Abstract
Examination of resting state brain activity using electrophysiological measures like complexity as well as functional connectivity is of growing interest in the study of autism spectrum disorders (ASD). The present paper jointly examined complexity and connectivity to obtain a more detailed characterization of resting state brain activity in ASD. Multi-scale entropy was computed to quantify the signal complexity, and synchronization likelihood was used to evaluate functional connectivity (FC), with node strength values providing a sensor-level measure of connectivity to facilitate comparisons with complexity. Sensor level analysis of complexity and connectivity was performed at different frequency bands computed from resting state MEG from 26 children with ASD and 22 typically developing controls (TD). Analyses revealed band-specific group differences in each measure that agreed with other functional studies in fMRI and EEG: higher complexity in TD than ASD, in frontal regions in the delta band and occipital-parietal regions in the alpha band, and lower complexity in TD than in ASD in delta (parietal regions), theta (central and temporal regions) and gamma (frontal-central boundary regions); increased short-range connectivity in ASD in the frontal lobe in the delta band and long-range connectivity in the temporal, parietal and occipital lobes in the alpha band. Finally, and perhaps most strikingly, group differences between ASD and TD in complexity and FC appear spatially complementary, such that where FC was elevated in ASD, complexity was reduced (and vice versa). The correlation of regional average complexity and connectivity node strength with symptom severity scores of ASD subjects supported the overall complementarity (with opposing sign) of connectivity and complexity measures, pointing to either diminished connectivity leading to elevated entropy due to poor inhibitory regulation or chaotic signals prohibiting effective measure of connectivity.
Keywords: Autism, Magnetoencephalography (MEG), Resting-state, Connectivity, Complexity, Synchronization likelihood (SL), Multi-scale entropy (MSE)
Introduction
Autism spectrum disorders (ASD) are a category of neurobiological developmental disorders characterized by social and communication impairments, as well as repetitive and restricted behaviors (APA 1994, 2000). ASD affects cognitive and language abilities, and has been shown to be associated with atypical sensory and motor functioning (Gidley Larson and Mostofsky 2006), visual perception (Simmons et al. 2009), perception of biological motion (Kaiser et al. 2010), auditory perception (Roberts et al. 2010), somatosensory integration (Russo et al. 2010), and reduced adaptability to environmental changes (Foley Nicpon et al. 2010). The above findings point to widespread brain anomalies.
Research suggests that many ASD symptoms are associated with abnormal structural and functional brain connectivity (Ghanbari et al. 2012; Vissers et al. 2012; Ghanbari et al. 2013). MRI offers insight into structural connectivity, primarily through the analysis of white matter pathways measured via diffusion weighted MRI (typically either diffusion tensor imaging or high angular resolution diffusion imaging), as well as an insight into functional connectivity (via fMRI) measured via assessment of correlated fluctuations in blood flow between brain regions while participants perform tasks and also while at rest. MRI structural connectivity studies suggest that ASD may be characterized by enhanced short-range and decreased long-range connectivity (Courchesne and Pierce 2005) (short and long-range defined terms of physical proximity; more generally, if connected regions are within the same lobe and hemisphere this is considered short-range connectivity). MRI functional connectivity studies also report abnormalities, with atypical connectivity between brain regions reported in fMRI studies of ASD in domains such as social interaction (Perkins et al. 2010), face processing (Critchley et al. 2000; Schultz et al. 2000), imitation (Williams et al. 2006), executive function and information processing (Schmitz et al. 2006), as well as in other cognitive tasks (Castelli et al. 2002; Just et al. 2004, 2007). The majority of fMRI connectivity studies, however, have assessed functional connectivity in ASD via examining connectivity during resting-state exams. These resting-state studies indicate that brain regions are less collaborative (measured in terms of synchronization or coherence) in ASD than typically developing controls (Calhoun et al. 2008; Monk et al. 2009; Assaf et al. 2010; Paakki et al. 2010; Weng et al. 2010; Wiggins et al. 2011).
Electroencephalography (EEG) and magnetoencephalography (MEG) have also examined resting-state activity in ASD. Such studies are of interest as ASD symptoms are increasingly thought to be due to a disruption in the excitatory/inhibitory balance of neural activity (Rubenstein and Merzenich 2003; Hughes 2008). Some of the most compelling support for an excitatory/inhibitory imbalance in ASD comes from the observation of epileptiform-like activity in EEG and magnetoencephalography (MEG) recordings of children with ASD during sleep. The few EEG resting-state ASD studies all report oscillatory anomalies. Specifically, eyes-open resting-state exams have shown greater relative delta and less relative alpha power in 4- to 12-year-old low-functioning children with ASD (Cantor et al. 1986), and greater 24–44 Hz power in 3- to 8-year-old boys with ASD (Orekhova et al. 2007). Eyes-closed exams have shown greater relative 3–6 Hz and 13–17 Hz power and less 9–10 Hz power in adults with ASD (Murias et al. 2007), and decreased delta (absolute and relative) and beta (absolute) power, as well as increased theta (relative) power, in 6- to 11-year-old children with ASD (Coben et al. 2008). Using MEG, Cornew et al. (2012) observed that children with ASD exhibited regionally specific elevations in delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and high frequency (20–120 Hz) power, supporting an imbalance of neural excitation/inhibition as a neurobiological feature of ASD. One putative hypothesis to explain this observation would be a lack of effective inhibition of the brain oscillations, leading to elevated, poorly regulated power levels. Mutations in genes involved in the expression of excitatory and inhibitory neurotransmitters (e.g. glutamate and gamma-aminobutyric acid (GABA)) have been identified in individuals with ASD (Ramoz et al. 2004; Collins et al. 2006), with a preliminary study using in vivo MR Spectroscopy reporting abnormal GABA levels in young children with ASD (Harada et al. 2010). In animal models of ASD, mutations in genes related to excitatory and inhibitory neurotransmitters have been associated with cognitive and social deficits (DeLorey et al. 2008).
Individual neurons and neural networks exhibit oscillatory activity over a wide range of frequencies: from delta-band activity (0–4 Hz) to at least gamma-band activity (approximately 30–80 Hz), with recent evidence of activity well above 100 Hz. Oscillatory activity within specific frequency bands is one of the most promising candidate mechanisms associated with information processing (see Basar et al. (2001); Klimesch et al. (2005)). For example, whereas cortical oscillations in the 30–50 Hz gamma band are thought to reflect early states of sensory perception (e.g., see Pantev et al. (1991)), later 14–30 Hz beta activity is thought to be associated with encoding the sensory percept (Kopell et al. 2000). Thus, a starting point for understanding brain processes is to examine the frequency components of brain processes, obtained through the spectral analysis of brain signals. An advantage of EEG and MEG over fMRI is that by virtue of the high (∼1 ms) temporal resolution of EEG and MEG, relationships between brain regions within specific frequency bands can be examined.
A few recent EEG and MEG studies have examined functional connectivity in ASD. Barttfeld et al. (2011) used resting-state EEG to evaluate dynamic brain connectivity in ASD with a focus on the low-frequency (delta) activity. They showed that the delta-band connectivity network in ASD does not fit into the small-world network model observed in controls, and that in ASD functional connectivity is deficient in long-range fronto-occipital connections and excessive in short-range frontal connections (Barttfeld et al. 2011). Coben et al. (2008) also investigated ASD and TD difference in inter-hemispheric and intra-hemispheric coherence in EEG resting-state delta, theta, alpha, and beta activity. They observed decreased intra-hemispheric delta and theta coherence across short to medium and long inter-electrode distances. Inter-hemispherically, delta and theta coherences were low across the frontal region in ASD (Coben et al. 2008). Finally, examining coherence, Murias et al. (2007) observed elevated EEG theta coherence in ASD in the left hemisphere frontal and temporal regions, and globally reduced coherence in frontal regions.
Magnetoencephalography has been used in only a few resting state ASD connectivity studies. Tsiaras et al. (2011) used resting-state MEG to create connectivity matrices by using three different techniques, with the aim of finding the optimal connectivity measure for classifying ASD versus TD. Automated classification based on graph measures computed from these matrices, identified robust interdependent measure as the measure with a classification performance over 90 %. Vissers et al. (2012), in reviewing findings in the brain connectivity literature, noted that different connectivity measures demonstrate complex patterns of abnormal connectivity. They thus noted that whereas the dominant theory in the literature is long distance under-connectivity and local over-connectivity of the frontal cortex, the reality might be more complex. Finally, Granger causality was used by Pollonini and Patidar (2010) in a MEG resting-state study of eight ASD and eight TD subjects. Using graph theoretical measures for quantifying connectivity, more than 85 % accuracy was obtained in separating the two groups.
In addition to the investigation of brain connectivity—a measure of synchronization/coherence between different brain regions—another line of research has investigated the integrity of the signal locally. For example, entropy based methods have captured EEG signal dynamics in autism (Catarino et al. 2011), showing a reduction in entropy in task-based studies. Similarly, Bosl et al. (2011) showed in resting state EEG studies of high-risk infant siblings versus low risk controls that entropy based complexity may predict autism risk. A limitation of these two EEG studies, however, is that the complexity of neurophysiological signals was studied only in the full (broad-band) spectrum. As noted above, given findings of ASD versus TD differences at specific frequencies, and given that activity at specific frequencies is related to different brain processes, a more detailed assessment of resting state complexity is needed.
As functional connectivity and entropy-based signal complexity have provided important putative markers of pathology in ASD, it is expected that a joint analysis of these two measures will reveal a more detailed picture of atypical resting state activity in ASD. Joint analysis may also reveal associations between the two measures. For example, it has been hypothesized that multi-scale signal complexity may be an indication of connectivity in a way that any change in multi-scale complexity is likely to indicate, to some degree, a change in the neural underpinnings of connectivity (Tononi et al. 1994; Friston et al. 1995; Takahashi et al. 2010).
This paper presents a joint analysis of electrophysiological complexity and connectivity in ASD. Multi-scale entropy (MSE) is used to characterize the complexity of resting state brain signals and functional connectivity is modeled using synchronization likelihood formalism. Connectivity and complexity are computed for delta, theta, alpha, beta, gamma and broadband activity. Secondary to the hypo/hyper-connectivity hypotheses in ASD, as well as arguments of excitatory/inhibitory imbalance, it is expected that whereas complexity and connectivity measures will differentiate ASD and TD, congruence between the two measures will yield insight into the neurobiological basis of ASD.
Groups examined in previous autism resting-state complexity studies include infants under 2 years old (Bosl et al. 2011) and adults 21–42 years old (Catarino et al. 2011). Resting-state EEG/MEG ASD connectivity studies have examined individuals aged 17–21 (Tsiaras et al. 2011), 16–38 (Barttfeld et al. 2011), 6–11 (Sheikhani et al. 2012), 18–38 (Murias et al. 2007), and 9–34 (Coben et al. 2008) years. The present study investigated brain activity in children aged 6–15 years, a unique period spanning late childhood and adolescence when significant connectivity changes are expected. Although functional connectivity studies primarily investigate connectivity at different frequencies, to our knowledge, sub-band investigations of complexity have not been previously conducted. Furthermore, the use of synchronization likelihood in MEG connectivity and its relationship with MSE complexity remains unique to this work.
Materials
Participants
Information on this population has been previously reported in Cornew et al. (2012). In brief, twenty-six individuals with ASD and 22 age-matched TD controls were recruited. Ages ranged from 6–15.5 years (mean = 10.1 years, SD = 2.3 years in ASD, and mean = 10.9 years, SD = 2.5 years in controls). Groups did not significantly differ in age (t = −1.3, p > 0.2). Participants with ASD were recruited from the Regional Autism Center of The Children’s Hospital of Philadelphia (CHOP), the Neuropsychiatry program of the Department of Psychiatry of the University of Pennsylvania School of Medicine, and from local and regional parent support groups. All children screened for inclusion in the ASD sample had received an ASD diagnosis prior to their involvement in the current research. This prior diagnosis was made by an expert clinician, typically a developmental pediatrician in the Regional Autism Center at CHOP, after an extensive clinical interview, documentation of DSM-IV criteria for ASD, and use of various ASD diagnostic tools, such as the Childhood Autism Rating Scale and, in many cases, the Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 2000). TD subjects were recruited through local newspaper advertisements and from pediatric practices of the CHOP primary care network.
Research participants made two visits to CHOP. Assessments were performed by a licensed child psychologist with expertise in autism. Several diagnostic scores were performed including ADOS (Lord et al. 2000), parent report on the Social Communication Questionnaire (SCQ) (Rutter et al. 2003) and Social Responsiveness Scale (SRS) (Constantino and Gruber 2005). Asperger’s disorder symptomatology was measured with the Krug Asperger’s Disorder Index (KADI) (Krug and Arick 2003). For final inclusion in the ASD group, children were required to have a confirmatory research diagnosis of ASD by exceeding established cut-offs on both the ADOS and SCQ. Mean of ASD diagnosis scores were: ADOS, 13 (SD = 4.8, range = 7–26); SCQ, 18.5 (SD = 5.2, range = 9–26); and SRS (raw), 87 (SD = 20, range = 49–118). Details of the population demographics and diagnostic scores are given in Table 1.
Table 1.
Population demographics and diagnostic scores
| TD controls (N = 22) Mean ± SD (range) |
ASD patients (N = 26) Mean ± SD (range) |
Group differences t values |
|
|---|---|---|---|
| Age | 10.9 ± 2.5 (6.0–15.5) | 10.1 ± 2.3 (6.9–14.5) | +1.3, p > 0.2 |
| ADOS | 2.2 ± 1.7 (0–7) | 13 ± 4.8 (7–26) | −10.7, p < 0.05 |
| SCQ | 3.8 ± 2.9 (0–9) | 18.5 ± 5.2 (9–26) | −12.3, p < 0.05 |
| SRS | 44 ± 9.8 (25–63) | 87 ± 20 (49–118) | −9.5, p < 0.05 |
| GAI | 105 ± 13 (85–132) | 104 ± 17 (67–138) | −0.2, p > 0.8 |
ADOS Autism Diagnostic Observation Schedule, SCQ Social Communication Questionnaire, SRS Social Responsiveness Scale, GAI General Ability Index
To rule out global cognitive delay, all subjects were required to score at or above the 5th percentile (SS > 75) on the Perceptual Reasoning Index (PRI) of the Wechsler Intelligence Scale for Children-IV (WISC-IV) (Wechsler 2003). In all subjects, the WISC-IV Verbal Comprehension Index was also obtained. Inclusion criteria for the TD control children included scoring below the cut-off for ASD on the ADOS as well as parent questionnaires. Per parent report, subjects also had never been diagnosed with the following: developmental delay, mental retardation, speech/language disorder/delay, communication disorder, language-based or other learning disability, ADHD, or psychiatric conditions (e.g., bipolar disorder, obsessive compulsive disorder, schizophrenia, conduct disorder, depression, or anxiety disorder). In addition to the inclusion/exclusion criteria outlined above, per parent report, all subjects and families were native English speakers and had no known genetic syndromes, neurological (e.g., cerebral palsy, epilepsy), or sensory (hearing, visual) impairments. Finally, given known associations between psychotropic medications and brain activity (Blume 2006), all subjects in this study were required to be medication-free. Parental report of medication use was obtained during the initial phone screening and again at the study visit to confirm that participants were medication-free from the time of recruitment and that their medication-free status had not changed between the phone screen and study visit. (Note that although participants were unmedicated for at least 2 months prior to MEG imaging, from our study records it is not possible to determine if subjects were taking medications prior to the phone screen. Nevertheless, 2 months is sufficiently long as to encompass medication washout periods). The study was approved by the CHOP Institutional Review Board and all participants’ families gave written informed consent. As indicated by institutional policy, where competent to do so, children over the age of seven additionally gave verbal assent.
The WISC-IV General Ability Index (GAI) of the WISC-IV (Wechsler 2003) was compared between groups to ensure that any observed group differences in oscillatory activity would not be attributable to group differences in cognitive ability. The mean GAI was 104 (SD = 17, range = 67–138) in the ASD group and 105 (SD = 13, range = 85–132) in the TD group. A t test confirmed that the groups did not differ on GAI (p > 0.8).
Data acquisition
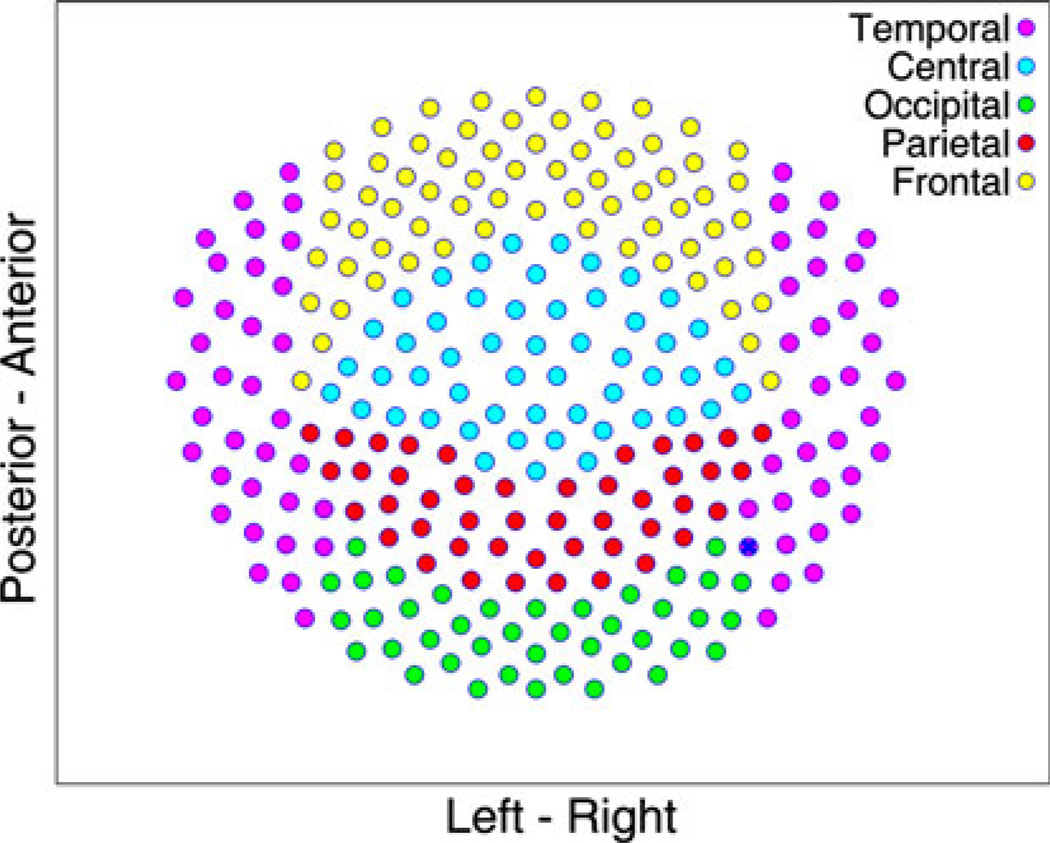
As reported in Cornew et al. (2012), data were collected using a 275-channel MEG system (VSM MedTech Inc., Coquitlam, BC), with 274 channels effective at the time of recording. More detailed acquisition methods may be found in that manuscript. The sensor map is shown in Fig. 1.
Fig. 1.
MEG sensor map with color-coded sensors corresponding to different brain regions. This is a 2D representation of the 3-D location of sensors within the head-shaped helmet (with e.g. blue “central” sensors vertically superior to purple “temporal” sensors)
Children were scanned in a supine position and instructed to lie still with their eyes gently closed during a 2-minute resting state exam. Three head-position indicator coils were attached to landmarks on the child’s head and foam wedges were inserted between the side of the participant’s head and the inside of the dewar to ensure immobility. The electro-oculogram was collected to ensure that participants’ eyes remained closed throughout the exam. After a band-pass filter (0.03–150 Hz), MEG signals were digitized at 1,200 Hz with 3rd-order gradiometer environmental noise reduction, and downsampled offline to 500 Hz (reducing temporal resolution to 2 ms). Head motion was continuously recorded for each subject across the two-minute scan. Differences between groups in maximum head displacement were not significant (TD 5.1 ± 7.6 mm, ASD 7.7 ± 6.8 mm, p>0.2).
Data Preprocessing
A clinical interpretation was performed to examine the data for gross abnormalities such as the presence of sub-clinical epileptiform discharges. All eye-closed recordings were read as normal by the clinical team. Prior to computing functional connectivity and complexity measures, a three-step process was employed to remove muscle and movement artifact. First, participants’ EOG data were visually examined and segments contaminated by blinks, saccades, or other significant EOG activity were removed. Second, blind to diagnosis, participants’ MEG data were visually inspected for muscle-related activity (focusing especially on data from sensors close to the temporalis muscles), and data containing muscle activity were removed. Third, any additional artifacts were rejected by amplitude and gradient criteria (amplitude 1,200 fT/cm, gradients 4,800 fT/cm/ sample). We have found this three-step approach to provide a more thorough artifact rejection than automated artifact-rejection routines. Finally, the signal was mean-subtracted and power line 60 Hz interference removed using second-order forward–backward notch filtering. At the conclusion of preprocessing, each participant’s time-series consisted of at least 40 s of artifact-free data, with a mean of 86 s (SD = 17) in the ASD group and a mean of 85 s (SD = 16) in the TD group.
Methods
Resting state data for each subject was analyzed at five standard frequency bands: δ (0.5–4 Hz), θ (4–8 Hz), α (8–13 Hz), β (13–30 Hz), γ (30–80 Hz), as well as BB (0.5–100 Hz). To isolate each frequency, a fourth order Butterworth bandpass filter was applied once in the forward and once in the backward direction to ensure zero phase-shift. MSE was computed as a measure of complexity and synchronization likelihood (SL) as a measure of connectivity.
Sample and Multi-scale entropy
Entropy-based measures of dynamic complexity have been considered robust algorithms for quantifying the predictability, regularity, and repeatability of biological signals (Takahashi et al. 2010). Complexity measures such as Kolmogorov complexity (Costa et al. 2005), approximate entropy (Pincus 1991), and sample entropy (SE) (Richman and Moorman 2000) were introduced and applied to EEG in Alzheimer’s disease (Abasolo et al. 2005) and individuals with seizures (Yum et al. 2008). More recently, an extension of the SE was introduced by Costa et al. (2002); Costa et al. (2005), a method that considers the fact that recurring patterns within a physiological signal may occur across a range of temporal scales and thus leads to the idea of MSE. Multi-scale analysis is essential in biological signals because biological signals operate across multiple temporal and spatial scales and, as such, their complexity is multi-scale (Costa et al. 2005). Since its introduction, MSE has been used for analysis of time series in EEG (Takahashi et al. 2010; Bosl et al. 2011; Catarino et al. 2011) and ECG (Costa et al. 2005, 2008).
Sample entropy measures the signal irregularity with the advantage of being less dependent on time series length, and is thus more robust than other entropy measures to the entropy estimate parameters. SE is defined as the logarithmic conditional probability that two similar time series of length m remain similar when one sample is appended to their series. Performing such operations on multiple coarsegrained time series produces the MSE measure. In the current study, MSE was calculated on 40-sec artifact-free resting-state segments for each frequency to produce MSE curves at 60 scales per sensor. The minimum, maximum, or average of the MSE measure along the scales can be used as different scalar measures of complexity. A higher MSE value implies lower repeatability and accordingly high signal complexity. Mathematical details of the computation of MSE and the technical details on the choice of parameters can be found in “Appendix 1”.
Synchronization Likelihood (SL) Based Functional Connectivity
Connectivity is a quantification of the mutual relationship between oscillatory signals at two nodes (e.g., two sensors representing two regions of the brain). Connectivity of a network of N nodes can be represented by an N × N symmetric matrix whose (i, j) element represents the connectivity between node i and node j. Several measures have been introduced for functional connectivity such as correlation (Zhou et al. 2009), mutual information (Zhou et al. 2009; Tsiaras et al. 2011), coherence (Zhou et al. 2009; Sakkalis 2011), and synchronization based methods (Sakkalis 2011). The present study adopted the SL method (Montez et al. 2006) as a robust measure of connectivity. The time–frequency SL technique assumes that two signals are synchronized if a pattern of one signal repeats itself at certain time instants for a number of times within a certain period, and another pattern in the other signal repeats itself at those same time instants (Montez et al. 2006), without the signals being necessarily identical. In the present study, SL was computed between band-specific time courses at sensors to quantify functional connectivity between the 274 sensors. Recording from 274 sensors, a symmetric 274 × 274 SL matrix per band was created whose elements range between 0 and 1, 1 being completely synchronous and 0 not at all. Mathematical details for computation of SL connectivity matrices can be found in “Appendix 2”.
Viewing the SL connectivity matrix of each subject at each band as a weighted connectivity graph, where the nodes are the sensors and non-zero SL between nodes represents an edge with that value of SL as the connection weight, then the connectivity strength of each sensor is quantified using a measure, Node Strength, which is the summation of the SL values between a given sensor and all other sensors (i.e. the summation of the corresponding row of the SL matrix to this sensor).
Results
MSE Analysis
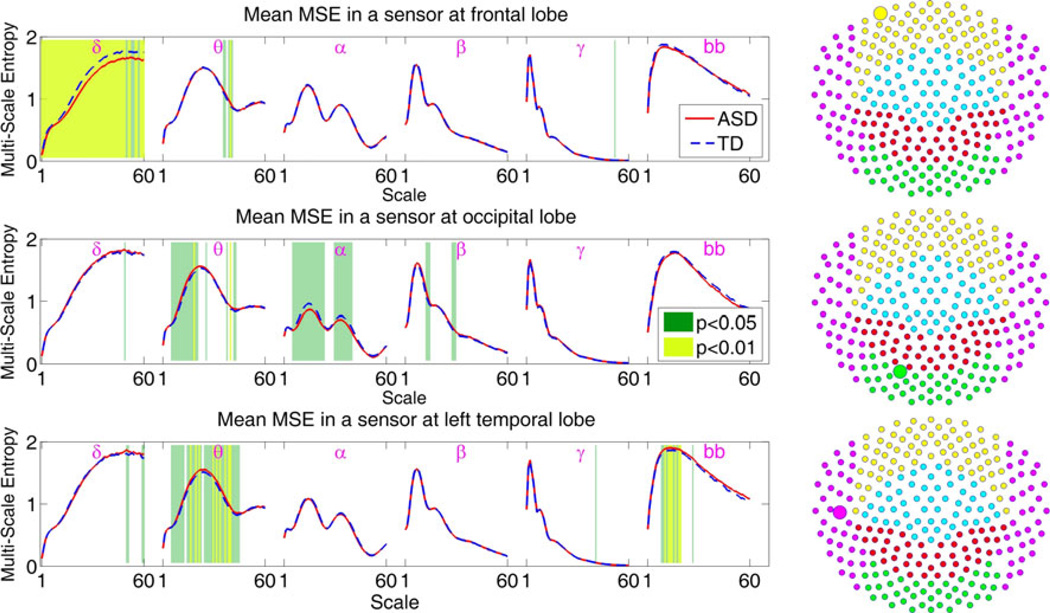
A sensor-wise MSE map was created for each subject. MSE curves at each frequency band were analyzed by plotting the curves averaged over ASD (red) and TD (blue) participants at three representative sensors (Fig. 2). A t-test between ASD and TD curves indicated the degree to which MSE differs between the two populations.
Fig. 2.
The group average MSE curves at each frequency for three representative sensors from frontal, temporal and occipital lobes (depicted by magnified circles on the right). Shown here are the average of group entropy values per all 60 scales, red for ASD and blue for TD. The results of the group analysis performed at each frequency and each scale with significance values denoted with color bars. The sensor locations were selected from the regions where significant differences were observed (see Fig. 3)
To determine a single MSE measurement per frequency (not 60 values), statistical group tests were performed at the maximum of the MSE curves. The local maximum in MSE curve represents two aspects of underlying signal dynamics: (1) the scale where the entropy reaches its maximum is closely related to signal’s spectral properties, and (2) the level of the maximum entropy indicates the signal’s complexity level (Park et al. 2007).
The ASD-TD group analysis results over maximum MSE are shown in Fig. 3. Results show that TD has significantly higher complexity than ASD in frontal regions in the delta band and occipital-parietal regions in the alpha band. ASD had higher complexity than TD in the delta (parietal regions), theta (central and temporal regions), gamma (frontal-central boundary regions), and broad-band (central plus left and right frontal-temporal boundary regions). Generally, with the exception of the alpha and beta bands, children with ASD exhibited elevated complexity over a broad range of space and frequencies. To address the problem of multiple comparisons, permutation testing was employed. A leave-one-subject-out approach was used and t-tests performed among the remaining subjects at each permutation. Thus, in each of the 48 permutations, one subject was taken out and t-tests performed using the remaining 47 subjects. This was done in every node-wise statistical analysis of MSE, and the resulting 48 t values from 48 permutations were then averaged to obtain the t value displayed at each node.
Fig. 3.
Group t tests of complexity results at different frequency bands. Statistical significance over the maximum MSE at each sensor and each frequency are shown in the left column, and the right column shows the unthresholded sensor map color-coded based on t values. T values 2 and 4.74 correspond to p values of 0.05 and 0.00002, respectively. Red indicates higher complexity for ASD and blue for TD
To exclude the possibility that MSE group differences were related to group differences in signal power (where low signal to noise ratio (SNR) would be expected to have increased complexity, simply as a function of relative noise level) (see Cornew et al. (2012)), a t-test examined group differences in power at each sensor. Sensor-level analyses (as in Fig. 4- top panel) showed group difference in the delta and alpha bands consistent with the source-space findings reported in Cornew et al. (2012). At the sensor level, frontal delta group differences were observed (ASD > TD) and at posterior sensors alpha group differences were observed (ASD > TD). At the sensor level, no significant differences were observed in the other frequencies. In addition, using power as a covariate in the MSE analyses did not show considerable effects on the MSE results shown in Fig. 3.
Fig. 4.
Group analysis of the power at delta and alpha bands. Red indicates higher power for ASD and blue for TD. Power differences were observed only in frontal delta and posterior alpha bands at specific sensors. The top panel shows all the sensors that demonstrate a power difference trend (p < 0.4), with group differences in these areas similar to the source group findings reported in Cornew et al. (2012). The bottom panel shows sensors with significant (p < 0.05) group difference. The affected sensors in the bottom panel were not included in the regions revealed by max-MSE (in Fig. 3), indicating that group MSE differences are not related to group power differences
Functional Connectivity Analysis
Figure 5 shows the connectivity of a region seeded at the left parietal lobe with the other 273 regions, at each of the six frequencies for a TD subject. Thus, each plot shows one row of the subject’s SL connectivity matrix (the row corresponding to the seed sensor) with the connectivity edges color-coded. Each vertex represents a sensor node with the color reflecting its connectivity magnitude with the sensor seed (red increased connectivity, blue less or no connectivity). Interpolation of the vertex colors determines the coloring of each face (i.e. triangles).
Fig. 5.

The SL connectivity of a sensor (shown by a black dot) in the left parietal lobe for the six frequency bands examined for a representative TD subject. Whereas dominant connectivity is local, patterns of inter-hemispheric connectivity are quite evident especially in the delta and alpha bands, as well as the broad-band signal
A group t-test was performed on each element of the SL connectivity matrices by a similar leave-one-out permutation testing approach as in MSE, and the significantly different connections (p < 0.05) between the two groups displayed in the left columns of Fig. 6. Red lines indicate connections with greater SL metrics in ASD than TD. Blue lines indicate connections with greater SL metrics in TD than ASD. Student t-tests were also performed at each sensor for the resting-state node strengths at each frequency by averaging the t values from leave-one-out permutation testing. T values are color-coded and shown in the right columns of Fig. 6. Hot (red/yellow) colors show an increase in SL in ASD relative to TD, whereas cold colors indicate a decrease in SL in ASD relative to TD. Using power as a covariate in the node strength difference did not lead to considerable effects on the results shown in Fig. 6. Inspection of Fig. 6 shows an increased short-range connectivity of children with ASD in the frontal lobe in the delta band, and increased long-range connectivity in the temporal, parietal and occipital lobes in the alpha band. All the other bands display decreased long-range connectivity in ASD.
Fig. 6.
Connectivity results at different frequency bands. The significant SL connections (p < 0.05) at each frequency are shown in the left column. The right column shows the t test over the SL connectivity node strengths over the whole brain, color-coded on the t value with red for higher in ASD and blue for TD. Results show differential short-range and long-range connectivity changes in ASD
Connectivity and Complexity Correlation Analysis
Figures 3 and 6 show the results of group differences between ASD and TD in complexity (maximum MSE) and connectivity (node strength). Observing similarities between the statistical differences in complexity (Fig. 3, right column) and connectivity maps (Fig. 6, right column), relationships between the two measures were examined by computing the correlation between the group differences in the mean of node strengths and MSE values along the group subjects (shown in Fig. 7, color-coded for each brain lobe). In Fig. 7, each dot represents a sensor node. Results show a high negative correlation between an ASD-related change in complexity and an ASD-related change in connectivity, with the highest correlations in delta, alpha, beta and broad-band. All correlations were significant (p < 0.0001).
Fig. 7.
Correlation between the ASD-TD group differences of resting state MSE and SL node strengths at each frequency. All the correlations are statistically significant (p < 0.0001). The high negative correlation in most frequency bands indicates that a decrease in connectivity in ASD is related to an increase in complexity in ASD (and vice versa). The least squares fit line and associated r value shows the best fit across all sensors
Correlation of Connectivity and Complexity with symptom severity
Figure 8 (top panel) shows the regions of significant differences (as differing levels of significance based on frequency) in connectivity between ASD and TD. At these regions, average node-strength (connectivity) as well as average max-MSE (complexity) across sensors was computed and this average measure correlated with SRS scores in the ASD subjects. Figure 8 (middle and bottom panels) shows the correlation values for each frequency bands. As shown in Fig. 8, the direction of correlation with SRS reversed between complexity and connectivity.
Fig. 8.
Correlation of autism severity score with average connectivity and complexity in selected regions for the five frequency bands (delta to gamma). Top panel shows regions of significant differences in connectivity between ASD and TD. Red and blue regions demonstrate whether that region has increased connectivity in ASD or TD, respectively. The middle panel shows the correlation of averaged connectivity node strength with SRS scores. The bottom panel shows the correlation of averaged complexity with SRS
Discussion
The main results of this study are:
MSE: TD subjects have significantly higher complexity than ASD in frontal regions in the delta band and occipital-parietal regions in the alpha band. ASD have higher complexity than TD in the delta (parietal regions), theta (central and temporal regions), gamma (frontal-central boundary regions) and broad-band (central plus left and right frontal-temporal boundary regions). (See Fig. 3).
FC: ASD subjects have increased short-range connectivity in the frontal lobe in the delta band, and in the temporal, parietal and occipital lobes in the alpha band. All the other bands display decreased long-range connectivity in ASD. Very few changes were observed in the gamma band (see Fig. 6).
Group differences between ASD and TD in complexity and FC were spatially complementary (see Figs 3 and 6 and e.g. alpha band of Fig. 7). Thus, in locations where FC is elevated in ASD, complexity is complementarily reduced (and vice versa).
Generally, across all frequency bands, complementarity between group differences in ASD and TD was observed with respect to connectivity and complexity. As discussed below, regional differences in delta, theta, and alpha connectivity (with commensurate changes in complexity) may reflect the different functional significance of activity in these different brain frequencies.
This latter observation of spatial complementarity is of novel significance. As discussed below, two hypotheses could account for such observations: diminished connectivity leading to poorer inhibitory regulation with subsequent elevated entropy, or complexity in regional signal. This is most likely considered at lower frequencies (delta, theta, alpha) at which longer range connectivity may be mediated. Conversely, it may be that endogenously chaotic signals prohibit effective measures of connectivity. It is tempting to speculate that this hypothesis may underlie the spatial complementarity between connectivity and complexity in the higher frequency beta and gamma bands.
Apparent local hyper connectivity in the frontal areas in the delta band may be consistent with reports of short range hyper connectivity from fMRI (Courchesne and Pierce 2005) and EEG (Barttfeld et al. 2011). On the other hand, the hypo connectivity in ASD seen in the theta band may be interpreted in terms of prevailing notions that longer range connectivity is mediated by theta oscillations (Khan et al. 2013). The posterior hyper connectivity in the alpha band in ASD is worthy of further study. The lack of connectivity in beta and gamma bands in ASD throughout the brain may reflect elevated “brain noise” in ASD, precluding the establishment of connectivity (Dominguez et al. 2013).
The applicability of MSE as a characterization of dynamic temporal complexity and its variability in ASD is investigated for the first time in this paper. Furthermore, we believe this may be the first demonstration of signal complexity based on MEG data. The necessity for multiple scales in the complexity analyses arises from the fact that pathology is not often observed at a single scale across all frequencies, as shown in Fig. 2. This figure also demonstrates that the MSE curves drop sharper as the frequency increases from delta toward gamma. The scale at which MSE reaches its peak also decreases from delta toward gamma. In fact, whereas low scales capture short-range temporal irregularity, high scales capture long-range temporal irregularity (Takahashi et al. 2010). Since the high-scale complexity abnormality in ASD was in the delta band, this may represent disturbed long-range temporal correlations, which in turn is perhaps an indication of the change in the temporal integration necessary for the flow of information in cognition (Breakspear 2006; Takahashi et al. 2010). Conversely, the complexity abnormalities may represent disruptions of comparatively shorter-range temporal signal correlations as the frequency contents increase towards gamma.
Multi-scale entropy characterizes the degree of repetition in the temporal patterns of a signal at multiple temporal scales. As such, MSE is a measure of the inherent complexity of signal dynamics. Analyses showed brain regions (represented by sensors) where the MSE of different frequencies is significantly affected in ASD. In particular, as shown in Fig. 3, in the delta band complexity is lower in ASD than TD in the left frontal lobe, whereas complexity is higher around the right temporal-parietal lobe. In the theta band, abnormalities are spread across the brain, although a significant complexity increase is seen in the central as well as left and right temporal regions. In the alpha band, the ASD group has diminished complexity bilaterally around the edge of the parietal and occipital lobes. In the beta band, a small region of the left frontal lobe is affected (demonstrated by diminished complexity), whereas for gamma, areas similar to beta band, as well as central and temporal regions show an increase in complexity in ASD.
A hypothesis could be made that the group differences in complexity are due to group differences in power. However, group differences in complexity remained after co-varying for power, indicating that information from MSE is due to more time-domain differences associated with ASD pathology rather than merely signal power. In addition, by examining Fig. 4 (top row), it can be seen that regional elevation of alpha power is observed in sensors spatially consistent with the brain sources (bilateral parietal) demonstrating the elevated alpha power in ASD reported by Cornew et al. (2012). However, the areas where significant group alpha power differences were observed (Fig. 4, bottom row), were not spatially identical to where complexity group differences were observed in Fig. 3. Given the above, the hypothesis that group differences in complexity are due to group differences in power is not strongly supported.
In addition to complexity, resting state functional connectivity in ASD and TD was assessed using SL to quantify linear and non-linear couplings between sensors. As is evident in Fig. 6, the ASD connectivity patterns indicate an increase in shorter-range (in terms of physical proximity of sensors) frontal networks in the delta band, whereas long-range connectivity was significant in the alpha band. However, long-range functional connectivity was significantly diminished in ASD in the delta, theta, and beta bands. Long-range connections are important in perception (Sporns et al. 2000), as well as information processing and integration (Just et al. 2004), and in providing a pathway for the neurons to quickly communicate with distant brain regions. Lack of such capability in individuals with ASD may prevent the integration of different sources of information necessary for coherent behavioral and cognitive states (e.g. see (Nicoll et al. 1993; Bressler 1995; Friston 2002; Sporns and Zwi 2004)). The short-range frontal connectivity increase in delta, as well as long-range under-connectivity observations here, are in line with other investigations (Barttfeld et al. 2011).
ASD-TD differences between node connectivity strength and MSE showed strong correlations across frequencies, ranging from r = −0.40 in theta to r = −0.82 in alpha. This suggests that complexity and connectivity have a joint interpretability, with a decrease in complexity associated with an increase in the ability of that region to network with other regions, and vice versa. Such a relationship has been hypothesized in earlier EEG complexity investigations of autism and schizophrenia (Takahashi et al. 2010; Bosl et al. 2011; Catarino et al. 2011), but these studies have all focused on complexity with no systematic investigation of relationships between complexity and connectivity. However, Friston (1996) used synthetic signal and showed that reduced connectivity increases the EEG signal complexity. This was then used by Takahashi et al. (2010) to infer that the increase in EEG complexity in the fronto-centro-temporal regions of individuals with schizophrenia may be rooted in abnormal network connectivity. Similarly, Catarino et al. (2011) investigated the EEG complexity change in ASD and interpreted their findings along the lines of Friston (1996), with increased complexity in ASD indicating atypical connectivity in autism. This association, however, was not directly investigated. The present finding of an inverse relationship between complexity and connectivity in ASD provides support for the existing work on brain functional complexity and connectivity investigations in pathology.
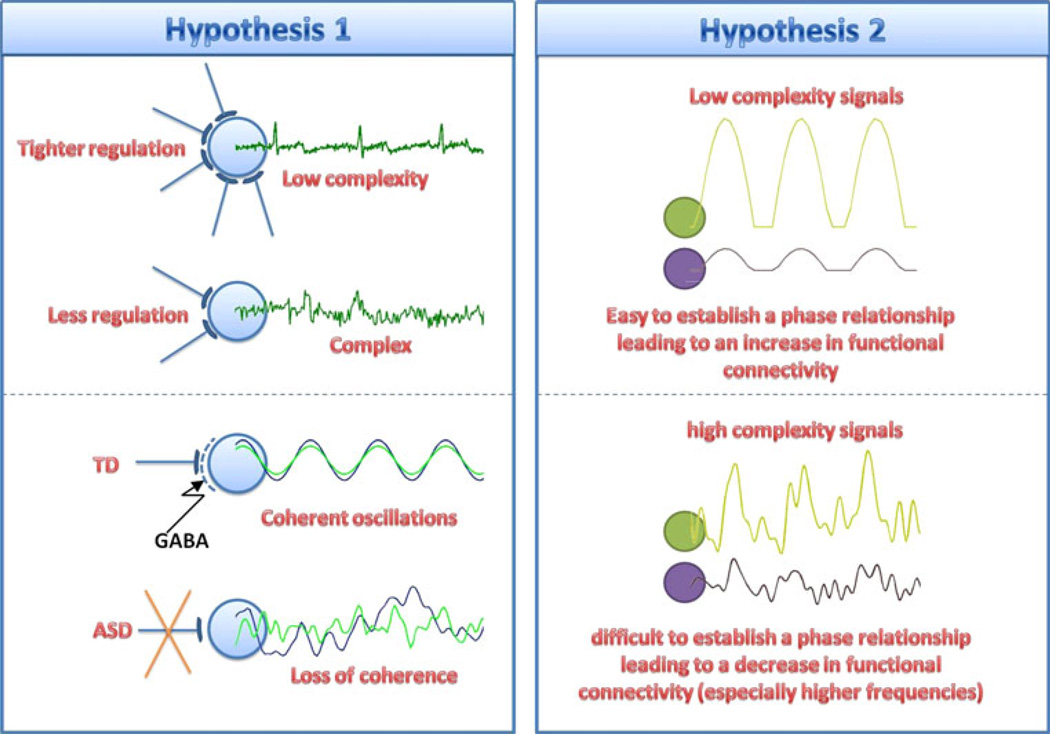
As alluded to above, there are two possible hypotheses to account for the significant spatial complementarities between complexity and connectivity changes (see Fig. 9). As shown in the left panel, it is perhaps the case that enhanced connectivity leads to better regulation of electrical activity, with subsequent reduced complexity. As shown in the right panel, it also may be that reduced complexity at a node indicates that the signal is more predictable, leading to a faster feedback loop, thereby facilitating the node’s enhanced capability in participating with widespread networks, leading to an increased connectivity. The first hypothesis of reduced connectivity in ASD as underlying increased complexity in ASD, may be the most biologically consistent with the notion of impaired inhibition in ASD and thus worth pursuing in larger more targeted studies. However, resolution of the two hypotheses (and their relative relevance at each frequency band) remains to be elucidated.
Fig. 9.
Two possible hypotheses of connectivity and complexity relationship. The left panel illustrates that the loss of GABA mediated inhibiting (inhibitory) regulation in ASD leads to less coherent oscillations and increasing local complexity. The right panel illustrates that decrease of GABA in ASD causes more complex signals leading to a decrease in functional connectivity
Association with the dimensional SRS total score, as an index of autism symptom severity, showed both features of spatial and spectral variation, as well as overall complementarity (with opposing sign) of connectivity and complexity measures. Detailed investigation of the spatial and spectral influences on relationships between connectivity and complexity measures versus specific symptom domains is motivated by such observations and further detailed studies are warranted.
Apparent discrepancies between results in the present study and those of resting state EEG study of Bosl et al. (2011) or the task-based EEG studies of Catarino et al. (2011) may be due to age differences in the populations. Additionally, Bosl et al. (2011) used broadband measures of complexity and stratified groups according to risk (but not diagnosis). EEG referencing and inherent differences between EEG and MEG may also contribute to study differences (Hornero et al. 2009).
Although differences in maximum head displacement during the exam between ASD and TD groups were not significant, a limitation of the present sensor-space analyses is the potential for differences across subjects in head position and thus the mapping of sensor to brain regions. Another limitation is the examination of sensor rather than source space measures. In future studies, we hope to use advanced source localization methods that provide accurate measures of brain activity to better examine functional connectivity and complexity in ASD.
In conclusion, present findings highlight the importance of a joint complexity and connectivity analysis in studies of resting state activity in ASD. These findings also revealed regions of the brain where ASD versus TD differences were observed. Functional connectivity at different frequencies showed that the long-range strength of connectivity decreases in the ASD at most frequencies, whereas short-range frontal connectivity increases in the delta band. Group differences obtained from connectivity and complexity were highly correlated in terms of spatial variation, a suggestion of the complexity conveying some information of connectivity strength and an indication of the role that the underlying neural feedback loops plays in complexity measures. Overall, the study emphasizes the need for a high temporal resolution characterization of brain resting activity when studying populations with ASD.
Acknowledgments
This work is supported in part by NIH R01DC008871 (PI: T. Roberts) and NIH P30HD026979 (Neuroimaging Core, Director: T. Roberts), NIH MH092862 (PI: R. Verma) and NIH MH098010 (PI: R. Verma), Pennsylvania Department of Health SAP # 4100042728 and SAP #4100047863 (PI: R. Schultz). Dr. Roberts would like to thank the Oberkircher Family for the Oberkircher Family Chair in Pediatric Radiology at the Children’s Hospital of Philadelphia.
Appendix 1: Computation of Multi-Scale Entropy
Let xN(ti) = [x(ti),x(ti + T),…,x(ti + (N – 1)T)] represent a vector of length N starting at time point ti of a signal (or a time series) recorded by the sampling period T. SE is defined as the logarithmic conditional probability that two similar sequences of length m, i.e. xm(tp) and xm(tq), remain similar when added one sample to their length, i.e. xm+1(tp) and xm+1(tq). This can be quantified as follows
where is the number of m-length vectors xm(tj) which are within a distance of r to xm(ti) when self matches are not counted (Richman and Moorman 2000; Richman et al. 2004; Costa et al. 2005). The distance based on which the similarity between the vectors xm(ti) and xm(tj) are calculated is defined as the maximum absolute difference between their elements (Costa et al. 2005). Figure 10 illustrates how this SE is calculated.
Fig. 10.
A simulated time series x(t) of 50 samples illustrating the SE calculation when m = 2 and r = 0.2σx. The green, pink, and red dashed lines around the first three datapoints x(0), x(0.02), and x(0.04) represent the area of ±r around these points, respectively. Here, a data point is determined matched with the first data point, if it is within the range of x(0) ± r. All those similar to the first data point are shown by green circles, and accordingly, pink and red circles are used for those matched with the second and third data points, respectively. In the segment of time series shown here, there are two two-component templates of green-pink matched with [x(0), x(0.02)] while there is only one three-component templates of subsequent green-pink-red points similar to [x(0), x(0.02), x(0.04)]. This procedure is repeated to calculate the ration of all two- and three-component template matches, from which the SE is calculated
Multi-scale entropy (MSE) is then defined as the sample entropies measured at consecutive coarse grained time series yτ corresponding to the scale τ. The coarse grained procedure is performed by
in which at each scale τ the data points are first divided into nonoverlapping segments of length τ, and then the data points inside each segment are averaged to calculate the corresponding . Figure 11 illustrates the coarse grained time series at the scale 3.
Fig. 11.
An illustration of the coarse-grained time series at the scale 3
By computing SE at various scales, MSE curves can be generated and used to compare the relative complexity of the power-normalized time series. The minimum, maximum, or average of the MSE measures along the scales can be used as different scalar measures of complexity. The two parameters m and r in MSE need to be set for proper analysis. It has been shown (Costa et al. 2005; Takahashi et al. 2010; Bosl et al. 2011; Catarino et al. 2011) that a good statistical validity of MSE is achieved when m = 1 or 2 and r = 0.1σ – 0.25σ where σ represents the SD of the time series at scale τ = 1 (i.e. SD of the original time series). In this work, we empirically chose m = 2 and r = 0.2σ after investigating different values. This procedure is performed for each sensor and each frequency band. A higher MSE value implies lower repeatability and accordingly higher signal complexity.
Appendix 2: Synchronization Likelihood
The time–frequency SL technique assumes that two signals are synchronized if a pattern of one signal repeats itself at certain time instants for a number of times within a certain period and another pattern in the other signal repeats itself at those same time instants (Montez et al. 2006).
For a given signal at channel k, i.e. xk(t), the signal pattern at any given time instant ti can be represented by an embedding vector xk,ti = [xk(ti),xk(ti+1),…,xk(ti+(m–1)l)] where l is the lag and m is the length of the embedding vector. l and m are typically set to and where fs is the sampling frequency, and hf and lf are the high and low frequency contents of the signal, respectively. At each time instant ti, the Euclidean distance is then measured between the reference embedding vector xk,ti and the set of all other embedding vectors at times tj, i.e. xk,tj, where tj lies in the range or where and tw1 < tw2. Then, nref nearest embedding vectors xk,tj are retained. This procedure is conducted for each channel k and each time instant ti. The SL between channel k1 and channel k2 at time instant ti is the number of simultaneous embedding vector recurrences in the two channels divided by the total number of recurrences, i.e. . Figure 12 illustrates how SL is calculated.
Fig. 12.
An illustration of synchronization likelihood between two signals from two channels plotted by different colors. A reference pattern was selected at the time 0.074 s (thick rectangle). The recurrences in both signals are shown by thin rectangles for nref = 10. Vertical arrows show simultaneous recurrences in both channels. SL at time 0.074 is therefore equal to the ratio between number of simultaneous recurrences and the number of nref, i.e. SL = 5/10 = 0.5
In this work, the parameters adjustments were performed for each frequency band according to the aforementioned equations, which are shown in Table 2.
Table 2.
The parameters in measuring the synchronization likelihood for connectivity networks
| Band (lf–hf) | l (sample) | m (sample) | tw1 (sec) | tw2 (sec) |
|---|---|---|---|---|
| δ (0.5–4 Hz) | 42 | 25 | 4.03 | 10 |
| θ (4–8 Hz) | 21 | 7 | 0.50 | 10 |
| α (8–13 Hz) | 13 | 6 | 0.26 | 10 |
| β (13–30 Hz) | 6 | 8 | 0.17 | 10 |
| γ (30–80 Hz) | 2 | 9 | 0.06 | 10 |
| BB (0.5–100 Hz) | 2 | 601 | 2.4 | 10 |
Recording from 274 sensors, we obtained a three dimensional SL matrix of 274 × 274 × T per recording per frequency, where T is the number of time instants at which SLti is calculated. The functional connectivity matrix is then defined as the average of all the T matrices along the time instants, yielding a symmetric 274 × 274 SL matrix (per band) whose elements range between 0 and 1.
The SL connectivity matrix of each subject at each band represents a weighted graph in which each sensor represents the graph node and each SL connection between two sensors represents the graph edge weighted by their SL value. The node strength in a graph is defined as the summation of the edges between a node and all other graph nodes, i.e. where NSi is the node strength of sensor i and SL(i,j) is the SL value between sensors i and j. Node strength of the connectivity graph can be interpreted as the relative strength of the corresponding node region to connect with other regions.
Contributor Information
Yasser Ghanbari, Section of Biomedical Image Analysis, Department of Radiology, University of Pennsylvania, Philadelphia, PA, USA.
Luke Bloy, Section of Biomedical Image Analysis, Department of Radiology, University of Pennsylvania, Philadelphia, PA, USA.
J. Christopher Edgar, Lurie Family Foundations MEG Imaging Center, Department of Radiology, Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Lisa Blaskey, Lurie Family Foundations MEG Imaging Center, Department of Radiology, Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Ragini Verma, Section of Biomedical Image Analysis, Department of Radiology, University of Pennsylvania, Philadelphia, PA, USA.
Timothy P. L. Roberts, Email: robertstim@email.chop.edu, Lurie Family Foundations MEG Imaging Center, Department of Radiology, Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
References
- Abasolo D, Hornero R, et al. Analysis of regularity in the EEG background activity of alzheimer’s disease patients with approximate entropy. Clinical Neurophysiology. 2005;116(8):1826–1834. doi: 10.1016/j.clinph.2005.04.001. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- APA. DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders. DC: Washington: American Psychiatric Association; 2000. [Google Scholar]
- Assaf M, Jagannathan K, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53(1):247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barttfeld P, Wicker B, et al. A big-world network in ASD: dynamical connectivity analysis reflects a deficit in long-range connections and an excess of short-range connections. Neuropsychologia. 2011;49(2):254–263. doi: 10.1016/j.neuropsychologia.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, et al. Gamma, alpha, delta, and theta oscillations govern cognitive processes. International Journal of Psychophysiology. 2001;39(2–3):241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Blume WT. Drug effects on EEG. Journal of Clinical Neurophysiology. 2006;23(4):306–311. doi: 10.1097/01.wnp.0000229137.94384.fa. [DOI] [PubMed] [Google Scholar]
- Bosl W, Tierney A, et al. EEG complexity as a biomarker for autism spectrum disorder risk. BMC Medicine. 2011;9:18. doi: 10.1186/1741-7015-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakspear M. The nonlinear theory of schizophrenia. Australian and New Zealand Journal of Psychiatry. 2006;40(1):20–35. doi: 10.1080/j.1440-1614.2006.01737.x. [DOI] [PubMed] [Google Scholar]
- Bressler SL. Large-scale cortical networks and cognition Brain Research. Brain Research Reviews. 1995;20(3):288–304. doi: 10.1016/0165-0173(94)00016-i. [DOI] [PubMed] [Google Scholar]
- Calhoun V, Kiehl K, et al. Modulation of temporally coherent brain networks estimated using ica at rest and during cognitive tasks. Human Brain Mapping. 2008;29(7):828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor DS, Thatcher RW, et al. Computerized EEG analyses of autistic children. Journal of Autism and Developmental Disorders. 1986;16:169–187. doi: 10.1007/BF01531728. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, et al. Autism, asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Catarino A, Churches O, et al. Atypical EEG complexity in autism spectrum conditions: A multiscale entropy analysis. Clinical Neurophysiology. 2011;122(12):2375–2383. doi: 10.1016/j.clinph.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Coben R, Clarke AR, et al. EEG power and coherence in autistic spectrum disorder. Clinical Neurophysiology. 2008;119(5):1002–1009. doi: 10.1016/j.clinph.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Collins AL, Ma D, et al. Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics. 2006;7(3):167–174. doi: 10.1007/s10048-006-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J, Gruber CP. Social Responsiveness Scale. Los Angeles: CA, Western Psychological Services; 2005. [Google Scholar]
- Cornew L, Roberts TP, et al. Resting-state oscillatory activity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42(9):1884–1894. doi: 10.1007/s10803-011-1431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Ary L, et al. Multiscale entropy analysis of biological signals. Physical Review E. 2005;71:021906. doi: 10.1103/PhysRevE.71.021906. [DOI] [PubMed] [Google Scholar]
- Costa M, Goldberger AL, et al. Multiscale entropy analysis of complex physiologic time series. Physical Review Letters. 2002;89(6):068102. doi: 10.1103/PhysRevLett.89.068102. [DOI] [PubMed] [Google Scholar]
- Costa MD, Peng CK, et al. Multiscale analysis of heart rate dynamics: Entropy and time irreversibility measures. Cardiovascular Engineering. 2008;8(2):88–93. doi: 10.1007/s10558-007-9049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but longdistance disconnection. Current Opinion in Neurobiology. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, et al. The functional neuroanatomy of social behaviour: Changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123(Pt 11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, et al. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: A potential model of autism spectrum disorder. Behavioural Brain Research. 2008;187(2):207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez LG, Velazquez JL, et al. A model of functional brain connectivity and background noise as a biomarker for cognitive phenotypes: Application to autism. PLoS ONE. 2013;8(4):e61493. doi: 10.1371/journal.pone.0061493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley Nicpon M, Doobay AF, et al. Parent, teacher, and self perceptions of psychosocial functioning in intellectually gifted children and adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2010;40:1028–1038. doi: 10.1007/s10803-010-0952-8. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Theoretical neurobiology and schizophrenia. British Medical Bulletin. 1996;52(3):644–655. doi: 10.1093/oxfordjournals.bmb.a011573. [DOI] [PubMed] [Google Scholar]
- Friston K. Beyond phrenology: What can neuroimaging tell us about distributed circuitry? Annual Review of Neuroscience. 2002;25:221–250. doi: 10.1146/annurev.neuro.25.112701.142846. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Tononi G, et al. Characterising the complexity of neuronal interactions. Human Brain Mapping. 1995;3(4):302–314. [Google Scholar]
- Ghanbari Y, Bloy L, et al. Dominant component analysis of electrophysiological connectivity networks. In: Ayache N, Delingette H, Golland P, Mori Nice K, editors. International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI 2012), Part III. LNCS. Heidelberg: Springer; 2012. pp. 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari Y, Smith AR, et al. International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI 2013), Nagoya, Japan. LNCS. Heidelberg: Springer; 2013. Connectivity subspace learning for pathology and developmental variations; pp. 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidley Larson JC, Mostofsky SH. Motor deficits in autism. In: Tuchman R, Rapin I, editors. Autism: a neurological disorder of early brain development. London: MacKeith Press; 2006. pp. 231–247. [Google Scholar]
- Harada M, Taki MM, et al. Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. Journal of Autism and Developmental Disorders. 2010;41:447–454. doi: 10.1007/s10803-010-1065-0. [DOI] [PubMed] [Google Scholar]
- Hornero R, Abasolo D, et al. Nonlinear analysis of electroencephalogram and magnetoencephalogram recordings in patients with Alzheimer’s disease. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2009;367:317–336. doi: 10.1098/rsta.2008.0197. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Gamma, fast, and ultrafast waves of the brain: Their relationships with epilepsy and behavior. Epilepsy and Behavior. 2008;13(1):25–31. doi: 10.1016/j.yebeh.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, et al. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127(Pt 8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, et al. Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser MD, Delmolino L, et al. Comparison of visual sensitivity to human and object motion in autism spectrum disorder. Autism Research. 2010;3:191–195. doi: 10.1002/aur.137. [DOI] [PubMed] [Google Scholar]
- Khan S, Gramfort A, et al. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proceedings of the National Academy of Sciences of USA. 2013;110:3107–3112. doi: 10.1073/pnas.1214533110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Schack B, et al. The functional significance of theta and upper alpha oscillations. Experimental Psychology. 2005;52(2):99–108. doi: 10.1027/1618-3169.52.2.99. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, et al. Gamma rhythms and beta rhythms have different synchronization properties. Proceedings of the National Academy of Sciences of USA. 2000;97(4):1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug D, Arick JR. Krug Asperger’s Disorder Index. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Lord C, Risi S, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47(2):764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montez T, Linkenkaer-Hansen K, et al. Synchronization likelihood with explicit time-frequency priors. Neuroimage. 2006;33(4):1117–1125. doi: 10.1016/j.neuroimage.2006.06.066. [DOI] [PubMed] [Google Scholar]
- Murias M, Webb SJ, et al. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biological Psychiatry. 2007;62(3):270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll A, Larkman A, et al. Modulation of EPSP shape and efficacy by intrinsic membrane conductances in rat neocortical pyramidal neurons in vitro. Journal of Physiology. 1993;468:693–710. doi: 10.1113/jphysiol.1993.sp019795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, et al. Excess of high frequency electroencephalogram oscillations in boys with autism. Biological Psychiatry. 2007;62:1022–1029. doi: 10.1016/j.biopsych.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Paakki JJ, Rahko J, et al. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Research. 2010;1321:169–179. doi: 10.1016/j.brainres.2009.12.081. [DOI] [PubMed] [Google Scholar]
- Pantev C, Makeig S, et al. Human auditory evoked gamma-band magnetic fields. Proceedings of the National Academy of Sciences of USA. 1991;88(20):8996–9000. doi: 10.1073/pnas.88.20.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Kim S, et al. Multiscale entropy analysis of EEG from patients under different pathological conditions. Fractals. 2007;15(04):399–404. [Google Scholar]
- Perkins T, Stokes M, et al. Mirror neuron dysfunction in autism spectrum disorders. Journal of Clinical Neuroscience. 2010;17(10):1239–1243. doi: 10.1016/j.jocn.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy as a measure of system-complexity. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(6):2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollonini L, Patidar U, et al. Functional connectivity networks in the autistic and healthy brain assessed using Granger causality. Conference Proceeding IEEE Engineering in Medicine and Biology Society (EMBS) 2010:1730–1733. doi: 10.1109/IEMBS.2010.5626702. [DOI] [PubMed] [Google Scholar]
- Ramoz N, Reichert JG, et al. Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. American Journal of Psychiatry. 2004;161(4):662–669. doi: 10.1176/appi.ajp.161.4.662. [DOI] [PubMed] [Google Scholar]
- Richman Joshua S, Lake Douglas E, et al. Sample entropy. Methods in Enzymology. 2004;384:172–184. doi: 10.1016/S0076-6879(04)84011-4. [DOI] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time series analysis using approximate entropy and sample entropy. American Journal of Physiology-Heart and Circulatory Physiology. 2000;278(6):H2039–H2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Roberts Timothy PL, Khan Sarah Y, et al. MEG detection of delayed auditory evoked responses in autism spectrum disorders: Towards an imaging biomarker for autism. Autism Research. 2010;3(1):8–18. doi: 10.1002/aur.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behaviour. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo N, Foxe JJ, et al. Multisensory processing in children with autism: High-density electrical mapping of auditory-somatosensory integration. Autism Research. 2010;3(5):253–267. doi: 10.1002/aur.152. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, et al. SCQ: Social Communication Questionnaire. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Sakkalis V. Review of advanced techniques for the estimation of brain connectivity measured with EEG/MEG. Computers in Biology and Medicine. 2011;41(12):1110–1117. doi: 10.1016/j.compbiomed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, et al. Neural correlates of executive function in autistic spectrum disorders. Biological Psychiatry. 2006;59(1):7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Sheikhani A, Behnam H, et al. Detection of abnormalities for diagnosing of children with autism disorders using of quantitative electroencephalography analysis. Journal of Medical Systems. 2012;36(2):957–963. doi: 10.1007/s10916-010-9560-6. [DOI] [PubMed] [Google Scholar]
- Simmons DR, Robertson AE, et al. Vision in autism spectrum disorders. Vision Research. 2009;49(22):2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Sporns O, Tononi G, et al. Connectivity and complexity: The relationship between neuroanatomy and brain dynamics. Neural Network. 2000;13(8–9):909–922. doi: 10.1016/s0893-6080(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Sporns O, Zwi JD. The small world of the cerebral cortex. Neuroinformatics. 2004;2(2):145–162. doi: 10.1385/NI:2:2:145. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Cho RY, et al. Antipsychotics reverse abnormal EEG complexity in drug-naive schizophrenia: A multi scale entropy analysis. Neuroimage. 2010;51(1):173–182. doi: 10.1016/j.neuroimage.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Sporns O, et al. A measure for brain complexity: Relating functional segregation and integration in the nervous system. Proceedings of the National Academy of Sciences. 1994;91(11):5033–5037. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiaras V, Simos PG, et al. Extracting biomarkers of autism from MEG resting-state functional connectivity networks. Computers in Biology and Medicine. 2011;41(12):1166–1177. doi: 10.1016/j.compbiomed.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, et al. Brain connectivity and high functioning autism: A promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neuroscience and Biobehavioral Reviews. 2012;36(1):604–625. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. San Antonio: The Psychological Corporation; 2003. [Google Scholar]
- Weng SJ, Wiggins JL, et al. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Research. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Peltier SJ, et al. Using a self-organizing map algorithm to detect age-related changes in functional connectivity during rest in autism spectrum disorders. Brain Research. 2011;1380:187–197. doi: 10.1016/j.brainres.2010.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, et al. Neuropsychologic functioning in children with autism: Further evidence for disordered complex information-processing. Child Neuropsychol. 2006;12(4–5):279–298. doi: 10.1080/09297040600681190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yum MK, Jung KY, et al. Effect of a ketogenic diet on EEG: Analysis of sample entropy. Seizure. 2008;17(6):561–566. doi: 10.1016/j.seizure.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Zhou D, Thompson WK, et al. MATLAB toolbox for functional connectivity. Neuroimage. 2009;47(4):1590–1607. doi: 10.1016/j.neuroimage.2009.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]