Abstract
As HAART becomes more accessible in sub-Saharan Africa, metabolic syndromes, body fat redistribution (BFR), and cardiovascular disease may become more prevalent. We conducted a 6-month, randomized controlled trial to test whether cardiorespiratory exercise training (CET), improves metabolic, body composition and cardiorespiratory fitness parameters in HAART-treated HIV+ African subjects with BFR. Six months of CET reduced waist circumference (−7.13 ± 4.4 cm, p < 0.0001), WHR (−0.10 ± 0.1, p < 0.0001), sum skinfold thickness (−6.15 ± 8.2 mm, p < 0.0001) and % body fat mass (−1.5 ± 3.3, p < 0.0001) in HIV+BFR+EXS. Hip circumference was unchanged in non-exercise control groups. CET reduced fasting total cholesterol (−0.03 ± 1.11 mM, p < 0.05), triglycerides (−0.22 ± 0.48 mM, p < 0.05) and glucose levels (−0.21 ± 0.71 mM, p < 0.05) (p < 0.0001). HDL-, LDL-cholesterol and HOMA values were unchanged after CET. Interestingly, HIV+ subjects randomized to non-exercising groups experienced increases in fasting plasma glucose levels, whereas HIV seronegative controls did not (p < 0.001). Predicted VO2 peak increased more in the HIV+BFR+EXS than in all other groups (4.7 ± 3.9 ml/kg/min, p < 0.0001). Exercise training positively modulated body composition and metabolic profiles, and improved cardiorespiratory fitness in HAART-treated HIV+ Africans. These beneficial adaptations imply that exercise training is a safe, inexpensive, practical, and effective treatment for evolving metabolic and cardiovascular syndromes associated with HIV and HAART exposure in resource-limited sub-Saharan countries, where treatment is improving, morbidity and mortality rates are declining, but where minimal resources are available to manage HIV- and HAART-associated cardiovascular and metabolic syndromes.
INTRODUCTION
Body composition and metabolic abnormalities associated with body fat redistribution (BFR) (central adiposity and/or peripheral lipoatrophy), glucose and lipid abnormalities, and hypertension1 have been reported in approximately 20–60%2,3 of HIV-positive (HIV+) patients receiving highly active antiretroviral therapy (HAART).4 Although treatment with potent HAART has improved the morbidity rate and well-being of HIV+ patients accessing these therapies,5 HAART-and HIV-related complications have been associated with increased cardiovascular disease (CVD) and diabetes risks.6,7 Framingham risk equations suggest increased risk for myocardial infarction8 and greater than a 20% increase in 10-year CVD risk9 in HIV+ patients receiving HAART compared to age-matched controls. Therefore HAART-treated HIV-infected patients represent an emerging population with increased risk for CVD and diabetes.
In developing countries, BFR and metabolic abnormalities have been reported in HAART-treated HIV patients10 and in HIV-infected Africans receiving first line World Health Organization (WHO)-recommended HAART.11 As HAART becomes more accessible to HIV-infected people in resource-limited regions of the world,12,13 and their quality of life improves,14 the challenge is how to manage HIV- and HAART-related metabolic syndromes. Subsequently, there is a growing concern that CVD and diabetes risks, the main causes of morbidity and mortality in the developed world, may emerge, along with infectious diseases, as significant health concerns in HIV+ individuals in sub-Saharan countries.15
Cardiorespiratory exercise training (CET) is an established, cost-effective, and efficacious lifestyle modification that improves insulin sensitivity16 and dyslipidemia17 and reduces central adiposity or trunk fat,18 leading to an improved cardiovascular and diabetic risk profile in HIV+ individuals from Western countries.19 Consequently, regular CET has been recommended in the guidelines for management of HIV-related dyslipidemia.20 Several nonrandomized controlled trials of aerobic and resistance exercise studies with small sample sizes and short training durations have reported improvement in lipid and body composition profiles in HIV+ individuals with BFR in Western countries.21–27 In resource-limited areas such as sub-Saharan Africa, CET may be a particularly important treatment for BFR and metabolic disorders in HIV+ individuals taking HAART. Therefore, we conducted a 6-month randomized controlled trial to test whether CET improves metabolic and anthropomorphic parameters and enhances cardiorespiratory fitness in HAART-treated HIV-infected African men and women with BFR in Rwanda.
MATERIALS AND METHODS
Study population
Participants were screened for eligibility from August to December 2005 at the Centre Hospitalier Universitaire de Kigali, Treatment and Research AIDS Centre, and HIV/AIDS clinics of Kimironko, Kicukiro, Bilyogo-Nyiranuma, Kinyinya, and Kacyiru health centers in Rwanda. Eligible HIV-positive volunteers were between 21 and 50 years old and on a stable WHO-recommended HAART regimen for ≥6 months. Participants had moderate to severe BFR, determined by physical examination and subjects’ self reporting, and rating changes in fat content using a validated questionnaire.28 The degree of body fat redistribution was rated as absent (score 0), mild (noticeable on close inspection, score 1), moderate (readily noticeable by the patient and the physician, score 2), or severe (readily noticeable to a casual observer, score 3). The overall score was the mean of the scores given by the participant and a score assigned to each participant by a consensus of three clinicians working in the field of HIV/AIDS. The presence and rating of BFR were confirmed in all participants by physical examination, in which 18% of HIV+ participants who self-reported moderate to severe BFR were excluded due to lack of confirmation from the clinicians. For the purposes of this study, a clinical diagnosis was given to HIV+ participants with moderate (score 2) to severe (score 3) BFR, and an overall mean score of ≥18 on a validated seven-item inventory for the face, neck, arms, breasts, abdomen, buttocks, and legs, with 21 as the highest score. All included HIV+ participants with BFR had a waist-to-hip ratio (WHR) ≥0.90 in men and 0.85 in women.
Subjects were excluded if they had emotional distress or psychosis, unstable angina, shortness of breath with exercise, a known medical history that would contraindicate exercise training, acute infections, or AIDS-defining opportunistic illnesses. Subjects were also excluded if they were obese [body mass index (BMI) ≥ 32 kg/m2], had musculoskeletal or neuromuscular disorders, participated in regular exercise (≥3 sessions/week, ≥30 min/session in the previous 4 weeks), were unwilling to exercise continuously for 6 months, or anticipated changes in HAART medications or were currently taking medications that affect lipid and glucose metabolism. Pregnant or breast-feeding women were excluded. Participants were receiving WHO-recommended first line HAART regimens, with 80% receiving stavudine, lamivudine, and nevirapine, a widely used first-line therapy in resource-limited regions of the world. Another 11% received zidovudine, lamivudine, and nevirapine and 5% received zidovudine, lamivudine, and efavirenz. A small percentage of subjects (3%) received stavudine, lamivudine, and efavirenz and only 1% received lamivudine, abacavir, and nevirapine. HAART regimen use was not different among the HIV+ participant groups.
One hundred and fifty-two HIV+ participants with moderate to severe BFR were screened and 52 were not eligible; 21 participants did not meet eligibility criteria for central obesity, 16 participants were determined unfit for the exercise program, 11 participants declined to participate, three anticipated changes in HAART medications, and one participant had epilepsy. One hundred HIV+ participants with moderate to severe BFR were stratified by gender and randomized to either an exercise group (n = 50; HIV+BFR+EXS) or a nonexercising control group (n = 50; HIV+BFR+noEXS). For comparative purposes, we also randomly recruited age, gender, and BMI-matched HIV-infected participants on stable HAART for ≥6 months, without BFR changes (as defined above) (n = 50; HIV+noBFR+ noEXS). Since there are few normative data on cardiorespiratory fitness and metabolic and body composition parameters in HIV-seronegative Rwandan adults, we also randomly recruited age, gender, and BMI-matched HIV-seronegative healthy adults as an additional comparative control group (n = 50; HIV-ve+noEXS). After obtaining ethical permission from the National Research Ethics Committee (Rwanda), written informed consent was obtained from all volunteers before participation.
Metabolic and anthropometrics
After an overnight fast, a venous blood sample was obtained and serum was isolated and analyzed for total cholesterol, high-density lipoprotein cholesterol (HDL cholesterol), triglycerides (TG), and glucose and insulin levels (Humalyzer 3000; Abbott Laboratories, Abbott Park, IL). The CD4+ count was quantified by flow cytometry using monoclonal antibodies (Simultest CD4, Beckon Dickinson). Low-density lipoprotein cholesterol (LDL cholesterol) was calculated from total cholesterol, HDL cholesterol, and TG using the Friedewald formula.29
Height, weight, and hip and waist circumferences were measured while the participant was standing, wearing light clothing and no shoes. Height and weight were measured to the nearest 0.1 kg and 0.1 cm, respectively. Waist and hip circumferences were assessed by two well-trained research associates. For reproducibility and to minimize interobserver variability, associates were trained on proper anatomic placement of the tape measure, and their measurements were cross-validated on a number of participants until the variability between duplicate measures among associates was minimal. Waist circumference was measured using a cloth tape measure at the narrowest circumference, halfway between the lowest ribs and iliac crests. Hip circumference was measured at the level of the anterior superior iliac spine, where this could be palpated, or at the broadest circumference below the waist. Two measurements were taken; if they differed by >2 cm, a third measurement was taken and the mean of the closest two measurements was used to calculate waist-to-hip ratio (WHR). Mid-triceps, mid-biceps, suprailiac, and subscapular skinfolds were measured using Lange skinfold callipers. For reproducibility, an experienced trained investigator (E.M.) located and measured all skinfolds in triplicate. Measurements were read to the nearest 0.2 mm and averaged for each skinfold site. The sum of four total skinfold measures was used to calculate percentage body fat (% BFM) and lean body mass (% LBM) using equations and formulas30,31 validated in a black population.32 All measurements were obtained at baseline and after 6 months of observation, placebo, or exercise intervention.
Exercise training protocol
HIV+BFR+EXS participated in a 6-month, supervised exercise training program (3 sessions/week, 1.5 h/session, alternating days) at Amahoro Fitness Club located in downtown Kigali. All participants were instructed on a progressive exercise program consisting of stretching exercises and 15 min of brisk walking followed by 45–60 min of jogging, running, stair climbing, low back and abdominal stabilization, and strengthening exercises. Participants were instructed to perform primarily jogging and running exercises with a goal of achieving at least 45% age-predicted maximal heart rate (HR) in the first 3 weeks, 60% age-predicted maximal HR in the next 6 weeks, and 75% age-predicted maximal HR by the end of the intervention. Heart rate was approximately assessed in one-third of participants in each training session with all participants age-predicted maximal HR assessed at least every week. This ensured that the exercise stimulus was adequate to elicit cardiorespiratory and musculoskeletal system adaptations. Each exercise training session ended with 15 min of cool down and stretching exercises. Participants’ attendance at each session was recorded and noncompliance was defined as 50% missed training sessions. Cardiorespiratory fitness was assessed at baseline and 6 months later using the 20-m multistage shuttle run test (20mMST)33 to predict maximum oxygen consumption (VO2 peak; ml/kg/min). During the 20mMST, participants ran continuously in an indoor fitness stadium on a flat hard synthetic surface between two points separated by 20 m at a pace that was incremented by 0.14 m/sec each minute or level. The required running pace was indicated by verbal cues and prerecorded audio signals that sounded when each 20-m “lap” should have been completed (much like a metronome). The inability to maintain the required pace for a particular increment was documented (level and number of 20-m laps completed) and represents the participants’ maximal effort, which is proportional to VO2 peak. The 20mMST assessment tool for cardiorespiratory fitness and exercise capacity has been validated in health and fitness settings.34 Each participant’s cardiorespiratory fitness was assessed individually during the 20mMST to minimize possible sources of error. Heart rate and blood pressure were taken immediately before and after the exercise test, and perceived exertion was evaluated with the Borg Rating of Perceived Exertion Scale (Borg RPE).
Three HIV+BFR+EXS participants did not complete the CET program: two dropped out, one died following surgery, and another one changed jobs to a distant location. One HIV+BFR+noEXS participant was lost to follow-up and three HIV+noBFR+noEXS subjects dropped out; one declined further participation and two started to obtain medications and social support from a rural nongovernmental organization. Seven HIV-ve+noEXS participants declined further participation due to lack of time, interest, family issues, or personal travel arrangements. Compliance with the exercise program was 82.2%. Figure 1 represents a flow diagram of study subjects.
FIG. 1.

Flow of participants through the trial; 21 did not meet the criteria for central obesity, 11 were determined unfit to exercise, 11 declined to participate, three anticipated changes in HAART regimens, and one had a neurological disorder.
Statistical analyses
Based on previous studies,22–24 we estimated that a sample of 37 individuals in each group would have a power of 90% to detect a 10% difference in triglycerides and total cholesterols for α = 0.05. Assuming a drop-out of 20% per group, at least 44 participants would be required per group. Since all subject groups were ≥44 per group after the intervention, the primary efficacy analyses were based on data from participants who completed the study at a 6-month period.
Between-group comparisons of the changes in the outcome variables were performed using analyses of covariance (ANCOVA). Analyses of covariance with the 6 month change as the dependent variable (absolute change from baseline), the treatment group as the independent variable, and baseline values as the covariates, were used to determine whether there were between-group differences at 6 months after adjusting for the initial baseline values. As part of the ANCOVA, statistical contrasts were used to make pairwise comparisons across groups. All analyses were performed using SAS software, version 9.1.3 of the SAS System for Linux (SAS Institute Inc., Cary, NC). All statistical tests were two-tailed, and significance was accepted at a p-value = 0.05. Data are presented in tables as arithmetic mean ± SD, or median (interquartile range), and in figures as mean ± SE, unless stated otherwise.
RESULTS
Cardiovascular outcomes
At baseline, participants with BFR randomized to the exercise or nonexercise group were well matched for age, CD4 cell count, body fat redistribution score, gender, body composition, and metabolic variables (Table 1). HIV-seronegative controls had lower diastolic blood pressure at the start of the 20mMST than HIV+noBFR+noEXS subjects (p < 0.05). At 6 months, HIV+BFR+EXS achieved a significantly higher HR and RPE at the end of the 20mMST (p < 0.001) than other subject groups. Likewise, predicted VO2 peak during the 20mMST increased more in the HIV+BFR+EXS group (4.7 ± 0.56 ml/kg/min, p < 0.0001) than in all other groups (Fig. 2). Following CET, the mean changes in CD4 cell count for HIV+BFR+EXS (19 ± 91 cells/μl) were not significantly different from that of HIV-infected subjects with BFR in the HIV+BFR+noEXS (34 ± 128 cells/μl) (p = 0.547) or HIV+noBFR+noEXS subjects (77 ± 160 cells/μl) (p = 0.079).
Table 1.
Baseline Characteristics, Body Composition, and Metabolic Dataa
| HIV+BFR+noE XS | HIV+BFR+EXS | p | |
|---|---|---|---|
| Age (years) | 37.5 ± 6.9 | 37.8 ± 5.5 | 0.807 |
| Number (% females) | 50 (60) | 50 (60) | — |
| CD4 cell count (cells/μl) | 348 ± 162 | 353 ± 168 | 0.882 |
| Height (m) | 1.63 ± 0.1 | 1.64 ± 0.1 | 0.898 |
| Weight (kg) | 64.8 ± 5.7 | 65.6 ± 10.4 | 0.891 |
| HAART duration (weeks) | 62.7 ± 27.6 | 71.4 ± 36.7 | 0.671 |
| BFR score(7-item) | 18.7 ± 2.3 | 19.3 ± 2.1 | 0.882′ |
| Body composition | |||
| Body mass index (kg/m2) | 24.4 ± 2.7 | 24.0 ± 3.1 | 0.406 |
| Waist (cm) | 92.3 ± 6.9 | 91.0 ± 8.4 | 0.341 |
| Hip (cm) | 93.7 ± 6.8 | 92.3 ± 7.6 | 0.183 |
| Waist-to-hip ratio | 0.98 ± 0.0 | 0.99 ± 0.1 | 0.796 |
| Skinfold (mm) | |||
| Triceps | 14.4 ± 3.4 | 14.4 ± 3.9 | 0.979 |
| Biceps | 10.8 ± 2.4 | 10.7 ± 72.9 | 0.848 |
| Subscapular | 18.7 ± 4.9 | 18.6 ± 5.2 | 0.884 |
| Suprailiac | 18.8 ± 5.1 | 18.7 ± 5.5 | 0.915 |
| Total skinfolds | 62.7 ± 12.9 | 62.3 ± 15.0 | 0.888 |
| Body fat mass (%) | 29.3 ± 4.3 | 29.4 ± 6.2 | 0.928 |
| Metabolic | |||
| Total cholesterol (mmol/liter) | 3.89 (1.04) | 3.78 (0.93) | 0.499 |
| HDL cholesterol (mmol/liter) | 1.27 (0.15) | 1.28 (0.19) | 0.684 |
| LDL cholesterol (mmol/liter) | 2.16 (0.77) | 2.10 (0.74) | 0.659 |
| Triglyceride (mmol/liter) | 1.34 (0.36) | 1.33 (0.39) | 0.941 |
| Glucose (mmol/liter) | 4.95 (0.74) | 4.76 (0.76) | 0.191 |
| Insulin (ρmol/liter) | 59 (28) | 67 (29) | 0.131 |
| HOMA | 1.87 (0.13) | 2.04 (0.14) | 0.196 |
Data expressed as mean ± SD or median (interquartile range); BFR score(7-item), 7-item body fat redistribution score; HOMAIR, homeostasis model assessment of insulin resistance.
FIG. 2.
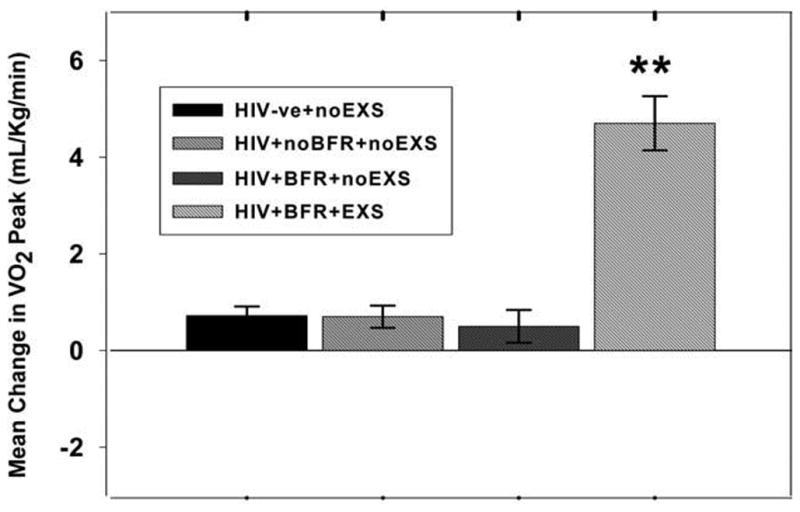
Mean change in VO2 peak predicted from a 20-m multistage shuttle run test. **p < 0.0001 versus HIV+BFR+EXS group; BFR, body fat distribution; VO2 peak max, maximum total body oxygen consumption; HIV-ve+noEXS, HIV-seronegative controls; HIV+noBFR+noEXS, nonexercise HIV+subjects with no BFR; HIV+BFR+noEXS, nonexercise HIV+ subjects with BFR; HIV+BFR+EXS, HIV+ subjects with BFR in the exercise group.
Body composition outcomes
After 6 months of CET, BMI, waist circumference (−7.13 ± 4.4) (Fig. 3) and WHR declined more in HIV+BFR+EXS, while it remained unchanged or increased in the other groups (Table 2). Hip circumference was unchanged after 6 months in all groups; therefore the decline in waist-to-hip ratio in HIV+BFR+EXS was attributed to the decline in waist circumference. In fact, waist circumference increased 1.84 ± 9.2 cm in HIV+noBFR+noEXS subjects. We also observed a decline in WHR in HIV+BFR+noEXS participants compared to HIV-ve+noEXS participants (0.00 ± 0.1 versus 0.01 ± 0.1; p < 0.05). After the CET, waist circumference in the exercise group was not different from that of HIV-seronegative subjects (Table 2). The BFR mean score significantly declined more in HIV+BFR+EXS than HIV+BFR+noEXS, while the BFR mean score significantly increased in HIV+noBFR+noEXS (Table 2). Subscapular (−1.9 ± 3.2 mm), suprailiac (−2.13 ± 3.5 mm), and sum skinfold thicknesses (−6.15 ± 8.2 mm) (Fig. 3) declined more in HIV+BFR+EXS than in all other groups (p < 0.0001). HIV+BFR+EXS experienced a significant decrease in % BFM (−1.5 ± 3.3, p < 0.001) and a significant increase in % LBM (1.5 ± 3.1, p < 0.0001) compared to all other groups (Table 2).
FIG. 3.

Mean changes in sum skinfolds, waist circumference, triglycerides, and fasting glucose in 6 months. *p < 0.05, **p < 0.0001 versus all subject groups; +p = 0.004 versus the HIV+BFR+EXS group; ++p < 0.0001 versus the HIV+BFR+noEXS group; NS, p = 0.359 versus the HIV+BFR+EXS group.
Table 2.
Changes in Body Composition from Baseline to 6 Months in Study Populationa
| HIV-ve+noEXS (n = 43, 63% females)
|
HIV+noBFR+noEXS (n = 47, 62% females)
|
HIV+BFR+noEXS (n = 49, 61% females)
|
HIV+ BFR+EXS (n = 48, 63% females)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Δ at 6 Months | Baseline | Δ at 6 months | Baseline | Δ at 6 months | Baseline | Δ at 6 months | |
| Body composition | ||||||||
| Body mass index (kg/m2) | 24.2 ± 2.4 | 0.02 ± 0.2 | 25.8 ± 2.8 | 0.14 ± 0.6 | 24.4 ± 2.7 | 0.06 ± 0.6 | 24.0 ± 3.1 | −0.53 ± 1.2**,‡ |
| Waist circumference (cm) | 83.1 ± 4.6 | 0.78 ± 6.5 | 85.0 ± 7.0 | 1.84 ± 9.2* | 92.3 ± 6.9 | 0.03 ± 10.7 | 91.0 ± 8.4 | −7.13 ± 4.4**,‡ |
| Hip circumference (cm) | 98.9 ± 4.6 | 0.14 ± 0.4 | 100.3 ± 4.3 | 0.05 ± 0.5 | 93.7 ± 6.8 | 0.22 ± 0.7 | 92.3 ± 7.6 | 1.13 ± 5.4 |
| Waist-to-hip ratio | 0.84 ± 0.1 | 0.01 ± 0.1* | 0.85 ± 0.1 | 0.02 ± 0.12 | 0.98 ± 0.0 | 0.00 ± 0.1 | 0.99 ± 0.1 | −0.10 ± 0.1** |
| BFR score(7-item) | 7.1 ± 3.44 | −0.12 ± 0.2 | 8.4 ± 3.1 | 1.51 ± 2.3† | 18.7 ± 2.3 | 0.51 ± 1.3 | 19.3 ± 2.1 | −4.7 ± 0.9**,‡ |
| Skinfold (mm) | ||||||||
| Triceps | 14.3 ± 3.8 | −0.19 ± 0.5 | 14.9 ± 3.7 | 0.09 ± 0.5 | 14.4 ± 3.4 | −0.20 ± 0.7 | 14.4 ± 3.9 | −1.42 ± 2.1** |
| Biceps | 10.6 ± 2.4 | 0.00 ± 0.0 | 11.3 ± 2.6 | 0.17 ± 0.6 | 10.8 ± 2.4 | −0.06 ± 0.3 | 10.7 ± 2.9 | −0.63 ± 1.6* |
| Subscapular | 19.7 ± 4.9 | −0.33 ± 0.9 | 17.7 ± 3.9 | −0.06 ± 0.4 | 18.7 ± 4.9 | −0.55 ± 1.7 | 18.6 ± 5.2 | −1.9 ± 3.2**,‡ |
| Suprailiac | 19.5 ± 4.7 | −0.30 ± 0.9 | 16.5 ± 2.9 | 0.04 ± 0.4 | 18.8 ± 5.1 | −0.43 ± 1.4 | 18.7 ± 5.5 | −2.1 ± 3.5**,‡ |
| Sum skinfolds | 64.1 ± 12.9 | −0.81 ± 1.5 | 60.4 ± 9.8 | 0.23 ± 1.2 | 62.7 ± 12.9 | −1.25 ± 3.5 | 62.3 ± 15.0 | −6.15 ± 8.2**,‡ |
| Body fat mass (%) | 29.5 ± 4.5 | −0.18 ± 0.6 | 29.1 ± 5.0 | −0.14 ± 0.9 | 29.3 ± 4.3 | −0.16 ± 0.7 | 29.4 ± 6.2 | −1.5 ± 3.3**,‡ |
Data expressed as mean ± SD;
p < 0.05,
p < 0.0001 versus HIV+subjects with BFR;
p < 0.05,
p < 0.0001 versus HIV+exercise group;
p < 0.0001 versus HIV+subjects with no B FR and HIV-seronegative subjects; BFR score(7-item), 7-tem body fat redistribution score.
Metabolic outcomes
At baseline, total cholesterol was significantly greater in HIV+BFR+EXS and HIV+BFR+noEXS than HIV+noBFR+noEXS and HIV-ve+noEXS (Table 3). Following CET, total cholesterol levels declined more in the HIV+BFR+EXS group than the HIV+BFR+noEXS group (p < 0.05). HIV-ve+noEXS showed significantly lower mean changes in total cholesterol than HIV+BFR+EXS (p < 0.0003). There were no significant changes in HDL cholesterol, LDL cholesterol, and HOMA values, but insulin levels declined more in the HIV+BFR+EXS than in the HIV+BFR+noEXS group (Table 3). Subjects in the exercise group demonstrated a significant decrease in fasting plasma glucose than HIV+ subjects with BFR in the nonexercise group [−0.21 (0.71) versus 0.39 (1.25), p < 0.0001] and HIV+noBFR+noEXS subjects (p < 0.002) (Fig. 3). Interestingly, HIV-infected subjects with BFR- and HIV-infected subjects with no BFR in the nonexercise groups had a significant increase in fasting plasma glucose, whereas HIV-seronegative subjects did not. Also the serum TG level declined more in HIV+BFR+EXS than in HIV+BFR+noEXS and was not changed in other subject groups (Time 3, Fig. 3).
Table 3.
Changes in Metabolic Profile from Baseline to 6 Months in Study Populationa
| Metabolic | HIV-ve+noEXS (n = 43, 63% females)
|
HIV+noBFR+noEXS (n = 47, 62% females)
|
HIV+BFR+noEXS (n = 49, 61% females)
|
HIV+BFR+EXS (n = 48, 63% females)
|
||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Δ at 6 months | Baseline | Δ at 6 months | Baseline | Δ at 6 months | Baseline | Δ at 6 months | |
| Total cholesterol (mmol/liter) | 3.00 (0.49) | 0.04 (0.41)† | 3.15 (0.50) | 0.17 (0.63) | 3.89 (1.05)‡‡ | 0.066 (1.28) | 3.78 (0.93)‡‡ | −0.03 (1.11)* |
| HDL cholesterol (mmol/liter) | 1.29 (0.16) | 0.01 (0.14) | 1.28 (0.17) | 0.06 (0.23) | 1.27 (0.15) | 0.07 (0.14) | 1.28 (0.19) | 0.03 (0.21) |
| LDL cholesterrol (mmol/liter) | 2.17 (0.70) | 0.01 (0.71) | 2.09 (0.74) | −0.01 (0.91) | 2.16 (0.77) | 0.19 (0.98) | 2.10 (0.74) | 0.14 (0.89) |
| Triglycerides (mmol/liter) | 1.20 (0.25) | −0.03 (0.24) | 1.20 (0.23) | 0.03 (0.34) | 1.34 (0.36)‡ | 0.07 (0.49) | 1.33 (0.39)‡ | −0.22 (0.48)*,‡ |
| Glucose (mmol/liter) | 4.44 (0.49) | 0.04 (0.27)‡‡ | 4.63 (0.74) | 0.23 (0.93) | 4.95 (0.74) | 0.39 (1.25) | 4.76 (0.76) | −0.21 (0.71)**,‡ |
| Insulin (ρmol/liter) | 45 (19) | 1 (19) | 54 (3.1) | 1 (21) | 59 (28) | 3 (23) | 67 (29) | −1 (18)* |
| HOMAIR | 1.28 (0.06) | 0.0 (0.0) | 1.60 (0.10) | 0.0 (0.1) | 1.87 (0.13) | 0.0 (0.2) | 2.04 (0.14) | 0.0 (0.1) |
Data expressed as mean ± SD;
p < 0.95,
p < 0.0001 versus HIV+BFR+noEXS;
p < 0.05,
p < 0.0001 versus HIV+noBFR+noEXS and HIV-ve+noEXS;
p < 0.0001 versus HIV+noBFR+noEXS;
p < 0.0001 versus HIV+BFR+noEXS and HIV+noBFR+noEXS; HOMAIR, homeostasis model assessment of insulin resistance.
DISCUSSION
Our findings indicate that CET positively modulates body composition, decreasing waist circumference, waist-to-hip ratio, and percentage body fat mass in WHO-recommended HAART-treated HIV+ African subjects with BFR in Rwanda. Exercise training further modulates a decline in total serum cholesterol, TG, and glucose, and also improves cardiorespiratory fitness. To our knowledge, this is the first study to demonstrate that CET is safe, practical, and effective at reducing central adiposity and cardiovascular risk factors and improving cardiovascular fitness in HAART-treated HIV+ sub-Saharan Africans with body fat alterations. Previous studies on the effects of CET on metabolic and anthropomorphic abnormalities in HAART-treated HIV+ patients were limited by small sample sizes, were disproportionate with respect to gender, and were not largely controlled.21–25 We believe our findings are particularly important due to increasing access to HAART in sub-Saharan countries and the potential for increased CVD and diabetes risks for HIV+ patients starting HAART.
HIV-infected participants with BFR who exercised showed a significant improvement in body composition, particularly resulting in a considerable reduction in waist circumference and WHR and an increase in lean body mass. Exercise training did not significantly affect hip circumference, but reduced waist circumference, which accounted for the decline in WHR. Both waist circumference and WHR are simple and inexpensive measurements used in normal populations to assess central adiposity, and are good predictors of CVD and diabetes risks.35
Elevated WHR in HAART-treated HIV-infected individuals is mainly due to a gradual increase in waist circumference rather than to changes in hip circumference.36 Our findings further showed that sedentary HAART-treated HIV+ African participants had an increase in waist circumference over time in comparison to age-, gender-, and BMI-matched HIV-seronegative participants. Others have reported a gradual increase in waist circumference in HIV+ patients with body fat redistribution.36 The cause and existence of this HIV- and HAART-associated central adiposity are under debate.37 Our findings suggest that waist girth increased over 6 months in HIV-infected African subjects treated with HAART, and that it is preventable with 6 months of aerobic exercise training. Further, the current study supports other studies that found a relationship between reductions in waist circumference and WHR and increases in lean body mass and reductions in body fat mass in subjects who participated in CET.22,23
Exercise training induced alterations in central adiposity and glucose and lipid parameters in the current study, and this translates to reduced cardiovascular disease risk in HAART-treated HIV-infected Africans, as seen in HIV-seronegative populations.38 Exercise training reduces fat mass and increases fat-free mass in other HIV+ cohorts;21–24 our findings support this notion and further indicate that CET attenuates fasting serum glucose and TG and improves cardiorespiratory fitness in HAART-treated HIV+ African men and women with body fat alterations. Thus, TG may reduce CVD morbidity and mortality rates and improve insulin sensitivity in HAART-treated HIV+ African men and women39 through its reduction of central adiposity, a strong predictor of insulin resistance in HIV+ patients.40
In the current study, exercise training improved the cardiorespiratory fitness of HIV+ African men and women with body fat alterations. At baseline, we did not observe differences in exercise capacity between HIV+ African subjects with or without body fat changes; however, when combined as one group, all HIV-infected subjects had lower baseline VO2max (ml/kg/min) compared to HIV-seronegative African control subjects. Cardiorespiratory fitness improved 19% in HIV-infected African subjects who exercised for 6 months, but did not achieve the value of the healthy, normal HIV-seronegative African controls in the current study. Lower cardiorespiratory fitness has been reported in HIV-infected subjects irrespective of HAART use,41 but improves with exercise training.25,42,43 This may further indicate inadequate aerobic capacity in HIV+ people, possibly due to the effects of HIV disease or HAART on tissue oxygen extraction and utilization. Low cardiorespiratory fitness has been associated with elevated cardiovascular disease morbidity and mortality, as a result central obesity and other risk factors in non-HIV-positive populations.44
The current study is limited by lack of more objective fat quantification methods, such as dual energy X-ray absorptiometry (DEXA) or magnetic resonance imaging (MRI) and computed tomography (CT) scans. Although expensive for large sample sizes, the use of MRI and/or CT scans would reveal the direct effects of exercise on visceral and subcutaneous fat content. Anthropometric measurements have proven sensitive and useful in assessing body composition changes in HIV-infected people.36 Further randomized controlled studies evaluating the effects of exercise training using modern body composition assessment tools are warranted, particularly in subjects whose metabolic variables indicate greater cardiovascular disease risk.
In summary, our findings provide evidence that exercise training is safe, practical, and efficacious in reducing central adiposity and CVD risk parameters and improving cardiovascular fitness in HAART-treated HIV-infected Africans with body fat alterations. The beneficial effects of exercise training are particularly important in sub-Saharan populations, where the accessibility to HIV treatment is improving, but where minimal resources and medications (antihypertensives, antidiabetics, antihyperlipidemics) are available to manage HIV- and HAART-associated metabolic syndromes that threaten to increase CVD risk.
Acknowledgments
We thank the participants in the study for their valuable time and commitment. We thank all research associates and the hospital and health centres’ administrative staff where the study took place. We value the support of the administrative and academic staff of Kigali Health Institute. We acknowledge and thank the Commission Nationale de Lutte Contre le SIDA (CNLS) and Multisectorial AIDS Program (MAP), Rwanda for funding this study. We thank Kenneth Schechtman, Ph.D., and Loralyn Benoit, Ph.D., for their statistical analyses.
References
- 1.Chen D, Mistra A, Garg A. Lipodystrophy in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab. 2002;87:4845–4856. doi: 10.1210/jc.2002-020794. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein KA, Ward DJ, Moorman AC. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15:1389–1398. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 3.Miller J, Carr A, Emery S, et al. HIV lipodystrophy: Prevalence, severity and correlates of risk in Australia. HIV Med. 2003;4:293–301. doi: 10.1046/j.1468-1293.2003.00159.x. [DOI] [PubMed] [Google Scholar]
- 4.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease inhibitor associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: A cohort study. Lancet. 1999;353:2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- 5.Sterne JA, Herman MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: A prospective cohort study. Lancet. 2005;366:378–384. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 6.Dolan SE, Hadigan C, Killilea KM, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr. 2005;39:44–54. doi: 10.1097/01.qai.0000159323.59250.83. [DOI] [PubMed] [Google Scholar]
- 7.Grover SA, Coupal L, Gilmore N, Mukherjee J. Impact of dyslipidemia associated with highly active antiretroviral therapy (HAART) on cardiovascular risk and life expectancy. Am J Cardiol. 2005;95:586–591. doi: 10.1016/j.amjcard.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Hadigan C, Meigs JB, Wilsonn PW, et al. Prediction of coronary heart disease in HIV-infected patients with fat redistribution. Clin Infect Dis. 2003;36:909–916. doi: 10.1086/368185. [DOI] [PubMed] [Google Scholar]
- 9.Bergersen BM, Sandvik L, Bruun JN, Tonstad S. Elevated Framingham risk score in HIV-positive patients on highly active antiretroviral therapy: Results from a Norwegian study of 721 subjects. Eur J Clin Microbiol Infect Dis. 2004;23:625–630. doi: 10.1007/s10096-004-1177-6. [DOI] [PubMed] [Google Scholar]
- 10.Pujari SN, Dravid A, Naik E, et al. Lipodystrophy and dyslipidaemia among patients taking first-line, World Health Organisation-recommended highly active antiretroviral therapy regimens in Western India. J Acquir Immune Defic Syndr. 2005;39:199–202. [PubMed] [Google Scholar]
- 11.Mutimura E, Stewart A, Crowther NJ. The prevalence and metabolic consequences of antiretroviral-associated lipodystrophy in a population of HIV-infected African subjects. Antiviral Ther; Program and Abstracts (Abstract #28) of the 8th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV; 24–26 September; San Francisco, CA. 2006. p. L20. [Google Scholar]
- 12.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: A collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 13.Gayle HD. Expanding access to HIV prevention. AIDS Res Ther. 2006;3:2. doi: 10.1186/1742-6405-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne JA, Herman MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: A prospective cohort study. Lancet. 2005;366:378–384. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 15.Kengne AP, Amoah AG, Mbanya JC. Cardiovascular complications of diabetes mellitus in sub-Saharan Africa. Circulation. 2005;112:3592–3601. doi: 10.1161/CIRCULATIONAHA.105.544312. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi Y, Nagasaka S, Takahashi N, et al. A single bout of exercise at higher intensity enhances glucose effectiveness in sedentary men. J Clin Endocrinol Metab. 2005;90:4035–4040. doi: 10.1210/jc.2004-2092. [DOI] [PubMed] [Google Scholar]
- 17.Katzmarzyk PT, Leon AS, Wilmore JH, et al. Targeting the metabolic syndrome with exercise: Evidence from the HERITAGE Family Study. Med Sci Sports Exerc. 2003;35:1703–1709. doi: 10.1249/01.MSS.0000089337.73244.9B. [DOI] [PubMed] [Google Scholar]
- 18.Watt K, Beye P, Siafarikas A, et al. Exercise training normalizes vascular dysfunction and improves central adiposity in obese adolescents. J Am Coll Cardiol. 2004;43:1823–1827. doi: 10.1016/j.jacc.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 19.Stewart KJ. Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: Plausible mechanisms for improving cardiovascular health. JAMA. 2002;288:1622–1631. doi: 10.1001/jama.288.13.1622. [DOI] [PubMed] [Google Scholar]
- 20.Dube MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: Recommendations of the HIV Medicine Association of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37:613–627. doi: 10.1086/378131. [DOI] [PubMed] [Google Scholar]
- 21.Roubenoff R. A pilot study of exercise training to reduce trunk fat in adults with HIV-associated fat redistribution. AIDS. 1999;13:1373–1375. doi: 10.1097/00002030-199907300-00015. [DOI] [PubMed] [Google Scholar]
- 22.Jones SP, Doran D, Leatt PB, Maher B, Pirmohamed M. Short-term exercise training improves body composition and hyperlipidaemia in HIV-positive individuals with lipodystrophy. AIDS. 2001;15:2049–2051. doi: 10.1097/00002030-200110190-00021. [DOI] [PubMed] [Google Scholar]
- 23.Yarasheski KE, Tebas P, Stanerson B, et al. Resistance exercise training reduces hypertriglyceridemia in HIV-infected men treated with anti-retroviral therapy. J Appl Physiol. 2001;90:133–138. doi: 10.1152/jappl.2001.90.1.133. [DOI] [PubMed] [Google Scholar]
- 24.Roubenoff R, Schmitz H, Bairos L, et al. Reduction of abdominal obesity in lipodystrophy associated with human immunodeficiency virus infection by means of diet and exercise: Case report and proof of principle. Clin Infect Dis. 2002;34:390–393. doi: 10.1086/338402. [DOI] [PubMed] [Google Scholar]
- 25.Scevola D, Di Matteo A, Lanzarini P, et al. Effect of exercise and strength training on cardiovascular status in HIV-infected patients receiving highly active antiretroviral therapy. AIDS. 2003;17:123–129. doi: 10.1097/00002030-200304001-00015. [DOI] [PubMed] [Google Scholar]
- 26.Driscoll SD, Meininger GE, Lareau MT, et al. Effects of exercise training and metformin on body composition and cardiovascular indices in HIV-infected patients. AIDS. 2004;18:465–473. doi: 10.1097/00002030-200402200-00013. [DOI] [PubMed] [Google Scholar]
- 27.Fitch K, Anderson EJ, Hubbard JL, et al. Effects of a lifestyle modification program in HIV-infected patients with metabolic syndrome. AIDS. 2006;20:1843–1850. doi: 10.1097/01.aids.0000244203.95758.db. [DOI] [PubMed] [Google Scholar]
- 28.Carr A, Emery S, Law M, et al. An objective case definition of lipodystrophy in HIV-infected adults: A case-control study. Lancet. 2003;361:726–735. doi: 10.1016/s0140-6736(03)12656-6. [DOI] [PubMed] [Google Scholar]
- 29.Bairaktari E, Hatzidimou K, Tzallas C, et al. Estimation of LDL cholesterol based on the Friedewald formula and on apo B levels. Clin Biochem. 2000;33:549–555. doi: 10.1016/s0009-9120(00)00162-4. [DOI] [PubMed] [Google Scholar]
- 30.Durnin JVGA, Rahaman MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr. 1967;21:681–689. doi: 10.1079/bjn19670070. [DOI] [PubMed] [Google Scholar]
- 31.Durnin JVGA, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16–72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 32.Zillikens MC, Conway JM. Anthropometry in blacks: Applicability of generalized skinfold equations and differences in fat patterning between blacks and whites. Am J Clin Nutr. 1990;52:45–51. doi: 10.1093/ajcn/52.1.45. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson DM, Fallowfield JL, Myers SD. A modified incremental shuttle run test for the determination of peak shuttle running speed and the prediction of maximal oxygen uptake. J Sports Sci. 1999;17:413–419. doi: 10.1080/026404199365939. [DOI] [PubMed] [Google Scholar]
- 34.Flouris AD, Metsios GS, Koutedakis Y. Enhancing the efficacy of the 20 m multi-stage run test. Br J Sports Med. 2005;39:166–170. doi: 10.1136/bjsm.2004.012500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meisinger C, Doring A, Thorand B, Heier M, Lowel H. Body fat distribution and risk of type 2 diabetes in the general population: Are there differences between men and women? The MONICA/KORA Augburg cohort study. Am J Clin Nutr. 2006;84:483–489. doi: 10.1093/ajcn/84.3.483. [DOI] [PubMed] [Google Scholar]
- 36.Brown T, Wang Z, Chu H, et al. Longitudinal anthropometric changes in HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr. 2006;43:356–362. doi: 10.1097/01.qai.0000243052.73321.8e. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson DL, Knox T, Spiegelman D, Skinner S, Gorbach S, Wanke C. Prevalence of, evolution of, and risk factors for fat atrophy and fat deposition in a cohort of HIV-infected men and women. Clin Infect Dis. 2005;40:1837–1845. doi: 10.1086/430379. [DOI] [PubMed] [Google Scholar]
- 38.O’Donovan G, Owen A, Bird SR, et al. Changes in cardiorespiratory fitness and coronary heart disease risk factors following 24 wk of moderate-or-high-intensity exercise of equal energy cost. J Appl Physiol. 2005;98:1619–1625. doi: 10.1152/japplphysiol.01310.2004. [DOI] [PubMed] [Google Scholar]
- 39.Palacios R, Merchante N, Macias J, et al. Incidence of and risk factors for insulin resistance in treatment-naïve HIV-infected patients 48 weeks after starting highly active antiretroviral therapy. Antiviral Ther. 2006;11:529–535. [PubMed] [Google Scholar]
- 40.Meininger G, Hadigan C, Rietschel P, Grinspoon SK. Body composition measurements as predictors of glucose and insulin abnormalities in HIV-positive men. Am J Clin Nutr. 2002;76:460–465. doi: 10.1093/ajcn/76.2.460. [DOI] [PubMed] [Google Scholar]
- 41.Cade WT, Fantry LE, Nabar S, Keyser RE. Impaired oxygen on-kinetics in persons with the human immunodeficiency virus are not due to highly active antiretroviral therapy. Arch Phys Med Rehabil. 2003;84:1831–1838. doi: 10.1016/j.apmr.2003.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Stringer WW, Berezovskava M, O’Brien WA, Beck CK, Casaburi R. The effects of exercise training on aerobic fitness, immune indices, and quality of life in HIV positive patients. Med Sci Sports Exerc. 1998;30:11–16. doi: 10.1097/00005768-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Thöni GJ, Fedou C, Brun JF, et al. Reduction of fat accumulation and lipid disorders by individualized light aerobic training by human immunodeficiency virus infected patients with lipodystrophy and/or dyslipidaemia. Diabetes Metabol Paris. 2002;28:397–404. [PubMed] [Google Scholar]
- 44.Carnethon MR, Gulati M, Greenland P. Prevalence and cardiovascular disease correlates of low cardiorespiratory fitness in adolescents and adults. JAMA. 2005;294:2981–2988. doi: 10.1001/jama.294.23.2981. [DOI] [PubMed] [Google Scholar]


