Abstract
MET, a replicated autism risk gene, encodes a pleiotropic receptor tyrosine kinase implicated in multiple cellular processes during development and following injury. Previous studies suggest that Met modulates excitatory synapse development in the neocortex and hippocampus, although the underlying mechanism is unknown. The peak of Met expression corresponds to the period of process outgrowth and synaptogenesis, with robust expression in hippocampal and neocortical neuropil. Resolving whether neuropil expression represents presynaptic, postsynaptic or glial localization provides insight into potential mechanisms of Met action. The subcellular distribution of Met was characterized using complementary ultrastructural, in situ proximity ligation assay (PLA) and biochemical approaches. At postnatal day (P) 7, immuno-electron microscopy revealed near-equivalent proportions of Met-immunoreactive pre- (axons and terminals) and post- (dendritic shafts and spines) synaptic profiles in the stratum radiatum in the hippocampal CA1 region. Staining was typically in elements in which the corresponding pre- or postsynaptic apposition was unlabeled. By P21, Met-immunoreactive presynaptic profiles predominated and approximately 20% of Met-expressing profiles were glial. A different distribution of Met-immunoreactive profiles was observed in layer V of somatosensory cortex: Met-labeled spines were rare and a smaller proportion of glial profiles expressed Met. Strikingly, Met-immunoreactive presynaptic profiles predominated over postsynaptic profiles as early as P7. PLA analysis of neurons in vitro and biochemical analysis of tissue subsynaptic fractions confirmed the localization of Met in specific synaptic subcompartments. The study demonstrates that Met is enriched at synapses during development and its activation may modulate synapse formation and stability through both pre- and post-synaptic mechanisms.
Keywords: axon, dendritic spine, synaptogenesis, receptor tyrosine kinase, hepatocyte growth factor
INTRODUCTION
The gene encoding the Met receptor tyrosine kinase has been associated with autism spectrum disorder (ASD), with biological data mounting that the receptor participates in several key neurodevelopmental processes that may be vulnerable (Judson et al., 2011b; Qiu et al., 2012). While well-characterized at the molecular level, Met-mediated cellular responses are particularly diverse and complex. In the forebrain, there is increasing evidence that Met activation modulates excitatory synapse maturation, function and plasticity. For example, in primary cultures of hippocampal neurons, activation of Met following addition of its ligand, hepatocyte growth factor (HGF), leads to an increase in the expression and clustering of synaptic proteins, including subunits of the glutamate receptor (Nakano et al., 2007; Tyndall and Walikonis, 2006). In hippocampal slice cultures, the same treatment results in an enhancement of long-term potentiation, mediated in part by potentiating glutamatergic N-methyl-D-aspartic acid (NMDA) receptor mediated currents, although long term depression remains unaffected (Akimoto et al., 2004). Consistent with these in vitro observations, mice in which HGF is over-expressed specifically in the nervous system exhibit increased levels of the NR2A and NR2B subunits of the NMDA receptor in the hippocampus (Kato et al., 2012). There also is evidence of altered excitatory circuit development in conditional knock-out mice, in which Met is deleted in progenitor cells arising from the dorsal pallium. Specifically, dendritic spine size in pyramidal cells residing in layers II/III and V in cingulate cortex is increased (Judson et al., 2010) and excitatory connectivity from layer II/III onto select pyramidal neurons in layer V in frontal cortex is strengthened (Qiu et al., 2011). These findings are relevant to studies in humans showing that a common functional variant in the MET promoter is associated with autism spectrum disorder risk (Campbell et al., 2006; Judson et al., 2011b), and that the promoter variant impacts functional and structural cortical connectivity in typical and ASD subjects (Rudie et al., 2012) and growth of gray matter specifically in neocortical regions that express MET in pre- and adolescent humans (Hedrick et al., 2012).
The temporal and spatial patterns of Met expression in the forebrain are consistent with its proposed modulatory role in synaptogenesis. In vitro, Met has been reported to be clustered at hippocampal excitatory synapses following addition of HGF (Tyndall and Walikonis, 2006), although it was not possible to resolve if this clustering represents expression in pre- or post-synaptic compartments, or both. In primate and rodent neocortex and hippocampus, peak Met expression corresponds to the robust period of axon growth and synapse formation (Judson et al., 2011a; Judson et al., 2009). At this time, Met-immunoreactivity can be observed throughout the neuropil and in specific axon tracts, including intensely labeled subregions of the corpus callosum and the fimbria. After synaptogenesis peaks, Met expression declines, such that immunoreactivity is essentially absent in axon tracts while retaining sparse to low intensity labeling of the neuropil (Judson et al., 2011a; Judson et al., 2009). The precise cellular (neuronal or glial) and subcellular (dendritic or axonal) localization of Met within the neuropil, however, are largely unknown. In situ hybridization indicates that, in the neocortex, Met is expressed almost exclusively in excitatory projection neurons (Eagleson et al., 2011). Electron microscopy (EM) revealed Met-immunoreactive postsynaptic terminals in the hippocampus (Tyndall and Walikonis, 2006). The developmental mapping studies in rodent and primate (Judson et al., 2011a; Judson et al., 2009), however, indicate that Met protein must be transported axonally and located in part presynaptically. Mechanistic insight regarding the role of Met in circuit formation and function will come in part from a more rigorous assessment of its distribution at the subcellular and subsynaptic levels in neocortex and hippocampus. In the present study, we have used complementary morphological and biochemical methods to assess the compartmentalization of Met during neocortical and hippocampal development.
MATERIALS AND METHODS
Animals
For the EM studies, C57Bl/6 mice, originally purchased from Jackson Laboratories (Bar Harbor, Maine), were bred in house at Weill Cornell Medical College. For the biochemical and cell culture studies, timed pregnant C57Bl/6 mice were purchased from Charles River (Wilmington, MA). Animals were provided free access to food and water and were housed in a 12 hour light:dark cycle. All research procedures using mice were approved by the Institutional Animal Care and Use Committee at Weill Cornell Medical College and the University of Southern California and conform to the 2011 Eighth Edition of the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Antibody Characterization
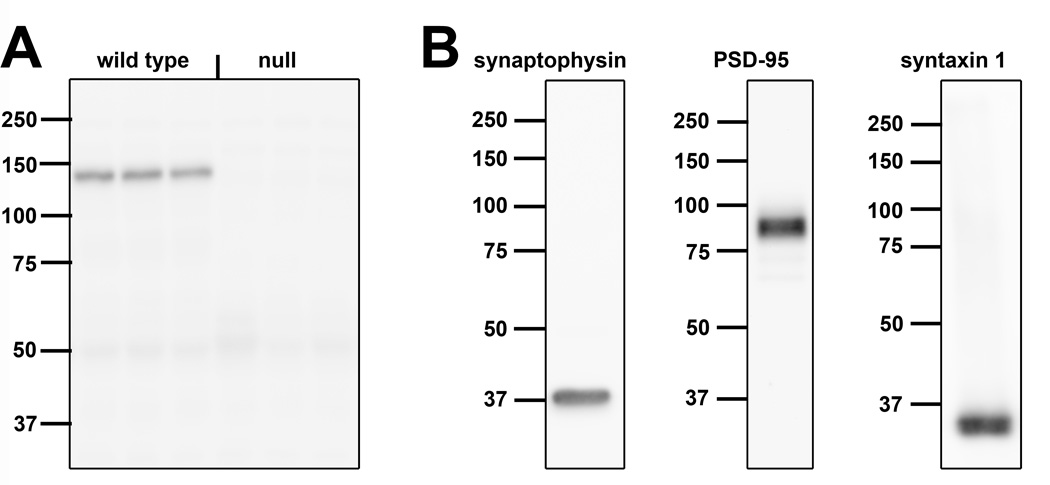
Primary antibodies used in this study are described in Table 1. The primary mouse anti-Met antibody used for immuno-EM and Western blotting has been shown to recognize Met in a variety of species, including mice and monkeys (Judson et al., 2011a; Judson et al., 2009). The antibody specificity was confirmed by the absence of signal following immunoblotting (Fig. 1A) and immunohistochemistry (Judson et al., 2011a; Judson et al., 2009), using tissue prepared from the cortex of mice in which the Met gene was deleted from the dorsal pallium.
Table 1.
Primary antibodies
| Antigen | Immunogen | Species | Manufacturer, catalog No |
|---|---|---|---|
| Met | Synthetic peptide, AA 1330–1379 of mouse C-terminus | Mouse monoclonal (B-2) | Santa Cruz Biotechnology (Santa Cruz, CA), sc-8057 |
| Met | Recombinant mouse Met, Glu25-Asn929 | Goat | R&D Systems (Minneapolis, MN), AF-527 |
| PSD-95 | Recombinant rat PSD-95 | Mouse | Millipore (Billerica, MA), MAB1596 |
| Syntaxin 1 | Full length rat syntaxin 1 | Mouse | Santa Cruz Biotechnology, sc-12736 |
| Synaptophysin | Vesicular fraction of bovine brain | Mouse | Millipore, MAB5258 |
| Synapsin 1 | Synapsin I (mixture of Ia & Ib) purified from bovine brain | Rabbit | Millipore, AB1543 |
| Neurexin 1 | KLH-conjugated linear peptide corresponding to human pan Neurexin-1-alpha | Rabbit | Millipore, ABN161 |
Figure 1. Specificity of the antibodies used in the present study.
A. Western blot of Met protein in homogenates of postnatal day (P) 14 mouse cortex from 2 wild type mice and 2 conditional null mice in which Met was deleted from cells arising from the dorsal pallium. Note the selective band at the expected molecular weight of the mature form of Met (145 kD) in wild type tissue and the absence of signal in null tissue for the antibody raised in mouse and the antibody raised in goat. B. Western blots of homogenates of P14 wild type mouse cortex. Each of the antibodies used to validate our biochemical fractionation procedure or to identify pre- and postsynaptic compartments in vitro recognizes a unique band at the expected molecular weight (synaptophysin: 38 kD, postsynaptic density-95 [PSD-95]: 95 kD, syntaxin 1: 31 kD, synapsin 1: 77 and 80kD). The pan neurexin 1 antibody recognized the β-forms of neurexin 1 (50–75kD), but not the α-forms (predicted size 160–170kD). Molecular weight sizes (kD) are indicated to the left of each blot.
Three mouse monoclonal antibodies were used to assess the efficacy of the subsynaptic fractionation procedure. Specifically, an anti-synaptophysin antibody was used as a marker of biochemical fractions containing synaptic vesicles, an anti-postsynaptic density protein 95 (PSD95) antibody was used as a marker of the postsynaptic density fraction and an anti-syntaxin 1 antibody was used as a marker of the presynaptic active zone fraction. For each antibody, we observed the same unique molecular weight band in Western blots of homogenates of postnatal day (P) 14 mouse cortex (Fig. 1B) as has been reported by the manufacturer (manufacturers’ information sheets) and in multiple publications (Anastasio et al., 2010; Bouvier et al., 2008; Louneva et al., 2008; Needleman et al., 2010; Phillips et al., 2001; Rebola et al., 2005).
For the PLA assay, three antibodies were used to label pre- and post-synaptic compartments in primary cultures of neocortical and hippocampal neurons. The same monoclonal anti-PSD-95 antibody described above was used to delineate the postsynaptic compartment. For presynaptic compartment labeling, polyclonal anti-neurexin 1 and anti-synapsin 1 antibodies were used to label the presynaptic compartment. The anti-synapsin 1 antibody recognizes the appropriate band that represents both the 1a and 1b forms at 77 and 80kD (Fig. 1B). The anti-pan neurexin 1 antibody is reported by the manufacturer to recognize multiple forms of neurexin 1. In Western blots of P14 mouse cortex, the antibody recognizes the β-forms of neurexin 1, but does not appear to recognize the α-forms (Fig. 1B). Staining of mouse primary neocortical and hippocampal neurons produced patterns of PSD-95, neurexin 1 and synapsin 1 immunoreactivity are identical with previous descriptions (Dean et al., 2003; Li et al., 2010; Mondin et al.; Zurner et al., 2011). We could not use the same monoclonal anti-Met antibody described above for the PLA assay as this assay requires two antibodies generated in different species. Therefore, we used a goat polyclonal anti-Met antibody: the antibody specificity was confirmed by the absence of signal following immunoblotting (Fig. 1A) using tissue prepared from the cortex of mice in which the Met gene was deleted from the dorsal pallium.
Immuno-electron microscopy
The distribution of Met in the neocortical and hippocampal neuropil was examined at P7, P14 and P21 using pre-embedding immuno-EM (Milner et al., 2011). Unless otherwise noted, all steps were performed at room temperature. Male mice (N = 3 at each age) were deeply anesthetized with sodium pentobarbital (150mg/kg i.p.) prior to transcardial perfusion first with 2ml 2% heparin in saline, followed by 10–20 ml 3.75% acrolein (Polysciences, Warrington, PA) and 2% paraformaldehyde (Electron Microscopy Sciences [EMS], Fort Washington, PA) in 0.1M phosphate buffer, pH 7.4 (PB). Brains were postfixed for 30 min in 2% acrolein and 2% paraformaldehyde in PB, and then stored in PB at 4°C until sectioned (less than 2 days). Coronal sections were cut (50µm thick) on a Leica VT1000S vibratome (Leica Microsystems, Deerfield, IL) and stored as free-floating sections in 30% ethylene glycol (Sigma, St Louis, MO)/ 30% sucrose (JT Baker, Phillipsburg, NJ) in PB at −20°C.
Sections that included the dorsal hippocampus and overlying somatosensory cortex (Fig. 2A) were processed for the immunohistochemical localization of Met. Sections were washed in PB, incubated for 30 min in 0.5% sodium borohydride (Sigma-Aldrich) in PB, and rinsed again in PB. After transfer to a cryoprotectant solution (25% sucrose, 10% glycerol [JT Baker] in 50mM phosphate buffer), sections underwent a freeze-thaw cycle, after which they were rinsed first in PB followed by 0.1M Tris-saline, pH 7.6 (TS), and blocked in 0.5% bovine serum albumin (BSA, Sigma) in TS for 30 min. Following two washes in TS, sections were incubated in the anti-Met primary antibody (1:300 in 0.1% BSA in TS) for 1 day at room temperature followed by 4 days at 4°C. After rinsing in TS, sections were incubated in biotinylated horse anti-mouse IgG (1:400 in 0.1% BSA in TS, Vector Laboratories, Burlingame, CA) for 30 min. Sections were rinsed in TS and processed using the ABC Vectastain Elite kit at twice the recommended dilution (Vector Laboratories). Met-immunolabeling was visualized with 3,3’-diaminobenzidine (Sigma) and hydrogen peroxide (EMS) in TS. Sections were rinsed in TS, then rinsed and stored in PB prior to embedding.
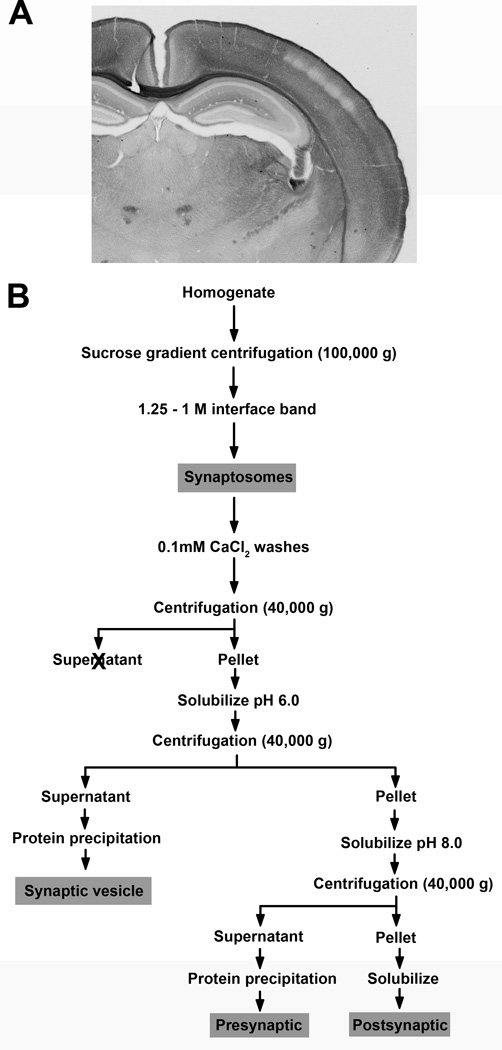
Figure 2. Samples for anatomical and biochemical studies.
A. Photograph of a coronal section through the mouse forebrain at P14 illustrating the distribution of Met immunoreactivity at the level at which the immuno-EM analyses were performed. Arrows denote the stratum radiatum of hippocampal CA1 and superficial layer V of somatosensory cortex (Ctx). Scale bar = 500µm. B. Schematic representation of the biochemical fractionation method used to generate synaptosomes and the subsequent separation into three subsynaptic fractions. Details are described in Methods. Grey boxes indicate fractions that were analyzed for Met expression.
Tissue sections were embedded and sectioned for EM using standard procedures (Milner et al., 2011). Briefly, sections were incubated in 2% osmium tetroxide (EMS) in PB for 1 hour, rinsed with PB, and dehydrated through a graded series of alcohol and propylene oxide (EMS). Sections were embedded in EMbed 812 (EMS) and flat mounted between Aclar sheets (Honeywell, Morristown, NH) at 60°C for 3 to 5 days. For each animal, the areas of interest (hippocampus and somatosensory cortex) were excised from one embedded tissue section, mounted on EMbed chucks, and sectioned on a Leica ultracut UCT ultratome (Leica Microsystems). The ultrathin sections (70nm thick) were collected on 400 mesh copper grids (EMS) and counterstained with uranyl acetate and Reynold’s lead citrate (EMS).
Sampling and analysis
Sections were viewed and photographed on a Tecnai Biotwin transmission electron microscope (FEI, Hillsboro, OR). For each mouse, one block from the hippocampus and one from the somatosensory cortex was analyzed. Met-immunolabeled profiles were counted and classified in the stratum radiatum, 55 µm distal from the hippocampal CA1 pyramidal cell layer, and in superficial layer V of the somatosensory cortex, approximately 350 (P7) to 400 (P14, P21) µm deep to the pial surface. To insure optimum labeling and to allow quantitative comparisons between groups, only grids near the plastic/tissue interface were selected (Milner et al., 2011). Within these areas, two to three grid squares, representing 6050 to 9075 µm2 of neuropil, were sampled; our previous studies have determined that this amount of sampling is sufficient to represent the population (Barker-Gibb et al., 2001; Milner et al., 2001b). Images of all fields containing immunolabeled profiles were acquired at 16,500× using Advanced Microscopy Techniques software (v3.2, Advanced Microscopy Techniques, Woburn, MA).
Cellular profiles were classified according accepted morphological criteria (Peters et al., 1991). Briefly, dendritic shafts contained regular microtubular arrays and were usually postsynaptic to axon terminal profiles. Dendritic spines also were usually postsynaptic to axon terminal profiles and sometimes contained a spine apparatus. Unmyelinated axons were smaller than 0.2 µm, round, contained a few small synaptic vesicles and lacked a synaptic junction in the plane of section (Milner and Bacon, 1989; Milner et al., 2001a). Axon terminals had numerous small synaptic vesicles and had a cross-sectional diameter greater than 0.2 µm. Glial profiles were distinguished by the presence of glial filaments and/or gap junctions (astrocytes), by the absence of microtubules, and/or by their tendency to conform to the boundaries of surrounding profiles (Barker-Gibb et al., 2001; Glass et al., 2001). Ambiguous profiles, which were more prevalent at P7, were classified as unknown. Asymmetric synapses were characterized by the larger size of the post-synaptic density whereas symmetric synapses had pre- and post-synaptic densities of equal size. Appositions were defined as those contacts between profiles in which no interposing glial processes were seen but that lacked any recognizable synaptic specializations. The number of each class of Met-immunolabeled profiles was tabulated and the data presented as mean ± SEM. Significant differences in the distribution between neocortex and hippocampus, and within a region across the three developmental ages, were determined using Pearson’s χ2 test. The cross-sectional area and minimum diameter of terminal profiles in which the entire perimeter could be distinguished were measured using Microcomputer Imaging Device software (MCID, Imaging Research Inc., Ontario, Canada).
Proximity Ligation Assay (PLA)
Primary neuronal cultures using tissue from embryonic day (E) 18 hippocampus or neocortex were prepared as described previously (Kaech and Banker, 2006). For each culturing session, tissue from a single litter (7–10 total, including male and female embryos) was pooled. Briefly, tissue was incubated in 0.25% trypsin-EDTA (Invitrogen Life Technologies, Grand Island, NY), then dissociated into a single cell suspension by mechanical trituration. Cells were plated at a density of 1 × 104 cells/cm2 onto coverslips (Carolina Biological Supply Company, Burlington, NC) coated with 100µg/ml poly-D-lysine (Sigma). Cells were cultured at 37°C in 5% CO2 in Dulbecco’s Modified Eagle’s Medium (Invitrogen) supplemented with 5% fetal bovine serum. After 2 hr, by which time the cells had adhered to the substrate, the coverslips were transferred to a new dish and the cells co-cultured above a layer of astrocytes in defined Neurobasal medium (Invitrogen) supplemented with 2% B27 (Invitrogen) and 1% L-glutamine (Invitrogen).
At 14 days in vitro (DIV), proximity ligation assays were performed using the Duolink in situ PLA kit (Olink Bioscience, Uppsala, Sweden (Soderberg et al., 2006) to assess the co-localization of Met with proteins found exclusively in the presynaptic or postsynaptic compartment. Coverslips were fixed with 4% PFA for 15 min at room temperature, washed in PBS, and permeabilized with 0.1% Triton X-100 in PBS for 15 min. The coverslips were incubated for 1 hr at room temperature in the blocking solution provided in the kit, followed by an overnight incubation with anti-Met (1:25) antibody in conjunction with anti-PSD-95 (1:100), anti-synapsin 1 (1:100) or anti-neurexin 1 (1:100) antibodies. For each culturing session, one primary antibody was omitted from some coverslips to control for potential nonspecific PLA signals generated by a single antibody. After two washes with PBS, coverslips were incubated with oligonucleotide-linked secondary antibodies (PLA probes) provided in the kit. Subsequent hybridization, ligation and amplification steps were performed according to the manufacturer’s protocol, after which coverslips were incubated with Cy2-labeled secondary antibodies (Jackson ImmunoReseach) for 20 min at room temperature. After two washes with PBS, coverslips were mounted with ProLong® Gold Antifade reagent (Invitrogen). A fluorescent signal is generated only when the two PLA probes are in close proximity (40nm).
For each labeling combination, five different fields per coverslip, derived from at least 3 independent culturing sessions, were examined using an Olympus Fluoview FV1000 confocal microscope (60× oil object). Digital images were collected and downloaded into Adobe Photoshop CS5 (v12.1, Adobe Systems Incorporated, San Jose, CA) to assess the presence or absence of the PLA signal. For quantification of PLA signal, the total number of PLA clusters in five random fields (900 µm2) was counted for each labeling combination. Typically, PLA signal has been expressed per cell. In our culture system, however, it is not possible to define the number of cells contributing to the processes in any one field. Therefore, we determined the area fraction of each box occupied by Met immunostaining using Image J. PLA signal was then expressed as the number of PLA clusters/Met signal in each sampled field. For each labeling combination, the PLA value for each field was averaged to give a single value for each culturing session. For each brain region, a one-way ANOVA was used to determine overall significance. If significant differences were observed, this analysis was complemented by a Tukey multiple comparison post-test.
Generation and characterization of subcellular fractions
Synaptomsome and subsynaptic fractions were generated according to published protocols (Louneva et al., 2008; Phillips et al., 2001). All procedures were performed on ice or at 4°C. All chemicals are from Sigma unless otherwise noted. Mice at P7, P14 and P21 were anesthetized with saturated Piramal Healthcare isoflurane (Clipper Distributing Company, St Joseph, MO), decapitated and the brains removed. The neocortex or hippocampus was dissected in freshly oxygenated artificial cerebrospinal fluid and processed immediately to generate synaptosomal and subsynaptic fractions (Fig. 2B). Tissue from multiple male and female mice (P7 cortex = 14; P7 hippocampus = 24; P14 and P21 cortex = 5; P14 and P21 hippocampus = 10) was pooled to yield 450–600mg (P7) or 250 – 350 mg (P14, P21) wet weight starting material. Pooled tissue was homogenized with a glass homogenizer (Wheaton, Millville, NJ) in 320mM sucrose, 0.1mM CaCl2, 1mM MgCl2 containing a protease inhibitor cocktail. The sucrose concentration of the homogenate was adjusted to 1.25M, overlaid with 1M sucrose, and centrifuged at 100,000 g. The synaptosome fraction, located at the sucrose interface, was recovered and washed twice in 0.1mM CaCl2, and a sample taken for analysis. The remaining synaptosomes were pelleted by centrifugation at 40,000 g. The pellet was solubilized by incubation in 20mM Tris-HCl, pH 6.0, 1% Triton-X 100, 0.1mM CaCl2, and then centrifuged at 40,000 g. The resulting supernatant is considered the synaptic vesicle fraction, whereas the pellet consists of pre- and post-synaptic membranes. The pellet was solubilized in 20mM Tris-HCl, pH 8.0, 1% Triton-X 100, 0.1mM CaCl2, and then centrifuged at 40,000 g. The supernatant contains presynaptic membranes, and the pellet contains the postsynaptic density fraction. The synaptic vesicle and presynaptic membrane fractions were acetone precipitated. The precipitates and the pellet containing the postsynaptic density fraction were resuspended in 5% sodium dodecyl sulfate (SDS). The protein concentration of each fraction (synaptosome, synaptic vesicle, presynaptic and postsynaptic density) was determined using the Dc protein assay (Bio-Rad, Hercules, CA).
Western blot analysis, using previously published protocols (Eagleson et al., 2011; Judson et al., 2009), was used to assess the distribution of Met in select subcellular compartments and the efficacy of the fractionation procedure. A sample of each fraction from the same region at the same age, as well as Precision Plus Protein Standards (Bio-Rad), was run on a single gel. Equal amounts of protein (30µg) were separated by SDS-polyacrylamide gel electrophoresis (7.5% gel) under reducing conditions and transferred overnight to a supported nitrocellulose membrane (Whatman Optitran BA-S85, Sigma). The membrane was stained for total protein with the Pierce Reversible Protein Stain Kit for Nitrocellulose Membranes (Pierce) to verify equal lane loading. Each membrane was probed sequentially with antibodies directed against Met (1:500), syntaxin-1 (1:2500), synaptophysin (1:1,000,000), and PSD-95 (1:2000), all diluted in blocking buffer - 5% blotto (nonfat dry milk, Cell Signaling Technology, Beverly, MA) in Tris buffered saline, 0.05% Tween-20 (TBST). Specifically, membranes were blocked in blocking buffer for 2 hours at room temperature, followed by incubation with primary antibody overnight at 4°C. Membranes were rinsed in TBST and incubated in a horseradish peroxidase-conjugated anti-mouse secondary antibody (1:5000 in blocking buffer, Jackson Immunoresearch, West Grove, PA) for one hour at room temperature. After rinsing in TBST, membranes were reacted with either Pierce SuperSignal West Dura Extended Duration Substrate (PSD-95, synpatophysin and syntaxin 1, Thermo Scientific, Rockford, IL) or Pierce SuperSignal West Femto Maximum Sensitivity Substrate (Met, Thermo Scientific) to visualize immunoreactive bands. Membranes were stripped between each primary antibody using OneMinute Western Blot Stripping Buffer (GM Biosciences, Rockville, MD).
Digital images of the Western blots were acquired and analyzed using a CCD camera coupled to a UVP BioImaging System using VisionWorksLS Image Acquisition and Analysis Software (v7.0.1, UVP, Upland, CA). The density of each band was measured, a global background subtraction applied, and the resulting values normalized for total protein loading (Brown et al., 2005; Gustin et al., 2010) based on the Pierce Reversible Protein Stain.
Digital illustrations
Images of tissue processed for immuno-EM were acquired using Advanced Microscopy Techniques software (v3.2, Advanced Microscopy Techniques). Images of primary cultures processed using the PLA method were acquired using an Olympus Fluoview FV1000 confocal microscope (60× oil object). Images of the Western blots were acquired using a CCD camera coupled to a UVP BioImaging System using UVP Imager software (v7.0.1, UVP, Upland, CA). Graphs were prepared in SigmaPlot (v7.0, SPSS Incorporated, Chicago, IL). Images and graphs were converted to TIFF format and imported into Adobe Photoshop CS5 (v12.1, Adobe Systems Incorporated, San Jose, CA), where they were cropped, adjusted for brightness and contrast, and sharpened for preparation of final figures.
RESULTS
Previous light microscopic (LM) studies revealed intense immunolabeling for Met in the neuropil of the early postnatal mouse neocortex and hippocampus (Judson et al., 2009). In the hippocampus, Met immunoreactivity (-ir) is mostly found in the CA1 subfield, with a gradient of most to least intense from stratum moleculare to the stratum pyramidale. In the somatosensory cortex, layers 2–3 and 5 are the most intensely labeled, with less labeling of layer 6 and no labeling of layer 4. At the LM level, it is not possible to resolve the specific cellular profiles that contain Met immunoreactivity. To address this, we examined the distribution of Met protein within developing neuropil at P7, P14 and P21 using complementary ultrastructural and biochemical approaches.
In general, Met-ir was limited to synaptic profiles (pre- and post), axons and glial processes. Vascular elements were unlabeled at all ages. The distribution of Met-ir in the pre- and postsynaptic elements and glia changed over development in both regions.
The distribution of Met-ir in hippocampal CA1 changes across development
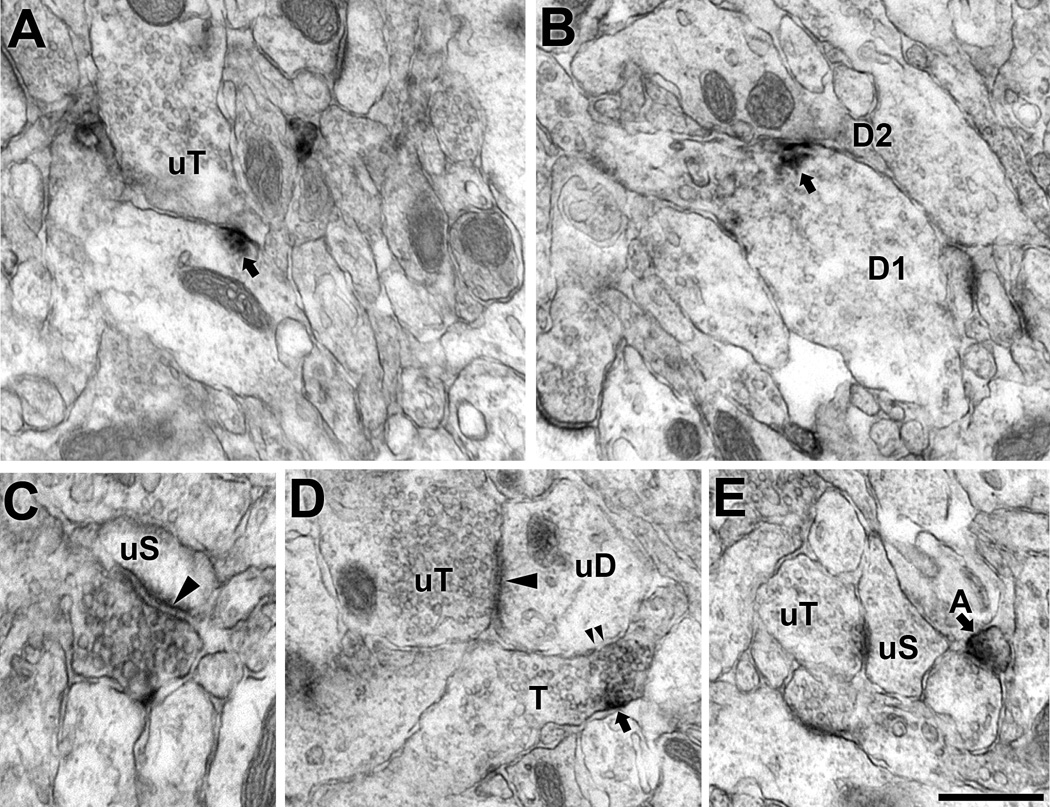
Regardless of age, Met-ir is present in dendrites, dendritic spines, axons, terminals and glia in the CA1 stratum radiatum in the dorsal hippocampus. In dendritic shafts, Met-ir is patchy and often associated with the plasma membrane or endomembrane structures in the cytoplasm (Fig. 3A). Occasionally, Met-ir is on the plasma membrane of dendritic profiles in the region in which they contact each other (Fig. 3B). In dendritic spines, Met-ir is distributed consistently in the head near the post-synaptic junction (Fig. 3C). Intermittent, narrow patches of Met-immunoreactive membranes also are detected in small unmyelinated axons (Fig. 3D and F); however, no Met-labeling was seen in unmyelinated axons. Axon terminals immunoreactive for Met contain numerous small synaptic vesicles; at all ages, the majority of Met-labeled terminals are less than 0.5 µm in diameter (Table 2; Fig. 3D). However, some terminals, especially at P7, have minimum diameters as large as 0.85 µm (Table 2; Fig. 3E). Within the large terminals, peroxidase reaction product is associated with clusters of vesicles near the synaptic specialization (Fig. 3E). There is limited glial cell immunolabeling, with these profiles usually conforming to the boundaries of the neuropil and lacked astrocytic fibrils (Fig. 3G).
Figure 3. Electron micrographs of Met-ir in pre- and post-synaptic profiles in CA1 stratum radiatum of the dorsal hippocampus.
A. Met-ir is found near the plasma membrane (top arrow) and endomembrane (em) in the cytoplasm of a dendrite which receives a contact (arrowhead) from an unlabeled terminal (uT). B. In this dendritic profile, Met-ir is on the region of the plasma membrane (arrow) that apposes an unlabeled dendritic profile (uD). The Met-labeled dendrite is likely a pyramidal cell as it contains a spine contacted (arrowhead) by an unlabeled terminal (uT). C. Met-ir is located in the head of a spine emanating for a dendritic shaft (D); the spine receives an asymmetric synapse (arrowhead) from an unlabeled terminal (uT). D. Met-ir is present in a small terminal (T) that forms a symmetric synapse (arrowhead) on an unlabeled dendrite (uD). A Met-labeled unmyelinated axon (Ax) also is adjacent to the unlabeled dendrite. E. A large Met-ir terminal (T) forms a synapse on an unlabeled spine (uS). The reaction product associated with clusters of vesicles near the synaptic specialization F. A longitudinal section of an axon, identified by the presence of small synaptic vesicles (ssv, example arrowhead), contains a cluster of Met-ir. G. Met-ir glial profile conforms to the boundaries of the neuropil. Bar = 500nm.
Table 2.
Size of Met-labeled terminals
| Age/region | N | Area (µm2) |
Minor axis (µm) |
Minor axis range (µm) |
|---|---|---|---|---|
| Hippocampus | ||||
| P7 | 39 | 0.27 ± 0.02 | 0.43 ± 0.17 | 0.17 – 0.85 |
| P14 | 32 | 0.22 ± 0.02 | 0.38 ± 0.08 | 0.22 – 0.56 |
| P21 | 34 | 0.18 ± 0.01 | 0.37 ± 0.07 | 0.24 – 0.59 |
| Cortex | ||||
| P7 | 81 | 0.24 ± 0.01 | 0.41 ± 0.12 | 0.23 – 0.96 |
| P14 | 44 | 0.24 ± 0.02 | 0.42 ± 0.10 | 0.25 – 0.66 |
| P21 | 16 | 0.24 ± 0.02 | 0.43 ± 0.10 | 0.28 – 0.58 |
Includes only terminals in which entire perimeters could be distinguished.
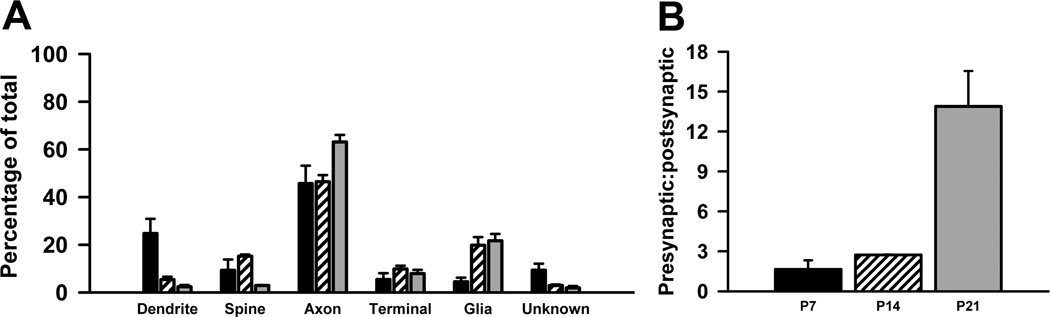
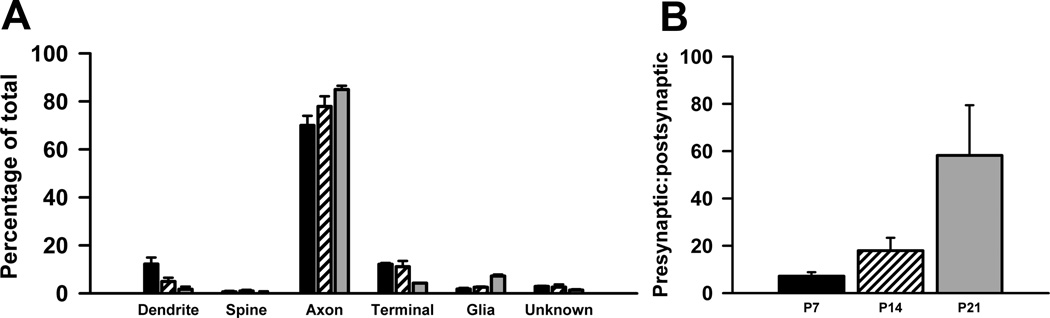
Quantitative analysis revealed that the distribution of Met-ir across different subcellular elements was significantly different across postnatal development (Table 3; Fig. 4A). At P7, postsynaptic profiles (i.e., dendrites and dendritic spines) account for approximately 1 in 3 labeled profiles, but their number decline such that they represent 1 in 5 at P14 and only 1 in 20 at P21 (Fig. 4A). In contrast, the percent of presynaptic profiles is relatively similar between the three ages. Specifically, axons are the most frequently labeled profile at all ages, ranging from 45% at P7 and P14 to 63% at P21 (Fig. 4A). Moreover, the proportion of labeled terminals remains constant over the three ages (Fig. 4A). As a consequence of the changes in the type of Met-ir profiles over the first three weeks postnatal, the ratio of pre- to post-synaptic profiles changes from near equivalence at P7 and P14 to predominantly presynaptic at P21 (Fig. 4B).
Table 2.
The average number of Met immunoreactive profiles/animal (N=3 for each age)
| Age | Dendrites | Spines | Axons | Terminals | Glia | Unknown |
|---|---|---|---|---|---|---|
| Hippocampus | ||||||
| P7 | 26.67 ±0.88 | 9.67 ±4.70 | 62.33 ±26.19 | 8.00 ±5.57 | 6.67 ±4.18 | 10.33 ±2.40 |
| P14 | 6.33 ±2.03 | 17.00 ±2.08 | 53.00 ±9.54 | 11.00 ±2.00 | 21.67 ±2.03 | 3.33 ±0.88 |
| P21 | 3.33 ±0.67 | 4.33 ±0.88 | 91.00 ±11.14 | 11.67 ±2.96 | 32.00 ±8.00 | 3.00 ±1.53 |
| Cortex | ||||||
| P7 | 28.00 ±1.00 | 1.33 ±0.67 | 182.00±48.52 | 30.67 ±7.26 | 4.00 ±0.00 | 7.00 ±1.15 |
| P14 | 6.67 ±1.76 | 1.67 ±0.88 | 121.00±31.07 | 15.67 ±1.33 | 3.67 ±0.33 | 3.33 ±0.88 |
| P21 | 2.33 ±1.45 | 0.67 ±0.33 | 106.33 ±8.74 | 5.33 ±0.67 | 9.00 ±1.00 | 1.67 ±0.33 |
Figure 4. Quantitative comparison Met-ir in identified profiles in the neuropil of the stratum radiatum of CA1 in dorsal hippocampus.
A. At P7 (black bars), P14 (striped bars) and P21 (grey bars) significant differences in the distribution of Met-labeled profiles were revealed across the three ages (P7 versus P14, p < 0.001, d.f. = 4, χ2; P7 versus P21, p < 0.001, d.f. = 4, χ2; P14 versus P21, p < 0.001, d.f. = 4, χ2) B. Graph illustrating the increase in the proportion of presynaptic to postsynaptic profiles from near equivalence at P7 and P14 to predominantly presynaptic at P21. Error bars represent standard error of the mean, N = 3 in each group.
At all ages, the majority of Met-immunoreactive terminals do not form a synaptic specialization in a single plane of section (Table 4). Of the terminals that do form synaptic specializations, the targets and types of synapse formed by Met-immunoreactive terminals varies depending on age (Table 2). At P7, most Met-labeled terminals form symmetric synapses on dendritic shafts. However, by P14 as well as P21, few Met-immunoreactive terminals synapse on dendrites. Instead, most of the Met-labeled terminals form asymmetric synapses on dendritic spines. At all three ages the number of terminals containing Met-ir is relatively constant.
Table 3.
Distribution of Met-labeled terminals
| Age/region | Dendrites | Spines | No Apps | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Asym | Sym | App | Asym | Sym | App | |||
| Hippocampus | ||||||||
| P7 | 8 | 11 | 1 | 19 | 39 | |||
| P14 | 2 | 10 | 1 | 5 | 14 | 32 | ||
| P21 | 1 | 1 | 3 | 12 | 1 | 16 | 34 | |
| Cortex | ||||||||
| P7 | 1 | 8 | 23 | 2 | 2 | 43 | 79 | |
| P14 | 1 | 7 | 14 | 3 | 21 | 46 | ||
| P21 | 9 | 5 | 14 | |||||
Asym: asymmetrical, Sym: symmetrical, App: apposition, No Apps: no apposition
In addition to neuronal elements, an increasing number of Met-immunoreactive glial profiles are found in the neuropil as development proceeds. At P7, glial cells represent only 3% Met-labeled profiles (Fig. 4A). At later ages, however, as many as 1 in 5 Met-immunoreactive profiles are glial (Fig. 4A).
At all developmental ages, Met-ir is predominantly located in presynaptic profiles in the somatosensory cortex
Like CA1 of the hippocampus, Met-immunoreactive profiles in superficial layer V of somatosensory cortex are found in pre- and postsynaptic profiles as well as glia (Fig. 5). Within dendritic shafts, Met-ir is found near the plasma membrane sometimes affiliated with the perisynaptic zone (Fig. 5A) or adjacent to the region contacted by another dendrite (Fig. 5B). Met-ir is located in small terminals that synapse on unlabeled dendritic spines (Fig. 5C) or in clusters of vesicles in terminals that appose terminal-spine synaptic complexes (Fig. 5D). As seen in the hippocampus, the majority of Met-labeled terminals in the cortex were less than 0.5 µm in diameter (Table 2). As noted for the hippocampus, Met-ir also labels limited membrane domains of small unmyelinated axons (Fig. 5E and F).
Figure 5. Electron micrographs illustrate Met-ir in pre- and post-synaptic profiles in the neuropil of superficial layer V in somatosensory cortex.
A. In this dendritic shaft, Met-ir is localized to the perisynaptic zone of an unlabeled terminal (uT) forming a symmetric synapse (arrowhead). B. A patch of Met-ir is found on the plasma membrane region of a dendrite (D1) that contacts another dendrite (D2). C. A Met-labeled terminal forms an asymmetric synapse (arrowhead) on a dendritic spine. D. A terminal with Met-ir affiliated with a cluster of synaptic vesicle (arrow) abuts (double arrowheads) an unlabeled dendritic (uD) that is also contacted (arrowhead) by an unlabeled terminal (uT). E. Met-ir is found in an unmyelinated axon (Ax) near a synaptic complex formed by an unlabeled terminal (uT) and unlabeled spine (uS). F. A longitudinal section of an axon, identified by the presence of small synaptic vesicles (ssv, example arrowhead), contains two patches of Met-ir. Bar = 500 nm in A (applies to A, B) and in C (applies to C–G).
The distribution of Met-ir across different types of profiles is overlapping, but not identical, between the two regions at all ages examined (Table 3; Fig. 6A; P7 hippocampus versus neocortex, p < 0.001, d.f. = 4, χ2; P14 hippocampus versus neocortex, p < 0.001, d.f. = 4, χ2; P21 hippocampus versus neocortex, p < 0.001, d.f. = 4, χ2). Indeed, Met-labeled presynaptic profiles already predominate in superficial layer V of neocortex at P7 (Fig. 6B), primarily due to an increased number of Met-labeled axons and the very limited staining of dendritic spines. It should be noted that even unlabeled spines are rare in this layer at P7, consistent with the onset of robust spinogenesis in the rodent neocortex during the second postnatal week (Meller et al., 1968; Miller and Peters, 1981). Nonetheless, even at P14 and P21, when spine number increases dramatically, Met-labeled spines are observed rarely, while the number of Met-labeled axons remains high. Thus, at all ages, axons represent the most frequently labeled profile, ranging from 70% at P7 to 85% at P21 (Fig. 6A), whereas spines represent the least (Fig. 6A, less than 1% at all ages). The proportion of Met-labeled dendrites and terminals are higher at P7 and P14 than at P21 (Fig. 6A). In contrast, Met-labeled glial profiles are uncommon during the first two postnatal weeks (Fig. 6A), but increase in number during the third postnatal week (Fig. 6A), although not to the same extent as seen in the hippocampus.
Figure 6. Quantitative comparison of Met-ir in identified profiles in the neuropil of superficial layer V in somatosensory cortex.
A. At P7 (black bars), P14 (striped bars) and P21 (grey bars) significant differences in the distribution of Met-labeled profiles were seen across the three ages (P7 versus P14, p < 0.001, d.f. = 4, χ2; P7 versus P21, p < 0.001, d.f. = 4, χ2; P14 versus P21, p < 0.001, d.f. = 4, χ2). B. Graph illustrating the increase in the proportion of presynaptic to postsynaptic profiles across development. Error bars represent standard error of the mean, N = 3 in each group.
Like the hippocampus, the majority of Met-labeled terminals do not form a synaptic specialization in a single plane of section (Table 4). Of the terminals that do form synaptic specializations, the targets and types of synapses formed by Met-labeled terminals vary depending on age (Table 4). At P7, most Met-labeled terminals form symmetric synapses on dendritic shafts. However, few to no Met-labeled terminals synapse on dendritic shafts at P14 and P21, respectively. As seen in the hippocampus, most of the Met-immunoreactive terminals form asymmetric synapses on dendritic spines at P14 and P21. Unlike the hippocampus, the number of terminals containing Met-ir greatly diminishes from P7 to P21.
PLA localizes Met presynaptically in growing neurons in vitro
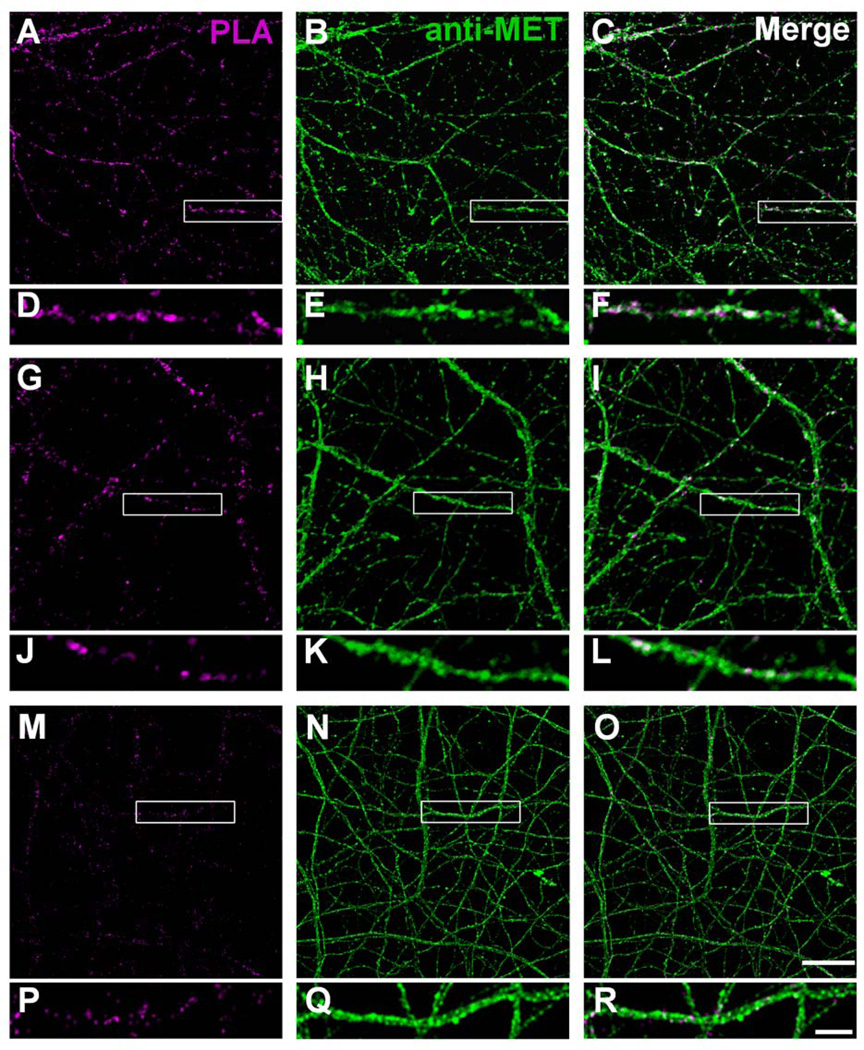
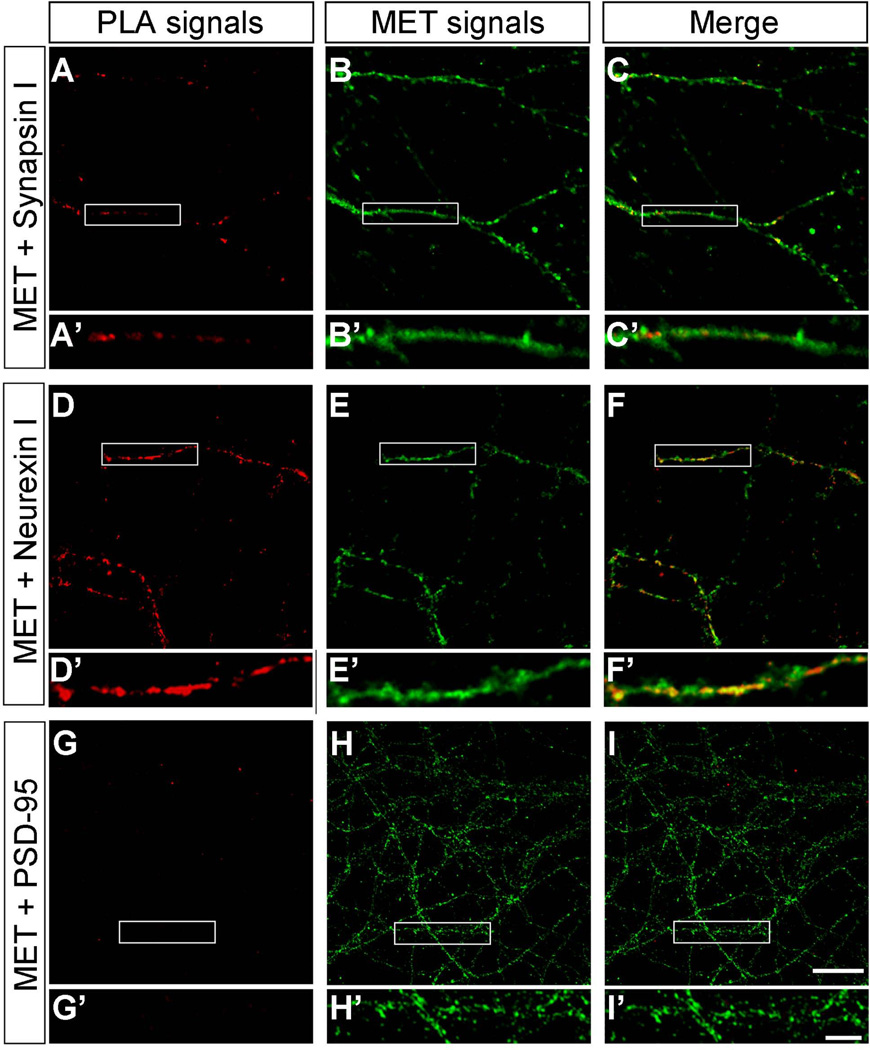
PLA provides a refined approach at the light microscope level to determine co-localization of Met with other proteins that are located in subsynaptic compartments. A fluorescent signal is only observed when the PLA probes used to detect the two proteins are within 40nm (Soderberg et al., 2006). In primary cultures of hippocampal (Fig. 7) and neocortical (Fig. 8) neurons after 14 days in vitro, there was extensive Met immunostaining of neuronal processes (anti-Met panels in Figs. 7 and 8). Co-localization of pre- and postsynaptic markers with Met was evident from strong PLA fluorescent signals in the presence of antibodies directed against Met and synapsin 1 (Figs. 7A–F, 8A–F) or Met and neurexin 1 (Figs. 7G–L, 8G–L), indicating the localization of Met in the presynaptic compartment. PLA using antibodies directed against Met and the postsynaptic marker PSD-95 revealed positive signals in hippocampal cultures (Fig. 7M–R). In contrast, no PLA signals using antibodies against Met and PSD-95 could be detected in neocortical cultures (Fig. 8M–R). Quantification of the PLA signal confirmed these observations (Fig. 9). Co-localization of Met differed significantly across the three markers in hippocampal [F(2,6) = 6.09, P = 0.04] and neocortical [F(2,6) = 9.58, P = 0.01] cultures. In the hippocampus, Tukey’s post-hoc comparisons of the three markers demonstrate that there is significantly more PLA signal for synapsin 1/Met than for PSD-95/Met at P < 0.05. A similar analysis for neocortex demonstrates significantly more PLA signal for synapsin 1/Met and for neurexin 1/Met than for PSD-95/Met at P < 0.05. Together, these data demonstrate a significant presynaptic localization of Met in vitro that has not been previously appreciated.
Figure 7. Representative confocal microscopy images of PLA staining in primary hippocampal neurons at 14 days in vitro.
The presynaptic compartment was labeled with synapsin 1 and neurexin 1 and the postsynaptic compartment with PSD-95. Magenta fluorescent profiles represent regions of PLA signal amplification denoting Met and synapsin 1 (A, D), Met and neurexin 1 (G, J), and Met and PSD-95 (M, P) co-localization. No PLA signal is observed in the presence of Met antibody alone (S, V). For comparison, corresponding total Met immunoreactivity in the same field is also illustrated (B, E; H, K; N, Q; T, W). The boxed regions are shown at higher magnification below each image. Bar = 20µm in U (applies to A–C, G–I, M–O, S–U), 5µm in X (applies to D–F, J–L, P–R, V–X).
Figure 8. Representative confocal microscopy images of PLA staining in primary neocortical neurons at 14 days in vitro.
The presynaptic compartment was labeled with synapsin 1 and neurexin 1 and the postsynaptic compartment with PSD-95. Magenta fluorescent profiles represent regions of PLA signal amplification denoting Met and synapsin 1 (A, D) and Met and neurexin 1 (G, J) co-localization. No signal was detected for Met and PSD-95 (M, P). No PLA signal is observed in the presence of Met antibody alone (S, V). For comparison, corresponding total Met immunoreactivity in the same field is also illustrated (B, E; H, K; N, Q; T, W). The boxed regions are shown at higher magnification below each image. Bar = 20µm in U (applies to A–C, G–I, M–O, S–U), 5µm in X (applies to D–F, J–L, P–R, V–X).
Figure 9. Quantitative analysis of PLA signals in primary hippocampal and neocortical cultures at 14 days in vitro.
PLA signal reflects co-localization of Met with the presynaptic markers synapsin 1 (Syn 1) and neurexin 1 (Nxn 1) or the postsynaptic marker PSD-95 in hippocampus (A) and neocortex (B). Error bars represent standard error of the mean, N = 3 independent culturing sessions in each group. * significantly different from Syn 1; ** significantly different from Syn 1 and Nxn 1.
As a negative control, no fluorescent signals could be detected in the presence of anti-Met (Fig. 7S–X, 8S–X), anti-synapsin 1, anti-neurexin 1 or anti-PSD-95 antibodies when used individually. As would be expected, strong fluorescent PLA signals could be detected in the presence of synapsin 1 and neurexin 1 antibodies in hippocampal and neocortical cultures, with no PLA signal detected in the presence of neurexin 1 and PSD-95 or synapsin 1 and PSD-95 antibodies in neocortical cultures (data not shown). In hippocampal cultures, however, occasional faint PLA signals could be observed in the presence of neurexin 1 and PSD-95 or synapsin 1 and PSD-95 antibodies (data not shown).
Biochemical distribution of Met in the pre- and post-synaptic compartments of the synapse
The immuno-EM and PLA analyses indicate that, in addition to its presence at extra-synaptic sites, such as axons, a significant proportion of Met-ir is associated with the developing synapse. Throughout the period of synaptogenesis in vivo, Met-ir is present specifically in axon terminals, spines and proximate to postsynaptic densities. A biochemical fractionation approach was used to expand the analysis of the localization of Met at the synapse and to determine, with a method that is not dependent upon tissue fixation methods, the relative distribution of the receptor across 1) presynaptic membranes associated with the active zones, 2) the postsynaptic density, and 3) synaptic vesicles and presynaptic membranes within the terminal but outside the active zones.
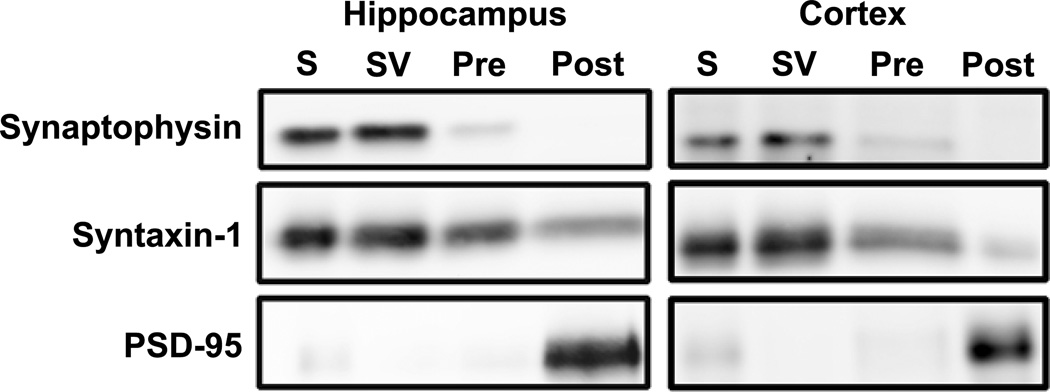
We first evaluated the efficacy of the fractionation method, using specific markers for different components of the synapse and found minor cross-contamination between the fractions (Fig. 10), consistent with previous reports (Anastasio et al., 2010; Bouvier et al., 2008; Lee et al., 2008; Louneva et al., 2008; Needleman et al., 2010; Phillips et al., 2001; Rebola et al., 2005; Rebola et al., 2003). In general, the signal for the synaptic vesicle membrane protein, synaptophysin, is enriched in the synaptic vesicle fraction. It should be noted, however, that this fraction likely also includes intracellular membranes from other intracellular organelles as well as presynaptic plasma membrane located outside the active zone. Less signal is found in the presynaptic active zone fraction (Fig. 10), and likely represents vesicles that had been docked at or fused with the active zone at the time of tissue harvest (Tao-Cheng, 2006). Little to no synaptophysin signal is detected in the postsynaptic fraction. As expected, syntaxin-1 is present in both presynaptic fractions (Fig. 10). Syntaxin-1, however, also is detected in the postsynaptic fraction, although at much lower levels. This indicates the presence of some presynaptic membranes in this fraction. The postsynaptic density protein PSD-95 is almost exclusively located in the postsynaptic fraction (Fig. 10), indicating little cross-contamination of postsynaptic membranes in either of the presynaptic fractions.
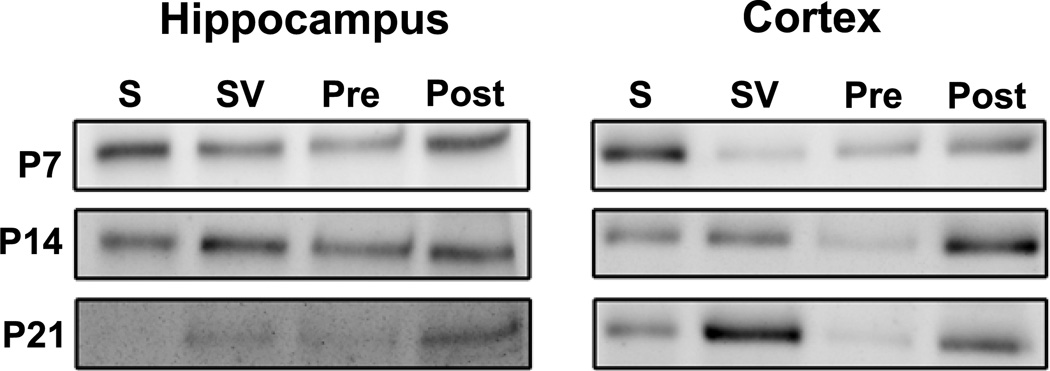
Figure 10. Western blots illustrating efficiency of isolating different synaptic fractionations in tissue prepared from P14 hippocampus and cortex.
Similar results were obtained at P7 and P21. Lanes were loaded with equal amounts of protein from synaptosome (S), synaptic vesicle (SV), presynaptic (PRE) and postsynaptic density (POST) fractions. Blots were probed sequentially with antibodies to the compartmentalized synaptic proteins synaptophysin (synaptic vesicles), syntaxin-1(presynaptic plasma membrane) and PSD-95 (postsynaptic density).
Met-ir is present in synaptic fractions generated from hippocampus and cortex at all ages examined (Fig. 11), consistent with an association of Met-ir with the synapse. Previous studies have emphasized the postsynaptic localization of Met in hippocampal synapses (Kawas et al., 2012; Tyndall and Walikonis, 2006). In contrast, we observed Met-ir associated with the presynaptic nerve terminal (synaptic vesicle and presynaptic membranes, including the active zone) fractions as well as the postsynaptic density fraction in the hippocampus at P7 and P14 (Fig. 11). By P21, although Met-ir is not visible in the synaptosomal fraction itself, Met-ir still is associated with both pre- and post-synaptic fractions. The localization of Met to cortical synapses has not been examined previously. Similar to the hippocampus, Met-ir can be observed in pre- and post-synaptic fractions across all stages of development (Fig. 11). These data highlight a significant distribution of Met protein to presynaptic nerve terminals during early postnatal development.
Figure 11. Relative distribution of Met in synaptosomes and subsynaptic fractions prepared from P7, P14 and P21 hippocampus and neocortex.
Similar results were observed in two independently derived preparations for each region at each age. Lanes were loaded with equal amounts of protein from synaptosome (S), synaptic vesicle (SV), presynaptic (PRE) and postsynaptic (POST) fractions. Note that different exposure times were used for each region at each age, so band intensities should only be compared within each blot, and not across different regions and ages.
DISCUSSION
The present findings highlight the localization of Met-ir in developing synaptic elements. These results provide a potential path for the morphological (Judson et al., 2010), electrophysiological (Qiu et al., 2011) and functional neuroimaging (Rudie et al., 2012) data that converge on altered Met expression during development leading to disturbances in connectivity. Use of complementary immuno-EM, PLA and biochemical fractionation approaches reveals the distribution of the Met receptor within select regions of the neuropil of the mouse hippocampus and neocortex during peak periods of synaptogenesis. At the ultrastructural level, Met-ir is observed mostly in neuronal processes, with axons representing the most numerously labeled profile. The relative distribution of Met-ir across different profiles, however, differs between the two regions and across development within a region. Using PLA and biochemical fractionation approaches, we confirmed the localization of Met at the synapse. Given its distribution, it is likely that both cell-autonomous (pre-) and non-cell autonomous (post-synaptic) mechanisms may underlie the modulation of synapse development by Met, a conclusion born out by previous morphological studies in the mouse neocortex and striatum of a dorsal pallium-specific conditional deletion of Met (Judson et al., 2010).
Regional and temporal variation in subcellular distribution of Met
Presynaptic (axons and terminals) and postsynaptic (dendrites and spines) neuronal elements are Met-immunoreactive, although the relative abundance varies between regions and postnatal ages that were examined. We note these differences with caution, as such variations may be dependent upon the specific neocortical layers and hippocampal subfields analyzed. In general, the analyses revealed that axons are the most abundant Met-labeled profile, consistent with previous light microscopic observations indicating that, during early periods of active axonal growth and synapse development, Met-ir is preferentially, although not exclusively, trafficked to the axonal compartment in restricted fiber tracts in the mouse and monkey forebrain (Judson et al., 2011a; Judson et al., 2009). This represents in part the transport of Met receptors to presynaptic terminals, but may also include positioning of Met in axonal membranes to influence other developmental processes, such as modulating the extent of axonal branching in terminal fields. It is unlikely that Met is involved in axon pathfinding, because in the absence of Met signaling, major forebrain fiber tracts still develop normally (Judson et al., 2010). We did not, however, examine terminal fields for possible arborization differences.
Based on the EM analyses, only a subpopulation of axons and terminals are Met-immunoreactive and we currently cannot identify the specific neuronal populations that give rise to the immunolabeled profiles. Axons and terminals present in the stratum radiatum include Schaffer collaterals of CA3 pyramidal cells, local GABAergic interneurons, as well as projections from the medial septum, ventral tegmental area, raphe and locus ceruleus (Amaral and Witter, 1989; Colom et al., 2005; Jones and Moore, 1977; Klausberger, 2009; Moore and Halaris, 1975; Swanson, 1982). Within superficial layer V of somatosensory cortex, axons and terminals arising from pyramidal neurons in the same region as well other cortical areas and local GABAergic interneurons are included. It is unlikely, however, that GABAergic neurons contribute to the Met-ir axons and terminals in either region, as cells arising from the ganglionic eminences do not express Met during normal development (Eagleson et al., 2011; Judson et al., 2009). Moreover, double label in situ hybridization in neocortex revealed excitatory neurons express Met, but GABAergic neurons do not (Eagleson et al., 2011). Similarly, pyramidal neurons located in the principal cell layer of CA1 or in infragranular layers of somatosensory cortex (Judson et al., 2009), rather than local interneurons, are the likely source of Met-immunoreactive dendrites and spines we observe in stratum radiatum and superficial layer V, respectively.
The development of Met-labeled terminals differs between the neocortex and hippocampus. Most notably, while the number of labeled terminals remains constant in the hippocampus between P7 and P21, there is a rapid and pronounced decline in Met-ir terminals in the neocortex over the same time period. Intriguingly, in both hippocampus and cortex, the majority of Met-labeled terminals do not form a synaptic specialization. Indeed, in both regions across all ages examined, about half the Met-labeled terminals are not found in close apposition to any other neuronal element. At P14 and P21, asymmetric synapses on dendritic spines represent the majority of synapses formed by Met-ir terminals in both hippocampus and neocortex. This is consistent with excitatory, rather than inhibitory, neurons contributing to Met-ir axons and terminals, as discussed above. In contrast, at P7, symmetric synapses on dendritic shafts predominate among Met-labeled terminals forming synaptic contacts. Although, in adults, such a morphology is typically associated with inhibitory synapses, it has been suggested that one of the first steps in spine formation in cortical pyramidal cells involves the formation of a symmetric contact between an axon and dendritic shaft that subsequently matures into an asymmetric synapse between a terminal and dendritic spine (Miller and Peters, 1981). Thus, the symmetric synapses formed by Met-ir terminals at P7 may represent the first stages in the maturation process of excitatory synapses. This would be consistent with the lack of co-localization of Met transcript with markers of inhibitory neurons in the developing neocortex (Eagleson et al., 2011).
The relative contribution of glial cells to Met-immunoreactive profiles within the neuropil varies across development and between regions. Few Met-labeled glial processes are present at the end of the first postnatal week. After this time, there is a marked increase in the proportion of Met-immunoreactive glial profiles in the hippocampus, but only a modest increase in the neocortex. Currently we cannot distinguish the specific type of glial cell associated with Met-ir in either region; however, the absence of fibrils suggests that they are not fibrous astrocytes. Previous analyses, using double-label in situ hybridization, indicated an absence of Met expression in astroglia and oligodendroglia in the P14 mouse somatosensory cortex, although specific microglial markers were not examined (Eagleson et al., 2011). Thus, the Met-ir observed in the current study may be associated microglial cells, an intriguing possibility as these cells have been implicated in synapse elimination and refinement during normal development (Hoshiko et al., 2012; Paolicelli et al., 2011; Schafer et al., 2012; Tremblay et al., 2010). Met expression also has been reported in astrocytes and oligodendrocytes, although usually either in vitro or in the context of an injury response (Kitamura et al., 2007; Lalive et al., 2005; Machide et al., 2000; Nagayama et al., 2004; Shimamura et al., 2007; Shimazaki et al., 2003; Yan and Rivkees, 2002). Future studies will aim to clarify the identity of Met-labeled glial processes.
We focused our EM analyses on the developing male neocortex and hippocampus, because in general, in vivo and electrophysiological analyses of Met expression and function only include males (Akimoto et al., 2004; Judson et al., 2009; Judson et al., 2010; Qiu et al., 2011). In vitro studies typically utilized primary cultures that include a mixed population of neurons from male and female embryos (Akimoto et al., 2004; Akita et al., 2008; Finsterwald and Martin, 2011; Lim and Walikonis, 2008; Nakano et al., 2007; Tyndall et al., 2007; Tyndall and Walikonis, 2006; 2007). However, sex differences in neuronal architecture, plasticity and circuitry, primarily arising from the influence of gonadal hormones, have been reported in the hippocampus (for example (Bian et al., 2012; Bowers et al., 2010; Cooke and Woolley, 2005; Fester et al., 2012; Gould et al., 1991; Isgor and Sengelaub, 2003; Lebron-Milad and Milad, 2012; Mitsushima et al., 2009; Romeo et al., 2004) and neocortex, including the somatosensory region (Dawson et al., 2009; Kritzer, 1998; Tobet et al., 1993; Urban-Ciecko and Mozrzymas, 2011). Thus, it is reasonable to hypothesize that there are sex differences in the expression and function of proteins involved in synapse and circuit formation, such as Met. Extensive age and sex-based analyses will address this hypothesis in future studies.
Met at the synapse
The demonstration of Met-ir in axon terminals and dendritic profiles at the ultrastructural level is consistent with the biochemical localization of Met in synaptosomes and across both pre- and post-synaptic compartments. We note there are technical considerations when comparing quantitative data across different methodological platforms that each has their own strengths and limitations. First, the ultrastructural analyses focused on discrete subregions of the hippocampus and neocortex, providing precise subcellular information on Met localization in the context of regional circuitry. In contrast, the entire neocortex and hippocampus from multiple mice were required to have sufficient starting tissue to generate biochemical subsynaptic fractions. This method is not impacted by factors such as antibody penetration and fixation, but does not provide anatomical information. It is likely that, using the biochemical fractionation method, at least some of the Met detected in the postsynaptic fractions may arise from contamination of this fraction with presynaptic proteins, as indicated by the presence of syntaxin 1 in this fraction. For the primary cell culture studies, embryonic neurons intrinsic to the neocortex or hippocampus were grown, but this excludes presynaptic contributions from subcortical sources. Finally, using the PLA method, there was no indication of postsynaptic localization in vitro in the neocortex; postsynaptic labeling in the hippocampus, however, was observed, which is consistent with previous reports (Kawas et al., 2012; Tyndall and Walikonis, 2006). We interpret the absence of postsynaptic labeling in cultures of neocortical neurons cautiously. The data clearly demonstrate that Met is located presynaptically in vitro, but the absence of postsynaptic localization may reflect a culture-specific phenomenon of a difference in synaptic maturation in vitro, or the possibility that Met distribution in neurons growing in vitro is not spatially associated with PSD-95, which is required for PLA to generate a signal. It is important to emphasize, however, that despite these caveats, the goal of the present study was to determine, with multiple methodologies, the presence of Met at synapses during peak periods of synaptogenesis in the neocortex and hippocampus. Each of the three different techniques provides evidence of a previously unappreciated presence of Met in presynaptic axon terminals.
TrkB, another member of the receptor tyrosine kinase family, also is present at pre- and postsynaptic sites in the hippocampus, although the majority of phosphorylated TrkB is localized to presynaptic profiles (Spencer-Segal et al., 2011). Thus, it will be important to determine if there is a similar differential profile of activated phospho- and total Met in the neocortex and hippocampus. Perhaps more intriguingly, the relative distribution of Met-ir is dependent on the stage of development and the brain region examined. Similar ultrastructural and biochemical analyses have been used to elucidate the subsynaptic localization of a variety of receptors and MHC class I proteins (Bouvier et al., 2008; Hettinger et al., 2001; Muly et al., 2007; Needleman et al., 2010; Rebola et al., 2005; Rebola et al., 2003). Several comparative examples are worth highlighting. Similar to our findings with Met, MHC I proteins are distributed pre- and postsynaptically, but appear to exhibit a more balanced distribution across synaptic compartments and do not show the dramatic reduction in expression during maturation (Needleman et al., 2010) that is seen with Met (Judson et al., 2009). In the adult, the subsynaptic localization of group II metabotropic glutamate receptors differs between the basolateral amygdala (BLA) and the bed nucleus of the stria terminalis (BNST), with the proportion of postsynaptic localization higher in the BLA; this difference corresponds to the ability to elicit more pronounced postsynaptic responses in the BLA compared to the BNST (Muly et al., 2007). Differences in the subsynaptic localization of the adenosine A2A receptor in the hippocampus and striatum also have been reported, with a highly enriched postsynaptic localization in striatum and presynaptic localization in hippocampus (Rebola et al., 2005). This likely reflects the predominant postsynaptic mechanism through which activation of the adenosine A2A receptor exerts its modulatory effect in the striatum, compared to a presynaptic mechanism in the hippocampus. Together with our data, and as noted in (Rebola et al., 2005), these studies indicate that it is not accurate to extrapolate data concerning receptor localization from one brain region to another or, indeed, from one stage of development to another. Our data also highlight potential differences in receptor localization in vitro and in vivo.
Multiple laboratories have now postulated a role for the Met receptor in excitatory synaptogenesis and plasticity. The specific molecular mechanisms through which Met exerts its modulatory effects, however, remain unknown. In studies focusing on the hippocampus, the localization of Met in the postsynaptic compartment has been highlighted (Kawas et al., 2012; Sharma, 2010; Tyndall and Walikonis, 2006), without reporting potential presynaptic contributions. The Met protein labeling in the neostriatum by corticofugal axons (Judson et al., 2009) raised the clear possibility that Met also is prominently localized presynaptically. The current report presents direct evidence that, even in neocortical and hippocampal regions, the presynaptic contribution of Met is substantial. Thus, to understand the role of Met in synapse development and function, and particularly related to pathogenesis in ASD, it will be critical to define the molecular interacting partners of Met and intracellular signaling cascades initiated in response to HGF pre- and post-synaptically.
Acknowledgments
Other acknowledgements
We thank Ms. Andreina Gonzalez for technical assistance with the electron microscopic immunocytochemical studies, Sara Le Van for technical assistance with the synaptosomal preparations and Western blots, Feng Wang for providing neocortical and hippocampal cultures and Tracey Van Kempen for assistance with the quantitative EM analysis of terminal size.
Grant Sponsor: NIH Grant R01 MH067842 (PL), NIH Grants R01 DA08259, HL 098351 and P01 HL096571 (TAM)
Footnotes
Conflict of interest
The authors state there is no conflict of interest.
Role of authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: K.L.E, T.A.M, P.L. Acquisition of data: K.L.E, T.A.M, Z.X. Analysis and interpretation of data: K.L.E, T.A.M, Z.X., P.L. Drafting of the manuscript: K.L.E. Critical revision of the manuscript for important intellectual content: T.A.M, Z.X., P.L. Statistical analysis: K.L.E. Obtained funding: T.A.M., P.L.
LITERATURE CITED
- Akimoto M, Baba A, Ikeda-Matsuo Y, Yamada MK, Itamura R, Nishiyama N, Ikegaya Y, Matsuki N. Hepatocyte growth factor as an enhancer of nmda currents and synaptic plasticity in the hippocampus. Neuroscience. 2004;128:155–162. doi: 10.1016/j.neuroscience.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Akita H, Takagi N, Ishihara N, Takagi K, Murotomi K, Funakoshi H, Matsumoto K, Nakamura T, Takeo S. Hepatocyte growth factor improves synaptic localization of the NMDA receptor and intracellular signaling after excitotoxic injury in cultured hippocampal neurons. Exp Neurol. 2008;210:83–94. doi: 10.1016/j.expneurol.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Anastasio NC, Lanfranco MF, Bubar MJ, Seitz PK, Stutz SJ, McGinnis AG, Watson CS, Cunningham KA. Serotonin 5-HT2C receptor protein expression is enriched in synaptosomal and post-synaptic compartments of rat cortex. J Neurochem. 2010;113:1504–1515. doi: 10.1111/j.1471-4159.2010.06694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker-Gibb AL, Dougherty KD, Einheber S, Drake CT, Milner TA. Hippocampal tyrosine kinase A receptors are restricted primarily to presynaptic vesicle clusters. J Comp Neurol. 2001;430(2):182–199. doi: 10.1002/1096-9861(20010205)430:2<182::aid-cne1024>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Bian C, Zhu K, Guo Q, Xiong Y, Cai W, Zhang J. Sex differences and synchronous development of steroid receptor coactivator-1 and synaptic proteins in the hippocampus of postnatal female and male C57BL/6 mice. Steroids. 2012;77(1–2):149–156. doi: 10.1016/j.steroids.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Bouvier D, Corera AT, Tremblay ME, Riad M, Chagnon M, Murai KK, Pasquale EB, Fon EA, Doucet G. Pre-synaptic and post-synaptic localization of EphA4 and EphB2 in adult mouse forebrain. J Neurochem. 2008;106:682–695. doi: 10.1111/j.1471-4159.2008.05416.x. [DOI] [PubMed] [Google Scholar]
- Bowers JM, Waddell J, McCarthy MM. A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol. Biol Sex Differ. 2010;1(1):8. doi: 10.1186/2042-6410-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Deutch AY, Colbran RJ. Dopamine depletion alters phosphorylation of striatal proteins in a model of Parkinsonism. Eur J Neurosci. 2005;22(1):247–256. doi: 10.1111/j.1460-9568.2005.04190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, Elia M, Schneider C, Melmed R, Sacco R, Persico AM, Levitt P. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103:16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom LV, Castaneda MT, Reyna T, Hernandez S, Garrido-Sanabria E. Characterization of medial septal glutamatergic neurons and their projection to the hippocampus. Synapse. 2005;58:151–164. doi: 10.1002/syn.20184. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64(1):34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Dawson N, Ferrington L, Olverman HJ, Harmar AJ, Kelly PA. Sex influences the effect of a lifelong increase in serotonin transporter function on cerebral metabolism. J Neurosci Res. 2009;87(10):2375–2385. doi: 10.1002/jnr.22062. [DOI] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson KL, Campbell DB, Thompson BL, Bergman MY, Levitt P. The autism risk genes MET and PLAUR differentially impact cortical development. Autism Res. 2011;4:68–83. doi: 10.1002/aur.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fester L, Prange-Kiel J, Zhou L, Blittersdorf BV, Bohm J, Jarry H, Schumacher M, Rune GM. Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. J Steroid Biochem Mol Biol. 2012;131(1–2):24–29. doi: 10.1016/j.jsbmb.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Finsterwald C, Martin JL. Cellular mechanisms underlying the regulation of dendritic development by hepatocyte growth factor. Eur J Neurosci. 2011;34:1053–1061. doi: 10.1111/j.1460-9568.2011.07839.x. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Huang J, Aicher SA, Milner TA, Pickel VM. Subcellular localization of alpha-2A-adrenergic receptors in the rat medial nucleus tractus solitarius: regional targeting and relationship with catecholamine neurons. J Comp Neurol. 2001;433(2):193–207. doi: 10.1002/cne.1135. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. The hippocampal formation: morphological changes induced by thyroid, gonadal and adrenal hormones. Psychoneuroendocrinology. 1991;16(1–3):67–84. doi: 10.1016/0306-4530(91)90071-z. [DOI] [PubMed] [Google Scholar]
- Gustin RM, Bichell TJ, Bubser M, Daily J, Filonova I, Mrelashvili D, Deutch AY, Colbran RJ, Weeber EJ, Haas KF. Tissue-specific variation of Ube3a protein expression in rodents and in a mouse model of Angelman syndrome. Neurobiol Dis. 2010;39(3):283–291. doi: 10.1016/j.nbd.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick A, Lee Y, Wallace GL, Greenstein D, Clasen L, Giedd JN, Raznahan A. Autism Risk Gene MET Variation and Cortical Thickness in Typically Developing Children and Adolescents. Autism Res. 2012 doi: 10.1002/aur.1256. 10.1002/aur.1256 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettinger BD, Lee A, Linden J, Rosin DL. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol. 2001;431:331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hoshiko M, Arnoux I, Avignone E, Yamamoto N, Audinat E. Deficiency of the Microglial Receptor CX3CR1 Impairs Postnatal Functional Development of Thalamocortical Synapses in the Barrel Cortex. J Neurosci. 2012;32:15106–15111. doi: 10.1523/JNEUROSCI.1167-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. J Neurobiol. 2003;55(2):179–190. doi: 10.1002/neu.10200. [DOI] [PubMed] [Google Scholar]
- Jones BE, Moore RY. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res. 1977;127:25–53. [PubMed] [Google Scholar]
- Judson MC, Amaral DG, Levitt P. Conserved subcortical and divergent cortical expression of proteins encoded by orthologs of the autism risk gene MET. Cereb Cortex. 2011a;21:1613–1626. doi: 10.1093/cercor/bhq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson MC, Bergman MY, Campbell DB, Eagleson KL, Levitt P. Dynamic gene and protein expression patterns of the autism-associated met receptor tyrosine kinase in the developing mouse forebrain. J Comp Neurol. 2009;513:511–531. doi: 10.1002/cne.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson MC, Eagleson KL, Levitt P. A new synaptic player leading to autism risk: Met receptor tyrosine kinase. J Neurodev Disord. 2011b;3:282–292. doi: 10.1007/s11689-011-9081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson MC, Eagleson KL, Wang L, Levitt P. Evidence of cell-nonautonomous changes in dendrite and dendritic spine morphology in the met-signaling-deficient mouse forebrain. J Comp Neurol. 2010;518:4463–4478. doi: 10.1002/cne.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kato T, Funakoshi H, Kadoyama K, Noma S, Kanai M, Ohya-Shimada W, Mizuno S, Doe N, Taniguchi T, Nakamura T. Hepatocyte growth factor overexpression in the nervous system enhances learning and memory performance in mice. J Neurosci Res. 2012;90:1743–1755. doi: 10.1002/jnr.23065. [DOI] [PubMed] [Google Scholar]
- Kawas LH, Benoist CC, Harding JW, Wayman GA, Abu-Lail NI. Nanoscale mapping of the Met receptor on hippocampal neurons by AFM and confocal microscopy. Nanomedicine. 2012 doi: 10.1016/j.nano.2012.08.008. 10.1016/j.nano.2012.08.008 [doi]. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Iwanami A, Nakamura M, Yamane J, Watanabe K, Suzuki Y, Miyazawa D, Shibata S, Funakoshi H, Miyatake S, Coffin RS, Nakamura T, Toyama Y, Okano H. Hepatocyte growth factor promotes endogenous repair and functional recovery after spinal cord injury. J Neurosci Res. 2007;85:2332–2342. doi: 10.1002/jnr.21372. [DOI] [PubMed] [Google Scholar]
- Klausberger T. GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur J Neurosci. 2009;30:947–957. doi: 10.1111/j.1460-9568.2009.06913.x. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Perinatal gonadectomy exerts regionally selective, lateralized effects on the density of axons immunoreactive for tyrosine hydroxylase in the cerebral cortex of adult male rats. J Neurosci. 1998;18(24):10735–10748. doi: 10.1523/JNEUROSCI.18-24-10735.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalive PH, Paglinawan R, Biollaz G, Kappos EA, Leone DP, Malipiero U, Relvas JB, Moransard M, Suter T, Fontana A. TGF-beta-treated microglia induce oligodendrocyte precursor cell chemotaxis through the HGF-c-Met pathway. Eur J Immunol. 2005;35:727–737. doi: 10.1002/eji.200425430. [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol Mood Anxiety Disord. 2012;2(1):3. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Raiker SJ, Venkatesh K, Geary R, Robak LA, Zhang Y, Yeh HH, Shrager P, Giger RJ. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci. 2008;28:2753–2765. doi: 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Serwanski DR, Miralles CP, Fiondella CG, Loturco JJ, Rubio ME, De Blas AL. Synaptic and nonsynaptic localization of protocadherin-gammaC5 in the rat brain. J Comp Neurol. 2010;518:3439–3463. doi: 10.1002/cne.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CS, Walikonis RS. Hepatocyte growth factor and c-Met promote dendritic maturation during hippocampal neuron differentiation via the Akt pathway. Cell Signal. 2008;20:825–835. doi: 10.1016/j.cellsig.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louneva N, Cohen JW, Han LY, Talbot K, Wilson RS, Bennett DA, Trojanowski JQ, Arnold SE. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer's disease. Am J Pathol. 2008;173:1488–1495. doi: 10.2353/ajpath.2008.080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machide M, Kamitori K, Kohsaka S. Hepatocyte growth factor-induced differential activation of phospholipase cgamma 1 and phosphatidylinositol 3-kinase is regulated by tyrosine phosphatase SHP-1 in astrocytes. J Biol Chem. 2000;275:31392–31398. doi: 10.1074/jbc.M002817200. [DOI] [PubMed] [Google Scholar]
- Meller K, Breipohl W, Glees P. Synaptic organization of the molecular and the outer granular layer in the motor cortex in the white mouse during postnatal development. A Golgi- and electronmicroscopical study. Z Zellforsch Mikrosk Anat. 1968;92:217–231. doi: 10.1007/BF00335649. [DOI] [PubMed] [Google Scholar]
- Miller M, Peters A. Maturation of rat visual cortex. II. A combined Golgi-electron microscope study of pyramidal neurons. J Comp Neurol. 1981;203:555–573. doi: 10.1002/cne.902030402. [DOI] [PubMed] [Google Scholar]
- Milner TA, Bacon CE. Ultrastructural localization of tyrosine hydroxylase-like immunoreactivity in the rat hippocampal formation. J Comp Neurol. 1989;281(3):479–495. doi: 10.1002/cne.902810311. [DOI] [PubMed] [Google Scholar]
- Milner TA, Drake CT, Aicher SA. Cellular relations between mu-opioid receptive, GABAergic and reticulospinal neurons in the rostral ventrolateral medulla. Brain Res. 2001a;917(1):1–14. doi: 10.1016/s0006-8993(01)02827-x. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001b;429:355–371. [PubMed] [Google Scholar]
- Milner TA, Waters EM, Robinson DC, Pierce JP. Degenerating processes identified by electron microscopic immunocytochemical methods. Methods Mol Biol. 2011;793:23–59. doi: 10.1007/978-1-61779-328-8_3. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Takase K, Takahashi T, Kimura F. Activational and organisational effects of gonadal steroids on sex-specific acetylcholine release in the dorsal hippocampus. J Neuroendocrinol. 2009;21(4):400–405. doi: 10.1111/j.1365-2826.2009.01848.x. [DOI] [PubMed] [Google Scholar]
- Mondin M, Labrousse V, Hosy E, Heine M, Tessier B, Levet F, Poujol C, Blanchet C, Choquet D, Thoumine O. Neurexin-neuroligin adhesions capture surface-diffusing AMPA receptors through PSD-95 scaffolds. J Neurosci. 31(38):13500–13515. doi: 10.1523/JNEUROSCI.6439-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Halaris AE. Hippocampal innervation by serotonin neurons of the midbrain raphe in the rat. J Comp Neurol. 1975;164:171–183. doi: 10.1002/cne.901640203. [DOI] [PubMed] [Google Scholar]
- Muly EC, Mania I, Guo JD, Rainnie DG. Group II metabotropic glutamate receptors in anxiety circuitry: correspondence of physiological response and subcellular distribution. J Comp Neurol. 2007;505:682–700. doi: 10.1002/cne.21525. [DOI] [PubMed] [Google Scholar]
- Nagayama T, Nagayama M, Kohara S, Kamiguchi H, Shibuya M, Katoh Y, Itoh J, Shinohara Y. Post-ischemic delayed expression of hepatocyte growth factor and c-Met in mouse brain following focal cerebral ischemia. Brain Res. 2004;999:155–166. doi: 10.1016/j.brainres.2003.11.052. [DOI] [PubMed] [Google Scholar]
- Nakano M, Takagi N, Takagi K, Funakoshi H, Matsumoto K, Nakamura T, Takeo S. Hepatocyte growth factor promotes the number of PSD-95 clusters in young hippocampal neurons. Exp Neurol. 2007;207:195–202. doi: 10.1016/j.expneurol.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Needleman LA, Liu XB, El-Sabeawy F, Jones EG, McAllister AK. MHC class I molecules are present both pre- and postsynaptically in the visual cortex during postnatal development and in adulthood. Proc Natl Acad Sci U S A. 2010;107:16999–17004. doi: 10.1073/pnas.1006087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay S, Webster H, editors. The fine structure of the nervous system: neurons and their supporting cells. 3rd Edition. New York: Oxford University Press; 1991. [Google Scholar]
- Phillips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W, Shan WS, Arndt K, Frank M, Gordon RE, Gawinowicz MA, Zhao Y, Colman DR. The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron. 2001;32:63–77. doi: 10.1016/s0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- Qiu S, Aldinger KA, Levitt P. Modeling of autism genetic variations in mice: focusing on synaptic and microcircuit dysfunctions. Dev Neurosci. 2012;34:88–100. doi: 10.1159/000336644. [DOI] [PubMed] [Google Scholar]
- Qiu S, Anderson CT, Levitt P, Shepherd GM. Circuit-specific intracortical hyperconnectivity in mice with deletion of the autism-associated Met receptor tyrosine kinase. J Neurosci. 2011;31:5855–5864. doi: 10.1523/JNEUROSCI.6569-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Canas PM, Oliveira CR, Cunha RA. Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience. 2005;132:893–903. doi: 10.1016/j.neuroscience.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Rebola N, Pinheiro PC, Oliveira CR, Malva JO, Cunha RA. Subcellular localization of adenosine A(1) receptors in nerve terminals and synapses of the rat hippocampus. Brain Res. 2003;987:49–58. doi: 10.1016/s0006-8993(03)03247-5. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Waters EM, McEwen BS. Steroid-induced hippocampal synaptic plasticity: sex differences and similarities. Neuron Glia Biol. 2004;1(3):219–229. doi: 10.1017/S1740925X05000086. [DOI] [PubMed] [Google Scholar]
- Rudie JD, Hernandez LM, Brown JA, Beck-Pancer D, Colich NL, Gorrindo P, Thompson PM, Geschwind DH, Bookheimer SY, Levitt P, Dapretto M. Autism-associated promoter variant in MET impacts functional and structural brain networks. Neuron. 2012;75:904–915. doi: 10.1016/j.neuron.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. Hepatocyte growth factor in synaptic plasticity and Alzheimer's disease. Scientific World Journal. 2010;10:457–461. doi: 10.1100/tsw.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura M, Sato N, Sata M, Wakayama K, Ogihara T, Morishita R. Expression of hepatocyte growth factor and c-Met after spinal cord injury in rats. Brain Res. 2007;1151:188–194. doi: 10.1016/j.brainres.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Yoshida K, Hirose Y, Ishimori H, Katayama M, Kawase T. Cytokines regulate c-Met expression in cultured astrocytes. Brain Res. 2003;962:105–110. doi: 10.1016/s0006-8993(02)03975-6. [DOI] [PubMed] [Google Scholar]
- Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- Spencer-Segal JL, Waters EM, Bath KG, Chao MV, McEwen BS, Milner TA. Distribution of phosphorylated TrkB receptor in the mouse hippocampal formation depends on sex and estrous cycle stage. J Neurosci. 2011;31:6780–6790. doi: 10.1523/JNEUROSCI.0910-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH. Activity-related redistribution of presynaptic proteins at the active zone. Neuroscience. 2006;141:1217–1224. doi: 10.1016/j.neuroscience.2006.04.061. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Roca AL, Crandall JE. Cellular organization in rat somatosensory cortex: effects of sex and laterality. Exp Neurol. 1993;121(1):65–76. doi: 10.1006/exnr.1993.1072. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndall SJ, Patel SJ, Walikonis RS. Hepatocyte growth factor-induced enhancement of dendritic branching is blocked by inhibitors of N-methyl-D-aspartate receptors and calcium/calmodulin-dependent kinases. J Neurosci Res. 2007;85:2343–2351. doi: 10.1002/jnr.21390. [DOI] [PubMed] [Google Scholar]
- Tyndall SJ, Walikonis RS. The receptor tyrosine kinase Met and its ligand hepatocyte growth factor are clustered at excitatory synapses and can enhance clustering of synaptic proteins. Cell Cycle. 2006;5:1560–1568. doi: 10.4161/cc.5.14.2918. [DOI] [PubMed] [Google Scholar]
- Tyndall SJ, Walikonis RS. Signaling by hepatocyte growth factor in neurons is induced by pharmacological stimulation of synaptic activity. Synapse. 2007;61:199–204. doi: 10.1002/syn.20362. [DOI] [PubMed] [Google Scholar]
- Urban-Ciecko J, Mozrzymas JW. Sex-specificity of associative learning-induced changes in GABAergic tonic inhibition in layer 4 neurons of mouse barrel cortex. Behav Brain Res. 2011;219(2):373–377. doi: 10.1016/j.bbr.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Yan H, Rivkees SA. Hepatocyte growth factor stimulates the proliferation and migration of oligodendrocyte precursor cells. J Neurosci Res. 2002;69:597–606. doi: 10.1002/jnr.10323. [DOI] [PubMed] [Google Scholar]
- Zurner M, Mittelstaedt T, tom Dieck S, Becker A, Schoch S. Analyses of the spatiotemporal expression and subcellular localization of liprin-alpha proteins. J Comp Neurol. 2011;519:3019–3039. doi: 10.1002/cne.22664. [DOI] [PubMed] [Google Scholar]