SUMMARY
Clear cell renal cell carcinoma (ccRCC) is the most common form of kidney cancer and is often linked to loss of chromosome 3p, which harbors the VHL tumor suppressor gene, loss of chromosome 14q, which includes HIF1A, and gain of chromosome 5q. The relevant target(s) on chromosome 5q is not known. Here we show that 5q amplification leads to overexpression of the SQSTM1 oncogene in ccRCC lines and tumors. Overexpression of SQSTM1 in ccRCC lines promoted resistance to redox stress and increased soft agar growth while downregulation of SQSTM1 decreased resistance to redox stress, impaired cellular fitness, and decreased tumor formation. Therefore the selection pressure to amplify 5q in ccRCC is driven, at least partly, by SQSTM1.
INTRODUCTION
Kidney cancer is a common cancer in the developed world. The most common form of kidney cancer is clear cell renal cell carcinoma (ccRCC), which is usually linked to biallelic inactivation of the VHL tumor suppressor gene located on chromosome 3p25 (Shen and Kaelin, 2012). Loss of chromosome 3p, which also harbors the renal cancer suppressors PBRM1, BAP1, and SETD2, is found in virtually all ccRCCs (Cancer Genome Atlas Research, 2013; Dalgliesh et al., 2010; Guo et al., 2011; Pena-Llopis et al., 2012; Sato et al., 2013; Varela et al., 2011). The VHL gene product (pVHL) targets the HIFα transcription factors for proteasomal degradation. Increased HIF2α promotes pVHL-defective tumorigenesis (Kondo et al., 2003; Raval et al., 2005; Shen and Kaelin, 2012).
In addition to 3p loss, ccRCCs often harbor large deletions of chromosome 14q (~40% of cases) and copy number gains of chromosome 5q (~70% of cases) (Beroukhim et al., 2009; Cancer Genome Atlas Research, 2013; Chen et al., 2009; Dondeti et al., 2012; Hagenkord et al., 2011; Krill-Burger et al., 2012; Sato et al., 2013; Shen et al., 2011). Loss of chromosome 14q and gain of chromosome 5q, considered either individually or together, are seen more often in kidney cancer than in other cancers and presumably reflect selection pressure to silence one or more 14q kidney cancer suppressor genes and to increase the expression of one or more 5q kidney cancer oncogenes (Shen et al., 2011). Interestingly, unbalanced translocations involving chromosomes 3p and 5q, including constitutional translocations, have been reported in kidney cancer that result in loss of chromosome 3p and gain of 5q (Bos et al., 1998; Iqbal et al., 1996; Kenck et al., 1997; Kovacs et al., 1991; Kovacs and Frisch, 1989; Kovacs and Kung, 1991; Kovacs et al., 1987; Presti et al., 1991).
HIF1α, which antagonizes HIF2α in certain settings, appears to be a target of the 14q deletions (Shen et al., 2011), while the relevant chromosome 5q gene(s) is/are unknown. We recently used high-density SNP arrays to measure copy number changes in 90 ccRCCs and 21 ccRCC cell lines (Beroukhim et al., 2009). Approximately 70% of the samples exhibited increased copies of a region of 5q with a peak at 5q35.3 that, allowing for possible passenger events, contained about 61 genes (Beroukhim et al., 2009). Notably, at least 12 of these genes were overexpressed relative to non-amplified tumors (p<0.05) including GNB2L1, MGAT1, RUFY1, RNF130, MAPK9, CANX, CNOT6, SQSTM1, LTC4S, TBC1D9B, HNRPH1, and FLT4 (Beroukhim et al., 2009). In this study we sought to identify the 5q amplicon gene underlying the selection pressure for 5q copy number gains in ccRCC.
RESULTS
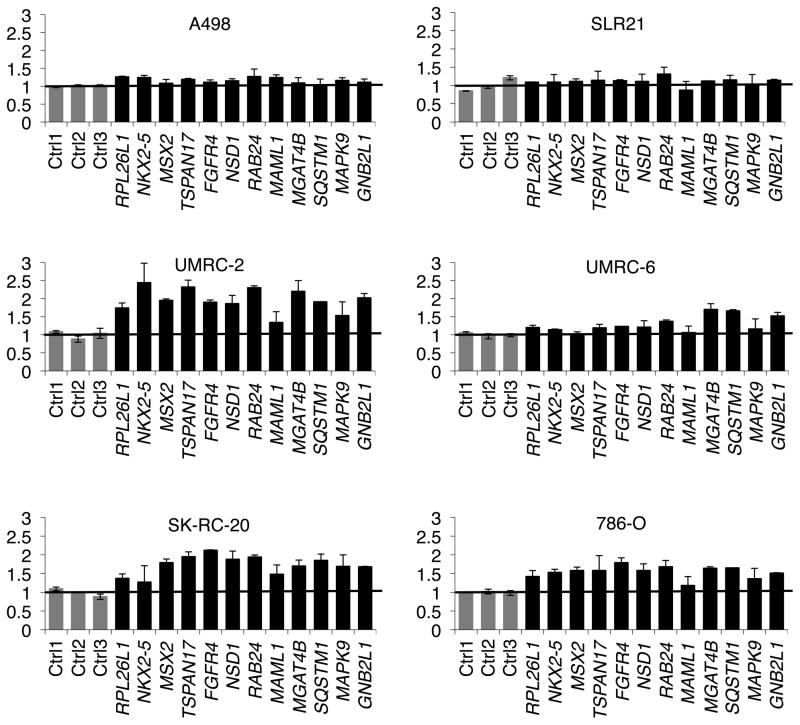
We interrogated the copy number changes in 16 ccRCC cell lines using multiplex ligation-dependent probe amplification (MLPA) of 12 randomly selected genes spanning the 5q amplicon (2–4 exons/gene) as well as 3 randomly selected control exons located elsewhere in the genome. Two cell lines (A498 and SLR21) did not exhibit 5q gains (MLPA score 1) relative to HK-2 immortalized renal epithelial cells (Ryan et al., 1994) while the remaining 14 lines exhibited low level copy gains suggestive of 3–6 copies (MLPA score 1.5–3) of 5q relative to a diploid cell with 2 copies (MLPA score 1)(Figures 1 and S1). These 5q copy numbers are consistent with earlier cytogenetic and Southern blot studies of ccRCCs (Kenck et al., 1997; Kovacs et al., 1991; Kovacs and Frisch, 1989; Kovacs and Kung, 1991). Interestingly, the 5q gain in one cell line (UMRC-6) appeared to be restricted to the telomeric genes MGAT4B, SQSTM1, MAPK9 and GNB2L1 while the other cell lines appeared, given the variability of the assay, to have sustained broader amplifications that encompassed all 12 genes (Figures 1 and S1).
Figure 1. Amplification of 5q Genes in ccRCC.
MLPA scores of selected 5q amplicon genes in the indicated cell lines normalized to HK-2 immortalized renal epithelial cells (diploid = 1). Grey bars = control exons on chromosomes 1 and 17. Error bars = SD.
See also Figure S1.
In parallel, we measured the mRNA levels of the 61 recurrently amplified 5q genes by quantitative real-time PCR in 4 of the 5q amplified cancer cell lines analyzed above as well as in HK-2 cells. As expected, multiple mRNAs were increased in the 4 5q amplified lines relative to HK-2 cells (Figure 2A and Table S1). Three mRNAs were induced greater than 12-fold but were of very low abundance (ADAMTS2, MSX2 and PCDH24). We elected to study SQSTM1 further because SQSTM1 overexpression was conspicuous when taking into account both fold-induction and absolute levels of the mRNA expression of the 61 genes, and because SQSTM1 is known or suspected of being an oncogene in other settings (Duran et al., 2008; Inami et al., 2011; Mathew et al., 2009; Nezis and Stenmark, 2012; Puissant et al., 2012).
Figure 2. Amplification and Increased Expression of SQSTM1 in ccRCC.
(A) Fold induction of mRNAs for genes located within the 5q amplicon in 5q amplified ccRCC lines (Caki-2, UMRC-2, UMRC-6 and 769-P) relative to HK-2 cells. Bubble sizes reflect mRNA abundance relative to ACTB mRNA in the 5q-amplified cells.
(B) MLPA scores of SQSTM1 (mean of 8 exons) in the indicated cell lines normalized to HK-2 immortalized renal epithelial cells (diploid = 1). Error bars = SD.
(C) SQSTM1 mRNA levels in the indicated cell lines, as determined by real-time PCR, relative to HK-2 cells.
(D) Anti-p62 immunoblot analysis of the indicated ccRCC cell lines. Loading was assessed by Ponceau S staining.
(E) SQSTM1 mRNA levels as a function of SQSTM1 copy number in TCGA dataset. ‘Gain’ refers to samples with one inferred additional DNA copy. ‘Amp’ refers to samples with ≥ 2 inferred additional copies. RPKM = reads per kilobase per million reads. Black horizontal bar = median.
(F and G) Box plots depicting correlation between p62 immunohistochemical staining in human ccRCC samples and tumor grade. Black horizontal bar = median. Black x = mean.
SQSTM1 copy number was increased in every 5q amplified cell line (Figures 2B and S2A) and was associated with increased SQSTM1 mRNA levels, as determined by real-time PCR (Figure 2C) and Northern blot analysis (Figure S2B), and protein (p62) levels (Figure 2D), relative to HK-2 cells. Notably, SQSTM1 mRNA levels, and particularly p62 protein levels, were also increased in A498 cells, which are not 5q-amplified (Figures 2C, 2D, and S2B). This suggests that A498 cells also upregulate p62 but through an alternative mechanism (see also below). Conversely, in 786-O and RCC4 cells, SQSTM1 mRNA and protein levels reverted toward those seen in HK-2 cells, possibly due to prolonged passage in culture (Figures 2C, 2D and S2B). Importantly, SQSTM1 mRNA levels are increased in 5q amplified kidney tumors compared to non-amplified kidney tumors (or normal kidney) [(Beroukhim et al., 2009; Cancer Genome Atlas Research, 2013) and Figure 2E], indicating that SQSTM1 mRNA expression level is regulated at the gene copy number level. However, SQSTM1 mRNA expression levels also showed considerable variation that cannot be attributed to gene copy number aberrations alone. Notably, increased levels of p62 are associated with increased tumor grade in ccRCCs (Figures 2F, 2G, and S2C–S2F).
p62 inactivates KEAP1, which is a negative regulator of NRF2 (Nezis and Stenmark, 2012; Puissant et al., 2012). Conversely, NRF2 can activate expression of SQSTM1 (Jain et al., 2010). Interestingly, deregulation of NRF2, which is a master regulator of the antioxidant response, plays an important role in papillary renal carcinoma as well as other tumors (Adam et al., 2011; Hayes and McMahon, 2009; Ooi et al., 2011). Moreover, NRF2 (official name NFE2L2) and KEAP1 mutations have been detected, albeit infrequently, in ccRCC (Figure 3A) (Cancer Genome Atlas Research, 2013; Sato et al., 2013). The NRF2 gene had inferred somatic mutations in 5 out of 368 cases and high-level DNA amplification in 2 additional cases. The KEAP1 gene had somatic missense mutations in 3 cases and a homozygous deletion in one additional case (Figure 3A). Notably, the NRF2 missense mutations would be predicted to disrupt KEAP1-binding and to thereby activate NRF2. Of note, NRF2 mutations, KEAP1 mutations, and high level SQSTM1 amplification (gain of 2 or more copies) appear to be mutually exclusive.
Figure 3. Activation of NRF2 in ccRCC.
(A) Genomic alterations of NRF2, KEAP1 and SQSTM1 in TCGA data (Cancer Genome Atlas Research, 2013).
(B–D) Gene set enrichment analysis (GSEA) of tumors with high SQSTM1 mRNA levels using gene sets associated with activation of NRF2 (B), response to oxidative stress (C) and mitochondrion organization and biogenesis (D).
We did not observe a strong NRF2 transcriptional signature in 5q amplified tumors relative to non-amplified tumors by gene set enrichment analysis (GSEA, data not shown), possibly because changes in SQSTM1 mRNA levels cannot be attributed to copy number aberrations alone or because non-amplified tumors can activate NRF2 by other means, such as described above. With respect to the former possibility, we reasoned that we could more directly probe the function of SQSTM1 in the TCGA data by correlating gene set expression with SQSTM1 mRNA expression levels. Indeed, we found that human ccRCCs with high levels of SQSTM1 mRNA exhibit transcriptional profiles that are consistent with activation of NRF2 as determined by GSEA using gene sets consisting of previously annotated NRF2 target genes (Figure 3B) and gene sets linked to NRF2-regulated processes, such as response to oxidative stress and mitochondrial biosynthesis (Figures 3C and 3D).
The sensitivity of ccRCC cell lines to oxidative stress mirrored their basal p62 levels and particularly their NRF2 levels (Figures 4A and 4B). A498 cells displayed high basal levels of NRF2 and modestly elevated p62 levels, suggesting that these cells harbor a mutation that drives the production of NRF2, which secondarily induces p62. SLR21 cells also displayed high NRF2 levels, possibly due to low levels of KEAP1. NRF2 and KEAP1 themselves, however, appear to be wild-type in A498 and SLR21 cells based on cDNA sequencing (data not shown). Exogenous expression of p62 in cells with low basal p62 levels induced NRF2 and reduced sensitivity to oxidative stress (Figures 4C, 4D and S3A). Conversely, knockdown of p62 with 3 distinct shRNAs reduced NRF2 levels and increased reactive oxygen species (ROS) sensitivity (Figures 4E, 4F, S3B and S3C). Collectively, these results support that 5q copy number gains in ccRCC increase p62 levels, which activate NRF2 and confer resistance to oxidative stress.
Figure 4. Increased Expression of SQSTM1 in ccRCC Confers Resistance to Redox Stress.
(A) Immunoblot analysis of the indicated cell lines. Equal loading was confirmed by Ponceau S staining of whole cell extracts (WCE) used for p62 and KEAP1 analysis and nuclear extracts (NE) used for NRF2 analysis.
(B) Normalized absorbance (A570) and representative images of the cell lines in (A) after treatment with increasing amounts of H2O2 for 4 hours followed by staining with crystal violet 3 days later. For each cell line the absorbance values for solubilized crystal violet were normalized based on cells that were not exposed to H2O2. Error bars = SD.
(C and D) Immunoblot analysis (C) and normalized absorbance (A570) and representative images of crystal violet staining (D) of 786-O and RCC4 cells infected with a lentivirus expressing SQSTM1 with a C-terminal V5 epitope tag or with the empty viral vector. Absorbance values were normalized as in (B). Error bars = SD. *p<0.05 compared to vector cells.
(E and F) Immunoblot analysis (E) and normalized absorbance (A570) and representative images of crystal violet staining (F) of UMRC-2 and UMRC-6 cells infected with lentiviruses expressing SQSTM1 shRNAs or a non-targeting control shRNA. 786-O, A498, SLR21 and HK-2 cells were included in (E) for comparison. Absorbance values were normalized as in (B). Error bars = SD. *p<0.05 compared to control shRNA.
See also Figure S3.
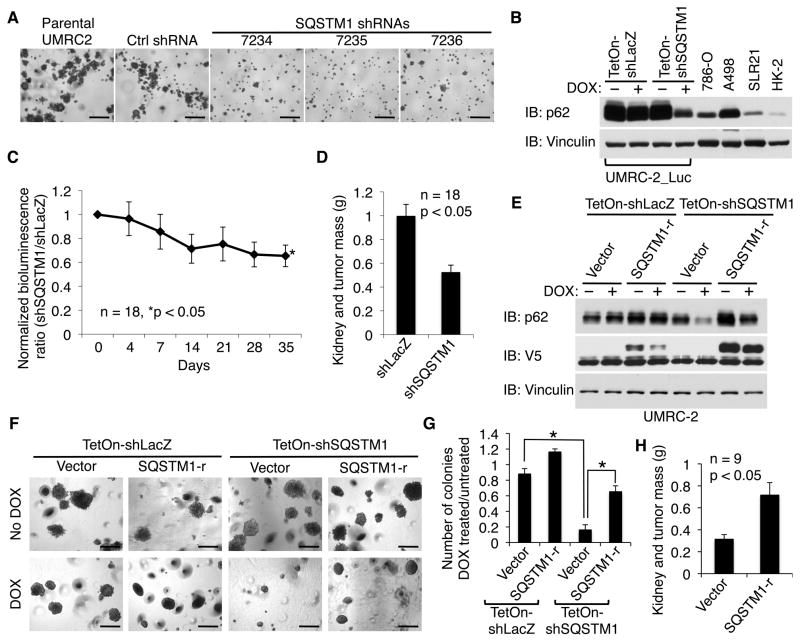
Acute VHL loss can induce senescence mediated, at least partly, by oxidative stress (Welford et al., 2010; Young et al., 2008), suggesting that p62 overexpression and VHL loss might cooperate with one another to promote renal carcinogenesis. In keeping with this view, we found that three independent SQSTM1 shRNAs, but not a control shRNA, impaired the ability of the 5q amplified VHL−/− ccRCC cell line UMRC-2 to grow in soft agar (Figures 5A and S4A). The same SQSTM1 shRNAs had only minimal effects on soft agar growth by 293 human embyronic kidney cells, which have low basal levels of p62 (Figures S4B–S4D). Induction of a SQSTM1 shRNA, but not a control shRNA, also decreased tumor formation by UMRC-2 cells (Figures 5B–5D) and UMRC-6 cells (Figures S4E and S4F). These phenotypic effects were specifically due to loss of p62 because they were reversed with a lentivirus encoding an shRNA-resistant SQSTM1 mRNA (Figures 5E–5H).
Figure 5. Downregulation of SQSTM1 Inhibits ccRCC Growth.
(A) Soft agar assay of parental UMRC-2 cells or cells infected with lentiviruses expressing SQSTM1 shRNAs or a non-targeting control shRNA. Scale bars = 0.5 mm.
(B) Immunoblot analysis of UMRC-2 cells infected with a virus encoding firefly luciferase and then with lentiviruses expressing a doxycycline (DOX)-inducible SQSTM1 shRNA (TetOn-shSQSTM1) or a control LacZ shRNA (TetOn-shLacZ). 786-O, A498, SLR21 and HK-2 cells were included for comparison.
(C and D) Orthotopic tumor growth by cells in (B) as determined by bioluminescent imaging (C) and total renal mass at necropsy (week 7) (D). Both kidneys of NOD scid gamma mice were injected with 2x106 viable tumor cells (LacZ shRNA cells in one kidney and SQSTM1 shRNA cells in the other). Following successful tumor engraftment, as determined by serial bioluminescent imaging, mice were begun on doxycycline-containing chow (day 0). The ratio of SQSTM1 shRNA tumor photons to LacZ shRNA tumor photons was measured weekly and normalized to day 0. Error bars = SEM.
(E–G) Immunoblot analysis (E), representative soft agar colonies (F), and soft agar colony quantification (G) of UMRC-2 cells infected with lentiviruses expressing a doxycycline (DOX)-inducible SQSTM1 shRNA (TetOn-shSQSTM1) or a control LacZ shRNA (TetOn-shLacZ) and later infected with a lentivirus expressing an shRNA-resistant SQSTM1 cDNA (SQSTM1-r) or the empty vector. Scale bars = 0.5 mm. Error bars = SD. *p<0.05.
(H) Orthotopic tumor growth of UMRC-2 cells expressing a doxycycline (DOX)-inducible SQSTM1 shRNA (TetOn-shSQSTM1) and subsequently infected with a lentivirus expressing an shRNA-resistant SQSTM1 cDNA (SQSTM1-r) or the vector (as in panel E) as determined by total renal mass at necropsy (week 6). Both kidneys of NOD scid gamma mice were injected with 1.5x106 viable tumor cells (SQSTM1-r cells in one kidney and vector cells in the other). Mice were begun on doxycycline-containing chow 10 days later. Error bars = SEM.
See also Figure S4.
The finding that p62 downregulation impaired ccRCC growth could simply indicate that SQSTM1 is a housekeeping gene and does not necessarily imply that supraphysiological levels of p62 promote tumor growth. Indeed, in parallel studies we infected multiple ccRCC cell lines with a custom shRNA library targeting all 61 5q amplicon genes (3–10 shRNAs/gene), monitored shRNA abundance over time by deep sequencing, and scored shRNA depletion/enrichment for each gene using the RIGER algorithm (Luo et al., 2008). We also evaluated the effects of shRNA-mediated loss of the 61 individual 5q genes on cellular fitness in a different experimental format wherein cells expressing red fluorescent protein and an effective shRNA targeting the 5q gene of interest were cocultured with cells expressing venus fluorescent protein and a control shRNA and monitored by FACS. shRNA-mediated knockdown of many other genes, in addition to SQSTM1, decreased the fitness of ccRCC cell lines (Figures S4G–S4J). Although some of these genes might be oncogenes, it is more likely that many of them are passenger housekeeping genes.
We therefore asked whether increased expression of SQSTM1 promotes ccRCC growth. Lentiviral expression of SQSTM1 in A498 and 786-O cells, did not affect proliferation under standard cell culture conditions (Figures 6A–6D, S5A and S5B) but promoted soft agar growth (Figures 6E–6G and S5C) and tumor formation in vivo (Figure 6H). These results were specific because the same vector did not affect UMRC-2 cells, which have high endogenous levels of p62 (Figures S5D and S5E). In addition, the SQSTM1 shRNAs that we documented impaired ccRCC fitness and tumor growth (Figures 4, 5 and S4) did so without lowering p62 levels below those seen in HK-2, 786-O, and SLR21 cells (Figures 4E, S3C, S4E and 5B). Collectively these results argue that supraphysiological p62 levels promote renal tumorigenesis.
Figure 6. SQSTM1 is a Renal Cancer Oncogene.
(A and B) Immunoblot analysis (A) and proliferation curves (B) of A498 cells infected with lentiviruses expressing SQSTM1 or LacZ, each with a C-terminal V5 epitope tag, or with the empty viral vector. Error bars = SD.
(C and D) Immunoblot analysis (C) and proliferation curves (D) of 786-O cells infected with a lentiviruse expressing SQSTM1 or with the empty viral vector. UMRC-2 cells were included in (C) for comparison. Error bars = SD.
(E) Soft agar assay of A498 cells analyzed in (A). Scale bars = 0.5 mm.
(F and G) Immunoblot analysis (F) and soft agar assay (G) of A498 cells infected with increasing amounts, as indicated by the triangle, of a lentivirus expressing SQSTM1 with a C-terminal V5 epitope tag or with the empty viral vector. UMRC-2 cells were included in (F) for comparison. Scale bars = 0.5 mm.
(H) Orthotopic tumor growth of 786-O cells analyzed in (C), as determined by total renal mass at necropsy (week 8). Both kidneys of Swiss nude mice were injected with 1x106 viable tumor cells (SQSTM1 cells in one kidney and vector cells in the other). Error bars = SEM.
See also Figure S5.
p62 is a multifunctional protein with multiple subdomains that interact with a variety of downstream effector molecules (Figure 7A) (Geetha and Wooten, 2002; Moscat and Diaz-Meco, 2009; Nezis and Stenmark, 2012; Puissant et al., 2012). We found that a previously described p62 mutant (“CT”) (Duran et al., 2011) lacking amino acids 1-229, and hence lacking both its PB1 (Phox/Bem1p) and ZZ (central zinc finger) domains as well as part of its TBS (TRAF6-Binding) domain, failed to promote soft agar growth in A498 cells (Figures 7A–7C) and did not rescue soft agar growth in UMRC-2 cells that were depleted of endogenous p62 with an inducible shRNA, despite retaining the ability to induce NRF2 (Figures 7D–7F and S6). Therefore promotion of soft agar growth by p62 requires certain functions mediated by its N-terminus. On the other hand, a p62 variant (“m6A”) bearing an inactivating mutation within its KEAP1-binding domain, and hence unable to induce NRF2, retained the ability to promote soft agar growth in A498 cells and could rescue UMRC-2 cells lacking endogenous p62 (Komatsu et al., 2010)(Figures 7A–7F and S6). Moreover, NRF2 levels in A498 cells are relatively high even in the absence of exogenous p62, as noted above. These two observations suggested that promotion of soft agar growth by p62 involves at least one effector other than NRF2.
Figure 7. Promotion of Soft Agar Growth and Activation of NRF2 are Dissociable Activities of p62.
(A) p62 Schematic. SQSTM1-CT mutant lacks the N-terminal 229 amino acids. SQSTM1-m6A replaces residues 347–352 with alanines. PB1 = Phox/Bem1p domain; ZZ = central zinc finger domain; TBS = TRAF6-binding domain; PEST= proline (P), glutamic acid (E), serine (S), and threonine (T) rich domain; LIR = LC3 interaction region; UBA = Ubiquitin-associated domain.
(B and C) Immunoblot analysis (B) and soft agar assay (C) of A498 cells infected with lentiviruses expressing wild-type SQSTM1 or mutant SQSTM1 (CT or m6A) or with the empty viral vector. Scale bars = 0.5 mm.
(D–F) Immunoblot analysis (D), representative soft agar colonies (E) and soft agar colony quantification (F) of UMRC-2 cells infected with lentiviruses expressing a doxycycline (DOX)-inducible SQSTM1 shRNA (TetOn-shSQSTM1) or a control LacZ shRNA (TetOn-shLacZ) and later infected with lentiviruses expressing an shRNA-resistant wild-type SQSTM1 cDNA (SQSTM1-r) or mutant SQSTM1 cDNA (CT-r or m6A-r), or with the empty viral vector. Scale bars = 0.5 mm. Error bars = SD. *p<0.05.
See also Figure S6.
It has been reported that wild-type p62, but not p62 CT, can activate mTOR (Duran et al., 2011). In addition, we found that activation of mTOR by Rheb2 or a constitutively active RagB mutant (RagBGTP), like overexpression of p62 itself, enhances the soft agar growth of A498 cells (Figures S7A–S7C). Moreover, some ccRCCs harbor mutations, such as TSC2 or MTOR mutations, that activate the mTOR pathway and such mutations, like NRF2 pathway mutations, are enriched in tumors that do not have high level chromosome 5q copy number gains, suggesting that p62 provides an alternative mechanism to activate mTOR (Cancer Genome Atlas Research, 2013). Although overexpression of p62 in ccRCC cells did not consistently activate mTOR under any condition tested (data not shown), we did observe that acute siRNA-mediated knockdown of p62 attenuated mTOR signaling (Figure S7D). Moreover, activation of mTOR with Rheb2 restored soft agar growth in UMRC-2 cells depleted of p62 (Figures 8A and 8B). Conversely, pharmacological blockade of mTOR with 1 nM rapamycin prevented soft agar growth driven by p62 or Rheb2 (Figure 8C). Therefore mTOR acts downstream or parallel of p62.
Figure 8. mTOR Acts Downstream or Parallel of p62.
(A and B) Immunoblot analysis (A) and soft agar assay (B) of UMRC-2 cells infected with lentiviruses expressing a doxycycline (DOX)-inducible SQSTM1 shRNA or a control LacZ shRNA and later infected with lentiviruses expressing EGFP or EGFP-Rheb2 fusion protein. Scale bars = 0.5 mm.
(C) Soft agar assay of A498 cells infected with lentiviruses expressing an empty vector or SQSTM1, or with lentiviruses encoding EGFP or EGFP-Rheb2. Cells were treated with either vehicle (DMSO) or rapamycin (1 nM). Scale bars = 0.5 mm.
(D) Signal transduction network involving p62 and kidney cancer mutational targets. Oncoproteins are shown in green and tumor suppressors are shown in red. For simplicity not every possible link is shown. PI3K = phosphoinositide 3-kinase; TSC = complex formed by tuberin and hamartin, which are mutated in tuberous sclerosis complex; mTOR = mammalian target of rapamycin; VHL = von Hippel-Lindau; HIF= hypoxia-inducible factor; REDD1 = regulated in development and DNA damage responses 1; CARD9= caspase recruitment domain containing protein 9; BNIP3 = BCL2/adenovirus E1B 19 kDa interacting protein 3; KEAP1 = kelch-like ECH-associated protein 1; NRF2 = nuclear factor erythroid 2-related factor 2; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells. FH = fumarate hydratase.
See also Figure S7.
DISCUSSION
Chromosome 5q is the region of the genome most frequently amplified in ccRCC and copy number changes involving chromosome 5q are more common in ccRCC than in any other cancer (Beroukhim et al., 2009; Chen et al., 2009; Dondeti et al., 2012; Hagenkord et al., 2011; Krill-Burger et al., 2012; Moore et al., 2012; Shen et al., 2011). Indeed, the triad of chromosome 3p loss, chromosome 14q loss, and chromosome 5q gain is a signature abnormality in ccRCC. Our studies strongly suggest that SQSTM1 is one of the genes underlying the recurrent 5q copy number gains in ccRCC.
SQSTM1 has been implicated as a potential oncogene in other settings, including human hepatocelluar carcinomas, lung carcinomas, pancreatic carcinomas, breast carcinomas, and in immortalized baby mouse kidney cells (Duran et al., 2008; Inami et al., 2011; Inoue et al., 2012; Mathew et al., 2009; Rolland et al., 2007; Thompson et al., 2003). The SQSTM1 gene product, p62, is a multifunctional protein that serves as an adaptor molecule to facilitate the degradation of specific proteins by autophagy (Geetha and Wooten, 2002; Moscat and Diaz-Meco, 2009; Nezis and Stenmark, 2012; Puissant et al., 2012). Of note, VHL−/− ccRCC have high basal rates of autophagy (Bray et al, 2012). The p62 protein also physically interacts with a number of signaling molecules to enhance the activity of downstream effectors such as NRF2, NF-κB, and mTOR. For example, p62 can bind to KEAP1 and prevent it from targeting NRF2 for degradation. There is increasing genetic and functional evidence that NRF2, which activates a transcriptional program that promotes resistance to redox stress, can promote tumor growth in some settings (DeNicola et al., 2011; Hayes and McMahon, 2009; Sporn and Liby, 2012). Acute VHL loss can, at least in some cells, cause senescence due, at least partly, to oxidative stress (Welford et al., 2010; Young et al., 2008). p62 might mitigate this effect as well as contribute to the resistance of ccRCCs to conventional cytotoxic therapy. Of note, SQSTM1 is itself an NRF2 target gene and hence potentially capable of participating in a positive feedback loop (Jain et al., 2010). This might contribute to the disproportionate increase in SQSTM1 mRNA levels relative to the other 5q amplicon genes observed in ccRCCs that have amplified chromosome 5q.
NRF2 pathway mutations occur, albeit rarely, in ccRCC (Cancer Genome Atlas Research, 2013; Sato et al., 2013) and, as shown here, are mutually exclusive with higher level SQSTM1 amplification (gain of 2 or more copies), further suggesting that NRF2 is likely an important target of p62 in ccRCC. On the other hand, we found that the KEAP1-binding domain of p62 was neither necessary nor sufficient for p62 to promote renal transformation, as measured in soft agar assays, indicating that additional p62 targets play roles in renal carcinogenesis. p62 also binds to several proteins upstream of NF-κB, including atypical PKC members and TRAF6, leading to enhanced NF-κB activity in certain settings (Nakamura et al., 2010; Nezis and Stenmark, 2012; Puissant et al., 2012). Interestingly, pVHL binds to and inhibits atypical PKC members and, through several mechanisms, suppresses NF-κB activity (An et al., 2005; An and Rettig, 2005; Datta et al., 2000; Datta et al., 2001; Lee et al., 2005; Okuda et al., 2001; Pantuck et al., 2010; Schermer et al., 2006; Yang et al., 2007) (Okuda et al., 1999). NF-κB promotes VHL−/− ccRCC growth in vivo (An et al., 2005; An and Rettig, 2005; Pantuck et al., 2010; Yang et al., 2007). Moreover, a recent report suggested that p62 binds to the Raptor complex and accentuates mTOR signaling under some experimental conditions (Duran et al., 2011). A significant fraction of ccRCCs have mutations that directly or indirectly activate mTOR and we found that forced activation of mTOR rescues soft agar growth in p62-depleted ccRCC cells, indicating that mTOR acts downstream or parallel of p62 (Cancer Genome Atlas Research, 2013).
As was true for NRF2, mTOR pathway mutations are mutually exclusive of high-level chromosome 5q gains in ccRCC (Cancer Genome Atlas Research, 2013). Several non-mutually exclusive explanations might account for this observation. First, as indicated above, p62 might enhance mTOR activity and decrease the selection pressure to mutate the mTOR pathway. Second, mTOR activation inhibits autophagy. This promotes the accumulation of p62 by preventing p62 from being destroyed together with its autophagocytic cargo. Therefore mTOR activation might decrease the selection pressure to increase SQSTM1 copy number. Finally, it is possible that p62 and mTOR operate on parallel, but redundant, pathways to promote renal carcinogenesis. Interestingly, a recent study showed that NRF2 activation in lung cancer cells renders them mTOR-dependent (Shibata et al., 2010).
mTOR is a validated therapeutic target in ccRCC. Notably, VHL−/− ccRCC cells are more sensitive than their pVHL-proficient counterparts to drugs, such as mTOR inhibitors, that promote autophagy (Hudes et al., 2007; Thomas et al., 2006; Turcotte et al., 2008). Moreover, a recent study showed that murine tumors linked to Tsc2 mutations, and hence mTOR activation, are p62-dependent (Parkhitko et al., 2011). The relationship between mTOR inhibition, induction of autophagy, and suppression of ccRCC is likely to be complex, however. In some preclinical models blocking autophagy actually enhances the activity of mTOR inhibitors by increasing mitochondrial ROS production in the face of impaired NRF2 activity stemming from the effects of mTOR on NRF2 nuclear localization (Bray et al., 2012). It will be important to ask whether the therapeutic efficacy of mTOR inhibitors is due, at least partly, to downregulation of p62 and, if so, whether p62 levels can be used as predictive or pharmocodynamic biomarkers.
Although SQSTM1 appears to be an important driver on chromosome 5q, the chromosomal gains and losses in renal cancer, including 5q gains, are non-focal, suggesting that they are driven by more than one gene. Indeed, 3p loss targets several ccRCC suppressor genes. In this regard, the 5q amplicon contains a number of other genes that could plausibly act as renal cancer oncogenes including MAML1, FGFR4 and MAPK9. These genes, however, did not consistently score in the oncogene assays described here. For example, FGFR4 shRNAs impaired cell fitness in vitro (Figure S4J) but promoted orthotopic tumor growth (data not shown). GNB2L1/RACK1 was, together with SQSTM1, recently highlighted as a potential ccRCC 5q oncogene because its protein product, like p62, activates mTOR (Duran et al., 2011; He et al., 2011) and because high-level 5q amplification is, as stated above, mutually exclusive with mTOR pathway mutations in ccRCC (Cancer Genome Atlas Research, 2013). Nonetheless, GNB2L1/RACK1 did not score as an oncogene in our assays (Figures S4G and S4I). It remains possible, however, that some of these genes confer a growth advantage under conditions that are not replicated by our assays. Knockdown of the 5q amplicon gene STC2 impaired ccRCC fitness in our hands (Figure S4J) and in a recent report (Dondeti et al., 2012). Whether STC2 is a housekeeping gene or an oncogene warrants further study.
In summary, enhanced expression of SQSTM1 promotes renal carcinogenesis in vitro and in vivo. The SQSTM1 gene product, p62, regulates NRF2, NF-κB, and mTOR, all 3 of which network with known renal carcinoma suppressor genes such as VHL, FH, TSC1, and TSC2 (Figure 8D). It will be important to determine which p62 effector pathways contribute to renal carcinogenesis and whether they can be pharmacologically attacked.
EXPERIMENTAL PROCEDURES
Cell Lines
A498, A704, Caki-2, HK-2, RCC4, 769-P and 786-O cells were purchased from the American Type Culture Collection. UMRC-2, UMRC-6 and UOK101 cells were provided by Drs. Bert Zbar and Martson Linehan (National Cancer Institute, Bethesda, MD). SK-RC-20 cells were provided by Drs. Gerd Ritter and Beatrice Yin (Memorial Sloan-Kettering Cancer Center, New York, NY). SLR20, SLR21, SLR23, SLR24, SLR25, and SLR26 cells were supplied by Drs. Mark A. Rubin and Kirsten Mertz (Weill Cornell Medical College, New York, NY). HK-2 cells, which are proximal renal tubular cells immortalized with human papilloma virus E6 and E7 proteins (Ryan et al., 1994), were maintained in keratinocyte serum-free medium supplemented with 0.05 mg/ml of bovine pituitary extract and 5 ng/ml of human recombinant epidermal growth factor (Invitrogen). A498, RCC4, UMRC-2, UMRC-6, UOK101 and 786-O cells were maintained in Dulbecco’s modified Eagle’s medium containing 10% FBS; 769-P, SLR20, SLR21, SLR23, SLR24, SLR25, and SLR26 in RPMI-1640 containing 10% FBS; A704 in Eagle’s Minimum Essential Medium (Lonza12-662) supplemented with 2 mM L-glutamine and 15% FBS; SK-RC-20 in RPMI-1640 containing 10%FBS and nonessential amino acids (Invitrogen), and Caki-2 in McCoy’s 5A containing 10% FBS. Following lentivirus infection, cells were maintained in the presence of puromycin (2 μg/ml) or blasticidin (10 μg/ml), depending on the vector. All cells were kept at 37°C in 5% CO2. VHL genotype for the renal cancer cell lines used in this study are listed in the matching section of Supplemental Experimental Procedures.
Plasmids
pLenti6/V5-DEST and pLenti6/V5-GW-LacZ were purchased from Invitrogen. The SQSTM1 cDNA is a generous gift from Dr. Eileen White (The Cancer Institute of New Jersey, New Brunswick, NJ) and was subcloned between the SpeI and XhoI sites in pLenti6/V5-DEST using the following primers: SQSTM1_For 5′-actgtcactagtgccaccatggcgtcgctcaccgtgaaggcc and SQSTM1_Rev 5′-atcacctcgaggccaacggcgggggatgctttg. pLenti6/V5-SQSTM1-CT was generated using SQSTM1_Rev and the following forward primer: SQSTM1-CT_For 5′-actgtcactagtgccaccatggaggatccgagtgtg. pLenti6/V5-SQSTM1-m6A was generated by site-directed mutagenesis using the following primers: SQSTM1-m_For 5′atctgtcttcaaaagaagtggccgcggctgcagctgcactccagtccctacagatgccag and SQSTM1-m_Rev 5′ctggcatctgtagggactggagtgcagctgcagccgcggccacttcttttgaagacagat. pLenti6/V5-SQSTM1-r was generated by site-directed mutagenesis using the following primers: SQSTM1-r_For 5′-gccacgcagtctctggcggaacaaatgaggaaaattgcattggagtccgaggggcgccct and SQSTM1-r_Rev 5′-agggcgcccctcggactccaatgcaattttcctcatttgttccgccagagactgcgtggc. SQSTM1-CT-r was generated using SQSTM1-r as the template and primers of SQSTM1-CT_For and SQSTM1_Rev. SQSTM1-m6A-r was generated by 2-step PCR. First, the 5′ fragment and the 3′ fragment were amplified using SQSTM1-r as the template and the following primer pairs, respectively: SQSTM1_For and SQSTM1-m_Rev, SQSTM1-m_For and SQSTM1_Rev. The 5′ fragment and the 3′ fragment were then mixed and amplified using primers SQSTM1_For and SQSTM1_Rev. pLKO-SQSTM1-7234, -7235, -7236 and -7237 were obtained from the TRC collection. For doxycycline-inducible shRNA expression, oligos for the corresponding shRNAs (SQSTM1-7235) were annealed and subcloned between the AgeI and EcoRI sites in pLKO-TetOn (Addgene). All plasmids were verified by DNA sequencing. pLJM1-EGFP, pLJM1-EGFP-Rheb2, pLJM1-Flag-RagBGTP and pLJM1-Flag-Metap2 are generous gifts from Dr. David M. Sabatini (Whitehead Institute for Biomedical Research, Cambridge, MA).
Multiplex Ligation-Dependent Probe Amplification
Multiplex ligation-dependent probe amplification was performed as described previously (Kozlowski et al., 2007; Shen et al., 2011) using reagents provided by MRC-Holland (http://www.mlpa.com). The probe sets are listed in the matching section in the Supplemental Experimental Procedures. The capillary electrophoresis and peak height determination of amplification products were performed by Mei Lin at the DNA Sequencing Facility, Brigham and Women’s Hospital, Boston, MA. Briefly, amplification products were 10× diluted in Hi-Di Formamide (ABI, Foster City, CA) containing 1/16 volume of ROX500 size standard (ABI) and then were separated by size on an ABI 3100 Genetic Analyzer (ABI). Electropherograms were analyzed by GeneMapper v3.5 (ABI), and peak height data were exported to an Excel table.
Sensitivity to H2O2
Cells were plated on 24-well plates in triplicates or quadruplicates. Cells were washed twice with PBS and incubated for four hours in DMEM without pyruvate (Invitrogen) supplemented with 1% FBS containing H2O2 at the concentrations indicated in figures. The media was then changed to complete growth medium appropriate for individual cell lines for 3 to 4 days prior to fixation and staining with crystal violet (4 mg/ml). To measure the relative density of cells, crystal violet was solubilized with 1% SDS and absorbance was measured at 570 nm. Each experiment was repeated at least 3 times. Results were evaluated using a 2-sided student t-test.
Orthotopic Xenograft and Bioluminescent Imaging
Immunodeficient mice were obtained from Taconic (Swiss nude mice) or The Jackson Laboratory (NOD scid gamma mice). Orthotopic xenograft was performed essentially as described previously (Li et al., 2007). Briefly, after the kidney was exposed, 1 to 2 × 106 viable cells were injected near the lower pole into the renal parenchyma. Tumors were monitored by palpation or by bioluminescent imaging. Bioluminescent detection and quantification of tumor burden were performed as described previously (Zhang et al., 2009). For each mouse, total photons from the kidney injected with cells expressing SQSTM1 shRNA were divided by total photons from the kidney injected with cells expressing LacZ shRNA and normalized to the ratio for that mouse on the day starting chow containing doxycycline (6 g doxycycline/kg chow (Bioserv)). Mice were sacrificed 6 to 8 weeks after injection as specified in figure legends. Total masses of kidney and tumor are presented as mean ± standard error of the mean (SEM) and evaluated statistically using a t test. All animal experiments complied with National Institutes of Health guidelines and were approved by Dana-Farber Cancer Institute Animal Care and Use Committee.
TCGA Data
mRNA expression and DNA copy number data was obtained for the TCGA clear cell renal cell carcinoma (KIRC) project through the cBio cancer genomics portal (http://www.cbioportal.org/public-portal/) (Cerami et al., 2012). We used the ‘core freeze’ set of samples, which had all types of data available for all samples (n = 368). We used log-transformed RPKM values for mRNA expression, and DNA copy number summarized at the gene level by the GISTIC algorithm (Mermel et al., 2011).
Gene Set Enrichment Analysis
We used gene set enrichment analysis (GSEA) (Subramanian et al., 2005) to identify pathways and gene sets associated with variation in SQSTM1 mRNA expression levels across TCGA tumor samples. Genes were sorted by their concordance (Pearson correlation) with SQSTM1 mRNA expression levels across tumors, and GSEA was used to evaluate gene sets enriched for either negatively or positively correlated genes. All genes on chromosome 5q were excluded from the analysis to avoid bias in the analysis related to the frequent broad copy number gains of chromosome 5q. All curated gene sets (MSigDB c2 collection) of size 5–500 genes (n = 3173 gene sets) were evaluated for enrichment. To account for gene-gene correlations in the enrichment analysis, GSEA p-values were computed with respect to a null distribution obtained from 1000 randomizations of the patient-phenotype labels.
NRF2 Target Gene Sets
We compiled two distinct NRF2 target gene sets based on previous studies of NRF2 function. The first set comprised a set of genes (n = 82) showing high confidence NRF2 binding sites in Chip-seq experiments and expression downregulation (log2 expression change > 0.15) in NRF2 silenced cell lines (Chorley et al., 2012). The second set of genes (n = 89) corresponded to a curated list of target genes summarized from multiple studies of NRF2 function (Hayes and McMahon, 2009). The two gene sets, listed in the matching section in the Supplemental Experimental Procedures, only showed moderate overlap (n = 11) and we used the union (n=160) of the two gene sets for gene set enrichment analysis. GSEA data can be found at http://cbio.mskcc.org/~ajac/sqstm1/gsea/sqstm1_expr/
Supplementary Material
Significance.
Kidney cancer is one of the ten most common cancers in the developed world. Inactivation of the VHL tumor suppressor gene on chromosome 3p is the signature lesion in clear cell renal cell carcinoma (ccRCC), which is the most common form of kidney cancer, but is not sufficient to cause this disease. Therefore there is a pressing need to understand the genetic events that cooperate with VHL loss to cause ccRCC. Many ccRCCs bear additional copies of chromosome 5q, together with chromosome 3p loss, including some with unbalanced translocations involving these two chromosomal arms. We show here that 5q copy number gains in renal cancer lead to SQSTM1 overexpression, which promotes renal carcinogenesis.
HIGHLIGHTS.
Copy number gains of chromosome 5q drive SQSTM1 overexpression
SQSTM1 gene product p62 activates NRF2 and promotes resistance to redox stress
mTOR acts downstream or parallel of p62 in renal cancer cells
p62 promotes renal cancer growth in vitro and in vivo
Acknowledgments
We thank members of the Kaelin Laboratory for useful comments and Drs. Eileen White and David M. Sabatini for reagents. W.G.K. is an HHMI investigator and supported by grants from the NIH.
Footnotes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam J, Hatipoglu E, O’Flaherty L, Ternette N, Sahgal N, Lockstone H, Baban D, Nye E, Stamp GW, Wolhuter K, et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer cell. 2011;20:524–537. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J, Fisher M, Rettig MB. VHL expression in renal cell carcinoma sensitizes to bortezomib (PS-341) through an NF-kappaB-dependent mechanism. Oncogene. 2005;24:1563–1570. doi: 10.1038/sj.onc.1208348. [DOI] [PubMed] [Google Scholar]
- An J, Rettig MB. Mechanism of von Hippel-Lindau protein-mediated suppression of nuclear factor kappa B activity. Molecular and cellular biology. 2005;25:7546–7556. doi: 10.1128/MCB.25.17.7546-7556.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beroukhim R, Brunet JP, Di Napoli A, Mertz KD, Seeley A, Pires MM, Linhart D, Worrell RA, Moch H, Rubin MA, et al. Patterns of gene expression and copy-number alterations in von-hippel lindau disease-associated and sporadic clear cell carcinoma of the kidney. Cancer research. 2009;69:4674–4681. doi: 10.1158/0008-5472.CAN-09-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos SD, van den Berg E, Dijkhuizen T, van den Berg A, Draaijers TG, Mensink HJ. Genetic analysis of 2 cases of clear cell renal cancer in 2 sisters. Int J Cancer. 1998;77:494–497. doi: 10.1002/(sici)1097-0215(19980812)77:4<494::aid-ijc3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Bray K, Mathew R, Lau A, Kamphorst JJ, Fan J, Chen J, Chen HY, Ghavami A, Stein M, DiPaola RS, et al. Autophagy suppresses RIP kinase-dependent necrosis enabling survival to mTOR inhibition. PloS one. 2012;7:e41831. doi: 10.1371/journal.pone.0041831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research N. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Ye Y, Yang H, Tamboli P, Matin S, Tannir NM, Wood CG, Gu J, Wu X. Genome-wide profiling of chromosomal alterations in renal cell carcinoma using high-density single nucleotide polymorphism arrays. Int J Cancer. 2009;125:2342–2348. doi: 10.1002/ijc.24642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorley BN, Campbell MR, Wang X, Karaca M, Sambandan D, Bangura F, Xue P, Pi J, Kleeberger SR, Bell DA. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res. 2012;40:7416–7429. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, Butler A, Davies H, Edkins S, Hardy C, Latimer C, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463:360–363. doi: 10.1038/nature08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta K, Nambudripad R, Pal S, Zhou M, Cohen HT, Mukhopadhyay D. Inhibition of insulin-like growth factor-I-mediated cell signaling by the von Hippel-Lindau gene product in renal cancer. The Journal of biological chemistry. 2000;275:20700–20706. doi: 10.1074/jbc.M909970199. [DOI] [PubMed] [Google Scholar]
- Datta K, Sundberg C, Karumanchi SA, Mukhopadhyay D. The 104–123 amino acid sequence of the beta-domain of von Hippel-Lindau gene product is sufficient to inhibit renal tumor growth and invasion. Cancer research. 2001;61:1768–1775. [PubMed] [Google Scholar]
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondeti VR, Wubbenhorst B, Lal P, Gordan JD, D’Andrea K, Attiyeh EF, Simon MC, Nathanson KL. Integrative genomic analyses of sporadic clear cell renal cell carcinoma define disease subtypes and potential new therapeutic targets. Cancer research. 2012;72:112–121. doi: 10.1158/0008-5472.CAN-11-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Amanchy R, Linares JF, Joshi J, Abu-Baker S, Porollo A, Hansen M, Moscat J, Diaz-Meco MT. p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol Cell. 2011;44:134–146. doi: 10.1016/j.molcel.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, Moscat J. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer cell. 2008;13:343–354. doi: 10.1016/j.ccr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Geetha T, Wooten MW. Structure and functional properties of the ubiquitin binding protein p62. FEBS Lett. 2002;512:19–24. doi: 10.1016/s0014-5793(02)02286-x. [DOI] [PubMed] [Google Scholar]
- Guo G, Gui Y, Gao S, Tang A, Hu X, Huang Y, Jia W, Li Z, He M, Sun L, et al. Frequent mutations of genes encoding ubiquitin-mediated proteolysis pathway components in clear cell renal cell carcinoma. Nature genetics. 2011;44:17–19. doi: 10.1038/ng.1014. [DOI] [PubMed] [Google Scholar]
- Hagenkord JM, Gatalica Z, Jonasch E, Monzon FA. Clinical genomics of renal epithelial tumors. Cancer Genet. 2011;204:285–297. doi: 10.1016/j.cancergen.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- He X, Wang J, Messing EM, Wu G. Regulation of receptor for activated C kinase 1 protein by the von Hippel-Lindau tumor suppressor in IGF-I-induced renal carcinoma cell invasiveness. Oncogene. 2011;30:535–547. doi: 10.1038/onc.2010.427. [DOI] [PubMed] [Google Scholar]
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O, Watanabe S, Ando J, Iwadate M, Yamamoto M, et al. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol. 2011;193:275–284. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue D, Suzuki T, Mitsuishi Y, Miki Y, Suzuki S, Sugawara S, Watanabe M, Sakurada A, Endo C, Uruno A, et al. Accumulation of p62/SQSTM1 is associated with poor prognosis in patients with lung adenocarcinoma. Cancer Sci. 2012;103:760–766. doi: 10.1111/j.1349-7006.2012.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal MA, Akhtar M, Ali MA. Cytogenetic findings in renal cell carcinoma. Hum Pathol. 1996;27:949–954. doi: 10.1016/s0046-8177(96)90223-3. [DOI] [PubMed] [Google Scholar]
- Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, McMahon M, Hayes JD, Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. The Journal of biological chemistry. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenck C, Bugert P, Wilhelm M, Kovacs G. Duplication of an approximately 1.5 Mb DNA segment at chromosome 5q22 indicates the locus of a new tumour gene in nonpapillary renal cell carcinomas. Oncogene. 1997;14:1093–1098. doi: 10.1038/sj.onc.1200915. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nature cell biology. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- Kondo K, Kim WY, Lechpammer M, Kaelin WG., Jr Inhibition of HIF2alpha Is Sufficient to Suppress pVHL-Defective Tumor Growth. PLoS Biol. 2003;1:439–444. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G, Emanuel A, Neumann HP, Kung HF. Cytogenetics of renal cell carcinomas associated with von Hippel-Lindau disease. Genes Chromosomes Cancer. 1991;3:256–262. doi: 10.1002/gcc.2870030404. [DOI] [PubMed] [Google Scholar]
- Kovacs G, Frisch S. Clonal chromosome abnormalities in tumor cells from patients with sporadic renal cell carcinomas. Cancer research. 1989;49:651–659. [PubMed] [Google Scholar]
- Kovacs G, Kung HF. Nonhomologous chromatid exchange in hereditary and sporadic renal cell carcinomas. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:194–198. doi: 10.1073/pnas.88.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G, Szucs S, De Riese W, Baumgartel H. Specific chromosome aberration in human renal cell carcinoma. Int J Cancer. 1987;40:171–178. doi: 10.1002/ijc.2910400208. [DOI] [PubMed] [Google Scholar]
- Kozlowski P, Roberts P, Dabora S, Franz D, Bissler J, Northrup H, Au KS, Lazarus R, Domanska-Pakiela D, Kotulska K, et al. Identification of 54 large deletions/duplications in TSC1 and TSC2 using MLPA, and genotype-phenotype correlations. Hum Genet. 2007;121:389–400. doi: 10.1007/s00439-006-0308-9. [DOI] [PubMed] [Google Scholar]
- Krill-Burger JM, Lyons MA, Kelly LA, Sciulli CM, Petrosko P, Chandran UR, Kubal MD, Bastacky SI, Parwani AV, Dhir R, et al. Renal cell neoplasms contain shared tumor type-specific copy number variations. Am J Pathol. 2012;180:2427–2439. doi: 10.1016/j.ajpath.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Nakamura E, Linggi MS, Sajan M, Farese R, Carter BLA, Kaelin W., Jr Dysregulation of JunB and Neural Crest Cell Apoptosis by Familial Pheochromocytoma VHL Alleles 2005 [Google Scholar]
- Li L, Zhang L, Zhang X, Yan Q, Minamishima YA, Olumi AF, Mao M, Bartz S, Kaelin WG., Jr Hypoxia-inducible factor linked to differential kidney cancer risk seen with type 2A and type 2B VHL mutations. Molecular and cellular biology. 2007;27:5381–5392. doi: 10.1128/MCB.00282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B, Cheung HW, Subramanian A, Sharifnia T, Okamoto M, Yang X, Hinkle G, Boehm JS, Beroukhim R, Weir BA, et al. Highly parallel identification of essential genes in cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20380–20385. doi: 10.1073/pnas.0810485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome biology. 2011;12:R41. doi: 10.1186/gb-2011-12-4-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LE, Jaeger E, Nickerson ML, Brennan P, De Vries S, Roy R, Toro J, Li H, Karami S, Lenz P, et al. Genomic copy number alterations in clear cell renal carcinoma: associations with case characteristics and mechanisms of VHL gene inactivation. Oncogenesis. 2012;1:e14. doi: 10.1038/oncsis.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kimple AJ, Siderovski DP, Johnson GL. PB1 domain interaction of p62/sequestosome 1 and MEKK3 regulates NF-kappaB activation. The Journal of biological chemistry. 2010;285:2077–2089. doi: 10.1074/jbc.M109.065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Network, T.C.G.A.R. Integrative Analysis of Genomic and Molecular Alterations in Clear Cell Renal Cell Carcinoma. Nature (Submitted) [Google Scholar]
- Nezis IP, Stenmark H. p62 at the interface of autophagy, oxidative stress signaling, and cancer. Antioxid Redox Signal. 2012;17:786–793. doi: 10.1089/ars.2011.4394. [DOI] [PubMed] [Google Scholar]
- Okuda H, Hirai S, Takaki Y, Kamada M, Baba M, Sakai N, Kishida T, Kaneko S, Yao M, Ohno S, et al. Direct interaction of the beta-domain of VHL tumor suppressor protein with the regulatory domain of atypical PKC isotypes. Biochem Biophys Res Commun. 1999;263:491–497. doi: 10.1006/bbrc.1999.1347. [DOI] [PubMed] [Google Scholar]
- Okuda H, Saitoh K, Hirai S, Iwai K, Takaki Y, Baba M, Minato N, Ohno S, Shuin T. The von Hippel-Lindau tumor suppressor protein mediates ubiquitination of activated atypical protein kinase C. The Journal of biological chemistry. 2001;276:43611–43617. doi: 10.1074/jbc.M107880200. [DOI] [PubMed] [Google Scholar]
- Ooi A, Wong JC, Petillo D, Roossien D, Perrier-Trudova V, Whitten D, Min BW, Tan MH, Zhang Z, Yang XJ, et al. An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer cell. 2011;20:511–523. doi: 10.1016/j.ccr.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Pantuck AJ, An J, Liu H, Rettig MB. NF-kappaB-dependent plasticity of the epithelial to mesenchymal transition induced by Von Hippel-Lindau inactivation in renal cell carcinomas. Cancer research. 2010;70:752–761. doi: 10.1158/0008-5472.CAN-09-2211. [DOI] [PubMed] [Google Scholar]
- Parkhitko A, Myachina F, Morrison TA, Hindi KM, Auricchio N, Karbowniczek M, Wu JJ, Finkel T, Kwiatkowski DJ, Yu JJ, et al. Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (SQSTM1)-dependent. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12455–12460. doi: 10.1073/pnas.1104361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Llopis S, Vega-Rubin-de-Celis S, Liao A, Leng N, Pavia-Jimenez A, Wang S, Yamasaki T, Zhrebker L, Sivanand S, Spence P, et al. BAP1 loss defines a new class of renal cell carcinoma. Nature genetics. 2012;44:751–759. doi: 10.1038/ng.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presti JC, Jr, Rao PH, Chen Q, Reuter VE, Li FP, Fair WR, Jhanwar SC. Histopathological, cytogenetic, and molecular characterization of renal cortical tumors. Cancer research. 1991;51:1544–1552. [PubMed] [Google Scholar]
- Puissant A, Fenouille N, Auberger P. When autophagy meets cancer through p62/SQSTM1. Am J Cancer Res. 2012;2:397–413. [PMC free article] [PubMed] [Google Scholar]
- Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Molecular and cellular biology. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland P, Madjd Z, Durrant L, Ellis IO, Layfield R, Spendlove I. The ubiquitin-binding protein p62 is expressed in breast cancers showing features of aggressive disease. Endocr Relat Cancer. 2007;14:73–80. doi: 10.1677/erc.1.01312. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney international. 1994;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- Sato Y, Yoshizato T, Shiraishi Y, Maekawa S, Okuno Y, Kamura T, Shimamura T, Sato-Otsubo A, Nagae G, Suzuki H, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nature genetics. 2013;45:860–867. doi: 10.1038/ng.2699. [DOI] [PubMed] [Google Scholar]
- Schermer B, Ghenoiu C, Bartram M, Muller RU, Kotsis F, Hohne M, Kuhn W, Rapka M, Nitschke R, Zentgraf H, et al. The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J Cell Biol. 2006;175:547–554. doi: 10.1083/jcb.200605092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Beroukhim R, Schumacher SE, Zhou J, Chang M, Signoretti S, Kaelin WG., Jr Genetic and Functional Studies Implicate HIF1alpha as a 14q Kidney Cancer Suppressor Gene. Cancer Discov. 2011;1:222–235. doi: 10.1158/2159-8290.CD-11-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Kaelin WG., Jr The VHL/HIF axis in clear cell renal carcinoma. Semin Cancer Biol. 2012 doi: 10.1016/j.semcancer.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Saito S, Kokubu A, Suzuki T, Yamamoto M, Hirohashi S. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathway. Cancer research. 2010;70:9095–9105. doi: 10.1158/0008-5472.CAN-10-0384. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GV, Tran C, Mellinghoff IK, Welsbie DS, Chan E, Fueger B, Czernin J, Sawyers CL. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–127. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- Thompson HG, Harris JW, Wold BJ, Lin F, Brody JP. p62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Oncogene. 2003;22:2322–2333. doi: 10.1038/sj.onc.1206325. [DOI] [PubMed] [Google Scholar]
- Turcotte S, Chan DA, Sutphin PD, Hay MP, Denny WA, Giaccia AJ. A molecule targeting VHL-deficient renal cell carcinoma that induces autophagy. Cancer cell. 2008;14:90–102. doi: 10.1016/j.ccr.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, Davies H, Jones D, Lin ML, Teague J, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469:539–542. doi: 10.1038/nature09639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford SM, Dorie MJ, Li X, Haase VH, Giaccia AJ. Renal oxygenation suppresses VHL loss-induced senescence that is caused by increased sensitivity to oxidative stress. Molecular and cellular biology. 2010;30:4595–4603. doi: 10.1128/MCB.01618-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Minamishima YA, Yan Q, Schlisio S, Ebert BL, Zhang X, Zhang L, Kim WY, Olumi AF, Kaelin WG., Jr pVHL acts as an adaptor to promote the inhibitory phosphorylation of the NF-kappaB agonist Card9 by CK2. Mol Cell. 2007;28:15–27. doi: 10.1016/j.molcel.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AP, Schlisio S, Minamishima YA, Zhang Q, Li L, Grisanzio C, Signoretti S, Kaelin WG., Jr VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nature cell biology. 2008;10:361–369. doi: 10.1038/ncb1699. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Gu J, Li L, Liu J, Luo B, Cheung HW, Boehm JS, Ni M, Geisen C, Root DE, et al. Control of cyclin D1 and breast tumorigenesis by the EglN2 prolyl hydroxylase. Cancer cell. 2009;16:413–424. doi: 10.1016/j.ccr.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.