Summary
Mitochondria are dynamic organelles, undergoing both fission and fusion regularly in interphase cells. Mitochondrial fission is thought to be part of a quality control mechanism, whereby damaged mitochondrial components are segregated from healthy components in an individual mitochondrion, followed by mitochondrial fission and degradation of the damaged daughter mitochondrion [1]. Fission also plays a role in apoptosis [2]. Defects in mitochondrial dynamics can lead to neurodegenerative diseases such as Alzheimer’s [3]. Mitochondrial fission requires the dynamin GTPase Drp1, which assembles in a ring around the mitochondrion and appears to constrict both outer and inner mitochondrial membranes [4]. However, mechanisms controlling Drp1 assembly on mammalian mitochondria are unclear. Recent results show that actin polymerization, driven by the endoplasmic reticulum-bound formin protein INF2, stimulates Drp1 assembly at fission sites [5]. Here, we show that myosin II also plays a role in fission. Chemical inhibition by blebbistatin or siRNA-mediated suppression of myosin IIA or myosin IIB causes an increase in mitochondrial length in both control cells and cells expressing constitutively active INF2. Active myosin II accumulates in puncta on mitochondria in an actin- and INF2-dependent manner. In addition, myosin II inhibition decreases Drp1 association with mitochondria. Based on these results, we propose a mechanistic model in which INF2-mediated actin polymerization leads to myosin II recruitment and constriction at the fission site, enhancing subsequent Drp1 accumulation and fission.
Results and Discussion
While Drp1 clearly is essential for mitochondrial fission, several studies have suggested that an initial Drp1-independent constriction step might be required prior to Drp1 accumulation [6–8]. In a previous publication, we presented data that actin polymerization at this site, initiated by INF2 on the ER membrane, provided the force to drive this initial constriction, with the pointed ends of these actin filaments pushing the mitochondrial outer membrane inward [5]. This model bears some resemblance to other polymerization-based force generation events, such as leading edge extension in cell motility or the initial steps of endocytosis [9]. Another actin-based mechanism for force generation, however, is the use of myosin II to constrict anti-parallel actin filament networks [10], such as in muscle contraction, stress fiber contraction [11], and possibly in cytokinesis [12]. Indeed, recent results suggest a role for myosins in mammalian mitochondrial fission, as suppression of myosin regulatory light chain or inhibition of myosin light chain kinase caused increases in mitochondrial length [13]. Here, we address the role of myosin II in this process, and its relationship to INF2 and Drp1.
Myosin II inhibition increases mitochondrial length
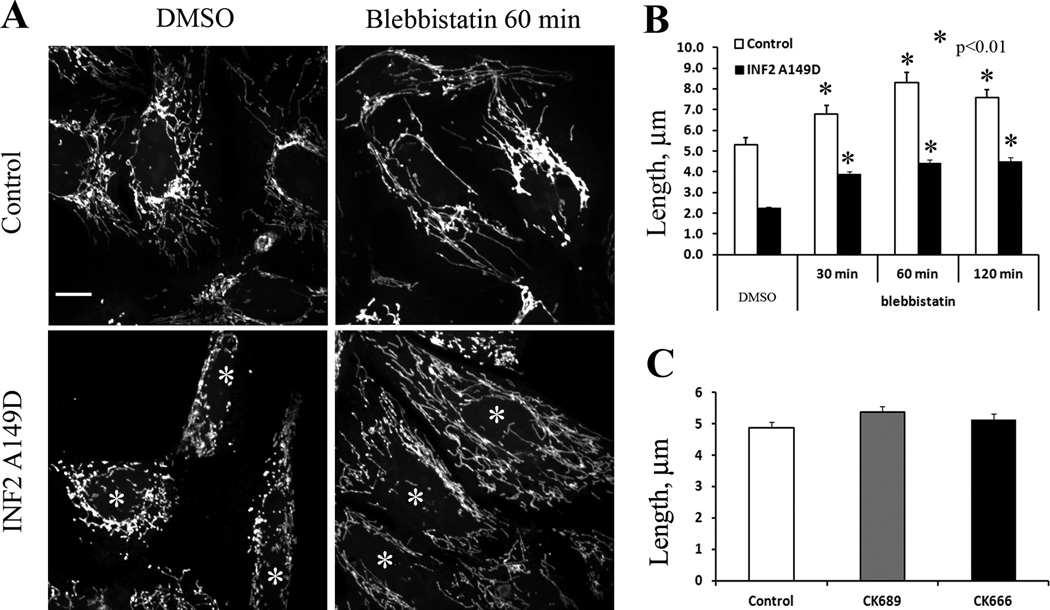
As an initial test of myosin II involvement in mitochondrial dynamics, we used the small molecule myosin II inhibitor, blebbistatin [14] on U2OS human osteosarcoma cells, and measured lengths of peripheral mitochondria. Short-term blebbistatin treatment results in decreased stress fiber density (Figure S1A) and a significant increase in mean mitochondrial length from 5.29 to 6.77, 8.28, and 7.57 µm at 30, 60 and 120 min respectively (Figure 1). This is likely an under-estimate of mitochondrial length change, because many of the mitochondria become too long to measure after blebbistatin treatment, with one extremity being obscured in the peri-nuclear region. Even so, analysis of the histogram of mitochondrial lengths shows a significant increase in the longest mitochondria upon blebbistatin, with the percentage of mitochondria >10 µm increasing from 13.6 to 29.2, 43.2, and 40.3 at the three time points tested (Figure S1B). Interestingly, inhibition of Arp2/3 complex with the small molecule inhibitor CK666 does not affect mitochondrial length (Figure 1C), despite its potent inhibition of lamellipodia under these conditions (Figure S1C, D). This result is significant, because it suggests that INF2 is acting in an Arp2/3-independent manner in producing the actin filaments that serve in mitochondrial fission, making the process different from others in which formins act in concert with Arp2/3 complex [15].
Figure 1. Blebbistatin treatment increases mitochondria length in U2OS cells.
(A) Control U2OS cells (top) or cells expressing GFP-INF2 A149D (bottom) were treated with DMSO (left) or 50 µM blebbistatin (right) for 60 min, then stained with Mitotracker. Asterisks indicate cells expressing INF2 A149D (See Figure S1 for GFP and actin staining). Scale bar, 10 µm.
(B) Quantification of mitochondrial length in control or GFP-INF2 A149D expressing cells, treated with DMSO or 50 µM blebbistatin for indicated times. N=103 to 344 mitochondria. Asterisks denote p values of < 0.01 by Student’s T-test. Error bars, SEM.
(C) Quantification of mitochondial length in control cells and cells treated with 200 µM CK689 (negative control compound) or CK666 (Arp2/3 complex inhibitor) for 60 min. N=223 to 289 mitochondria. Error bars, SEM.
We have previously shown that the constitutively active A149D mutant of INF2-CAAX (INF2 A149D) causes a significant mitochondrial length decrease and enriches at fission sites [5]. To test whether myosin II inhibition reverses this effect, we treated INF2-A149D-transfected U2OS cells with blebbistatin and measured peripheral mitochondrial length. As observed previously, INF2-A149D expression decreases mitochondrial length by > 2-fold (Figure 1B). Blebbistatin treatment partially reverses this effect, with mitochondrial lengths of 2.18, 3.87, 4.42 and 4.49 µm at 0, 30, 60 and 120 min respectively (Fig 1A, B). The percentage of mitochondria >10 µm increases from 0% (INF2-A149D alone) to 7.3, 10.1 and 11.8% for 30, 60, and 120 min respectively.
While blebbistatin displays specificity for myosin II over three other myosin classes (l, V and X, [14]), most of the 20+ classes of mammalian myosins have not been tested for blebbistatin sensitivity, raising the question of whether myosin II is the target for blebbistatin’s effect on mitochondrial length. For this reason, we also used an RNAi approach to suppress expression of the two predominant non-muscle myosin II proteins, IIA and IIB, in U2OS cells. Treatment with specific siRNA oligonucleotides results in a >80% reduction in the targeted myosin II, with no significant effect on levels of the other myosin II or on two other myosins (Figure S2).
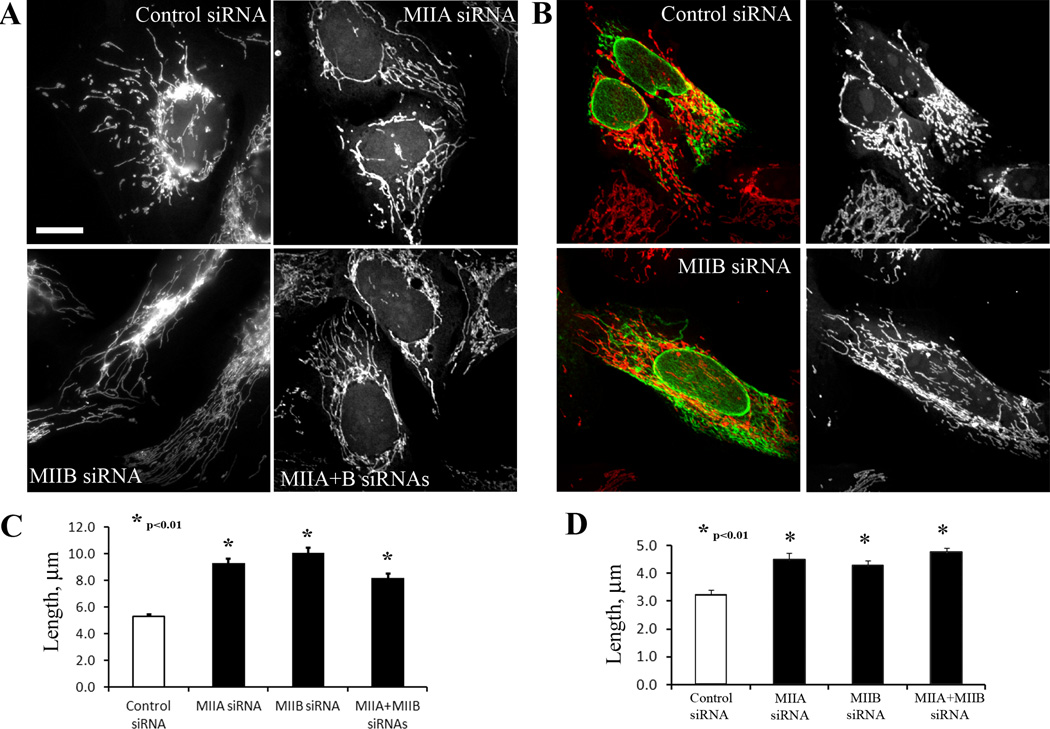
Suppression of either myosin IIA or IIB results in a significant increase in mitochondrial length, from 5.21 µm to 9.46 and 9.15 µm for IIA or IIB respectively (Figure 2A, C). In addition, suppression of MIIA or MIIB partially reverses the effect of INF2-A149D expression in U2OS cells (Figure 2B, D), increasing average length by 37% and 31%, respectively. Interestingly, combined suppression of MIIA and MIIB does not cause an additive increase in mitochondrial length above that caused by suppression of either myosin individually (Figure 2B, D). However, limitations in measuring long mitochondria, described above, might hinder detection of such length changes. These results suggest that myosin II plays a role in mitochondrial fission, possibly utilizing the actin filaments generated by INF2.
Figure 2. Depletion of myosin IIA or IIB increases mitochondrial length.
(A) Mitotracker staining of U2OS cells expressing indicated siRNAs. Scale bar, 10 µm.
(B) U2OS cells expressing control or myosin IIB siRNA were transfected with GFPINF2 A149D (green) and stained with mitotracker (red). Mitochondria are alone also shown separately in the right panels.
(C) and (D) Quantification of mitochondrial lengths for experiments depicted in A and B, respectively. Data from three experiments, n = 261 to 368 mitochondria for C, and 329 to 407 mitochondria for D. Error bars, SEM.
Localization of myosin II to fission sites
We next asked whether myosin II enriched at mitochondrial fission sites, initially conducting immunofluorescence localization of endogenous protein. One issue with analyzing myosin II localization is protein abundance, causing extensive staining throughout the cell and being particularly intense on stress fibers. In this respect, we found that an antibody against serine 19-phosphorylated (activated) myosin light chain (anti-P-MRLC) gave the lowest background staining, thus was most suitable for examining mitochondrial localization against a high background of stress fibers. Anti-P-MRLC staining is detected at sites of mitochondrial constriction (Figure 3A, B). In cells co-stained for P-MRLC, mitochondria, and ER, P-MRLC accumulates at constriction sites also enriched for ER (Figure 3A).
Figure 3. Myosin II enriches at mitochondrial constriction sites.
(A) U2OS cell transfected with mito-BFP and ER-green, then fixed and stained with anti-P-MRLC (myosin regulatory light chain, phosphoserine 19). Arrowhead shows mitochondrial constriction site. Scale bar, 10 µm.
(B) U2OS cells labeled with mitotracker (red), then fixed, stained with anti-PMRLC (green), and imaged by spinning disc confocal microscopy with 5–7 z-sections taken (200 nm z steps). Arrowheads denote constriction sites. Two examples shown. For each, left is maximum intensity image and right is 3D reconstruction. Scale bar, 2 µm.
(C) U2OS cell transfected with mito-dsRed and GFP-MIIA, then imaged live by confocal microscopy (single Z-plane). Arrow points to MIIA accumulation at fission site. Scale bar, 2 µm. See also Movie S1.
(D) Single confocal slices of “apical” mitochondria, located adjacent to the nucleus at > 1.4 µm from the ventral surface of the cell, avoiding interference from abundant ventral stress fibers. U2OS cells labeled with mitotracker (red), then fixed and stained with anti-P-MRLC (green). Cells either untreated (left), transfected with siRNA oligos against INF2 for 72 hrs (middle), or treated with 0.5 µM Latrunculin B for 60 min (right). Scale bar, 20 µm. See Figure S3 for quantification of mitochondria-associated P-MRLC puncta upon Myosin II and mitochondrial fission these treatments, as well as close-up image of apical mitochondria, P-MRLC, and actin filaments.
We also examined myosin II localization to mitochondria in live cells, using a GFP-fusion of myosin IIA expressed on a low-expressing “speckle” promoter, since low expression was found to be important in limiting artifactual myosin II-containing structures in previous studies [16]. Despite the confounding influence of extensive accumulation on stress fibers, GFP-myosin IIA accumulates transiently at mitochondrial constriction sites undergoing fission (Figure 3C, movie S1).
In another strategy to examine myosin II enrichment on mitochondria, we used confocal microscopy to examine more apical regions, avoiding the ventral surface that contains a high density of acto-myosin-based structures. The peri-nuclear region contains abundant mitochondria and is relatively devoid of stress fibers. Though their lengths are difficult to quantify due to their high density in this location, these mitochondria appear generally shorter than peripheral mitochondria, similar to observations made in other cells [17]. Anti-P-MRLC puncta enrich on these mitochondria (Figure 3D, Figure S3A). Both actin depolymerization by Latrunculin B treatment and INF2 inhibition by siRNA cause significant decreases in the number of these puncta (Figure 3D, Figure S3B). Taken together, these results suggest that INF2-assembled actin filaments are required for active myosin II association with mitochondria.
Myosin II inhibition results in decreased mitochondrially-associated Drp1
We have previously shown that INF2 suppression results in decreased mitochondrially-associated Drp1, suggesting that INF2 acts up-stream of Drp1 [5]. To test whether myosin II acts at a similar stage in the process, we examined Drp1 distribution after myosin II suppression. In control cells, Drp1 accumulates as puncta on the mitochondrial surface (Figure 4A). Suppression of either myosin IIA or myosin IIB reduces the number of mitochondrially-associated Drp1 puncta significantly (Figure 4). Previous results show that myosin regulatory light chain (MRLC) inhibition, either by siRNA suppression or by an MLCK inhibitor, increases mitochondrial length and decreases mitochondrially-associated Drp1 puncta [13]. Since MRLC interacts with several classes of myosin ([18], J. Sellers, personal communication), it was not clear which myosin was the relevant motor. Our work shows that myosin II specifically plays a role in this process.
Figure 4. Localization of DRP1 to mitochondria is reduced upon myosin II suppression.
(A) U2OS cells transfected with control, MIIA or MIIB siRNAs were treated with mitotracker (red), then fixed and immunostained for DRP1 (green). Zoomed regions shown on bottom row. Scale bar, 10 µm.
B) Quantification of mitochondrial-associated DRP1 puncta in control and myosin II suppressed cells. Data from two experiments, n= 87 to 110 mitochondria. Error bars, SEM.
Based on these results, we propose an alternative to our previous actin polymerization-based model for initial mitochondrial constriction (Figure S4). INF2 activation at the ER-mitochondrial interface results in actin filament assembly. These filaments assume mixed orientations around the mitochondrion. Myosin II mini-filaments are recruited to these actin filaments, and their motor activity results in contraction of the actin filament network either parallel or perpendicular to the fission plane, constricting the mitochondrion. This initial constriction allows Drp1 to bind and oligomerize at the constriction site, as such a pre-constriction event has been suggested by previous findings [6–8]. Another possibility is that actin and/or myosin II might help recruit Drp1 to the fission site by direct binding, perhaps in conjunction with one of the outer mitochondrial membrane proteins purported to act as a Drp1 receptor[19, 20]. Such binding has been suggested by cellular experiments[13], and is consistent with direct binding between dynamin and actin filaments[21].
The myosin II might be recruited from another structure. We do not think that this structure would be existing dorsal or ventral stress fibers, since serum starvation in DMEM, which strongly reduces these structures, actually causes mitochondria to become shorter (data not shown), and since previous reports fail to detect any association between mitochondria and stress fibers[22]. Still, other myosin II-based structures, such as cortical acto-myosin[23], might contribute.
There are parallels between this model and assembly of other contractile bundles requiring both a formin and myosin II, such as cytokinetic ring assembly [12, 24]. Based on these similarities, we refer to the process of INF2- and myosin II-mediated mitochondrial constriction as “mitokinesis”. It is possible that myosin II is not required for mitochondrial constriction per se but for organization and stabilization of the ring structure, similar to some models of cytokinesis [25–27]. One clear difference between this process and cytokinesis is that myosin recruitments to mitochondria depends upon actin filaments and formin, whereas myosin II is recruited to the site of cytokinesis independently of actin where tested[28–30].
It might seem curious that both MIIA and MIIB appear to play roles in mitochondrial fission, but a series of studies shows that the two myosins also play complementary roles in acto-myosin fiber assembly during fibroblast polarization [16]. MIIA is the more dynamic protein, assembling into short acto-myosin structures early in polarization, while MIIB stabilizes these structures at later timepoints. Studies with genetically-modified mice suggest that MIIA can replace some but not all physiological functions of MIIB [31]. Thus, it is possible that MIIA and MIIB play partially redundant roles also in mitochondrial fission, perhaps forming hetero-oligomers within the same mini-filament. Alternately, it is possible that there are separate pools of MIIA- and MIIB-dependent fission events, but this seems less likely, particularly since simultaneous suppression of both proteins does not result in measurably longer mitochondria.
However, it is worth remembering that mitochondrial populations are heterogeneous, both within individual cell types and between cell types [17]. There might also be multiple mechanisms for inducing mitochondrial fission, although all are likely to go through Drp1 ultimately. Our results suggest that myosin II and INF2 act in the same fission mechanism, but we do not discount the fact that other mechanisms, independent of myosin II, INF2, and actin, might also exist for this important process.
Finally, we do not rule out other configurations of actin filaments and myosin that might produce similar constrictive forces. One issue that might compromise a cytokinetic-like acto-myosin ring during mitochondrial fission is that the purified non-muscle myosin II mini-filament has been measured at between 230 and 320 nm in length[32, 33], about the same size as the width of a mammalian mitochondrion. However, considerably smaller myosin II oligomers appear to exist in cells[34].
Myosin II involvement in mitochondrial fission is the latest in a growing relationship between myosins and mitochondrial dynamics in general. Myosin V is required for proper mitochondrial distribution in budding yeast, although there is vigorous debate as to whether this myosin acts to transport mitochondria along actin cables [35] or acts as a mitochondrial retention factor [36]. Interestingly, myosin V depletion actually stimulates axonal mitochondrial motility (both anterograde and retrograde) in Drosophila neurons, while myosin VI depletion stimulates only retrograde motility [37]. In the same system, myosin V depletion (but not that of myosin VI or myosin II) increases mitochondrial length, possibly suggesting a role for myosin V in mitochondrial fission in axonal mitochondria. Another widely expressed myosin, myosin 19, enriches strongly on mitochondria and acts in mitochondrial motility in cultured cells, although there is no evidence that it acts in mitochondrial fission [38]. Finally, myosin II has been implicated in maintenance of the mitochondrial genome, with the suggestion that both actin and myosin II might be present in the mitochondrial matrix [39].
The examples described above highlight the potential for multiple mechanisms controlling mitochondrial dynamics. For example, the effects of myosin V on mitochondrial size in Drosophila axons [37] might reflect a fundamentally different fission mechanism than that observed in this paper. The possibility of multiple mechanisms will be useful to keep in mind in the continued elucidation of fission pathways.
Experimental Procedures
Plasmids and siRNA oligonucleotides
The human full length GFP-INF2 CAAX A149D construct was described previously [5]. The ER-green construct, containing the ER-targeting sequence (amino acids 233–250) of budding yeast UBC6 [40], was a gift from Victoria Allan (University of Manchester, Manchester, United Kingdom). Further details are in Supplementary Information.
Cell culture, transfections and drug treatment
U2OS cell lines were grown in DMEM (Invitrogen) supplemented with 10% calf serum (Atlanta Biologicals). Plasmid transfections were performed in OPTI-MEM media (Invitrogen) with Lipofectamine 2000 (Invitrogen). siRNA transfections used RNAmax (Invitrogen). Cells were analyzed 24 hr and 72–80 hr post-transfection for DNA and RNAi respectively. Cells were treated with MitoTracker Red CMXRos (Invitrogen) at 100 nM in DMEM 20 min prior to fixation. Chemical inhibitor treatments were 50 µM blebbistatin (Sigma-Aldrich), 200 µM CK666 or CK689 (Calbiochem), or 0.5 µM Latrunculin B (Calbiochem). Further details are in Supplementary Information.
Antibodies
Antibodies against nonmuscle myosin IIA, myosin IIB, P-MRLC, and Drp1 were from Cell Signaling. Monoclonal anti-tubulin DM1-α was from Sigma. Secondary antibodies used were TexasRed, Cy5 or Fluorescein conjugated anti-rabbit IgG, (Jackson Immunoresearch and Vector Laboratories, respectively). Further details are in Supplementary Information.
Immunofluorescence Microscopy
Cells were fixed with 4% formaldehyde (Electron Microscopy Sciences, PA) in phosphate-buffered saline (PBS) for 30 min at room temperature prior to primary and secondary antibody treatment. When needed, AlexaFluor450-phalloidin (Invitrogen), TRITC-phalloidin (Sigma/Aldrich), and/or 4,6-diamidino-2-phenylindole (DAPI) were added to secondary antibody solution. Samples were mount on polyvinyl alcohol-DABCO. Further details are in Supplementary Information.
Live imaging and confocal microscopy
Imaging of live and fixed cells was performed using a temperature-controlled spinning disk confocal system from Quorum Technologies, Inc. (Guelph, Canada) on a Nikon Eclipse Ti microscope. Images and movies were processed using Nikon Elements and Photoshop CS (Adobe, San Jose, CA). Further details are in Supplementary Information.
Measurements and image analysis
To measure mitochondrial length, maximum intensity projections of z-series with 0.2 µm increments for red channel (Mitotracker or mito-dsRed) were created. The flat regions of cells with clearly resolved mitochondria were selected, and 25–30 mitochondria per cell were measured using the line tool in Nikon Elements software. Drp1 puncta were counted on fixed cells labeled with anti-Drp1 antibody. To assess P-MRLC localization at sites of apical mitochondria, a representative confocal slice at least 1.4 microns from the ventral surface was chosen, and a 5 × 5 micron box within the region containing apical mitochondria was made. The number of P-MRLC puncta within this box was Myosin II and mitochondrial fission counted manually, with a positive punctum defined as a region of < 5 pixels in either dimension whose intensity was clearly above background. Statistic analysis was performed in Excel (Microsoft), data presented as mean ± standard error from at least two experiments. Unpaired Student’s t-test was used to compare values with p<0.01 considered significant. Further details are in Supplementary Information.
Supplementary Material
Highlights.
-
-
myosin II inhibition by blebbistatin or siRNA increases mitochondrial length
-
-
myosin II acts in the same fission pathway as INF2 and Drp1
-
-
myosin II accumulation on mitochondria depends on INF2-mediated actin polymerization
-
-
Short-term Arp2/3 complex inhibition does not affect mitochondrial size
Acknowledgments
We thank Vinay Ramabhadran for advice and reagent preparation, Rick Horwitz for providing the GFP-myosin IIA expression plasmid, Tatyana Svitkina for several useful reagents, and Amali Namm for her highly evolved brain. This work was funded by grants from the National Institutes of Health (GM069818 and DK088826). Myosin II and mitochondrial fission
References
- 1.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoppins S, Nunnari J. Cell Biology. Mitochondrial dynamics and apoptosis--the ER connection. Science. 2012;337:1052–1054. doi: 10.1126/science.1224709. [DOI] [PubMed] [Google Scholar]
- 3.DuBoff B, Feany M, Gotz J. Why size matters - balancing mitochondrial dynamics in Alzheimer's disease. Trends Neurosci. 2013;36:325–335. doi: 10.1016/j.tins.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci. 2010;1201:34–39. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science. 2013;339:464–467. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell. 1999;4:815–826. doi: 10.1016/s1097-2765(00)80391-3. [DOI] [PubMed] [Google Scholar]
- 7.Legesse-Miller A, Massol RH, Kirchhausen T. Constriction and Dnm1p recruitment are distinct processes in mitochondrial fission. Mol Biol Cell. 2003;14:1953–1963. doi: 10.1091/mbc.E02-10-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Sun Y, Oster GF, Drubin DG. Mechanochemical crosstalk during endocytic vesicle formation. Curr Opin Cell Biol. 2010;22:36–43. doi: 10.1016/j.ceb.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reymann AC, Boujemaa-Paterski R, Martiel JL, Guerin C, Cao W, Chin HF, De La Cruz EM, Thery M, Blanchoin L. Actin network architecture can determine myosin motor activity. Science. 2012;336:1310–1314. doi: 10.1126/science.1221708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellegrin S, Mellor H. Actin stress fibres. J Cell Sci. 2007;120:3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- 12.Balasubramanian MK, Srinivasan R, Huang Y, Ng KH. Comparing contractile apparatus-driven cytokinesis mechanisms across kingdoms. Cytoskeleton (Hoboken) 2012;69:942–956. doi: 10.1002/cm.21082. [DOI] [PubMed] [Google Scholar]
- 13.Duboff B, Gotz J, Feany MB. Tau Promotes Neurodegeneration via DRP1 Mislocalization In Vivo. Neuron. 2012;75:618–632. doi: 10.1016/j.neuron.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 15.Block J, Breitsprecher D, Kuhn S, Winterhoff M, Kage F, Geffers R, Duwe P, Rohn JL, Baum B, Brakebusch C, et al. FMNL2 drives actin-based protrusion and migration downstream of Cdc42. Curr Biol. 2012;22:1005–1012. doi: 10.1016/j.cub.2012.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vicente-Manzanares M, Newell-Litwa K, Bachir AI, Whitmore LA, Horwitz AR. Myosin IIA/IIB restrict adhesive and protrusive signaling to generate front-back polarity in migrating cells. J Cell Biol. 2011;193:381–396. doi: 10.1083/jcb.201012159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins TJ, Berridge MJ, Lipp P, Bootman MD. Mitochondria are morphologically and functionally heterogeneous within cells. Embo J. 2002;21:1616–1627. doi: 10.1093/emboj/21.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzik-Lendrum S, Heissler SM, Billington N, Takagi Y, Yang Y, Knight PJ, Homsher E, Sellers JR. Mammalian myosin-18A, a highly divergent myosin. J Biol Chem. 2013;288:9532–9548. doi: 10.1074/jbc.M112.441238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2008;19:2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer CS, Elgass KD, Parton RG, Osellame LD, Stojanovski D, Ryan MT. Adaptor Proteins MiD49 and MiD51 Can Act Independently of Mff and Fis1 in Drp1 Recruitment and Are Specific for Mitochondrial Fission. J Biol Chem. 2013;288:27584–27593. doi: 10.1074/jbc.M113.479873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu C, Yaddanapudi S, Weins A, Osborn T, Reiser J, Pollak M, Hartwig J, Sever S. Direct dynamin-actin interactions regulate the actin cytoskeleton. Embo J. 2010;29:3593–3606. doi: 10.1038/emboj.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minin AA, Kulik AV, Gyoeva FK, Li Y, Goshima G, Gelfand VI. Regulation of mitochondria distribution by RhoA and formins. J Cell Sci. 2006;119:659–670. doi: 10.1242/jcs.02762. [DOI] [PubMed] [Google Scholar]
- 23.Salbreux G, Charras G, Paluch E. Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 2012;22:536–545. doi: 10.1016/j.tcb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Wu JQ, Sirotkin V, Kovar DR, Lord M, Beltzner CC, Kuhn JR, Pollard TD. Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast. J Cell Biol. 2006;174:391–402. doi: 10.1083/jcb.200602032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proctor SA, Minc N, Boudaoud A, Chang F. Contributions of turgor pressure, the contractile ring, and septum assembly to forces in cytokinesis in fission yeast. Curr Biol. 2012;22:1601–1608. doi: 10.1016/j.cub.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma X, Kovacs M, Conti MA, Wang A, Zhang Y, Sellers JR, Adelstein RS. Nonmuscle myosin II exerts tension but does not translocate actin in vertebrate cytokinesis. Proc Natl Acad Sci U S A. 2012;109:4509–4514. doi: 10.1073/pnas.1116268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lord M, Laves E, Pollard TD. Cytokinesis depends on the motor domains of myosin-II in fission yeast but not in budding yeast. Mol Biol Cell. 2005;16:5346–5355. doi: 10.1091/mbc.E05-07-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, Yeh E, Pringle JR. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J Cell Biol. 1998;142:1301–1312. doi: 10.1083/jcb.142.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lippincott J, Li R. Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J Cell Biol. 1998;140:355–366. doi: 10.1083/jcb.140.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naqvi NI, Eng K, Gould KL, Balasubramanian MK. Evidence for F-actin-dependent and -independent mechanisms involved in assembly and stability of the medial actomyosin ring in fission yeast. Embo J. 1999;18:854–862. doi: 10.1093/emboj/18.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang A, Ma X, Conti MA, Adelstein RS. Distinct and redundant roles of the non-muscle myosin II isoforms and functional domains. Biochemical Society transactions. 2011;39:1131–1135. doi: 10.1042/BST0391131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niederman R, Pollard TD. Human platelet myosin. II. In vitro assembly and structure of myosin filaments. J Cell Biol. 1975;67:72–92. doi: 10.1083/jcb.67.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinard JH, Stafford WF, Pollard TD. The mechanism of assembly of Acanthamoeba myosin-II minifilaments: minifilaments assemble by three successive dimerization steps. J Cell Biol. 1989;109:1537–1547. doi: 10.1083/jcb.109.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shutova M, Yang C, Vasiliev JM, Svitkina T. Functions of nonmuscle myosin II in assembly of the cellular contractile system. PLoS One. 2012;7:e40814. doi: 10.1371/journal.pone.0040814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fortsch J, Hummel E, Krist M, Westermann B. The myosin-related motor protein Myo2 is an essential mediator of bud-directed mitochondrial movement in yeast. J Cell Biol. 2011;194:473–488. doi: 10.1083/jcb.201012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boldogh IR, Pon LA. Mitochondria on the move. Trends Cell Biol. 2007;17:502–510. doi: 10.1016/j.tcb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Pathak D, Sepp KJ, Hollenbeck PJ. Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J Neurosci. 2010;30:8984–8992. doi: 10.1523/JNEUROSCI.1621-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quintero OA, DiVito MM, Adikes RC, Kortan MB, Case LB, Lier AJ, Panaretos NS, Slater SQ, Rengarajan M, Feliu M, et al. Human Myo19 is a novel myosin that associates with mitochondria. Curr Biol. 2009;19:2008–2013. doi: 10.1016/j.cub.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reyes A, He J, Mao CC, Bailey LJ, Di Re M, Sembongi H, Kazak L, Dzionek K, Holmes JB, Cluett TJ, et al. Actin and myosin contribute to mammalian mitochondrial DNA maintenance. Nucleic Acids Res. 2011;39:5098–5108. doi: 10.1093/nar/gkr052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wozniak MJ, Bola B, Brownhill K, Yang YC, Levakova V, Allan VJ. Role of kinesin-1 and cytoplasmic dynein in endoplasmic reticulum movement in VERO cells. J Cell Sci. 2009;122:1979–1989. doi: 10.1242/jcs.041962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.