasmid containing two transgene expression cassettes, the CD1d gene driven by a CMV promoter and a Blasticidin resistance gene driven by a SV40 promoter, was initially used to produce lentivector, LV.pCMV.CD1d (Fig. 1A). The viral transduction activity at an MOI of 10 was evaluated using flow cytometry (Fig. 1B). The results showed that up to 99% of the U87 cells displayed CD1d expression 2 days after transduction; however, only 3% of the H1 cells expressed CD1d 5 days after transduction (Fig. 1B). This observation suggests that H1 cells are not susceptible to transduction by LV.pCMV.CD1d, probably because of the low activity of CMV promoter in hESCs [26, 27]. To enrich the CD1d-expressing H1 cells, Blasticidin was used to select the transduced hESCs. Although Blasticidin-resistant colonies were generated after 2-week selection, the CD1d expression remained at a low level in these drug-resistant H1 cells (Fig. 1C), indicating the separation of CD1d and drug resistance gene expression using this construct. Interestingly, when these Blasticidin-resistant H1 cells were used to generate DCs, we were able to obtain a substantial amount of CD1d-overexpressing hESC-DCs, but not with the unmodified parental H1 cells (supplemental online Fig. 1); however, the yields of these CD1d-overexpressing hESC-DCs were inconsistent among different batches of differentiation.
Figure 1.
The transgene expression cassette is crucial in hESC engineering. (A): Structure of lentivector LV.pCMV.CD1d. (B): Transient CD1d expression in U87 and H1 after transduction with LV.pCMV.CD1d. The CD1d expression in U87 and H1 after transduction at a multiplicity of infection of 10 were analyzed by flow cytometry on day 2 and 5 respectively. (C): The drug-resistant H1 line was generated using LV.pCMV.CD1d. The transduced H1 cells were selected for 2 weeks with Blasticidin. A phase contrast image shows the morphology of the Blasticidin-selected H1 cells. The CD1d expression in these drug-resistant H1 cells was analyzed by flow cytometry. Histograms in (B) and (C) show staining by antibody against CD1d (black lines) and its isotype control (dotted lines). The percentages of the positive cells is indicated. Scale bar = 200 μm.
Generation of hESC Lines With Stable CD1d Expression Using an Optimized Transgene Expression Construct
Based on the above observation, we optimized the transgene expression construct for genetic modification of hESCs (Fig. 2A). In this optimized construct, an EF1α promoter instead of a CMV promoter was used to drive CD1d expression; a puromycin resistance gene and the CD1d gene separated by IRES are expressed under the EF1α promoter in a single expression cassette. Lentivector LV.pEF1α.CD1d (Fig. 2A) was produced to transduce H1 cells. Using this lentivector, we were able to improve the CD1d expression in hESCs. As shown in Figure 2B, a dose-response CD1d expression was observed after transduction with the indicated MOIs; with an MOI of 10, up to 19% of H1 cells became CD1d+ 3 days after transduction, suggesting that this new construct is more suitable for genetic modification of hESCs.
Figure 2.
Generation of hESC lines with stable CD1d expression. (A): Structure of lentivector LV.pEF1α.CD1d. (B): Transient CD1d expression in H1 cells after transduction with LV.pEF1α.CD1d. The CD1d expression was analyzed by flow cytometry 3 days after transduction at the indicated MOI. (C–E): Generation of homogeneous modified H1 lines. To increase the homogeneity of the modified H1 lines, small H1 clumps were seeded at a low density as shown in (C). Scale bars = 500 μm. These H1 cells were then transduced with LV.pEF1α.CD1d at an MOI of 0.1 and selected with puromycin for 1 week. The resulting drug-resistant H1 colonies are indicated by arrows (D). To further increase the purity of the modified H1 lines, the H1 colonies were dissected into small clumps. The resultant clumps were further expanded individually. A typical H1 line generated from a single clump is shown in (E). (F): Long-term CD1d expression in genetically modified H1 lines C1–C4. The CD1d expression was analyzed by flow cytometry from 4 to 22 weeks after selection with 1 μg/ml puromycin. (G): Stabilized CD1d expression by increased selection pressure. Modified H1 lines C1 and C2 were further selected using 3 μg/ml puromycin. The CD1d expression in the resultant sublines C1.3 and C2.3 was analyzed by flow cytometry after 4-week selection. Histograms in (B), (F), and (G) show staining by antibody against CD1d (black lines) and its isotype control (dotted lines). The percentages of the positive cells are indicated. In (F) and (G), the changes in mean fluorescence intensity are also shown in parentheses. Abbreviations: hESC, human embryonic stem cell; IRES, internal ribosome entry site; MOI, multiplicity of infection; WPRE, woodchuck post-transcriptional response element.
To derive hESCs with stable CD1d expression, it is desirable to reduce the copy number of the integrated transgene and increase the homogeneity of the transduced hESCs. Thus, the following method was used to achieve these properties. First, small H1 clumps were seeded at a low density so that the cells were approximately 1% confluent at the time of transduction 2 days later (Fig. 2C). An MOI of 0.1 and a short incubation period of 6 hours with the vectors were used to minimize the integrated transgene copy, and the drug selection process was started 3 days after transduction with 1 μg/ml puromycin. One week after drug selection, puromycin-resistant H1 colonies were observed (Fig. 2D). Those small and separated H1 colonies were further dissected into clumps of approximately 100 cells and transferred to organ culture dishes for amplification under puromycin; Figure 2E shows an expanded colony that originated from one such produced small H1 clump. Such generated H1 cell lines were then monitored for their CD1d expression along the stem cell maintenance process. As shown in Figure 2F, some of these lines, such as the C1 and C2 lines, had a higher percentage of CD1d expression than other lines, such as the C3 and C4 lines. The surface CD1d molecules in the C1 and C2 lines were stably expressed during the long-term maintenance, as demonstrated by flow cytometry analysis from 4 to 22 weeks after drug selection (Fig. 2F). These modified H1 lines showed normal hESC morphology and stained positive for pluripotency markers (supplemental online Fig. 2). Further selection of the C1 and C2 lines under 3 μg/ml puromycin for 4 weeks were able to generate sublines C1.3 and C2.3, which possessed a higher CD1d expression level than their parental lines (Fig. 2G).
Derivation of CD1d-Overexpressing Human DCs From Modified hESCs
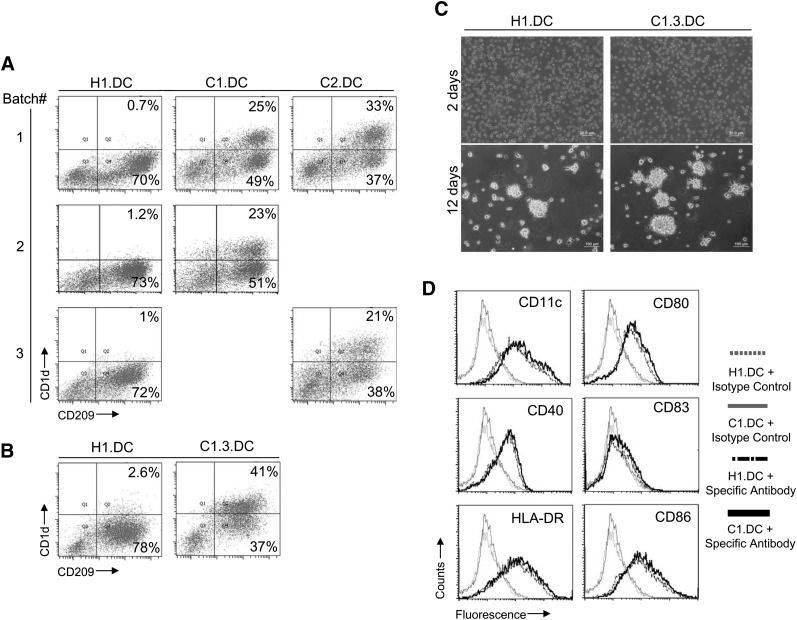
To investigate the feasibility of generating CD1d-overexpressing human DCs from the CD1d-overexpressing hESCs, the modified hESCs were differentiated into DCs using a three-step protocol [18, 22]. Flow cytometry analysis showed that the modified hESC lines, C1, C2, and C1.3, were able to differentiate into DCs as efficiently as the unmodified H1 line (Fig. 3A, 3B); generally, approximately 60%–80% of the total cells were positively stained by an antibody against CD209 after DC differentiation, a DC surface marker (Fig. 3A, 3B). Moreover, starting with the C1 and C2 lines, approximately 21%–33% of the total cells were CD209+CD1d+, which was significantly higher than the 1%–2% from the parental H1 line (Fig. 3A). A further increase in the yield of the CD1d-overexpressing human DCs was achieved by using the C1.3 subline, which was selected to have a higher CD1d expression level (Fig. 3B). Starting with the C1.3 line, up to 41% of the total cells were CD209+CD1d+, which was more than 50% of the generated DCs (Fig. 3B).
Figure 3.
Derivation of CD1d-overexpressing human DCs from modified hESCs. (A): CD1d expression in human DCs generated from modified H1 lines. The modified H1 lines, C1 and C2, were used to generate human DCs. The total cells after differentiation were stained with anti-CD209 and anti-CD1d antibodies and analyzed by flow cytometry. The numbers in the dot plots indicate the percentages of CD1d+CD209+ and CD1d-CD209+ cells in the total cells. (B): Increased yield of CD1d-overexpressing human DC using modified H1 line C1.3. (C): Morphology of human DCs derived from hESC lines H1 and C1.3 during myeloid differentiation process. Scale bar = 200 μm (2 days) and 100 μm (12 days). (D): Phenotypes of human DCs derived from hESC lines H1 and C1. The DCs were stained and analyzed by flow cytometry. Histograms show the staining by specific antibodies against indicated markers and their isotype controls. Abbreviations: DC, dendritic cell; hESC, human embryonic stem cell.
Morphologically, there was no difference between the DCs generated from the modified hESC line and those generated from the parental line (Fig. 3C). As demonstrated in Figure 3C, the myeloid cells derived from both the H1 line and its modified version C1.3 line grew as separated cells after culturing in DC differentiation medium for 2 days, whereas typical clusters of DCs were observed after culturing for 12 days. Phenotypically, the human DCs generated from the modified hESC line were similar to those from the parental line (Fig. 3D). They all expressed typical DC surface markers, such as CD11c, CD40, HLA-DR, CD80, CD86, and a small amount of CD83 (Fig. 3D). These results imply that hESCs stably modified with the CD1d gene are able to generate a substantial amount of CD1d-overexpressing human DCs.
Expansion of Human iNKT Cells Using DCs Derived From CD1d-Overexpressing hESCs
To study the function of human DCs derived from the stably modified CD1d-overexpressing hESCs, the generated hESC-DCs were pulsed with αGC and used to induce iNKT cell expansion by coculturing with human PBLs. As shown in Figure 4A, the frequency of iNKT cells remained very low after culturing for 7 days without DC stimulation. DC stimulation alone with the H1-derived DCs (H1.DCs) or with the C1.3-derived DCs (C1.3.DCs) induced no observable iNKT cell expansion; pulsation with αGC was necessary to induce significant iNKT cell expansion with the H1.DCs or the C13.DCs. More importantly, the CD1d-overexpressing C1.3.DCs derived from the stably modified hESC line C1.3 were more efficient than the H1.DCs derived from the unmodified parental line H1 in presenting αGC for iNKT cell expansion. Thus, stable modification of hESCs with the CD1d gene enabled the generation of DCs that were more efficient at inducing iNKT cell expansion. Furthermore, the efficiency of such modified hESC-derived human DCs to induce iNKT cell expansion was compared with that of conventionally used monocyte-derived DCs, and the results showed that the C1.3.DCs were functionally comparable to the autologous moDCs generated from healthy donors (Fig. 4B).
Figure 4.
Expansion of human iNKT cells using DCs derived from CD1d-overexpressing hESCs. (A): Expansion of human iNKT cells induced by CD1d-overexpressing human DCs generated from modified hESC line C1.3. The αGC-pulsed DCs were cocultured with PBLs to expand iNKT cells. The samples were then stained and analyzed by flow cytometry 7 days after coculture. Quantitative analysis of the experiments (mean ± SD, n = 4) is shown. The statistical significance of differences was determined by the two-sided Student’s t test. A p value of <.05 was considered statistically significant. (B): Comparison of CD1d-overexpressing C1.3-derived DCs and healthy autologous moDCs for iNKT cell expansion. Quantitative analysis of the experiments (mean ± SD, n = 4) is shown. The statistical significance of differences was determined by two-sided Student’s t test. Abbreviations: DC, dendritic cell; αGC, α-galactosylceramide; hESC, human embryonic stem cell; iNKT, invariant natural killer T; moDC, monocyte-derived dendritic cell; PBL, peripheral blood lymphocyte.
Surface CD1d Expression on Modified hESC-Derived DCs Is Important for Enhancing iNKT Cell Expansion
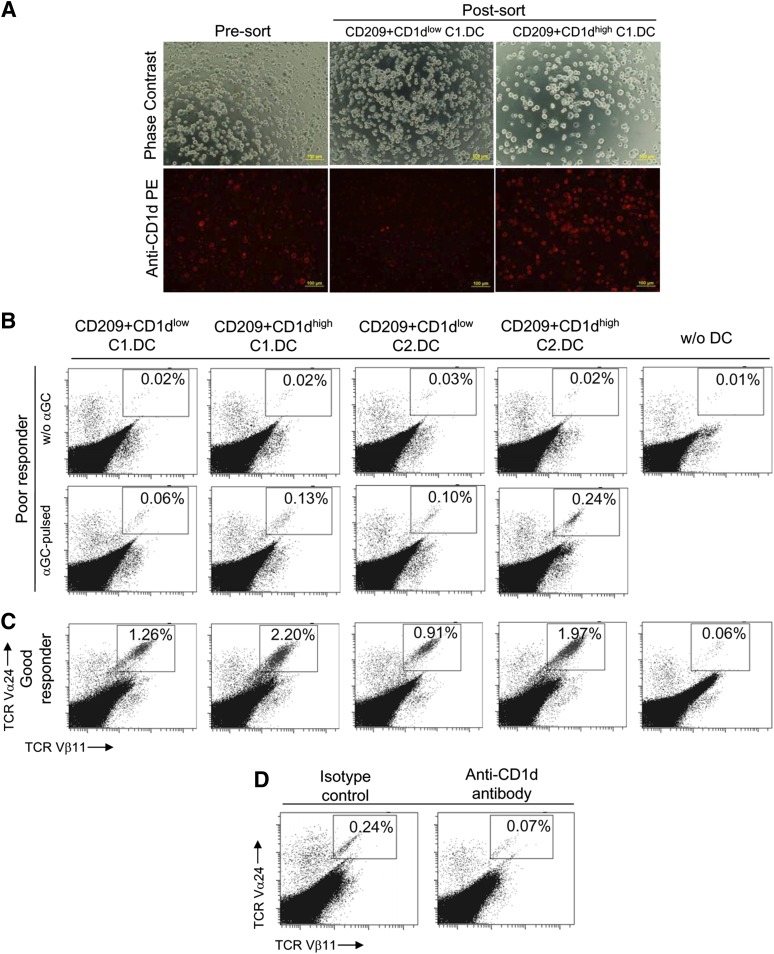
To understand the relationship between the surface CD1d expression on hESC-DCs and the ability to induce iNKT cell expansion, the DCs derived from the stably modified hESCs were further sorted into a CD1dlow subset with low-level to undetectable CD1d expression and a CD1dhigh subset with high-level CD1d expression before using for iNKT cell stimulation. In Figure 5A, phase contrast and fluorescence images of live cells showed the presort C1-derived DCs (C1.DCs) and the postsort CD209+CD1dlow and CD209+CD1dhigh C1.DC subpopulations. The efficiencies of these two subpopulations in inducing iNKT cell expansion were compared. As demonstrated using PBLs from a donor with low iNKT cell frequency (Fig. 5B), pulsing with αGC was important for inducing iNKT cell expansion. Pulsed with αGC, the sorted DCs were able to induce iNKT cell expansion. The CD1dhigh DC subsets were more efficient than the CD1dlow DC subsets, as shown with hESC-DCs derived from two different modified hESC lines, C1 and C2. Similar results were obtained using PBLs from a donor with higher initial iNKT cell frequency (Fig. 5C), wherein robust iNKT cell expansions were observed, which again demonstrated the advantage of using the CD1dhigh DC populations in amplifying the iNKT cells over the CD1dlow populations. Moreover, blocking the surface CD1d molecules on unsorted C1.DCs with anti-CD1d antibody reduced the DC efficacy for iNKT cell expansion (Fig. 5D). These results indicate the importance of surface CD1d expression on DCs for enhancing iNKT cell response.
Figure 5.
Surface CD1d expression on modified hESC-derived DCs is important for enhancing iNKT cell expansion. (A): Isolation of CD1dhigh and CD1dlow human DCs generated from modified hESC line C1 using flow cytometry. Phase contrast and florescence images of live cells show the presort and postsort populations. Scale bar = 100 μm. (B, C): Comparison of CD1dhigh- and CD1dlow-modified hESC-derived DCs for iNKT cell expansion. The two populations were sorted, pulsed with αGC and cocultured with PBLs to expand iNKT cells. The samples were stained and analyzed by flow cytometry 7 days after coculture. The dot plots show the iNKT cell expansion using PBLs from donors with low iNKT cell frequency (B) and high iNKT cell frequency (C). (D): Reduction of iNKT cell expansion ability of CD1d-overexpressing C1-derived DCs by blocking CD1d. The unsorted C1-derived DCs were pretreated with anti-CD1d blocking antibody or its isotype control before using for iNKT cell expansion. The dot plots in (B–D) show the iNKT cell expansion, and the percentages of iNKT cells in total T cells are indicated. Abbreviations: αGC, α-galactosylceramide; DC, dendritic cell; hESC, human embryonic stem cell; iNKT, invariant natural killer T; PBL, peripheral blood lymphocyte; w/o, without.
Cytotoxicity of iNKT Cells Expanded by DCs Derived From CD1d-Overexpressing hESCs Against Tumor Cells
To test the tumor lysis activity of iNKT cells expanded by CD1d-overexpressing modified hESC-derived DCs, bulk coculture of PBLs with αGC-pulsed hESC-DCs was used to expand iNKT cells. As shown in Figure 6A, marked iNKT cell expansion was observed in the 10-day bulk culture after an initial stimulation with the CD1d-overexpressing C1.3.DCs, but less with the unmodified H1.DCs. These 10-day bulk cultures were then subjected to a second stimulation with their respective αGC-pulsed hESC-DCs. During the restimulation, the C1.3.DCs continued to induce intensive iNKT cell expansion, whereas only modest expansion was observed with H1.DCs, as demonstrated by flow cytometry analysis of the 13-day bulk culture (Fig. 6A). Bulk culture of PBLs alone without αGC-pulsed hESC-DCs showed no obvious expansion of iNKT cells (Fig. 6A). This result suggests that the CD1d-overexpressing modified hESC-derived DCs can be useful in large-scale generation of human iNKT cells by repetitive in vitro stimulation.
Figure 6.
Cytotoxicity of iNKT cells expanded by DCs derived from CD1d-overexpressing hESCs against tumor cells. (A): Expansion of iNKT cells in bulk coculture by CD1d-overexpressing C1.3-derived DCs. Bulk cocultures were set up using αGC-pulsed hESC-DCs and PBLs. After incubation for 10 days, the bulk cocultures were washed and restimulated with the respective αGC-pulsed hESC-DCs. Bulk culture of PBLs alone without αGC-pulsed hESC-DCs was used as a control. The percentages of iNKT cells in the bulk cocultures were analyzed by flow cytometry at the indicated time points. The percentages of iNKT cells in total T cells are shown in the dot plots. (B): Cytotoxicity of expanded iNKT cells against glioma cell lines. Fourteen-day bulk cocultures were harvested and used as effector cells to lyse glioma cells T98G or A172 at the indicated E:T ratios. Abbreviations: DC, dendritic cell; E:T, effector to target; αGC, α-galactosylceramide; hESC, human embryonic stem cell; iNKT, invariant natural killer T; PBL, peripheral blood lymphocyte; TCR, T-cell receptor; w/o, without.
Direct tumor lysis function of such expanded iNKT cells was further tested 4 days after restimulation against CD1d-positive T98G and CD1d-negative A172 [28]. The iNKT cells expanded by C1.3.DCs showed more cytotoxicity against CD1d-expressing T98G cells than those expanded by H1.DCs, whereas neither iNKT cell population was able to lyse CD1d-negative A172 cells (Fig. 6B). This result indicates the potential of direct cytotoxicity of such expanded iNKT cells against CD1d-expressing tumors.
Immunostimulatory Functions of DCs Derived From CD1d-Overexpressing hESCs
To further assess the immunostimulatory function of DCs derived from CD1d-overexpressing hESCs, the ability of hESC-DCs to stimulate allogeneic T-cell proliferation was measured by a CFSE-based assay. C1.3.DCs derived from the modified hESCs were able to stimulate allogeneic T cell proliferation in a dose-response manner similar to that induced by H1.DCs (Fig. 7A). To investigate the cytokine profile induced by C1.3.DCs, hESC-DCs were cocultured with PBLs for 3 days, and the supernatants were analyzed by CBA assay. The results showed that C1.3.DCs induced more IFN-γ production than H1.DCs (Fig. 7B). In terms of IL-4 and TNF production, there was no difference between H1.DCs and C1.3.DCs (Fig. 7B). To evaluate the capability of C1.3.DCs to present antigens other than αGC, hESC-DCs were pulsed with MART-1 peptide with or without preloading of αGC. These DCs were then cocultured with HLA-A2+ PBLs for 9 days and the MART-1 peptide-specific CTL responses were analyzed by pentamer staining. Without preloading of αGC, C1.3.DCs were more efficient than H1.DCs in inducing MART-1 peptide-specific CD8+ T cell response; in contrast, the preloading of αGC obviously reduced T cell priming ability of C1.3.DCs (Fig. 7C). These results are similar to our previous observations using H1.DCs with CD1d-upregulation mediated by baculoviral vector [22].
Figure 7.
Immunostimulatory functions of DCs derived from CD1d-overexpressing hESCs. (A): Allostimulatory function of CD1d-overexpressing C1.3-derived DCs. The hESC-DCs were incubated with CFSE-labeled pan-T cells at the indicated ratios. After incubation for 5 days, the T cell proliferation was measured by CFSE dilution via flow cytometry. The percentages of the divided T cells are indicated. (B): Cytokine profile induced by CD1d-overexpressing C1.3-derived DCs. The hESC-DCs were incubated with PBLs for 3 days. The supernatants were collected and the cytokine concentration was measured by cytometric bead array assay. Results represent mean ± SD, n = 4. The p values are derived from the two-sided Student’s t test. (C): Induction of tumor antigen-specific T-cell response by CD1d-overexpressing C1.3-derived DCs. The hESC-DCs were first pulsed with or without αGC for 24 hours and then pulsed with MART-1 peptide for 4 hours. These hESC-DCs were cocultured with HLA-A2+ PBLs for 9 days. The samples were stained with anti-CD3, anti-CD8, and A*0201/ELAGIGILTV Pentamer and analyzed by flow cytometry. The numbers in the representative contour plots indicate the percentage of Pentamer+ CD8+ cells in the CD3+ population. Abbreviations: CFSE, carboxyfluorescein succinimidyl ester; DC, dendritic cell; αGC, α-galactosylceramide; hESC, human embryonic stem cell; IFN, interferon; IL, interleukin; PBL, peripheral blood lymphocyte; TNF, tumor necrosis factor.
Discussion
Several antitumor mechanisms of the activated iNKT cells have been proposed. (a) Direct cytotoxicity against CD1d-positive tumors is one possible mechanism because the fully activated iNKT cells express a large number of cell-death-inducing molecules, such as FasL, TRAIL, and perforin/granzyme [29], which directly kill tumor cells in hematological malignancies (e.g., leukemia, lymphoma, and myeloma) as well as in some solid tumors (e.g., glioma and medulloblastoma) [30]. (b) The activation of other immune cells with antitumor activities has also been proposed. The activated iNKT cells produce a large amount of INF-γ, which induces antitumor responses of NK cells and CTLs [31]. (c) Reversal of the immunosuppressive state of tumor microenvironment by activated iNKT cells is another possible mechanism. It has been reported that iNKT cells inhibit the function of immunosuppressive cells such as myeloid-derived suppressor cells [32] and tumor-associated macrophages [33]. (d) Finally, inhibition of tumor angiogenesis has been considered. The activated iNKT cells inhibit intradermal tumor angiogenesis in mice by activating NK cells that subsequently release higher levels of IFN-γ [34]. In human gliomas, the CD1d-expressing neovasculature could be directly targeted by activated iNKT cells [28]. Clinical data suggest that infiltration of iNKT cells in tumors is significantly related to an improved long-term disease-free survival in patients with neuroblastoma [35] and colon cancer [36]; in contrast, a severe deficiency in peripheral blood iNKT cells is associated with poor prognosis in patients with head and neck cancer [37]. Moreover, induction of NKT cell-dependent immune responses is significantly associated with survival benefit in patients with lung cancer [14]. Thus, immunological manipulation of iNKT cell response via active immunization may provide a promising therapeutic strategy for cancer patients.
To exploit the antitumor activities of iNKT cells, endogenous iNKT cell activation using human APCs (such as DCs) loaded with iNKT cell ligand is a popular strategy in clinical trials [10–14]. However, it is still challenging to harness the antitumor properties of iNKT cells efficiently in a clinical setting. This is because the iNKT cell frequency in humans is much lower than in mice [38] and the cell number is further decreased in cancer patients [39–44]. Hence, a large number of competent DCs are necessary to target and activate human iNKT cells. To address this challenge, we have demonstrated that CD1d overexpression in human DCs using baculoviral vector promotes human iNKT cell expansion [22]. Although it is possible to apply this strategy to enhance patient-derived in vitro generated moDCs for iNKT cell activation, such patient-originated DCs are limited in number and may inherit the same poor function of their circulating counterparts in terms of activating iNKT cells [45, 46]. To this end, an unlimited DC source of consistent quality is desirable, and human pluripotent stem cells such as hESCs can be used to derive such DCs [17–22, 24, 25]. Although baculoviral transduction is capable of overexpressing the CD1d gene in hESC-DCs for efficient activation of human iNKT cells [22], the involvement of genetic modification for each batch of hESC-DCs reduces the attractiveness of such an approach for large-scale industrial production and clinical application. Therefore, to fully use the unique properties of human pluripotent stem cells, we explored the possibility of using hESCs genetically modified with the CD1d gene to generate the CD1d-overexpressing human DCs.
To therapeutically improve hESC-derived DCs, integrating a therapeutic gene into the hESC genome and then deriving DCs from these modified hESCs is a rational approach. It is crucial to design expression construct for stem cell engineering so that the transgene can resist silencing during the differentiation process to express sufficiently in DCs. Thus, we generated various lentivectors to modify hESCs to determine a suitable transgene expression construct for this application. In our initial study, a lentivector containing two independent expression cassettes, the CMV promoter-driven human CD1d gene and the SV40 promoter-driven Blasticidin resistance gene, was used. This vector efficiently expressed CD1d in U87 cells but not in hESCs. Although Blasticidin-resistant hESCs were obtained after drug selection, the percentage of CD1d+ cells remained low in these selected hESCs. This disparity in the expression of CD1d gene and drug resistance gene is possibly the result of the differential strength of their respective promoters. To solve this problem, we used another vector in which the CD1d gene and a puromycin resistance gene separated by IRES were expressed under an EF1α promoter in a single expression cassette. This optimized lentivector improved CD1d expression in hESCs. After puromycin and mechanical selection, homogeneous modified hESC lines were generated, in which up to 87% of the cells were CD1d+. The constitutive expression of CD1d, a nonclassical class I-like major histocompatibility complex molecule, did not affect the morphology and phenotype of the modified hESCs or their subsequent differentiation into human DCs. The CD1d expression appeared stable in these modified hESC lines during long-term maintenance. Moreover, the CD1d-overexpressing hESCs were able to generate DCs as efficiently as the unmodified hESCs. Most importantly, more than 50% of the derived DCs remained CD1d+. These results suggest that optimization of transgene expression construct is important for stem cell engineering to efficiently derive functionally modified DCs.
The human DCs generated from the stably modified hESCs were similar to those from the parental hESCs in terms of morphology and phenotype. Functionally, these modified DCs were more efficient in inducing iNKT cell expansion. The hESC-DCs with high-level expression of CD1d were more competent to stimulate iNKT cell response than those with low-level expression; blocking the surface CD1d using antibody reduced the efficiency of CD1d-overexpressing hESC-DCs. These observations suggest that CD1d introduced via genetic manipulation at the hESC stage is crucial for enhancing iNKT cell ligand presentation in the hESC-DCs. In addition, the use of these CD1d-overexpressing hESC-DCs did not cause iNKT cell anergy, which was demonstrated by the responsiveness of the activated iNKT cells to restimulation and the cytotoxicity of the expanded iNKT cells against the target tumor cells. In addition to endogenous iNKT cell activation, adoptive transfer of in vitro expanded iNKT cells is another possible strategy for iNKT cell-based cancer immunotherapy. Its combination with the use of iNKT cell ligand-pulsed DCs may provide better clinical outcome [47, 48]. To produce the required amount of human iNKT cells for adoptive immunotherapy, multiple stimulations with iNKT cell ligand-loaded human DCs are necessary. The CD1d-overexpressing hESC-DCs may provide an accessible DC source for this purpose, as demonstrated by their iNKT cell expansion ability in the bulk cell culture in this study.
Conclusion
In summary, our study demonstrated that human DCs generated from stably modified hESCs using the CD1d gene may provide a convenient, unlimited, and competent DC source for iNKT cell-based cancer immunotherapy.
Supplementary Material
Acknowledgments
This work was supported by the Institute of Bioengineering and Nanotechnology (Biomedical Research Council, Agency for Science, Technology and Research, Singapore). We thank other laboratory members for helpful discussion and support.
Author Contributions
J.Z.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; S.W.: data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeFrancesco L. Landmark approval for Dendreon’s cancer vaccine. Nat Biotechnol. 2010;28:531–532. doi: 10.1038/nbt0610-531. [DOI] [PubMed] [Google Scholar]
- 3.Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 4.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 5.Kawano T, Nakayama T, Kamada N, et al. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NKT cells. Cancer Res. 1999;59:5102–5105. [PubMed] [Google Scholar]
- 6.Nicol A, Nieda M, Koezuka Y, et al. Human invariant valpha24+ natural killer T cells activated by alpha-galactosylceramide (KRN7000) have cytotoxic anti-tumour activity through mechanisms distinct from T cells and natural killer cells. Immunology. 2000;99:229–234. doi: 10.1046/j.1365-2567.2000.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieda M, Nicol A, Koezuka Y, et al. TRAIL expression by activated human CD4(+)V alpha 24NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells. Blood. 2001;97:2067–2074. doi: 10.1182/blood.v97.7.2067. [DOI] [PubMed] [Google Scholar]
- 8.Metelitsa LS, Naidenko OV, Kant A, et al. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167:3114–3122. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- 9.Giaccone G, Punt CJ, Ando Y, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 10.Okai M, Nieda M, Tazbirkova A, et al. Human peripheral blood Valpha24+ Vbeta11+ NKT cells expand following administration of alpha-galactosylceramide-pulsed dendritic cells. Vox Sang. 2002;83:250–253. doi: 10.1046/j.1423-0410.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- 11.Nieda M, Okai M, Tazbirkova A, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 12.Chang DH, Osman K, Connolly J, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishikawa A, Motohashi S, Ishikawa E, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 14.Motohashi S, Nagato K, Kunii N, et al. A phase I-II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol. 2009;182:2492–2501. doi: 10.4049/jimmunol.0800126. [DOI] [PubMed] [Google Scholar]
- 15.Parekh VV, Wilson MT, Olivares-Villagómez D, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaura A, Hotta C, Nakazawa M, et al. Human invariant Valpha24+ natural killer T cells acquire regulatory functions by interacting with IL-10-treated dendritic cells. Blood. 2008;111:4254–4263. doi: 10.1182/blood-2007-04-085142. [DOI] [PubMed] [Google Scholar]
- 17.Zhan X, Dravid G, Ye Z, et al. Functional antigen-presenting leucocytes derived from human embryonic stem cells in vitro. Lancet. 2004;364:163–171. doi: 10.1016/S0140-6736(04)16629-4. [DOI] [PubMed] [Google Scholar]
- 18.Slukvin II, Vodyanik MA, Thomson JA, et al. Directed differentiation of human embryonic stem cells into functional dendritic cells through the myeloid pathway. J Immunol. 2006;176:2924–2932. doi: 10.4049/jimmunol.176.5.2924. [DOI] [PubMed] [Google Scholar]
- 19.Senju S, Suemori H, Zembutsu H, et al. Genetically manipulated human embryonic stem cell-derived dendritic cells with immune regulatory function. Stem Cells. 2007;25:2720–2729. doi: 10.1634/stemcells.2007-0321. [DOI] [PubMed] [Google Scholar]
- 20.Su Z, Frye C, Bae KM, et al. Differentiation of human embryonic stem cells into immunostimulatory dendritic cells under feeder-free culture conditions. Clin Cancer Res. 2008;14:6207–6217. doi: 10.1158/1078-0432.CCR-08-0309. [DOI] [PubMed] [Google Scholar]
- 21.Tseng SY, Nishimoto KP, Silk KM, et al. Generation of immunogenic dendritic cells from human embryonic stem cells without serum and feeder cells. Regen Med. 2009;4:513–526. doi: 10.2217/rme.09.25. [DOI] [PubMed] [Google Scholar]
- 22.Zeng J, Shahbazi M, Wu C, et al. Enhancing immunostimulatory function of human embryonic stem cell-derived dendritic cells by CD1d overexpression. J Immunol. 2012;188:4297–4304. doi: 10.4049/jimmunol.1102343. [DOI] [PubMed] [Google Scholar]
- 23.Choi KD, Vodyanik MA, Slukvin II. Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J Clin Invest. 2009;119:2818–2829. doi: 10.1172/JCI38591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senju S, Haruta M, Matsumura K, et al. Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther. 2011;18:874–883. doi: 10.1038/gt.2011.22. [DOI] [PubMed] [Google Scholar]
- 25.Haruta M, Tomita Y, Yuno A, et al. TAP-deficient human iPS cell-derived myeloid cell lines as unlimited cell source for dendritic cell-like antigen-presenting cells. Gene Ther. 2013;20:504–513. doi: 10.1038/gt.2012.59. [DOI] [PubMed] [Google Scholar]
- 26.Zeng J, Du J, Zhao Y, et al. Baculoviral vector-mediated transient and stable transgene expression in human embryonic stem cells. Stem Cells. 2007;25:1055–1061. doi: 10.1634/stemcells.2006-0616. [DOI] [PubMed] [Google Scholar]
- 27.Du J, Zeng J, Zhao Y, et al. The combined use of viral transcriptional and post-transcriptional regulatory elements to improve baculovirus-mediated transient gene expression in human embryonic stem cells. J Biosci Bioeng. 2010;109:1–8. doi: 10.1016/j.jbiosc.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Dhodapkar KM, Cirignano B, Chamian F, et al. Invariant natural killer T cells are preserved in patients with glioma and exhibit antitumor lytic activity following dendritic cell-mediated expansion. Int J Cancer. 2004;109:893–899. doi: 10.1002/ijc.20050. [DOI] [PubMed] [Google Scholar]
- 29.Motohashi S, Nakayama T. Clinical applications of natural killer T cell-based immunotherapy for cancer. Cancer Sci. 2008;99:638–645. doi: 10.1111/j.1349-7006.2008.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metelitsa LS. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin Immunol. 2011;140:119–129. doi: 10.1016/j.clim.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motohashi S, Okamoto Y, Yoshino I, et al. Anti-tumor immune responses induced by iNKT cell-based immunotherapy for lung cancer and head and neck cancer. Clin Immunol. 2011;140:167–176. doi: 10.1016/j.clim.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 32.De Santo C, Salio M, Masri SH, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song L, Asgharzadeh S, Salo J, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119:1524–1536. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayakawa Y, Takeda K, Yagita H, et al. IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood. 2002;100:1728–1733. [PubMed] [Google Scholar]
- 35.Metelitsa LS, Wu HW, Wang H, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004;199:1213–1221. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tachibana T, Onodera H, Tsuruyama T, et al. Increased intratumor Valpha24-positive natural killer T cells: A prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11:7322–7327. doi: 10.1158/1078-0432.CCR-05-0877. [DOI] [PubMed] [Google Scholar]
- 37.Molling JW, Langius JA, Langendijk JA, et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25:862–868. doi: 10.1200/JCO.2006.08.5787. [DOI] [PubMed] [Google Scholar]
- 38.Kronenberg M. Toward an understanding of NKT cell biology: Progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 39.Tahir SM, Cheng O, Shaulov A, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 40.Yanagisawa K, Seino K, Ishikawa Y, et al. Impaired proliferative response of V alpha 24 NKT cells from cancer patients against alpha-galactosylceramide. J Immunol. 2002;168:6494–6499. doi: 10.4049/jimmunol.168.12.6494. [DOI] [PubMed] [Google Scholar]
- 41.Molling JW, Kölgen W, van der Vliet HJ, et al. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116:87–93. doi: 10.1002/ijc.20998. [DOI] [PubMed] [Google Scholar]
- 42.Dhodapkar MV, Krasovsky J, Osman K, et al. Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J Exp Med. 2003;198:1753–1757. doi: 10.1084/jem.20031030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujii S, Shimizu K, Klimek V, et al. Severe and selective deficiency of interferon-gamma-producing invariant natural killer T cells in patients with myelodysplastic syndromes. Br J Haematol. 2003;122:617–622. doi: 10.1046/j.1365-2141.2003.04465.x. [DOI] [PubMed] [Google Scholar]
- 44.Motohashi S, Kobayashi S, Ito T, et al. Preserved IFN-alpha production of circulating Valpha24 NKT cells in primary lung cancer patients. Int J Cancer. 2002;102:159–165. doi: 10.1002/ijc.10678. [DOI] [PubMed] [Google Scholar]
- 45.van der Vliet HJ, Wang R, Yue SC, et al. Circulating myeloid dendritic cells of advanced cancer patients result in reduced activation and a biased cytokine profile in invariant NKT cells. J Immunol. 2008;180:7287–7293. doi: 10.4049/jimmunol.180.11.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imataki O, Heike Y, Makiyama H, et al. Insufficient ex vivo expansion of Valpha24(+) natural killer T cells in malignant lymphoma patients related to the suppressed expression of CD1d molecules on CD14(+) cells. Cytotherapy. 2008;10:497–506. doi: 10.1080/14653240802072747. [DOI] [PubMed] [Google Scholar]
- 47.Kunii N, Horiguchi S, Motohashi S, et al. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009;100:1092–1098. doi: 10.1111/j.1349-7006.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamasaki K, Horiguchi S, Kurosaki M, et al. Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol. 2011;138:255–265. doi: 10.1016/j.clim.2010.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.