Abstract
Yeast D-amino acid oxidase (DAO) can serve as a genetically encoded producer of reactive oxygen species (ROS) in redox signaling studies. However, dynamics of hydrogen peroxide production and its sensitivity to externally added D-alanine (D-Ala) in cells have not been determined. Here we show that DAO, fused to a genetically encoded H2O2 indicator HyPer, can be used for controlled production of ROS in living eukaryotic cells. We found a clear heterogeneity in ROS production dynamics between individual cells. Moreover, different cell lines demonstrated distinct sensitivity to added D-Ala. Finally, by comparing signals generated by the HyPer-DAO fusion protein versus coexpressed HyPer and DAO proteins, we show that the fusion system is more sensitive to hydrogen peroxide production. Our results show the utility of the HyPer-DAO genetically encoded system for redox signaling studies and suggest that H2O2 produced by DAO in the cytoplasm acts locally in close proximity to the enzyme. Antioxid. Redox Signal. 20, 1039–1044.
Introduction
Cells produce reactive oxygen species (ROS) during metabolism and ROS emerge as crucial signaling molecules. Hydrogen peroxide (H2O2) plays an important regulatory role in cell signaling cascades by oxidizing critical allosteric and catalytic Cys residues of a range of proteins (9). At high concentrations it also becomes involved in pathological processes, damaging cellular structures through Fenton chemistry. H2O2 is rapidly degraded by antioxidant enzymes that localize ROS signaling in time and space and minimize their harmful effects (9).
In many studies of ROS-mediated signaling, H2O2 and other pro-oxidative agents are applied externally, without estimating their actual intracellular concentration. This may result in multiple protein oxidation and cause oxidative stress; conversely, apparent lack of effect may be due to numerous reasons, ranging from the oxidant degradation in the media to poor cell membrane permeability. Together, this complicates interpretation of experimental results. Besides, this approach does not allow targeted oxidation of specific proteins, which would be very useful for evaluating the role of ROS in a specific pathway. Therefore, developing a targeted ROS production system would provide a novel approach for redox signaling studies. It would be further advantageous if this approach would allow tracing of intracellular H2O2 dynamics.
D-amino acid oxidase (DAO) is an enzyme that oxidizes the NH2- group of D-amino acid, producing corresponding α-keto acid, ammonia, and hydrogen peroxide (7). The yeast DAO gene from Rhodotorula gracilis (red yeast) has been previously cloned and the enzyme characterized in vitro (5). It is highly specific to D-amino acids, has relatively low affinity to D-alanine (D-Ala) (about 1 mM) and high enzymatic activity in comparison with known DAO from other organisms (8). DAO can serve as a convenient genetically encoded source for controlled D-Ala-dependent H2O2 production in mammalian cells (3). However, the estimation of the intracellular H2O2 production requires extracellular measurements of the released oxidant (3). The rate of H2O2 release as well as the rate of the initial D-amino acid uptake may vary between cells. Therefore, the requirement of measuring the outcome of DAO reaction would be better suited by an intracellular reporter of H2O2..
Innovation.
D-amino acid oxidase (DAO) has been frequently used as a genetically encoded reactive oxygen species producer, but methods for estimating the amount of DAO-generated H2O2 inside cells are lacking. We addressed this problem by fusing DAO with HyPer, which allowed direct visualization of the intracellular DAO activity. By comparing signals from HyPer-DAO fusion and from HyPer coexpressed with DAO, we revealed important differences in the indicator oxidation kinetics, which indicates that the efficiency of redox-active Cys oxidation depends on proximity to the H2O2 source.
To trace intracellular DAO-driven H2O2 production, we used the genetically encoded fluorescent indicator HyPer (1). HyPer is based on the circularly permutated yellow fluorescent protein inserted into a regulatory domain of Escherichia coli H2O2-sensitive transcription factor OxyR. The indicator is sensitive to physiological concentrations of H2O2 and has a ratiometric fluorescent readout, making it a useful tool for studying redox signaling and oxidative stress in cultured cells (3) and live animals (2). Therefore, we examined whether H2O2 produced by DAO in mammalian cells can be visualized by combining the generator (DAO) and the indicator (HyPer) to produce a single fusion protein.
Results, Discussion, and Future Directions
To produce a genetically encoded system for visually controlled H2O2 production, we engineered a fusion protein from DAO and HyPer polypeptides. Initially, we produced both the HyPer-DAO and DAO-HyPer variants and expressed them in cultured HeLa-Kyoto cells. DAO-HyPer was not responsive to externally added D-Ala, while having unperturbed response to H2O2, thus indicating that DAO, but not the HyPer, function was impaired in the fusion protein. In contrast, HyPer-DAO (Fig. 1A) responded to D-Ala addition (Fig. 1B) indicating that N-terminal fusion of DAO preserved the function of the enzyme intact. Therefore, we used HyPer-DAO in further experiments and compared it to DAO and HyPer coexpressed.
FIG. 1.
HyPer-D-amino acid oxidase (DAO) produces and detects H2O2 in response to D-alanine (D-Ala). (A) Scheme of HyPer-DAO fusion. (B) Time course of H2O2 production by HyPer-DAO in HeLa-Kyoto cells upon addition of 4 mM D-Ala. Mean values represent measurements of seven individual cells, with error bars showing standard error of the mean. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
We first examined whether the HyPer response to DAO was pH driven. HyPer is pH sensitive and since the DAO reaction produces ammonia ions, changes in the HyPer ratio might reflect alkalinization of the cytoplasm rather than changes in H2O2 production. To determine whether ammonia released by DAO affects intracellular pH, we used a version of HyPer with C199S substitution, which makes the protein nonsensitive to H2O2, but preserves its sensitivity to pH. We observed no change in signal intensity in HyPerC199S-DAO-transfected cells in response to 8 mM D-Ala, whereas HyPer-DAO responded to the amino acid. These results indicate that production of H2O2, but not the change in local pH, was the cause of the signal change (Fig. 1B). Moreover, they indicate that the amount of ammonia released from the DAO is not sufficient to produce detectable changes of pH.
Because of the close proximity of HyPer to the source of the oxidant in the HyPer-DAO fusion, its response kinetics may differ from that of the DAO and HyPer coexpression system. To examine this, we cotransfected HeLa-Kyoto (Fig. 2) and NIH-3T3 (Fig. 3) cells with DAO and HyPer plasmids and monitored H2O2 production using the fluorescent ratiometric readout of the indicator. In both cell lines, DAO produced detectable amounts of H2O2 in response to 4–8 mM D-Ala added to the imaging medium (Figs. 2A, B and 3A, B), with no response to the addition of 8 mM L-alanine (not shown). Since the signals were within the previously determined range of HyPer sensitivity (1), we conclude that in our experiments, the amount of H2O2 produced in cells did not exceed 50–100 nM, a value that is within the physiological range for H2O2. As we did not observe any signs of cellular stress like cell shrinkage or plasma membrane blebbing during 45 min after addition of D-Ala, products of the DAO activity are likely not toxic to the cells. Notably, in contrast to previous observation (3), D-Ala alone was sufficient to induce sustained H2O2 production by the cells and did not required external addition of DAO cofactor flavin adenine dinucleotide to the cells.
FIG. 2.
HyPer-DAO fusion versus HyPer & DAO coexpression in HeLa-Kyoto cells. (A) Widefield ratiometric images of HeLa-Kyoto cells cotransfected with HyPer and DAO encoding vectors. Numbers indicate time points in minutes. (B) Time course of HyPer ratio changes in HeLa-Kyoto cells coexpressing HyPer and DAO upon addition of different concentrations of D-Ala. (C) Time course of HyPer-DAO fusion ratio changes in HeLa-Kyoto cells upon addition of different concentrations of D-Ala. (D) Widefield ratiometric images of HeLa-Kyoto cells transfected with HyPer-DAO fusion encoding vector. Numbers indicate time points in minutes. In (B) and (C), mean values represent at least five individual cells, with error bars showing standard error of the mean. Lookup table reflects the HyPer or HyPer-DAO ratio. Scale bars are 20 μm. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 3.
HyPer-DAO fusion versus HyPer & DAO coexpression in NIH-3T3 cells. (A) Widefield ratiometric images of NIH-3T3 cells cotransfected with HyPer and DAO encoding vectors. Numbers indicate time points in minutes. (B) Time course of HyPer ratio changes in NIH-3T3 cells coexpressing HyPer and DAO upon addition of different concentrations of D-Ala. (C) Time course of HyPer-DAO fusion ratio changes in NIH-3T3 cells upon addition of different concentrations of D-Ala. (D) Widefield ratiometric images of NIH-3T3 cells transfected with HyPer-DAO fusion encoding vector. Numbers indicate time points in minutes. In (B) and (C), mean values represent at least five individual cells, with error bars showing standard error of the mean. Lookup table reflects HyPer or HyPer-DAO ratio. Scale bars are 20 μm. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Next, we analyzed the dependence of H2O2 production in HeLa-Kyoto and NIH-3T3 cells on extracellular D-Ala concentrations. In NIH-3T3 fibroblasts, we observed a prominent HyPer signal at 1–2 mM external D-Ala and above, while no H2O2 production was detected in HeLa-Kyoto cells at these concentrations of D-Ala. H2O2 production in HeLa-Kyoto cells was observed when D-Ala concentrations in the media were elevated to 4 mM and above. This may indicate that different cell lines have different D-Ala uptake rates, which would explain the differences in the indicator response between the cell lines.
In both lines, further increasing the D-Ala concentration leads to an increased number of responding cells, but did not necessarily increase the rate of hydrogen peroxide production. In fact, in 3T3 cells, we observed some inhibition of H2O2 production at concentrations of D-Ala above 4 mM, probably due to the substrate-dependent inhibition of the DAO enzyme. Taken together, our results suggest that optimal D-Ala concentrations should be determined specifically for each cell type at given experimental conditions.
Since active oxygen forms degrade rapidly in the cytoplasm, we asked whether a fusion protein, containing both DAO and HyPer, would lead to increased HyPer oxidation and thus be a more efficient indicator of DAO activity. As expected, signals from cells expressing the HyPer-DAO fusion protein were about 1.5-2-fold higher on average compared to the cells coexpressing separate DAO and HyPer proteins (Figs. 2C, D and 3C, D). This improvement was not due to the altered dynamic range of HyPer in fusion with DAO as both HyPer and HyPer-DAO respond equally to external H2O2 (Fig. 4). Therefore, fusing DAO with a protein of interest makes the target oxidation selectively more efficient compared to other cytoplasmic proteins. This approach may be useful for targeted oxidation of a specific protein of interest, for separation of a specific cascade from other redox signaling pathways, or for limiting the oxidative damage to a specific cell structure.
FIG. 4.
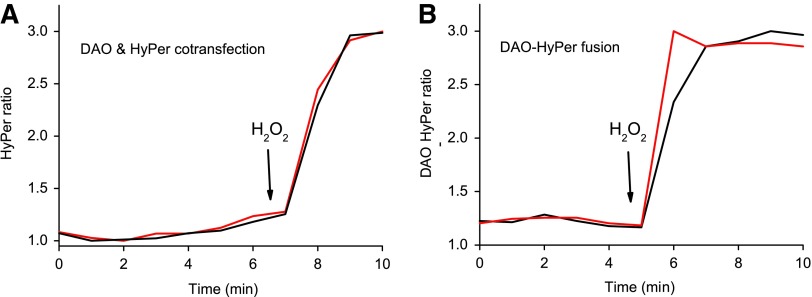
Dynamic range of HyPer is not altered in fusion with DAO. Addition of 100 μM H2O2 to HeLa-Kyoto cells either (A) coexpressing DAO and HyPer or (B) expressing HyPer-DAO leads to similar HyPer ratio changes. Black and red lines show the response of individual cells. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
There was a clear heterogeneity in hydrogen peroxide production dynamics among individual cells (Figs. 2 and 3). From our observation, H2O2 production not only depends on the HyPer-DAO fusion protein expression level, but also on some other cell properties, perhaps on the activity of amino acid transporters. Together, our results indicate that it is important to use either HyPer/DAO cotransfection or HyPer-DAO fusion in live cell imaging to ensure that H2O2 production actually takes place in studied cells.
How much H2O2 is produced by the DAO in mammalian cells? Despite millimolar concentrations of D-Ala added, H2O2 concentration in the cytoplasm does not exceed the 50–100 nM range as the ratio of HyPer coexpressed with DAO changes for up to 1.5-fold. These ratio changes correspond to ∼50 nM H2O2, as determined by in vitro calibration of purified HyPer protein [Fig. 1B in (1)]. This further suggests that even in the presence of millimolar D-Ala, oxygen is a limiting substrate, since the concentration of O2 in cell culture media is ∼200 μM and even less in animal tissues (∼13–15 μM).
Finally, can the HyPer-DAO system be used in vivo? We suggest that it can be used without any restrictions in relatively small organisms suitable for whole body imaging, such as Danio rerio and Caenorhabditis elegans. HyPer demonstrated superior performance in both species (4, 6). D-amino acids can be delivered with food. Even higher levels of precision of H2O2 production/detection can be achieved by using cell type-specific promoters and subcellular localization tags. In bigger organisms, such as mice, HyPer-DAO in vivo imaging may be more problematic due to the low transparency of tissues and relatively poor brightness of HyPer. We believe that substitution of HyPer with its brighter versions in the future would enable a wider use of the HyPer-DAO system in vivo.
In summary, we successfully combined DAO and HyPer indicator to make a genetically encoded system for controlled H2O2 production/detection. This system can be useful in in situ and in vivo studies of redox signaling. The presence of the H2O2 indicator allows visualizing H2O2 production by DAO and makes it easier to adjust the optimal D-Ala concentration for specific experimental conditions. Additional benefits and more efficient oxidation of a protein of interest may be achieved by generating a fusion construct of this protein with DAO.
Notes
Materials used
H2O2, D-Ala, and L-alanine were purchased from Sigma. The Dulbecco's modified Eagle's medium (DMEM), Opti–minimal essential medium (opti-MEM), MEM, HEPES, and fetal calf serum (FCS) were from Invitrogen. X-tremeGene 9 DNA transfection reagent was from Roche. Glass-bottomed dishes and eight-well slides were from MatTek, LabTech, and WPI. NIH-3T3 cells were from ATCC. The HeLa-Kyoto cell line was provided by Carsten Schultz, EMBL. Encyclo polymerase chain reaction (PCR) kit was from Evrogen. Restriction endonucleases were from SibEnzyme.
DNA constructs
The DAO sequence was obtained from the EMBL-EBI database (accession number U60066), modified for optimal mammalian translation, and synthesized using GenScript, with the 2-amino acid potential microbody targeting signal removed.
To make a HyPer-DAO fusion construct, DAO was amplified from a synthetic DAO encoding vector using the primers 5′- AGTCAAGCTTACACAGCCAGAAGAGGGTG-3′ and 5′- AGTCAAGCTTAGAGCTCGAGATGCCGCTC-3′. The PCR product was digested with HindIII and BamHI and cloned into the pHyPer vector (Evrogen). To make a pH control construct, we used HyPer with C199S mutation, previously introduced by site-directed mutagenesis in our laboratory (1). All constructs were confirmed by sequence analysis.
Cell culture and transfection
NIH-3T3 and HeLa-Kyoto cells were cultured in the DMEM supplemented with 10% FCS at 37°C in an atmosphere containing 95% air and 5% CO2. Cells were split every second day and seeded on glass bottom dishes or eight-well slides. Twenty-four hours later, cells were transfected by the mixture of vector DNA and X-tremeGene6 transfection reagent according to the manufacturer's recommendations.
Imaging
Cells were transferred to an environmental chamber in the MEM with HEPES at 37°C and imaged using a Leica 6000 widefield microscope equipped with a 20×air objective and HCX PL APO lbd.BL 63×1.4NA oil objective. Fluorescence was excited sequentially using 427/10 and 504/12 band-pass excitation filters. Emission of the indicator was collected every 60 s using a 525/50 bandpass emission filter. After five images were acquired, 0.5–8.0 mM D-Ala, 8 mM L-alanine, or MEM was added.
Time series processing
Time series were analyzed using ImageJ software. For H2O2 dynamics calculation, stacks corresponding to 420 nm and 500 nm excitation peaks of HyPer were converted to 32 bit after background subtraction. A 420-nm stack was thresholded to remove pixel values from the background. A 500-nm stack was divided by the corresponding 420-nm stack frame by frame. Time course of HyPer fluorescence was calculated for regions of interest inside the imaged cell.
Abbreviations Used
- D-Ala
D-alanine
- DAO
D-amino acid oxidase
- DMEM
Dulbecco's modified Eagle's medium
- FCS
fetal calf serum
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- MEM
minimal essential medium
- PCR
polymerase chain reaction
- ROS
reactive oxygen species
Acknowledgments
We thank Arcady Fradkov for his help with the codon-optimized DAO gene design. The work was supported by the Russian Foundation for Basic Research (EMBL-RFBR grant 12-04-92427 and 13-04-40333-H to V.V.B.), the National Institute of Aging (grant R01AG040209 to G.E.), Russian Ministry of Education and Science (grant 11.G34.31.0071 to G.E.; contract #8269), and Molecular and Cell Biology Program of Russian Academy of Sciences.
References
- 1.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, and Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3: 281–286, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Fang J, Deng D, Nakamura H, Akuta T, Qin H, Iyer AK, Greish K, and Maeda H. Oxystress inducing antitumor therapeutics via tumor-targeted delivery of PEG-conjugated D-amino acid oxidase. Int J Cancer 122: 1135–1144, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Haskew-Layton RE, Payappilly JB, Smirnova NA, Ma TC, Chan KK, Murphy TH, Guo H, Langley B, Sultana R, Butterfield DA, Santagata S, Alldred MJ, Gazaryan IG, Bell GW, Ginsberg SD, and Ratan RR. Controlled enzymatic production of astrocytic hydrogen peroxide protects neurons from oxidative stress via an Nrf2-independent pathway. Proc Natl Acad Sci U S A 107: 17385–17390, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knoefler D, Thamsen M, Koniczek M, Niemuth NJ, Diederich AK, and Jakob U. Quantitative in vivo redox sensors uncover oxidative stress as an early event in life. Mol Cell 47: 767–776, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YH. and Chu WS. D-Amino acid oxidase activity form Rhodosporiduim toruloides. Lett Appl Microbiol 23: 283–286, 1996 [Google Scholar]
- 6.Niethammer P, Grabher C, Look AT, and Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459: 996–999, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollegioni L, Diederichs K, Molla G, Umhau S, Welte W, Ghisla S, and Pilone MS. Yeast D-amino acid oxidase: structural basis of its catalytic properties. J Mol Biol 324: 535–546, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Pollegioni L, Piubelli L, Sacchi S, Pilone MS, and Molla G. Physiological functions of D-amino acid oxidases: from yeast to humans. Cell Mol Life Sci 64: 1373–1394, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4: 278–286, 2008 [DOI] [PubMed] [Google Scholar]