Abstract
To determine whether A3 adenosine receptor (A3AR) signaling modulates myocardial function, energetics, and cardioprotection, hearts from wild-type and A3AR-overexpressor mice were subjected to 20-min ischemia and 40-min reperfusion while 31P NMR spectra were acquired. Basal heart rate and left ventricular developed pressure (LVDP) were lower in A3AR-overexpressor hearts than wild-type hearts. Ischemic ATP depletion was delayed and postischemic recoveries of contractile function, ATP, and phosphocreatine were greater in A3AR-hearts. To determine the role of depressed heart rate and to confirm A3AR-specific signaling, hearts were paced at 480 beats/min with or without 60 nmol/l MRS-1220 (A3AR-specific inhibitor) and then subjected to ischemiareperfusion. LVDP was similar in paced A3AR-overexpressor and paced wild-type hearts. Differences in ischemic ATP depletion and postischemic contractile and energetic dysfunction remained in paced A3AR-overexpressor hearts versus paced wild-type hearts but were abolished by MRS-1220. In summary, A3AR overexpression decreased basal heart rate and contractility, preserved ischemic ATP, and decreased postischemic dysfunction. Pacing abolished the decreased contractility but not the ATP preservation or cardioprotection. Therefore, A3AR overexpression results in cardioprotection via a specific A3AR effect, possibly involving preservation of ATP during ischemia.
Keywords: cardioprotection, ischemia, nuclear magnetic resonance spectroscopy
Adenosine is an endogenous metabolite that is released from the myocardium during ischemia-reperfusion. Exogenous administration of adenosine or adenosine receptor agonists has been shown to be cardioprotective (20, 26). Because of its potent cardioprotective effects, much attention has focused on the use of adenosine as a cardiotherapy. Thus far the main approach in therapy development has been to administer adenosine, adenosine receptor agonists, or modulators of adenosine degradation (15, 22). Clinical use of such approaches is complicated by the effects of these agents on noncardiac tissue and by progressive desensitization of adenosine receptors. Cardiac-specific targeting could alleviate some of these problems. Therefore, a transgenic mouse model was recently developed with cardiac-specific overexpression of the Gi/o-coupled A1 adenosine receptor (A1AR) (23). Hearts from these mice were inherently resistant to ischemia, indicating that increasing the number of functional adenosine receptors alone can enhance endogenous adenosine signaling and lead to cardioprotection.
Although it is thought that the cardioprotective effects of adenosine are mediated primarily via the A1AR, recent evidence suggests that signaling through the A3 adenosine receptor (A3AR), which also couples to Gi/o, could be protective (1, 28, 31). Although levels of the A3AR are low in cardiac tissue from most species (29, 32), A3AR overexpression could be useful as a genetic therapy. To determine whether cardiac-specific overexpression of the A3AR protects against ischemic injury, transgenic mice were created with a gene construct consisting of the canine A3AR cDNA under the control of the α-myosin heavy chain promoter (2). It was demonstrated that, after in vivo regional ischemia and reperfusion, infarct size was lower in low copy number A3AR overexpressors than in wild-type mice, confirming that A3AR signaling could protect against myocardial necrosis (2). In the present study, we determined whether cardiac-specific overexpression of the A3AR could also protect against postischemic contractile dysfunction after short periods of ischemia. Perfused hearts from A3AR-overexpressor and wild-type mice were subjected to 15- or 20-min global ischemia and 40-min reperfusion. Because adenosine has negative chronotropic effects (14) and cardioprotection has been related to preservation of ischemic ATP (8, 24, 27) and intracellular pH levels (5, 7, 19), contractile parameters and energetics were also studied in the A3AR-overexpressor hearts. Furthermore, the canine A3AR-specific inhibitor, MRS-1220, was used to confirm that any observations in the A3AR-overexpressor hearts were indeed due to A3AR signaling.
MATERIALS AND METHODS
Animals
Transgenic mice exhibiting cardiac-specific overexpression of the A3AR were created by microinjection of a transgene construct, containing canine A3AR cDNA under the control of the α-myosin heavy chain promoter, into the pronuclei of fertilized FVB/N mouse oocytes (2). After embryo implantation, the presence of the transgene in the resultant founder mice was confirmed by Southern blot analysis and expression of canine A3AR mRNA was determined by Northern blot analysis. A3AR levels were assessed in the transgenic lines by radioligand binding assays (2).
In the present study, 15 heterozygous low copy number transgenic mice (A3tg.1) and 16 of their wild-type littermates were used; all animals were treated in accordance with National Institutes of Health (NIH) guidelines and the “Guiding Principles for Research Involving Animals and Human Beings” of the American Physiological Society. Binding assays revealed 12.7 fmol/mg of G protein-coupled A3ARs in A3tg.1 hearts and negligible levels in wild-type littermates. A3tg.1 hearts exhibited no evidence of cardiac abnormalities, as determined from morphological and histochemical analysis and determination of expression of hypertrophic marker genes (2).
Ischemia-Reperfusion Protocol
Hearts were isolated and perfused in the Langendorff mode as described previously (4). Hearts were then subjected to 20 min of no-flow ischemia followed by 40-min reperfusion. Left ventricular developed pressure (LVDP), rate of contraction (+dP/dt), rate of relaxation (−dP/dt), and heart rate were monitored via a water-filled latex balloon in the left ventricle. Recovery of contractile function was assessed by measurement of LVDP at the end of reperfusion and expressed as a percentage of preischemic LVDP.
Control Hearts, Pacing Protocol, and MRS-1220 Treatment
A control group of untreated A3tg.1 (n = 5) and wild-type (n = 6) hearts was allowed to beat at their intrinsic heart rate, and another group of A3tg.1 (n = 5) and wild-type (n = 5) hearts was paced throughout preischemia, and reperfusion at 8 Hz with a 4-ms square pulse generated by a Grass stimulator (Grass-Telefactor). We found that pacing itself increased ischemic injury (unpublished observations); therefore, the ischemic period was reduced to 15 min in the paced hearts to provide a suitable level of postischemic recovery of contractile function. A further group of A3tg.1 (n = 5) and wild-type (n = 5) hearts was paced at 8 Hz and then treated with 60 nmol/l of the canine A3AR-specific inhibitor MRS-1220, beginning 5 min before the 15-min ischemic period and continuing throughout reperfusion.
NMR Spectroscopy
Relative changes in concentrations of phosphorus metabolites were observed during the ischemia-reperfusion protocols by acquiring consecutive 31P NMR spectra as described previously (6). The areas of the spectral peaks were expressed as a percentage of the peak areas of an initial, preischemic control spectrum from each heart. Intracellular pH was estimated from the chemical shift of the Pi peak relative to phosphocreatine (PCr) with previously obtained titration curves.
Statistics
Results are expressed as means ± SE. Significance (P ≤ 0.05) was determined for discrete variables by one-way analysis of variance and for continuous variables by analysis of variance for repeated measures; both analyses were followed by a Fisher's post hoc test.
RESULTS
Basal Contractile Function
During the preischemic period, LVDP was lower in unpaced A3AR-overexpressor hearts (87 cmH2O) than in unpaced wild-type hearts (110 cmH2O) (P < 0.0001; Table 1). Heart rate was also lower 319 beats/minute in unpaced A3AR-overexpressor hearts than in unpaced wild-type hearts (390 beats/min). +dP/dt (2.8 cmH2O/ms in A3AR overexpressors vs. 3.9 cmH2O/ms in wild type) and −dP/dt (−2.1 cmH2O/ms in A3AR overexpressors vs. −3.0 cmH2O/ms in wild type) (P < 0.001; Table 1) were also lower in unpaced A3AR-overexpressor hearts than in unpaced wild-type hearts. Thus basal myocardial contractility was decreased by overexpression of the A3AR.
Table 1.
Effect of A3AR overexpression and A3AR inhibition on basal myocardial contractile function
| Preinfusion |
Postinfusion |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Heart rate, beats/min |
LVDP, cmH2O |
+dP/dt, cmH2O/ms |
−dP/dt, cmH2O/ms |
Heart rate, beats/min |
LVDP, cmH2O |
+dP/dt, cmH2O/ms |
−dP/dt, cmH2O/ms |
n |
| Unpaced | |||||||||
| Wild type | 390 ± 11 | 110 ± 6 | 3.9 ± 0.2 | −3.0 ± 0.1 | 6 | ||||
| A3 | 319 ± 16* | 87 ± 1* | 2.8 ± 0.1* | −2.1 ± 0.1* | 5 | ||||
| Paced | |||||||||
| Wild type | 480 ± 0 | 113 ± 2 | 3.9 ± 0.1 | −3.2 ± 0.1 | 5 | ||||
| A3 | 480 ± 0 | 109 ± 2 | 3.8 ± 0.1 | −2.8 ± 0.3 | 5 | ||||
| Paced + MRS 1220 | |||||||||
| Wild type | 480 ± 0 | 103 ± 1 | 3.7 ± 0.2 | −2.7 ± 0.3 | 480 ± 0 | 105 ± 3 | 3.5 ± 0.3 | −2.7 ± 0.2 | 5 |
| A3 | 480 ± 0 | 109 ± 3 | 3.6 ± 0.1 | −2.8 ± 0.1 | 480 ± 0 | 109 ± 1 | 3.7 ± 0.1 | −2.9 ± 0.1 | 5 |
Data are means ± SE, A3AR, A3 adenosine receptor; A3, A3AR overexpressor; LVDP, left ventricular developed pressure; +dP/dt, rate of contraction; −dP/dt, rate of relaxation.
Significant difference from corresponding wild-type group (P < 0.001)
†significant difference between postinfusion and corresponding preinfusion value (P < 0.05).
On pacing at 480 beats/min, LVDP increased in A3AR-overexpressor hearts to 109 cmH2O (P < 0.0001 vs. unpaced A3AR overexpressors), whereas LVDP did not differ in paced versus unpaced wild-type hearts (Table 1). +dP/dt and −dP/dt were also increased by pacing in A3AR-overexpressor hearts, to 3.8 cmH2O/ms and −2.8 cmH2O/ms, respectively. The increase in LVDP, +dP/dt, and −dP/dt in paced A3AR-overexpressor hearts resulted in these values not being significantly different from those of paced or unpaced wild-type hearts (Table 1).
Pretreatment of paced hearts with 60 nmol/l of the A3AR-specific inhibitor MRS-1220 had no effect on LVDP, +dP/dt, or −dP/dt in either A3AR-overexpressor or wild-type hearts (Table 1).
Postischemic Contractile Recovery
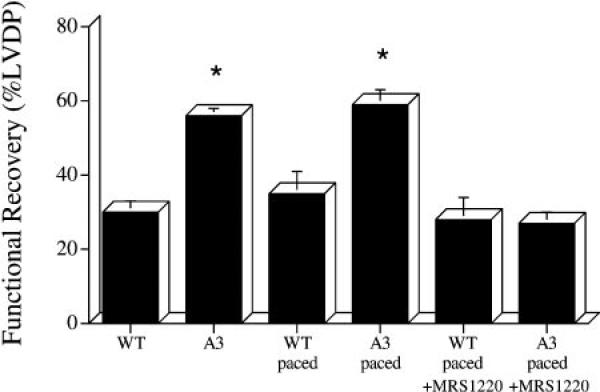
Recovery of contractile function after 20-min ischemia and 40-min reperfusion was higher in unpaced A3AR-overexpressor hearts (56% initial LVDP) than in unpaced wild-type hearts (30% initial LVDP) (P < 0.0001; Fig. 1). Postischemic contractile function was also higher in absolute terms in unpaced A3AR hearts (49 ± 2 cmH2O) than in unpaced wild-type hearts (33 ± 4 cmH2O) (P < 0.05).
Fig. 1.
Recovery of contractile function after 15-min (paced groups) or 20-min (unpaced groups) ischemia and 40-min reperfusion in wild-type (WT) and A3AR-overexpressor (A3) mouse hearts. WT, unpaced WT hearts; WT paced, WT hearts paced at 480 beats/min; A3, unpaced A3 hearts; A3 paced, A3 hearts paced at 480 beats/min; WT paced+MRS 1220, WT paced hearts pretreated with A3AR inhibitor MRS-1220; A3 paced+MRS 1220, A3 paced hearts pretreated with MRS-1220; LVDP, left ventricular developed pressure. Data are means ± SE. *Significant difference from corresponding WT group (P < 0.001).
In paced hearts, recovery of contractile function after 15-min ischemia and 40-min reperfusion was also higher in A3AR overexpressors (60% initial LVDP) than in wild type (35% initial LVDP) (P < 0.001; Fig. 1). Postischemic contractile function was also higher in absolute terms in paced A3AR hearts (65 ± 4 cmH2O) than in paced wild-type hearts (39 ± 6 cmH2O) (P < 0.001).
Pretreatment with 60 nmol/l MRS-1220 lowered functional recovery after 15-min ischemia and 40-min reperfusion in paced A3AR-overexpressor hearts to 27% initial LVDP (P < 0.0001 vs. untreated paced A3AR overexpressors), which was not significantly different from that observed in untreated paced wild-type hearts (35% initial LVDP) or MRS-1220-treated paced wild-type hearts (28% initial LVDP) (Fig. 1). Postischemic LVDP was also lower, at 40 ± 6 cmH2O, in MRS-1220-treated versus untreated paced A3AR-overexpressor hearts (P < 0.0001). Pretreatment with MRS 1220 had no significant effect on percent recovery of contractile function (28% initial LVDP) or absolute LVDP (29 ± 6 cmH2O) in paced wild-type hearts.
Phosphate Metabolites and Intracellular pH
Phosphate metabolites and intracellular pH were measured in the transgenic and wild-type hearts to determine whether overexpression of the A3AR altered myocardial energetics and pH regulation.
ATP levels
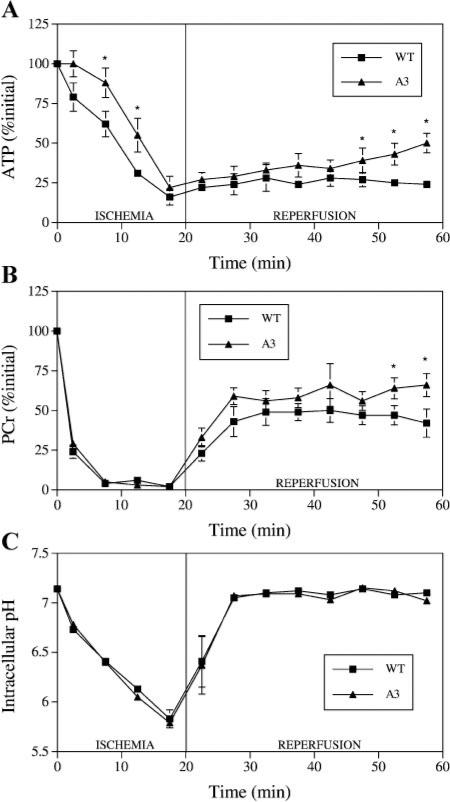
During ischemia, ATP depletion was delayed in the unpaced A3AR-overexpressor hearts compared with wild-type hearts (Fig. 2A), but by the end of 20-min ischemia, ATP levels were the same in unpaced A3AR-overexpressor and wild-type hearts, both at ~20% initial ATP. By the end of reperfusion, ATP had increased to a greater extent in unpaced A3AR-overexpressor hearts, which reached 50% initial ATP, than in unpaced wild-type hearts, which reached 24% initial ATP (P < 0.01; Fig. 2A).
Fig. 2.
Myocardial intracellular levels of ATP (A) and phosphocreatine (PCr) (B) and intracellular pH (C) during 20-min ischemia and 40-min reperfusion in unpaced WT and A3 mouse hearts. Points are means ± SE. *Significant difference from corresponding WT group (P < 0.05).
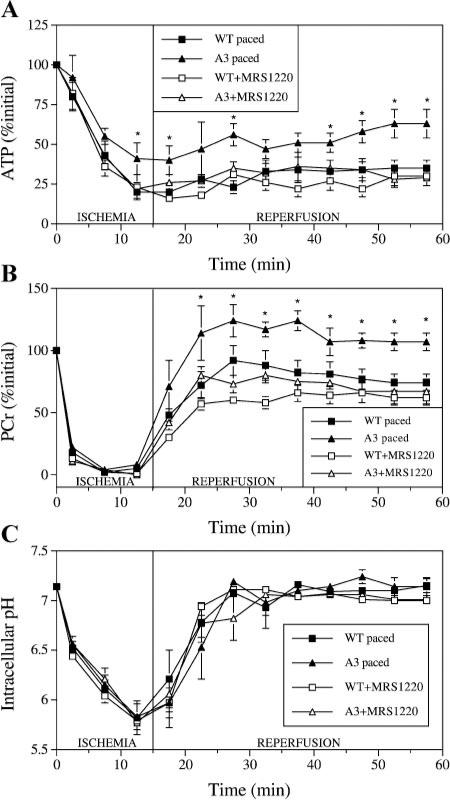
In hearts paced at 480 beats/min, similar to the unpaced hearts, ischemic ATP depletion was delayed in the A3AR overexpressors compared with wild type. However, the shorter 15-min ischemic period used in the paced heart experiments resulted in ATP levels being significantly higher at the end of ischemia in paced A3AR-overexpressor hearts (41% initial ATP) than in paced wild-type hearts (20% initial ATP) (P < 0.05; Fig. 3A). During reperfusion, ATP increased to a greater extent in paced A3AR-overexpressor hearts, which reached 63% initial ATP by the end of 40-min reperfusion, than in paced wild-type hearts, which reached 35% initial ATP (P < 0.01; Fig. 3A).
Fig. 3.
Myocardial intracellular levels of ATP (A) and PCr (B) and intracellular pH (C) during 15-min ischemia and 40-min reperfusion in paced WT and A3 mouse hearts with and without pretreatment with the A3AR inhibitor MRS-1220. Points are means ± SE. *Significant difference from corresponding WT group (P < 0.05).
Pretreatment with 60 nmol/l MRS-1220 abolished the delay in ATP depletion during ischemia in the paced A3AR-overexpressor hearts (Fig. 3A). Correspondingly, ATP levels were 22% of initial ATP by the end of 15-min ischemia in the paced A3AR-overexpressor hearts (P < 0.05 vs. untreated paced A3AR overexpressors), which was not significantly different from that observed in untreated paced wild-type hearts (20% initial ATP) or MRS-1220-treated paced wild-type hearts (23% initial ATP). Pretreatment with MRS-1220 had no significant effect on ischemic ATP levels in paced wild-type hearts. Pretreatment with MRS-1220 also abolished the increased ATP recovery during reperfusion in paced A3AR-overexpressor hearts, end-reperfusion ATP levels being 28% of initial ATP in MRS-1220-treated paced A3AR-overexpressor hearts (P < 0.001 vs. untreated paced A3AR overexpressors), which was not significantly different from that observed in untreated paced wild-type hearts (35% initial ATP) or MRS-1220-treated paced wild-type hearts (30% initial ATP). Pretreatment with MRS-1220 had no significant effect on reperfusion ATP levels in paced wild-type hearts.
To further analyze the delay in ATP depletion in paced and unpaced A3AR-overexpressor hearts compared with wild-type hearts, the rates of ATP depletion during the first 15 min of ischemia were calculated. Pacing increased the rate of ATP depletion in both A3AR-overexpressor and wild-type hearts from −3.6 ± 0.8% initial ATP/min in unpaced A3AR-overexpressor hearts to −5.4 ± 0.4% initial ATP/min in paced A3AR-overexpressor hearts (P < 0.05) and from −5.1 ± 0.2% initial ATP/min in unpaced wild-type hearts to −7.1 ± 0.4% initial ATP/min in paced wild-type hearts (P < 0.05). Confirming the delay in ATP depletion in A3AR-overexpressor versus wild-type hearts, the rate of ATP depletion was significantly lower in both unpaced A3AR-overexpressor versus unpaced wild-type hearts (P < 0.05) and paced A3AR-overexpressor versus paced wild-type hearts (P < 0.05).
PCr levels
During ischemia, PCr levels fell in all hearts. At the end of ischemia, there were no differences in PCr levels (~5% initial PCr) between unpaced A3AR-overexpressor and wild-type hearts (Fig. 2B) or between any of the paced groups, all also at ~5% of initial PCr (Fig. 3B). Although PCr levels were not different during early reperfusion, by the end of reperfusion PCr levels had increased to a greater extent in unpaced A3AR-overexpressor hearts, which reached 66% of initial PCr, than in unpaced wild-type hearts, which reached 42% of initial PCr (P < 0.05; Fig. 2B).
In paced hearts, reperfusion PCr levels recovered to a greater extent in A3AR-overexpressor hearts, which reached 107% of initial PCr by the end of reperfusion, than in wild-type hearts, which reached 74% of initial PCr (P < 0.001; Fig. 3B).
Pretreatment with MRS-1220 abolished the increased PCr recovery during reperfusion in paced A3AR-overexpressor hearts, end-reperfusion PCr levels being 67% of initial PCr in MRS-1220-treated paced A3AR-overexpressor hearts (P < 0.001 vs. untreated paced A3AR overexpressors), which was not significantly different from that observed in untreated paced wild-type hearts (74% initial PCr) or MRS-1220-treated paced wild-type hearts (62% initial PCr). Pre-treatment with MRS-1220 had no significant effect on reperfusion ATP levels in paced wild-type hearts.
Intracellular pH
During ischemia, pH decreased in all hearts. At the end of ischemia, there were no differences in pH levels, at pH ~5.80, between unpaced A3AR-overexpressor and wild-type hearts (Fig. 2C) or between any of the paced groups, all also at pH ~5.80 (Fig. 3C). During reperfusion, pH increased in all hearts back to preischemic values. At the end of reper-fusion, there were also no differences in pH levels, at pH ~7.10, between unpaced A3AR-overexpressor and wild-type hearts (Fig. 2C) or between any of the paced groups, all at pH ~7.10 (Fig. 3C).
DISCUSSION
Effect of A3AR Overexpression on Heart Rate, Basal Contractility, Energetics, and Postischemic Dysfunction
In the present study, preischemic heart rate, peak contraction (LVDP), +dP/dt, and −dP/dt were lower in perfused hearts from A3AR-overexpressor mice than wild-type mice (Table 1). This observation of A3AR-induced negative chronotropy and inotropy is reminiscent of that induced by exogenous adenosine or by overexpression of the A1AR (14, 23). The A3AR-overexpressor and wild-type hearts were then subjected to 20-min ischemia and 40-min reperfusion. During ischemia, the decrease in PCr and intracellular pH were similar in A3AR-overexpressor and wild-type hearts (Fig. 2). However, ischemic ATP depletion was delayed in the A3AR-overexpressor hearts compared with wild-type hearts, the rate of fall of ATP being −3.6% initial ATP/min in A3AR-overexpressor hearts compared with −5.1% initial ATP/min in wild-type hearts during the first 15 min of ischemia. During reperfusion, recovery of the energy metabolites ATP and PCr was faster in A3AR-overexpressor hearts than wild-type hearts (Fig. 2). In addition, postischemic contractile dysfunction was lower in A3AR-overexpressor hearts than wild-type hearts (Fig. 1). It appears, therefore, that overexpression of the A3AR results in short-term preservation of ATP levels during ischemia and protects against postischemic contractile and energetic dysfunction.
Effect of Pacing on Contractility, Energetics, and Postischemic Dysfunction in A3AR-Overexpressor Versus Wild-Type Hearts
Depressed preischemic heart rate and contractility can lead to decreased ATP utilization and increased postischemic recovery (17). To determine whether depressed heart rate was responsible for the ischemic ATP preservation and cardioprotection in the A3AR overexpressors, hearts were paced at 480 beats/min throughout the protocol. Pacing increased LVDP, +dP/ dt, and −dP/dt in A3AR-overexpressor hearts but, surprisingly, had no effect on these parameters in wild-type hearts (Table 1). Although surprising, this finding is consistent with recent studies indicating that the force-frequency response is biphasic in mouse hearts. It has been shown that increasing heart rate in the range of 300–400 beats/min results in increased developed pressure, whereas increasing heart rate from 400 beats/min upward either has no effect or decreases developed pressure (9, 16). The peak of the force-frequency curve in mice varies slightly with different experimental models (9, 16). Our results would suggest a positive force-frequency relationship in the 300- to 400 beats/min range and a plateau in the 400- to 500 beats/min range in isolated mouse hearts, consistent with a recent study by Headrick et al. (13).
Pacing itself increased the rate of ischemic ATP depletion from −3.6 to −5.4% initial ATP/min in A3AR-overexpressor hearts and from −5.1 to −7.1% initial ATP/min in wild-type hearts over the 15-min ischemic period. As can be seen from these values, despite the equivalent contractility in paced A3AR-overexpressor hearts compared with paced wild-type hearts, ATP depletion remained slower in the A3AR-overexpressor hearts at −5.4% initial ATP/min in paced A3AR-over-expressor hearts compared with −7.1% initial ATP/ min in paced wild-type hearts. Likewise, the postischemic recovery of ATP, PCr, and contractile function remained greater in the paced A3AR-overexpressor hearts compared with paced wild-type hearts (Figs. 1 and 3). From these results, we can conclude that the preservation of ischemic ATP and the reduced postischemic contractile and energetic dysfunction in the A3AR-overexpressor hearts were not due to the decreased basal heart rate and contractility induced by A3AR overexpression.
Effect of A3AR Inhibition on Contractility, Energetics, and Postischemic Dysfunction in A3AR-Overexpressor Versus Wild-Type Hearts
To determine whether the ischemic ATP preservation and cardioprotection observed in A3AR-overexpressor hearts was due specifically to A3AR signaling, and not to secondary alterations induced by the presence of the transgene, paced hearts were pretreated with 60 nmol/l of a canine A3AR-specific inhibitor, MRS-1220. The concentration of MRS-1220 was chosen on the basis of the MRS-1220 inhibitor constant for the canine A3AR of 6 nmol/l (18) and on preliminary dose-response studies in isolated hearts. The 60 nmol/l concentration had no effect on contractile function; LVDP, +dP/dt, and −dP/dt being the same before and after infusion of 60 nmol/l MRS-1220 in both paced wild-type and paced A3AR-overexpressor hearts (Table 1).
Pretreatment with MRS-1220 completely abolished the ischemic ATP preservation and the greater postischemic recovery of ATP, PCr, and contractile function in the A3AR-overexpressor compared with wild-type hearts (Figs. 1 and 3). It appears, therefore, that the preservation of ischemic ATP levels and the protection from postischemic contractile and energetic dysfunction observed in the A3AR-overexpressor hearts were mediated specifically by the A3AR and were not due to adaptive alterations induced by long-term A3AR over-expression.
In summary, we have demonstrated that cardiac-specific overexpression of the A3AR results in decreased basal heart rate and contractility, short-term ischemic ATP preservation, and protection from postischemic contractile and energetic dysfunction. Depressed preischemic contractility can itself be protective. However, by pacing hearts at a rate that resulted in similar contractile function in the A3AR-overexpressor hearts as in wild-type hearts, we demonstrated that the depressed contractility had no role in the ischemic ATP preservation and cardioprotection in the A3AR-overexpressor hearts. By pretreating hearts with the A3AR-specific inhibitor MRS-1220, we also demonstrated that the energetic and functional effects observed in the A3AR-overexpressor hearts were mediated specifically by the A3AR. Together, these results imply that myocardial A3AR signaling is cardioprotective and leads to preservation of ischemic ATP levels. Because cardioprotection was coincident with decreased ATP depletion in all our experiments, the decreased ATP depletion may be the mechanism by which A3AR signaling leads to cardioprotection. Interestingly, decreased ATP depletion during ischemia was also found to be coincident with protection in A1AR-overexpressor hearts (12), suggesting that the A3AR and A1AR may mediate protection via a common mechanism of energy preservation.
To our knowledge, this is the first study to assess the energetic consequences of cardiac-specific overexpression of the A3AR and is also the first study to determine the effects of A3AR overexpression on postischemic contractile dysfunction after short-term ischemia. Our results support the use of cardiac-specific expression techniques. Previous studies showed that, although A3AR agonists can have direct beneficial effects on isolated hearts (11), A3AR signaling in mast cells may be detrimental to the myocardium via proinflammatory mechanisms (21, 25, 30), such that systemic A3AR inhibition can actually be protective (3, 10). These studies highlight the necessity for cardiac-specific A3AR targeting in future therapeutic approaches and also highlight the possible complications of using systemic A3AR agonists as cardioprotective agents.
Acknowledgments
The authors thank Robert E. London for use of the NMR facilities and Scott A. Gabel for assistance with the pacing apparatus.
This work was supported by National Institutes of Health grants (to C. Steenbergen and J. Auchampach).
REFERENCES
- 1.Auchampach JA, Rizvi A, Qiu Y, Tang XL, Maldonado C, Teschner S, Bolli R. Selective activation of A3 adenosine receptors with N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide protects against myocardial stunning and infarction without hemo-dynamic changes in conscious rabbits. Circ Res. 1997;80:800–809. doi: 10.1161/01.res.80.6.800. [DOI] [PubMed] [Google Scholar]
- 2.Black RG, Guo Y, Ge ZD, Murphree SS, Prabhu SD, Jones WK, Bolli R, Auchampach JA. Gene dosage dependent effects of cardiac-specific overexpression of the A3 adenosine receptor. Circ Res. doi: 10.1161/01.res.0000028007.91385.ee. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerniway RJ, Yang Z, Jacobson MA, Linden J, Math-erne GP. Targeted deletion of A3 adenosine receptors improves tolerance to ischemia-reperfusion injury in mouse myocardium. Am J Physiol Heart Circ Physiol. 2001;281:H1751–H1758. doi: 10.1152/ajpheart.2001.281.4.H1751. [DOI] [PubMed] [Google Scholar]
- 4.Cross HR, Lu L, Steenbergen C, Philipson KD, Murphy E. Overexpression of the cardiac Na+/Ca2+ exchanger increases susceptibility to ischemia/reperfusion injury in male, but not female, transgenic mice. Circ Res. 1998;83:1215–1223. doi: 10.1161/01.res.83.12.1215. [DOI] [PubMed] [Google Scholar]
- 5.Cross HR, Opie LH, Radda GK, Clarke K. Is a high glycogen content beneficial or detrimental to the ischemic rat heart? A controversy resolved. Circ Res. 1996;78:482–491. doi: 10.1161/01.res.78.3.482. [DOI] [PubMed] [Google Scholar]
- 6.Cross HR, Steenbergen C, Lefkowitz RJ, Koch WJ, Murphy E. Overexpression of the β2-adrenergic receptor and a βARK1 inhibitor both increase contractility but have differential effects on susceptibility to ischemic injury. Circ Res. 1999;85:1077–1084. doi: 10.1161/01.res.85.11.1077. [DOI] [PubMed] [Google Scholar]
- 7.De Albuquerque CP, Gerstenblith G, Weiss RG. Importance of metabolic inhibition and cellular pH in mediating preconditioning contractile and metabolic effects in rat hearts. Circ Res. 1994;74:139–150. doi: 10.1161/01.res.74.1.139. [DOI] [PubMed] [Google Scholar]
- 8.Fralix TA, Murphy E, London RE, Steenbergen C. Protective effects of adenosine in the perfused rat heart: changes in metabolism and intracellular ion homeostasis. Am J Physiol Cell Physiol. 1993;264:C986–C994. doi: 10.1152/ajpcell.1993.264.4.C986. [DOI] [PubMed] [Google Scholar]
- 9.Georgakopoulos D, Kass D. Minimal force-frequency modulation of inotropy and relaxation of in situ murine heart. J Physiol. 2001;534:535–545. doi: 10.1111/j.1469-7793.2001.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Y, Bolli R, Bao W, Wu WJ, Black RG, Jr, Murphree SS, Salvatore CA, Jacobson MA, Auchampach JA. Targeted deletion of the A3 adenosine receptor confers resistance to myocardial ischemic injury and does not prevent early preconditioning. J Mol Cell Cardiol. 2001;33:825–830. doi: 10.1006/jmcc.2001.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison GJ, Cerniway RJ, Peart J, Berr SS, Ashton K, Regan S, Matherne GP, Headrick JP. Effects of A3 adenosine receptor activation and gene knock-out in ischemicreperfused mouse heart. Cardiovasc Res. 2002;53:147–155. doi: 10.1016/s0008-6363(01)00424-2. [DOI] [PubMed] [Google Scholar]
- 12.Headrick JP, Gauthier NS, Berr SS, Morrison RR, Matherne GP. Transgenic A1 adenosine receptor overexpression markedly improves myocardial energy state during ischemia-reperfusion. J Mol Cell Cardiol. 1998;30:1059–1064. doi: 10.1006/jmcc.1998.0672. [DOI] [PubMed] [Google Scholar]
- 13.Headrick JP, Peart J, Hack B, Flood A, Matherne GP. Functional properties and responses to ischaemia-reperfusion in Langendorff perfused mouse heart. Exp Physiol. 2001;86:703–716. doi: 10.1111/j.1469-445x.2001.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 14.Headrick JP, Peart J, Hack B, Garnham B, Matherne GP. 5′-Adenosine monophosphate and adenosine metabolism, and adenosine responses in mouse, rat and guinea pig heart. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:615–631. doi: 10.1016/s1095-6433(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 15.Heidland UE, Heintzen MP, Michel CJ, Strauer BE. Intracoronary administration of dipyridamole prior to percutaneous transluminal coronary angioplasty provides a protective effect exceeding that of ischemic preconditioning. Coron Artery Dis. 2000;11:607–613. doi: 10.1097/00019501-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Hoit BD, Ball N, Walsh RA. Invasive hemodynamics and force-frequency relationships in open- versus closed-chest mice. Am J Physiol Heart Circ Physiol. 1997;273:H2528–H2533. doi: 10.1152/ajpheart.1997.273.5.H2528. [DOI] [PubMed] [Google Scholar]
- 17.Huizer T, de Jong JW, Achterberg PW. Protection by bepridil against myocardial ATP-catabolism is probably due to negative inotropy. J Cardiovasc Pharmacol. 1987;10:55–61. doi: 10.1097/00005344-198707000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Jordan JE, Thourani VH, Auchampach JA, Robinson JA, Wang NP, Vinten-Johansen J. A3 adenosine receptor activation attenuates neutrophil function and neutrophil-mediated reperfusion injury. Am J Physiol Heart Circ Physiol. 1999;277:H1895–H1905. doi: 10.1152/ajpheart.1999.277.5.H1895. [DOI] [PubMed] [Google Scholar]
- 19.Kida M, Fujiwara H, Ishida M, Kawai C, Ohura M, Miura I, Yabuuchi Y. Ischemic preconditioning preserves creatine phosphate and intracellular pH. Circulation. 1991;84:2495–2503. doi: 10.1161/01.cir.84.6.2495. [DOI] [PubMed] [Google Scholar]
- 20.Leesar MA, Stoddard M, Ahmed M, Broadbent J, Bolli R. Preconditioning of human myocardium with adenosine during coronary angioplasty. Circulation. 1997;95:2500–2507. doi: 10.1161/01.cir.95.11.2500. [DOI] [PubMed] [Google Scholar]
- 21.Linden J. Cloned adenosine A3 receptors: pharmacological properties, species differences and receptor functions. Trends Pharmacol Sci. 1994;15:298–306. doi: 10.1016/0165-6147(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 22.Mahaffey KW, Puma JA, Barbagelata NA, DiCarli MF, Leesar MA, Browne KF, Eisenberg PR, Bolli R, Casas AC, Molina-Viamonte V, Orlandi C, Blevins R, Gibbons RJ, Califf RM, Granger CB. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol. 1999;34:1711–1720. doi: 10.1016/s0735-1097(99)00418-0. [DOI] [PubMed] [Google Scholar]
- 23.Matherne GP, Linden J, Byford AM, Gauthier NS, Headrick JP. Transgenic A1 adenosine receptor overexpression increases myocardial resistance to ischemia. Proc Natl Acad Sci USA. 1997;94:6541–6546. doi: 10.1073/pnas.94.12.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66:913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- 25.Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. Disruption of the A3 adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem. 2000;275:4429–4434. doi: 10.1074/jbc.275.6.4429. [DOI] [PubMed] [Google Scholar]
- 26.Stambaugh K, Jacobson KA, Jiang JL, Liang BT. A novel cardioprotective function of adenosine A1 and A3 receptors during prolonged simulated ischemia. Am J Physiol Heart Circ Physiol. 1997;273:H501–H505. doi: 10.1152/ajpheart.1997.273.1.H501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steenbergen CS, Perlman ME, London RE, Murphy E. Mechanisms of preconditioning: ionic alterations. Circ Res. 1993;72:112–125. doi: 10.1161/01.res.72.1.112. [DOI] [PubMed] [Google Scholar]
- 28.Strickler J, Jacobson KA, Liang BT. Direct preconditioning of cultured chick ventricular myocytes. Novel functions of cardiac adenosine A2a and A3 receptors. J Clin Invest. 1996;98:1773–1779. doi: 10.1172/JCI118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takano H, Bolli R, Black RG, Jr, Kodani E, Tang XL, Yang Z, Bhattacharya S, Auchampach JA. A1 or A3 adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res. 2001;88:520–528. doi: 10.1161/01.res.88.5.520. [DOI] [PubMed] [Google Scholar]
- 30.Tilley SL, Wagoner VA, Salvatore CA, Jacobson MA, Koller BH. Adenosine and inosine increase cutaneous vasopermeability by activating A3 receptors on mast cells. J Clin Invest. 2000;105:361–367. doi: 10.1172/JCI8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tracey WR, Magee W, Masamune H, Kennedy SP, Knight DR, Buchholz RA, Hill RJ. Selective adenosine A3 receptor stimulation reduces ischemic myocardial injury in the rabbit heart. Cardiovasc Res. 1997;33:410–415. doi: 10.1016/s0008-6363(96)00240-4. [DOI] [PubMed] [Google Scholar]
- 32.Zhou QY, Li C, Olah ME, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of an adeno-sine receptor: the A3 adenosine receptor. Proc Natl Acad Sci USA. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]